Abstract
The effects of flow on endothelial cells have been widely examined for the ability of fluid shear stress to alter cell morphology and function; however, the effects of endothelial cell morphology without flow have only recently been observed. An increase in lithographic techniques in cell culture spurred a corresponding increase in research aiming to confine cell morphology. These studies lead to a better understanding of how morphology and cytoskeletal configuration affect the structure and function of the cells. This review examines endothelial cell micropatterning research by exploring both the many alternative methods used to alter endothelial cell morphology and the resulting changes in cellular shape and phenotype. Micropatterning induced changes in endothelial cell proliferation, apoptosis, cytoskeletal organization, mechanical properties, and cell functionality. Finally, the ways these cellular manipulation techniques have been applied to biomedical engineering research, including angiogenesis, cell migration, and tissue engineering, is discussed.
Keywords: Endothelial cell, micropatterning, cytoskeleton, tissue engineering, angiogenesis
Introduction
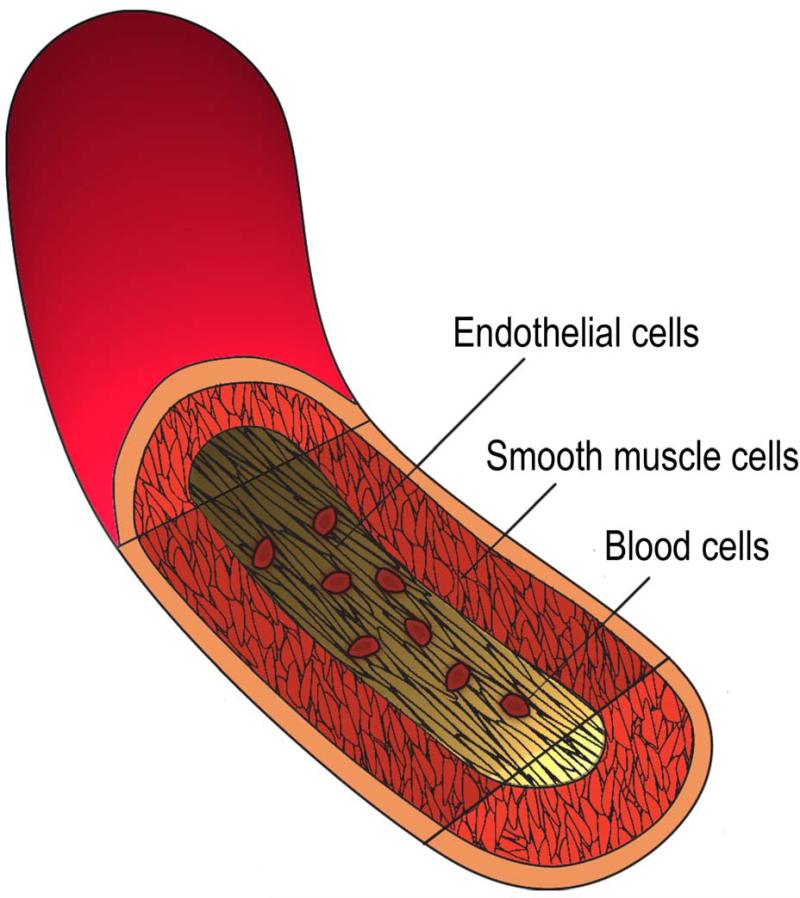
Endothelial cells (ECs) are a critical component of blood vessels, functioning at the interface between the constituents of the blood and the vessel wall (see Fig. 1). The body's maintenance of a healthy endothelial layer is a critical aspect of avoiding vascular disease. Atherosclerosis and thrombosis have been linked to endothelial cell injury or dysfunction.89 These pathologies can cause vessel blockage, which can lead to heart attack or stroke. The most recent data from the American Heart Association reported that cardiovascular disease accounted for 33.6% of all deaths in the United States in 2007.71 With this high prevalence, the importance of understanding EC function (or dysfunction) has never been more critical.
Fig 1.
Diagram of the basic anatomy of the artery illustrating the primary cell types that interface with the endothelial layer.
A large body of work has demonstrated that EC morphology and phenotype are dramatically altered under fluid shear stress. Under unidirectional flow, endothelial cells exhibit an elongated morphology which corresponds with a healthy, atheroprotective phenotype, while statically-cultured cells or cells stimulated by disturbed flow exhibit a cobblestone morphology and a more thrombogenic and inflammatory phenotype. While these changes are well documented, the effects of the cells’ morphological changes in the absence of flow have only recently been elucidated. Encouraging cells into a specific morphology can be technically challenging, but the application of lithographic techniques in biology encouraged cell micropatterning, which has lead to a better understanding of how morphology alone alters cell phenotype. Soft lithography, however, is not the only method that has been used for altering EC morphology. Other methods include bioprinting and photopolymerization. Using these techniques, micropatterning of endothelial cells causes changes in the cytoskeleton and cellular morphology. In addition to altering cytoskeletal features, endothelial cell alignment can change cell phenotype, specifically proliferation, apoptosis, cell migration, and functionality.
The application of some of these techniques increases the interest in utilizing micropatterning for biomaterial design and clinical applications. Researchers used micropatterning to study cell migration and cell-cell interactions. Attempts were also made to use micropatterning to improve the angiogenic potential of cells, build tissue engineered constructs, and improve biomaterials. While many of these applications are in the early stages of development, they represent novel ways for improving current understanding and treatment options for cardiovascular health.
1. Methods of alignment
Table 1 compares the more common methods for micropatterning endothelial cells and lists some of the advantages and disadvantages seen with these techniques. Many of the methods have been and can be altered for an experimenter's specific desires, which will be discussed in detail in the following sections. While soft lithography is perhaps the most common method for aligning ECs, this term encompasses a large and varied set of work. The idea of using polymer molds from photo-patterned wafers has been expanded dramatically. Other methods, such as direct photopolymerization or photodecomposition, also encompass a large variety of work. For this reason we examine the micropatterning methods in two groups: those that physically confine the cells to wells or channels and those that pattern biochemical layers to create a cytophilic and cytophobic surface on which cell growth and migration is limited.
Table 1.
Comparison of micropatterning techniques used with endothelial cells
| General method | References | Approx. resolution | Advantages | Disadvantages |
|---|---|---|---|---|
| Soft lithography | Many1,5-7,10,13,17,19-23,25,27,28,30,31,39,42,45,46,51,53,54,63,66-69,71,76-86,88 | 1-2μm | Inexpensive (after mask is created) High resolution Readily available Commonly used Option for topographical or biochemical features |
Repeatability Difficult to adapt to large, 3D surfaces Difficult and time consuming to do complex patterns (requires multiple layers/applications) |
| Photochemistry | Many2,3,8,16,18,33-38,40,43,47,48,50,55-58,62,65,70,74,92 | 2-8μm | Very flexible Reproducible High resolution Large surface area “All-in-one” systems exist |
Often uses specialized equipment/set up Requires photosensitive material Difficult to avoid some topographical features (on the order of tens of nm) |
| Inkjet printing | Gauvreau et al.26 | 30-100μm | Computer-aided so it is flexible and reproducible Simple, inexpensive instrumentation Fast Can use multiple biomolecules at one time |
Low resolution Difficult to adapt to large, 3D surfaces |
| Laser bioprinting | Guillemot et al.29 | 1-5μm | Flexible, complex patterns Reproducible Fast Can pattern cells and biomaterials |
Specialized system Expensive |
| Laser guided direct writing | Nahmias et al.59 | 10μm | Can directly pattern cells Uses arbitrary patterns and surfaces (including gels) |
Requires special instrumentation Expensive Slow Requires round 3D structures (cells, beads, etc) |
| Microscale direct writing | Huang et al.32 | 6-9μm | Precise computer control Large area Multiple components Multiple patterns of varying size and shape |
Requires special, expensive instrumentation (AFM) Slow Best for circular and repeating designs Patterns based on dots--max diameter of 60μm |
a. Topographical cell restriction
i. Lithographic techniques
Standard lithographic techniques have been used to create design masters on silicon13,46,66 or chrome81 wafers. The most basic application of these techniques seeded cells directly into the silicon master.46 While the goal was to observe EC migration toward a chemoattractive agent, the cells’ survival throughout seven day experiment suggests the potential of this method for other applications. A more common approach to micropattern with lithographic techniques used a silicon wafer as a mold for soft polymers, gels, or occasionally more rigid polymer materials. Polyurethane (PU),13 poly-L-lactic acid (PLA),66 poly-DL-lactic acid,66 and polycaprolatone66 were each cast on silicon wafer molds, allowed to polymerize, and removed, resulting in channels of dimensions around or greater than the size of the cells. Once seeded, the ECs were primarily observed within the channels of the polymers. A three-dimensional construct was created by clamping multiple “sheets” from the mold technique or by rolling a single sheet, although cells were not tested on either of these construct designs.66
One of the most common mold materials used for the topographical restriction of ECs with soft lithography is polydimethylsiloxane (PDMS).22,78-80 The PDMS, like other polymers, can be formed into a large variety of geometries and dimensions and can be protein-coated to enhance cell attachment.22,78,80 The PDMS was also modified to exhibit a variety of elastic moduli to study the influence of stiffness on cells.22 In 2010, the PDMS mold method was applied to a three-dimensional approach. PU was electrospun onto a rotating cylinder covered with the patterned PDMS mold, which resulted in a vessel-shaped construct with grooves on the interior surface.79
PDMS molds were also used to confine ECs within a gel. Collagen type I and ECs were gelled together in 100μm PDMS channels.68 Similarly, 1mm PDMS channels were injected with cells suspended in a thrombin solution. Mixing the cell solution with fibrinogen resulted in a fibrin matrix. The distance between channels (0.5, 1, or 2mm) allowed for different cell types, including stem cells and human umbilical vein endothelial cells (HUVECs), to be put in different channels. Solidified cell channels were then surrounded with fibrin.77
Using the PDMS as masters to mold other materials has the advantage of preserving the original silicon wafer master, which can lose its features after many repeated uses. Gao et al.25 used this approach to create a gelatin mold. Then they used a flat PDMS layer to stamp PEG-PLA to the mold ridges to discourage EC attachment outside of the gelatin channels. Wang and Ho85 created chitosan gel and gelatin molds using this method, and similarly used a flat PDMS stamp to apply Pluronic® (a product composed of block copolymers of ethylene oxide and propylene oxide) to the ridges. The PDMS master technique was applied in a slightly different way to create an agarose mold. PDMS channels were placed on lysine-coated glass. Then, agarose was poured around and through the PDMS. Upon hardening of the agarose and removal of the PDMS, the resulting pattern had a cytophilic surface (the coated glass) surrounded by cytophobic agarose.75 This approach was also used to create islands for single cells.27,62
Janakiraman et al.39 modified the basic soft lithography technique in a unique way by coating a collagen/gylcosaminoglycan (specifically chondroitin 6-sulfate) gel with acetic acid. A patterned silicon wafer was placed on top of the gel and weighted down to imprint the pattern into the partially dissolved gel. Gluteraldehyde solution was then used to crosslink and solidify the gel. The imprinted pattern allowed for complex patterns with 2-3μm resolution. The authors argued that the biodegradability and resulting mechanical properties of the gel made this an improved method for creating tissue engineered scaffolds.
ii. Bioprinting
Bioprinting is the direct application of a desired biological material, such as polymers, proteins, and/or cells, to a surface, yet consists of a very diverse group of methods. Most groups have modified a standard, commercial inkjet or laser printer to function with these materials, rather than ink. One major advantage of these methods is the precise computer control, which increases repeatability. Recent work demonstrated the ability to confine cells with bioprinting by printing cells simultaneously with gelling liquids. Sodium alginate63, thrombin11,90, and fibrinogen90 were each combined with cells and printed onto a substrate which allowed a solid biomaterial to form, in these cases alginate or fibrin gels. Hyaluronan was also suggested as a potential material with this approach.63 Repeating layers of printing with this technique could form reasonably large solid materials (e.g. 1×0.5×0.2cm) in which cells were confined.90
iii. Photopolymerization
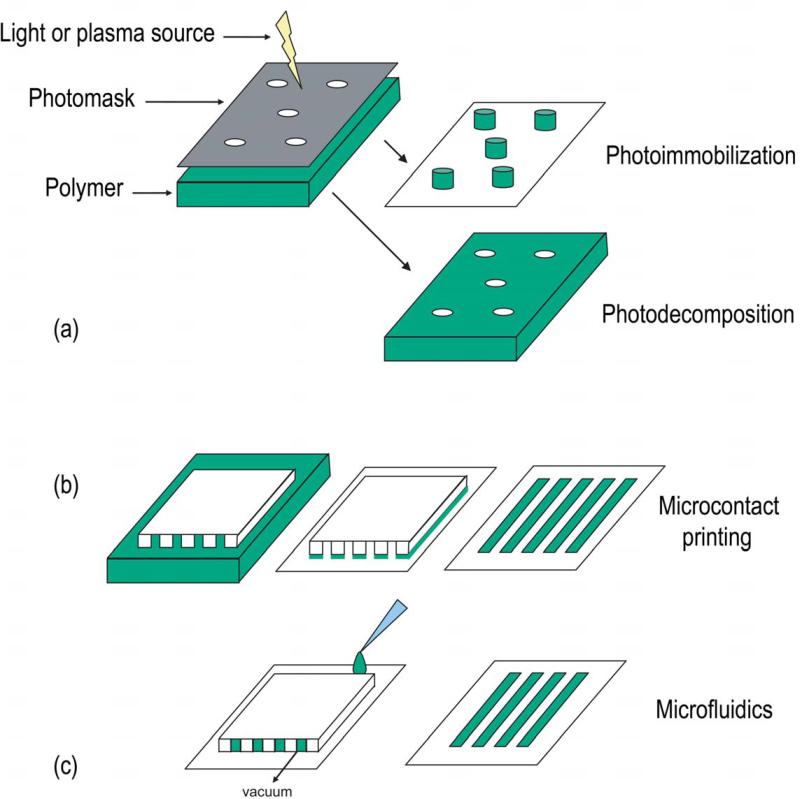
Photopolymerization (see Fig. 2a) is a common method for micropatterning topographical features. Itoga et al. developed and improved photopolymerizable polyacrylamide (PAA) systems, which were capable of creating complex patterns over large areas (50×50mm) with high resolution (2-8μm).34-37 Another approach to create both channels and circles coated PEG on tissue culture-treated polystyrene (TCP), polymerized the PEG, rinsed the excess, and allowed cells to attach to the underlying TCP.43 This method was also used to photoimmobilize sulphated hyaluronic acid2 or hyaluronan-Cu (II) complex3 on silanized glass. Chen et al.8 combined photopolymerization with microfluidics by carving a pattern into glass with a diamond point, then filling it with a photopolymerizable, cytophilic polymer, on top of a PAA gel. The glass mold was removed after polymerization with UV. Given the low resolution of this approach (182μm), the method was modified so that the cytophilic polymer was combined with the PAA and then polymerized out of the PAA with a laser, increasing resolution to 11μm. Laser speed determined the channel width. In similar approaches, laser were used to ablate PAA-coated TCP (only 34nm deep)38 and form grooves on poly(ethylene terephtalate) (PET).18
Fig 2.
Schematics of common micropatterning methods used with endothelial cells.
Jiang et al.41 developed another unique method for making microscale topographical features that did not use lithography or photopolymerization. A PDMS sheet was mechanically stretched, oxidized with plasma, and relaxed to form a wave structure that could be used as a mold. Lengthening the oxidation time increased the wavelength and the amplitude. Changing the mechanical properties of the original PDMS also altered the resulting final structure. While this is the only method that generated rounded structures, there was a limited range of sizes that could be created, and the generated wavelength and amplitude were not independent.
b. Biochemical cell restriction
Biochemical cell restriction relies on the use of cytophilic and cytophobic adsorbed coatings to guide cell attachment. While many of the methods described for topographical restriction of cells are similar to those used for biochemical restriction, the resulting cellular environment can be quite different. The basic methods that have been used for creating biochemical micropatterns for ECs are microcontact printing, microfluidics, bioprinting, and photochemistry.
i. Microcontact printing
Microcontact printing is a soft lithography technique, which uses a material (often PDMS created from a Si wafer) as a stamp (see Fig. 2b). The material is coated with a solution and then the raised, coated pattern of the stamp is pressed onto the final surface. The transferred pattern results in a cytophilic or cytophobic domain, around which the original surface remains unchanged.
One of the earliest applications of microcontact printing for ECs used methyl-terminated groups stamped onto glass surfaces with hydroxl-terminated groups. Coating with fibronectin (FN) and blocking with bovine serum albumin (BSA) created FN areas where the stamp was, on which the cells could attach. This approach has been used to make circles, squares, and lanes with up to 1-2μm resolution.5-7,17 Stamps have also been used for creating regions of thiol-terminated groups1,53 and polyethylene oxide, which were also coated with FN.67 More commonly, FN was stamped directly onto a surface, including glass,23,42,45,76 polystyrene,76 silicone,76 Flexcell® surface,54 cell culture plastic,20 and PDMS.21,70 When using PDMS as a stamp, plasma treatment was used to improve the adsorption of FN onto the PDMS. The stamps or molds created by soft lithography do create consistent patterning material, but the reproducibility of the system relies on the ability of the researcher to apply consistent pressure to each stamp rather than the computer-aided control afforded to some of the other patterning methods presented here. More recent work eliminated some of the batch variation, seen with microcontact printing, with a small, portable device which prints a solution at a set pressure.19
Blocking the background surface after microcontact printing is a common step to avoid cell binding or migration off of the printed pattern. BSA is a useful non-toxic blocking agent, but some non-specific binding does occur.23 One group incubated the surface with lipid vesicles to prevent cell adhesion outside of the desired area.42 Various forms of poly(ethylene glycol) (PEG) are also a strong deterrent for cell adhesion.1,10,25,53 Pluronic® (BASF), has been used frequently.54,70,76 Tan et al. determined, with various concentrations of hydroxyl-terminated surfaces, that a surface above 40% -OH was needed for protein adsorption, but the Pluronic® did not adsorb at higher than 70% -OH.76 They preferred the F127 product to the shorter, less hydrophobic version, F108, and found the printed and blocked surfaces were stable for at least 4wks. Stamping of cytophobic materials was accomplished with PEG on chitosan.25 The cells attached onto the chitosan, after which, the PEG was washed off. The revealed unseeded chitosan was then seeded with a second cell type.10 Multiple cell types on a single surface was also accomplished by consecutive seeding onto temperature-sensitive, microcontact printed, polymer dishes.20
ii. Microfluidics
Microfluidics is another common soft lithographic method for confining cells biochemically. Molds are made as described for microcontact printing, but rather than using the mold for stamps, the molds are placed on a surface through which a fluid can be pulled with a vacuum (see Fig. 2c). All the microfluidic approaches discussed here for controlling ECs used a PDMS mold. Most surfaces on which the mold was placed were glass or TCP, but one group used silicone.87 Protein solutions were often used for encouraging cell attachment, including FN65,87 and collagen I.30,31,51,82,83 As with microcontact printing, surfaces were blocked with BSA30,31,82,83 or Pluronics®.51,65,87 Lane widths ranged from 5μm82 to 80μm.31
In addition to creating cytophilic and cytophobic surfaces, microfluidics was used to create biochemical and mechanical variations. To study cell migration, biochemical variations were created by pulling a high concentration of collagen through a mold, removing the mold, and then adding a low concentration of collage over the entire surface.30,31 To create varying mechanical stiffnesses, an acrylamide solution was pulled through a PDMS mold, cured with a UV light, and the mold was removed. Another acrylamide solution was then added to surround the entire original area. After curing the second solution, the entire surface was coated with FN.28 A similar approach was used to form mechanical variations with PDMS surfaces. Rather than using a mold, solidified PDMS was cut into squares, heated and adhered to a sucrose-coated substrate before pouring a second PDMS solution around the squares. After the second solution cured, the sucrose was dissolved and the surface was coated with FN.28 Creating these mechanical variations in the surface is neither topographical confinement nor biochemical restriction. This unique type of micropatterning occurs through cells’ preference to migrate from compliant to stiff regions of the material over a period of days (rather than hours as seen in biochemical patterning). The mechanical variations methods resulted in about 70% of cells in the stiffer regions, versus around 95% of cells staying within a biochemically-restricted region.28
iii. Bioprinting
Bioprinting has occasionally been used to physically confines cells, as discussed previously; yet, the more common usage is the direct application of a desired solution onto a flat surface. One of the earlier uses of inkjet printing with ECs used cells in culture medium to print small groups of vascular ECs (1-4 cells per drop).60 Using a modified Hewlett-Packard inkjet printer, Gauvreau et al. were able to print CRGD, GRGDS and WQPPRARI peptides as well as their combinations onto plasma-treated expanded polytetrafluoroethylene (ePTFE).26 Similar results were obtain by Woodrow et al. using a modified robotic microarray printer (Virtek ChipWriter Pro, BioRad, Hercules, CA) to print proteins singularly and in combination, specifically collagen I, collagen III, collagen IV, FN, laminin, and Matrigel (a solution of basement membrane proteins), onto electrospun PLA scaffolds or onto a HydroGel slide (a commercially available acrylamide surface on glass).86 These researchers showed the preferential attachment of several cell types onto these protein-coated scaffolds for the purpose of tissue engineering.
Two similar methods, laser guided direct writing and biological laser printing, were used for writing materials and cells. Laser guided direct writing was developed based on the principles of optically trapping cells. Like optical tweezers, cells are forced into a position with a laser beam, but rather than holding the cell in a fixed position, the laser guided direct writing technique forces the cells along the beam onto an arbitrary surface.58,59 This technique was used to ‘write’ cells onto Matrigel, and by writing on a collagen gel layer above a previously written layer, they created a three-dimensional construct.59 While other cells types could be written directly, HUVECs had to be attached to beads for use in this system due to the cells’ low refractive index. Biological laser printing was used to print a polymer (sodium alginate), a biomaterial (hydroxyapatite), and ECs.29 In this system, a laser transferred materials and cells from a thin layer (adhered to a glass substrate) to the final substrate in a controlled pattern. Guillemot et al. optimized their system to print from multiple material types without reloading the system.
A process called microscale direct writing was also used to pattern ECs.32 This method employed an atomic force microscope (AFM) to put gelatin, collagen IV, FN, or fluorescent dyes on an epoxy-modified glass surface, which allowed for covalent bonding of the molecules. The AFM cantilever had a reservoir for the biomolecules, which were transferred to the surface through direct contact or capillary action.
iv. Photochemistry
The use of light (most often UV) to polymerize or degrade various materials from a surface has gained in popularity in recent years (see Fig. 2a). This approach has similar features to microcontact printing or microfluidics in that the resulting surface usually has two constituents, a cytophilic and a cytophobic region. Matsuda et al.55,56 first developed this technology for ECs. By using a photomask, UV irradiation allowed cells to attach where the mask was, or was not, based on the materials used. For example, cells adhered on TCP when the poly(dimethyl acrylamide) was photopolymerized, preventing cell attachment to the irradiated region. Alternatively, when styrene copolymer was cast on polyvinyl alcohol, cells adhered on the irradiated regions.55,56 As illustrated by these initial studies, decomposition or immobilization can each result in either cytophilic or cytophobic regions.
Photodecomposition was used to create two- and three- dimensional structures as well as micropatterning of single or multiple cells types. In one study, photodecomposition removed the PEG coating from a silanized glass surface.44 Similarly, tetraethyleneglycol was photodecomposed to reveal a silane layer, and protein attached to the glass on which the ECs were seeded.64 After cells were allowed to attach in the lanes they could be transferred to a Matrigel or collagen I gel, which encouraged tube formation. In another variation, TiO2 nanoparticles were patterned onto a glass slide, covered with photoreactive hyaluronan, photoetched to remove the hyaluronan, and seeded with cells, which preferentially attached to the TiO2 pattern.48 This photodecomposition approach was also used to seed multiple cell types. A photoreactive polymer-coated glass slide was stimulated with UV through a photomask so that the polymer was only removed from one area. After seeding the first cell type (on the newly exposed glass), the photomask was moved to remove the polymer from another area. This second area then allowed for the attachment of a second cell type.40
Photoimmobilization was also used for micropatterning. UV was used to photoimmobilize gelatin [combined with VEGF (vascular endothelial growth factor)] onto polystyrene or silanized glass either as drops of solution or through a photomask to give a grid pattern on the surface.33 The VEGF encouraged cell attachment and proliferation in a dose dependant manner. Hyaluronan was also used on silanized glass (as described above), but instead of coating the surface and decomposing the hyaluronan, it was immobilized on the surface.16,47 This resulted in some depth to the features, but the 40nm depth was minimal compared to the depths of topographical cell restriction. Few other users of these techniques measured or commented on any resulting differences in height within their work. Moon et al. photopolymerized a PEG base layer onto a glass surface, and then patterned a cell adhesive ligand (RGDS)-conjugated PEG polymer using a photomask.57 This group made a more complex and precise structure by using laser scanning lithography to bind VEGF and RGDS to their PEG base.50 A similarly unique method used PEG photopolymerized on glass. Interestingly, cells would attach to the glass and not the PEG when the PEG was dissolved in methanol, but would attach to the PEG and not the glass when the PEG was dissolved in a water/methanol mixture.91 Nakayama et al. developed a semi-automated process to produce micropatterned chips (20 samples at a time). The poly(ethylene terephthalate) film was grafted with three polymers onto three lanes using photopolymerization: polyAANa (stained for negative ions), polyDMAPAAm (stained for positive ions), and polyAAm (nonionic polymer). Cells were confluent on the polyDMAPAAm and the non-reacted surface, could migrate onto the polyAANa region, but would not adhere to the polyAAm.61
Plasma etching and plasma polymerization have also been used to create micropatterned surfaces. Satomi et al. plasma etched PEG off of gold-coated surfaces.73 By using multiple sizes of PEG chains, this work determined that shorter chains led to a higher PEG surface density and lower nonspecific protein binding to the gold surface which resulted in less cell spreading outside the desired region.73 Reactive oxygen plasma etching was used on poly-L-lysine coated glass or polystyrene. By using a PDMS mold on the surface, the contacted areas resulted in unetched regions.69 The etched polystyrene and the unetched poly-L-lysine (surrounded by etched glass) supported cell attachment.
v. Other techniques
A few other unique variations of what has been described previously have been tested for micropatterning ECs. Gray et al. modified their method of pouring agarose around a PDMS mold by relying not only on the plated FN areas, but also adding an electrode to the base of the area. By flowing the cells across the surface, only one cell was allowed to attach to each electrode.27 While this approach ultimately resulted in a physical restriction of the cells, the use of electrically trapping could allow for patterned cells without the agarose mold. In another variation on the methods described above, the standard soft lithography approach was modified for use on a glass surface, where photoresist was patterned onto glass, collagen coated the non-patterned areas, and then the photoresist was washed away. This allowed for the seeding of multiple cell types, and since the photoresist was removed little topographical restriction remained.93 Gagne et al. micropatterned peptides (CGRGDS and CWQPPRARI) onto PTFE samples. One peptide was sprayed onto the surface and then soaked in a solution with the other peptide to give dots of ~10μm in diameter of the sprayed peptide. The dots and background coatings were made using each peptide. The dot size could be controlled but the position was random.24
2. Effects of micropatterning
The huge diversity of micropatterning techniques discussed here illustrates their potential use in a variety of biological systems. Micropatterning has allowed researchers to distinguish differences in cell alignment, proliferation, and function of the cells. Appreciating the changes that occur with ECs, through alterations in cell morphology or cell-cell contacts, can not only improve the understanding of this crucial cell, but also aid in the discovery of future clinical applications.
While ECs were used in all the studies described here, it is important to note that ECs come from a variety of sources from throughout the body. Large arteries (i.e., aorta, pulmonary, or carotid) were frequently used to harvest mature ECs from a variety of species. HUVECs are also a very common endothelial cell model, which are a highly-proliferative, readily available source for healthy, human ECs. HUVECs are preferable to immortalized cells lines,52 but behave quite differently from aortic ECs.74 Substantial differences were found between the ECs of arteries and veins,9,15,49 as well as the ECs of large vessels versus small ones.9 Therefore, throughout this section the specific cell source used in each experiment is identified, particularly when results differ between cell sources.
a. Morphology
i. Cell shape
Cell confinement on micropatterned surfaces was accomplished with both the topographical and biochemical restriction methods described previously. Cell alignment in micropatterned lanes was not only repeatedly observed but also quantified. Researchers utilized a width to length ratio of the cells40 and a surface area to perimeter ratio or “shape index” to indicate an elongated morphology.53,82,83 Li et al. used the latter method to determine alignment and measured the angle of alignment from the intended direction by calculating the absolute value of the cosine of the angle.51 Using these methods, elongation was observed within 24hrs and maintained for at least another 24hrs with a microfluidic method of attaching carotid ECs on collagen I.83 Cell density53 and cell area80 were unchanged between the micropatterned and non-micropatterned aortic ECs. A nuclear form factor analysis was also used to quantify alignment in micropatterned lanes.22 This method compared the log of the length over width of the nucleus, where the dimensions were relative to the pattern's axes. Feinberg et al.22 argued that because the nuclear and cell alignment correlated well, the nuclear form factor analysis incorporated geometrical alignment and orientation using one metric.
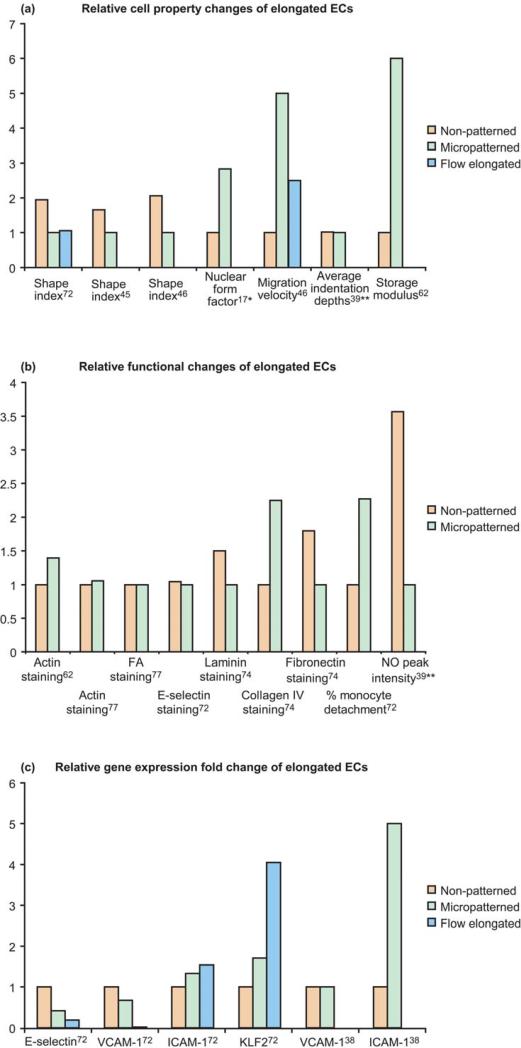
While the quantification of cell alignment can be a time-consuming process, the resulting data is quite compelling (see Fig. 3a). Vartanian et al. used the shape index to confirm that the cell alignment seen with biochemical micropatterning was the same as that seen from subjecting the ECs to fluid shear stress. These values were significantly different from the non-patterned, control group.82 This confirmed the validity of using micropatterning techniques to mimic the EC alignment induced by flow.
Fig 3.
Relative changes for non-patterned, micropatterned, or fluid shear stress elongated endothelial cells. Data in (a) and (b) are divided by the lowest value in each data set, resulting in a baseline of 1. Data in (c) are taken directly from the published work. Vartanian et al.72 calculated direct fold changes (2-ddCt); whereas Kato et al.38 used relative differences from gel electrophoresis.
* indicates data from the greatest treatment difference
** indicates the average from three patterns
The micropatterned lane size significantly affected the alignment of the ECs. In general, decreasing lane width increased the percentage of aligned cells or the extent of alignment. The maximum width for cell alignment appears to be approximately 200μm. Jang et al. found that vascular ECs aligned with 150μm channels but less so with 200μm channels.40 At 182μm8 and 115μm53 widths, cell alignment was only observed on the edges. Decreasing FN lane width also increased alignment with HUVECs.87 Li et al.51 observed aortic ECs on 15, 30, and 60μm lanes, where the angle of alignment was significantly different from controls for all micropatterned groups. On 60μm lanes, where cells were not touching the edge, there was often still alignment (likely through cell-cell communication). The 15μm lanes caused the most elongation while the 30 and 15μm also had lead to significantly lower spreading areas.51 Increasing channel depth (from 200nm to 1 μm) also increased cell alignment and elongation.80 On a surface with wavy topography, greater capillary EC alignment occurred with greater wavelength and amplitude.41
The spacing between the lanes of confined cells can alter their morphology as well. Even with a non-fouling surface between cytophilic areas, cells can remodel adhesive proteins, lay down new proteins, or simply bridge the gaps between areas of attachment. Chen et al. found that bovine capillary ECs bridged PEG gaps up to 10μm and human capillary ECs bridged gaps up to 20μm,7 which was similar to the crossover range seen with bovine pulmonary ECs blocked with a lipid membrane.42 With topographical patterning, depth became an important factor in cell orientation; HUVECs bridged 3μm deep grooves, yet attached to the floor of 10μm deep grooves.18 When collagen I lanes were blocked with BSA, endothelial progenitor cells derived from blood frequently crossed over the 20μm or 45μm wide gaps and decreasing the lane width or spacing led these cell to spread within 4 hours.84
Kofron et al. determined that for their model of microcontact printing with FN, the protein width contributed the most to HUVEC alignment (47.3%), while the spacing width contributed 32.6%, and the plating density contributed 20.1%.45 These quantitative contributions would certainly vary for other cell types or micropatterning techniques. Feinberg et al. systematically and rigorously observed the effects of elastic modulus, surface chemistry, ridge height, and lane spacing on the nuclear alignment of porcine vascular pulmonary ECs. The ridge height and surface chemistry, and to a lesser extent the width of the ridges, affected the nuclear alignment. By isolating the effects of material stiffness, they found that there was a small increase in alignment with stiffer materials, but only with the 1.5μm tall ridges.22 In many cases, topographical micropatterning had greater nuclear alignment than micropatterning without ridges; however, alignment was the same with 5μm ridges or 50μm lanes of patterned FN. The greatest alignment was seen on 5μm ridges with FN, suggesting that the combination of biochemical signals and microtopography may have the most profound effects on ECs; however, since the combination of the two was not an additive or synergistic effect and was not significantly different from the 5μm ridges or 50μm lanes of patterned FN alone, the combination may hinder the individual effects.
There can be little doubt that micropatterning with lanes leads to cellular alignment along the lanes, but this occurred with both subcellular and cellular-sized lanes. Microvessel ECs formed a single cell width on lanes about 10-20μm wide, and a 2 cell width was observed at 30μm.25,85 Interestingly, pulmonary ECs cultured on 5μm wide FN lanes separated by 5μm spacing did not align,22 but when HUVECs were plated on FN lanes of 10μm with 10μm spacing, the cells stayed aligned even when they crossed over the protein lanes.45 Di Canio et al. determined that 9.2μm is the minimum width in which human coronary artery ECs can align. They explained that this minimum length restriction is due to the size of the nucleus.16,48 While this may be true for biochemically restricted cells, topographical patterns can influence cells even when the features are subcellular-sized.78
ECs have also been confined to micropatterned islands. This afforded control of single cell spreading and number of cell-cell contacts.6,27,62 When squares of FN were patterned, the larger ones (40 and 20μm in width) yielded primarily flattened cells, while the 5μm wide squares gave completely rounded cells.42
ii. Cytoskeletal alignment
In addition to observing and quantifying cell and nuclear alignment, specific cytoskeletal elements were altered during micropatterning compared to non-patterned controls. Changes were observed in actin, 5,32,41,53,70,78,80,82,83 focal adhesions (FAs),5,41,53,78,80,81 and microtubules,83 but not in vimentin70 or VE-cadherin (see Fig. 3b).53
Actin quantities increased significantly with micropatterning compared to controls and the fibers have aligned in the direction of the topographical or biochemically-restricted patterns.17,32,53,70,82 Alternatively, one study found with HUVECs that the amount of actin staining significantly decreased with the smallest width lane of 15μm, while on larger lanes there was no difference in actin compared to controls.87 With topographical patterning at the sub-cellular scale, the actin fibers were co-located with the ridge features.41,78,80 When ECs were seeded on very small cytophillic circles (diameter=3μm), such that single cells would bind to multiple circles, actin was localized around the circles with fibers stretching between them.5 The actin staining for the biochemical micropatterning of ECs was similar to that seen for ECs applied to fluid shear stress.82 However, a dense peripheral band of actin was observed with micropatterned ECs but not with flow-induced alignment.83 Additionally, actin alignment on micropatterned cells began within the first hour after seeding, was largely completed by 24hrs, and was maintained for at least another 24hrs, whereas flow-induced actin alignment was not seen until 8-12hrs.82 This substantial change between flow-stimulated and micropatterned cellular structure could have profound differences related to cell functionality. Cell structure relates to cell signaling,12 mechanical properties,14 and motility5 suggesting that all these areas may be altered between micropatterned and flow-stimulated cells.
Focal adhesions increased with micropatterning and were seen along the edges of the pattern.53 Like with actin, sub-cellular topography caused the FAs to clearly align along the edge of the pattern.78,80,81 With a sub-cellular wavy topography, however, no changes were observed in FAs among the crests or troughs on which the cell was attached.41 A single study using HUVECs on 15μm lanes indicated decreased FA with micropatterning,87 but with aortic ECs, more FAs were seen on 30 and 60μm lane than on 15μm lanes.51 When capillary ECs were seeded on square and circle patterns, the FAs were observed primarily around the edges where the tension was highest.5 With increased cell area (from 10-50μm wide squares), the FAs and phosphotryosine increased. Similar results were seen when contact area was limited but cell area was still allowed to increase.5 However, conflicting results have been found regarding the effect of cell density. One group found that increasing cell density decreased FAs62 and another found it increased.21 FAs organized within the first 24hrs after seeding.81
Microtubules aligned with the micropatterned lanes.83 As with actin, this alignment was seen within the first hour, completed by 24hrs, and maintained for at least another 24hrs. The microtubule-organizing center was not affected by micropatterning, whereas under fluid shear stress, the microtubule-organizing center moved to the upstream position.83 Interestingly, disruption of the microtubules, but not the actin, prevented EC alignment on the micropatterned lanes.
b. Proliferation and apoptosis
Micropatterning has been used for the examination of cell-cell contact for studying proliferation. By forming limited cell contacts, specifically 0 to 4 neighbors, Gray et al. found that ECs with cell-cell contact had increased proliferation compared to no cell contact. With 2-4 cell contacts proliferation increased but less than with a single cell contact.27 This suggests that cells in a single cell width lane would have higher proliferation, but typically, the extent of alignment did not affect proliferation.80 Elongated cells also had the same DNA synthesis and nuclear volume as rounded cells.70 Dike et al. found that ECs in 10μm lanes stopped proliferating by 72hrs.17 Perhaps these results are not related to the cell contacts, but rather the spreading area, which has been linked to proliferation. A surface area of 1500μm2 encouraged spreading and proliferation, and an even higher growth rate was seen in cells confined to 3000μm2.6
Likewise, limiting spreading appears to increase apoptosis.7 When EC surface area was limited to 500μm2 with micropatterned islands apoptosis increased.6 This was also true on 10μm-diameter micropatterned circles, which resulted in substantial cell death.23 Wu et al. found that 15μm lanes confined HUVECs such that more than 50% of the cells were apoptotic or dead after 12hrs. The decrease in cell spreading and the presence of annexin V (an early apoptosis marker) confirmed the observed cell death.87 Interestingly, the application of flow parallel to the 15μm micropatterned HUVECs reversed most of these changes. The authors suggested that the increase in Rho activation limited apoptosis and that “both parallel flow and RhoV14 exert their anti-apoptotic effects through the formation/organization of cytoskeleton-FA.”87 When capillary ECs were patterned on 10μm or 30μm lanes, however, apoptosis was not observed even though smaller width lanes did result in a lower spreading area.17 These conflicting results may illustrate one of the potential differences between these cell types.
c. Functional
i. Cell migration and signaling
Migration of ECs on micropatterned surfaces has been examined primarily through time lapse microscopy.28,31,51,78 When ECs were patterned on 15μm collagen lanes, they migrated significantly faster than on 30 or 60μm.51 Lin et al. also showed a dependence on lane width for the migration of ECs within micropatterned lanes (see Fig. 3a). The migration patterns of ECs on 20μm lanes were similar to the cells located on the edges of 115μm micropatterned lanes, while the cells in the center of the 115μm lanes migrated similarly to non-patterned ECs. The migration observed within the lane, however, was decreased when flow was applied either parallel or perpendicular to the lanes.53 Alternatively, Uttayarat et al. found that micropatterning can encourage or discourage migration when flow is parallel or perpendicular, respectively. The shear stress threshold where flow overcomes patterning in regard to migration appears to be between 13.5 and 58 dyn/cm2.78 Similarly, the chemotaxis driven by collagen (where cells migrated to a higher concentration of collagen) was overcome at a flow rate of 3 dyn/cm2 after which migration rates were dominated by the fluid shear stress.31 The micropatterning of two mechanically different substrates led to cell migration from the less stiff to the more stiff regions. The researchers blocked proliferation in these experiments to confirm that the observed cells were driven by mechanotaxis and did not just grow more readily in the stiffer regions.28
While cell spreading has largely not been examined in micropatterned lanes, it has been a focus of ECs confined to single cells or small micropatterned islands. Having cell-cell contacts reduced the EC adhesion to, and spreading on, the extracellular matrix (ECM).62 Cells with limited spreading ability had increased RhoA signaling, which regulates cytoskeletal dynamics, but lower ROCK activity, which can inhibit integrin activation and cytoskeletal contraction, suggesting that the RhoA activity did not result in ROCK and myosin activation.4 While these results are important for cell regulation, the specific consequences of these results are unknown; however, they clearly emphasize the importance of cell spreading and attachment in regulating the cells’ mechanical and chemical signals.
A detailed look at focal adhesions and migration was performed with the aid of fibroblasts seeded on subcellular-sized micropatterns.88 When spacings between areas of potential binding were increased, spreading speed and extent of elongation decreased. Cell migration was directionally oriented with the cells spreading their membrane processes in the direction of newly formed focal adhesions. Xia et al.88 developed a computer model, based on the focal adhesion driven migration, which was capable of predicting cell orientation and elongation during migration on micropatterned islands. The results of their computer model and experimental cell studies were highly correlated, which illustrates the power and potential for future work of combining micropatterning and modeling.
ii. Gene and protein
The results for the changes in gene expression and protein production due to micropatterning of ECs are limited (see Fig. 3 b and c). Krüppel-like factor 2 (KLF2) gene expression (a transcription factor known for its fluid shear stress dependence) was increased not only to ECs under fluid shear stress, but also for cells that were elongated by micropatterning. However, the cells under shear stress (either patterned or not) had higher KLF2 expression than the micropatterned cells. When microtubules but not when actin were disrupted, the KLF2 was significantly downregulated.82 Therefore, the increase in KLF2 due to micropattern elongation was dependent on microtubule elongation, but not actin elongation.
The components of the basement membrane on which ECs reside vary depending on the location and flow conditions. Collagen IV and laminin are considered atheroprotective ECM while FN and collagen I are considered atheroprone matrix proteins. Carotid ECs, aligned with micropatterning, deposited significantly more collagen IV and less FN compared to the non-patterned controls.84 When HUVECs were cultured in lanes, they produced laminin and FN, but not collagen I after 14 days in culture.57 As a component of the ECM in wound healing, it is unsurprising that FN was deposited by HUVECs around the dramatic topography, like in grooves and around pillar and well structures, suggesting that the cells use the FN to stabilize themselves.81 Therefore, FN may increase with cells on topographical features rather than biochemical ones.
E-selectin, VCAM, and ICAM are important surface bound markers which promote leukocyte adhesion. E-selectin gene expression was significantly decreased when flow was applied to either micropatterned or non-micropatterned cells, but protein was unchanged between the micropatterned and control vascular ECs.82 Lymphatic ECs, which were confined to small micropatterned circles (such that spreading was limited), had significantly more VCAM than control cells, but no difference in VCAM gene expression was observed with cells aligned in channels.43 Vartanian et al. however, did observe significantly less VCAM gene expression with biochemically-patterned ECs as well as ECs stimulated by fluid shear stress. Additionally VCAM gene expression synergistically decreased when fluid shear stress was applied to micropatterned cells.82 ICAM gene expression was significantly increased in ECs under fluid shear stress alone; however, no change was observed with aligned micropatterned cells or even micropatterned cells with flow applied.82 Conversely, aligned HUVECs expressed significantly more ICAM gene than control cells at 12hrs and nearly significantly (p=0.08) more at 24 and 48hrs. Circular patterned HUVECs also had more ICAM gene expression than control.43 In general, these results suggest that elongated cells are less prone to immunogenic stimulation.
iii. Immunogenic response and hemostasis
Some research has directly observed the immunogenic responses of micropatterned cells (see Fig. 3b). In one study, monocytes were attached to carotid ECs and detachment was observed with time-lapse microscopy. Greater detachment was observed on aligned ECs compared to non-patterned cells.82 Kidoaki and Matsuda observed the nitric oxide (NO) production from HUVECs patterned in round and spindle shapes. Endothelial cell release of NO can inhibit leukocyte adhesion and is a potent regulator of platelet aggregation. In their study, control cells (non-patterned) had the greatest NO production followed by the round pattern cells. The lowest NO production was seen in the spindle-shaped cells. This is unlike that seen with flow-aligned cells.44 While many of the other changes that have been observed with micropatterned ECs suggest their improved immune and hemostasis functions compared to non-patterned cells, their inability to increase NO production does not.
iv. Mechanical properties
While the mechanical properties of individual cells are not often tested, early work found that the application of fluid shear stress significantly increased the mechanical stiffness of aortic ECs, which may be attributed to the observed alignment of actin filaments.72 Similarly, when HUVECs were micropatterned onto either rounded morphologies (90 or 120μm diameters) or elongated morphologies (20, 30, or 40μm widths), rounded cells were less stiff than control (non-patterned) cells with the smaller 90μm diameter being less stiff than the 120μm one. The 40μm elongated cells had the greatest stiffness, with the 30 and 20μm widths being similar to control values. The elongated cells had denser stress fibers compared to round and control ECs.44 In a similar study with microvessel ECs, different results were obtained (see Fig. 3a). When cells were elongated with micropatterning they exhibited a slight increase in stiffness at the center of the cell compared to ECs micropatterned into 2500 μm2 area circles, but a significant decrease in stiffness at the edge. The overall storage modulus (G’) was significantly lower from rounded ECs.70 This may appear opposite from what was seen previously; however, the results of the elongated cells of the latter study were compared to cells that are physically confined into a rounded micropatterned area that was substantially smaller than the previous work. While the surface area was the same for the elongated, micropatterned cells, the strain within the rounded cells was substantially higher.70 It appears that cells which are allowed to spread more extensively, do not exhibit such dramatic internal strains.
3. Applications for regenerative medicine
a. Cell-cell interaction and migration
The study of cell-cell interactions has been greatly enhanced through micropatterning. Micropatterning has been useful for limiting cell contacts and confining cells or chemoattractive agents to a particular region. Hepatocyte function was improved through contact with ECs. By micropatterning lanes that allow the seeding of both cell types in different regions, the contact and cellular signaling was improved.93 Consecutive seeding allowed for multiple cell types to be seeded on the exact same surface through the use of photomasks and the micropatterning techniques.40
Micropatterning has been very useful for studying cell migration. Two studies used micropatterned topographical molds to seed cells into lanes with gels. By encapsulating them in soft materials their migration, interactions, and angiogenic potential was observed. This basic approach was used to study HUVECs under mechanical strain92 and the migration of various types of stem cells toward HUVECs.77
b. Tubulogenesis/vasculogenesis
Perhaps one of the most widely interesting applications of micropatterning endothelial cells has been angiogenesis or vasculogenesis for tissue engineering. It is generally accepted that the majority of tissue engineered constructs will require some type of vascular network to allow for nutrient diffusion and waste removal for the cells; therefore, the interest in creating vessels and capillary networks extends to a large range of applications. Micropatterning has been used toward this end in a variety of studies to stimulate the spontaneous tubulogenesis of endothelial cells. 10μm FN lanes made from microcontact printing stimulated human capillary ECs to form tubes by 72hrs, and the tubes detached from this surface around 4 days. PECAM (CD31) staining was seen as a line along the lumen. FN and laminin were also reorganized to a central line by 72hrs.17 Dike et al. indicated that this micropattern-induced tubulogenesis followed the steps of previous in vitro angiogenesis. After cell alignment, a cellular cord formed and ECM tendrils formed on the cell surface. Subsequently, the adherent cells enveloped the ECM tendrils and generated tension causing detachment. The last step of removal of the central ECM thread and ECM accumulation on the abluminal side, which causes reversal of EC polarity, did not occur in the micropatterned cells. 17 Gao et al. were also able to form tubes on 10μm and 20μm gelatin surface patterns after 3 days using human microvascular ECs. In addition they used topographically restricted gelatin molds with 20μm and 30μm width grooves of 4.6μm depth for tublogenesis.25 HUVECs were also cultured to form tubes by optimizing the concentration of RGDS peptide on an RDGS-PEG polymer surface and the width of the channel,57 and were further enhanced through the addition of patterned VEGF.50 Another group micropatterned HUVECs and stimulated tube formation by transferring the patterned lanes of cells onto a matrigel or collagen layer.64 After using micropatterning for tubulogenesis, one technique was developed to pattern a second cell type around the EC tubes.10 This study used fibroblasts as a proof-of-concept work of this technique and showed that the cells would form around the previous one; however, much work is still needed to optimize and test the benefits of this co-culture scheme. Most recently, bovine adrenal microvascular ECs or HUVECs were gelled in collagen I within a PDMS micropatterned mold and stimulated with bFGF (basic fibroblast growth factor) and VEGF. This approach created tubes in 24hrs and even allowed for some control of the diameter. With a fixed channel width, higher collagen concentrations formed larger diameter tubes with the HUVECs. Also, with a constant collagen concentration, larger tubes were seen with increasing channel width.68
c. Tissue engineering
In addition to creating a vascular network for supplying blood to other organs, there is also a strong interest in creating vascular constructs for grafting around diseased and blocked vessels. The goal is to provide better alternatives to current grafting techniques. Current graft materials include autologous veins, which cause donor site morbidity and are not a viable option in all patients, or synthetic replacements, which are successful only at larger diameters. The work that sprayed ePTFE with various cell adhesive peptides (described at the end of section 2) has implications for improving the endothelialization of Gore-Tex grafts.24 Having an endothelial layer may improve the integration and integrity of the grafts by decreasing tissue ingrowth and thrombus formation. However, the technique for spraying the peptides was developed for a flat surface and was not applied to the vessel lumen, indeed, no proposal for an appropriate way to transition this technology from flat surface to tubes was made by the authors.
Similarly, the modified laser printing technique developed by Guillemot et al. allowed for printing of polymers, biomaterials, and cells from a single system. They also found that the cell death was reduced from previous methods of laser printing.29 While these researchers discuss the potential of this method to create 3D structures with multiple components, they have yet to demonstrate this application of the technique. A modified microcontact printing method was used to plate a layer of two cell types (seeded consecutively) onto a heat-sensitive polymer which was then transferred as a single layer onto another surface. This technique also has the potential to build 3D structures with multiple cell types, but again, this was not tested.20
The use of collagen/gylcosaminoglycan gels for micropatterning ECs was suggested as a potentially useful biomaterial for tissue engineering because the weighted patterning technique allowed for the creation of complex branching patterns. The authors also argued that the material is biodegradable and has appropriate mechanical properties for tissue engineering. However, this is still in the early stages of work and requires substantial optimization and testing.39
One group that has created a three-dimensional construct used micropatterned sheets that could be clamped together or rolled to create channel structures, but cell-seeding was not described.66 Another method, while also still in the early stages, does seem closer to creating a three dimensional construct than some of the other work described here. By using a PDMS mold to micropattern channels into PU electrospun tube-shaped grafts, a 3D vessel was formed which had grooves that could encourage endothelial cell alignment.79 This ability to align the ECs may generate a healthier endothelialized layer. Additionally, the PU material had material properties more similar to arterial tissue than some of the currently available synthetic graft materials. Electrospun PLA scaffolds have also been proposed as a potential tissue engineered scaffold. Various ECM proteins as well as their combinations have been inkjet printed onto this potential scaffold.86 The combination of micropatterning with these scaffolds may also improve the endothelial layer; however, as with the ePTFE printing, the ability to print these proteins onto the abluminal surface was not shown.
While this review has focused on the micropatterning of endothelial cells, many other types of cells have been micropatterned, including various stem cells. Mesenchymal stem cells,77 embryonic stem cells,32 and endothelial progenitor cells82 have all been micropatterned and represent potential alternative cell sources for tissue engineering of vascular grafts. Since endothelial cells are isolated from vessels, they are somewhat impractical for autologous use in a patient. As with autologous vessel usage, autologous cell isolation would cause some donor site morbidity; therefore, stem or progenitor cells may be preferable to mature ECs.
4. Conclusion
The ability to confine and alter endothelial cell morphology has clearly been accomplished through a large variety of micropatterning techniques. While some methods have been used quite commonly, others have been uniquely modified for a specific outcome or to improve upon an existing method for technical purposes. Different methods have set out to improve automation, resolution, repeatability, and cost from other techniques. The resulting patterned cells have altered cell shape, cytoskeletal alignment, migration, gene expression, protein production, immunological response, and mechanical properties. ECs that were micropatterned into an elongated morphology showed many similarities to the well-established phenotype of ECs elongated by fluid shear stress; however, more work is necessary to continue to tease out the differences between cells elongated by biochemical or topographical cues and those elongated by flow.
Additionally, the ability to apply the information learned with micropatterning has great potential, but is yet to be realized. There are numerous studies which have used micropatterning to learn more about EC behavior, tubulogenesis, and vascular tissue engineering, yet there is still much more to discover. While many of the studies described here for tubulogenesis and tissue engineering with micropatterning are still in the early stages of development and are far from a practical solution, the potential for EC guidance through a 3D structure or a functional vascular graft is supported by early micropatterning work.
Acknowledgements
The authors gratefully acknowledge funding from the American Heart Association grant 09BGIA2260384 and National Institutes of Health grants R01HL103728 and R01HL 095474.
Footnotes
Conflict of Interest Statement
There are no conflicts of interest.
References
- 1.Amirpour ML, Ghosh P, Lackowski WM, Crooks RM, Pishko MV. Mammalian cell cultures on micropatterned surfaces of weak-acid, polyelectrolyte hyperbranched thin films on gold. Anal Chem. 2001;73:1560–6. doi: 10.1021/ac000907f. [DOI] [PubMed] [Google Scholar]
- 2.Barbucci R, Lamponi S, Magnani A, Pasqui D. Micropatterned surfaces for the control of endothelial cell behaviour. Biomol Eng. 2002;19:161–70. doi: 10.1016/s1389-0344(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 3.Barbucci R, Lamponi S, Magnani A, Piras FM, Rossi A, Weber E. Role of the Hyal-Cu (II) complex on bovine aortic and lymphatic endothelial cells behavior on microstructured surfaces. Biomacromolecules. 2005;6:212–9. doi: 10.1021/bm049568g. [DOI] [PubMed] [Google Scholar]
- 4.Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res. 2007;313:3616–23. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun. 2003;307:355–61. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 7.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog. 1998;14:356–63. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- 8.Chen YM, Shen KC, Gong JP, Osada Y. Selective cell spreading, proliferation, and orientation on micropatterned gel surfaces. J Nanosci Nanotechnol. 2007;7:773–9. doi: 10.1166/jnn.2007.517. [DOI] [PubMed] [Google Scholar]
- 9.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100:10623–8. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Co CC, Wang YC, Ho CC. Biocompatible micropatterning of two different cell types. J Am Chem Soc. 2005;127:1598–9. doi: 10.1021/ja044382a. [DOI] [PubMed] [Google Scholar]
- 11.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–7. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 12.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daxini SC, Nichol JW, Sieminski AL, Smith G, Gooch KJ, Shastri VP. Micropatterned polymer surfaces improve retention of endothelial cells exposed to flow-induced shear stress. Biorheology. 2006;43:45–55. [PubMed] [Google Scholar]
- 14.del Alamo JC, Norwich GN, Li YS, Lasheras JC, Chien S. Anisotropic rheology and directional mechanotransduction in vascular endothelial cells. Proc Natl Acad Sci U S A. 2008;105:15411–6. doi: 10.1073/pnas.0804573105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng DX, Tsalenko A, Vailaya A, Ben-Dor A, Kundu R, Estay I, Tabibiazar R, Kincaid R, Yakhini Z, Bruhn L, Quertermous T. Differences in vascular bed disease susceptibility reflect differences in gene expression response to atherogenic stimuli. Circ Res. 2006;98:200–8. doi: 10.1161/01.RES.0000200738.50997.f2. [DOI] [PubMed] [Google Scholar]
- 16.Di Canio C, Lamponi S, Barbucci R. Spiral and square microstructured surfaces: the effect of the decreasing size of photo-immobilized hyaluronan domains on cell growth. J Biomed Mater Res A. 2010;92:276–84. doi: 10.1002/jbm.a.32317. [DOI] [PubMed] [Google Scholar]
- 17.Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim. 1999;35:441–8. doi: 10.1007/s11626-999-0050-4. [DOI] [PubMed] [Google Scholar]
- 18.Duncan AC, Rouais F, Lazare S, Bordenave L, Baquey C. Effect of laser modified surface microtopochemistry on endothelial cell growth. Colloids Surf B Biointerfaces. 2007;54:150–9. doi: 10.1016/j.colsurfb.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Elloumi-Hannachi I, Maeda M, Yamato M, Okano T. Portable microcontact printing device for cell culture. Biomaterials. 2010;31:8974–9. doi: 10.1016/j.biomaterials.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Elloumi Hannachi I, Itoga K, Kumashiro Y, Kobayashi J, Yamato M, Okano T. Fabrication of transferable micropatterned-co-cultured cell sheets with microcontact printing. Biomaterials. 2009;30:5427–32. doi: 10.1016/j.biomaterials.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg AW, Schumacher JF, Brennan AB. Engineering high-density endothelial cell monolayers on soft substrates. Acta Biomater. 2009;5:2013–24. doi: 10.1016/j.actbio.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg AW, Wilkerson WR, Seegert CA, Gibson AL, Hoipkemeier-Wilson L, Brennan AB. Systematic variation of microtopography, surface chemistry and elastic modulus and the state dependent effect on endothelial cell alignment. J Biomed Mater Res A. 2008;86:522–34. doi: 10.1002/jbm.a.31626. [DOI] [PubMed] [Google Scholar]
- 23.Flusberg DA, Numaguchi Y, Ingber DE. Cooperative control of Akt phosphorylation, bcl-2 expression, and apoptosis by cytoskeletal microfilaments and microtubules in capillary endothelial cells. Mol Biol Cell. 2001;12:3087–94. doi: 10.1091/mbc.12.10.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagne L, Rivera G, Laroche G. Micropatterning with aerosols: application for biomaterials. Biomaterials. 2006;27:5430–9. doi: 10.1016/j.biomaterials.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Gao D, Kumar G, Co C, Ho CC. Formation of capillary tube-like structures on micropatterned biomaterials. Adv Exp Med Biol. 2008;614:199–205. doi: 10.1007/978-0-387-74911-2_23. [DOI] [PubMed] [Google Scholar]
- 26.Gauvreau V, Laroche G. Micropattern printing of adhesion, spreading, and migration peptides on poly(tetrafluoroethylene) films to promote endothelialization. Bioconjug Chem. 2005;16:1088–97. doi: 10.1021/bc049717s. [DOI] [PubMed] [Google Scholar]
- 27.Gray DS, Liu WF, Shen CJ, Bhadriraju K, Nelson CM, Chen CS. Engineering amount of cell-cell contact demonstrates biphasic proliferative regulation through RhoA and the actin cytoskeleton. Exp Cell Res. 2008;314:2846–54. doi: 10.1016/j.yexcr.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray DS, Tien J, Chen CS. Repositioning of cells by mechanotaxis on surfaces with micropatterned Young's modulus. J Biomed Mater Res A. 2003;66:605–14. doi: 10.1002/jbm.a.10585. [DOI] [PubMed] [Google Scholar]
- 29.Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, Pippenger B, Bareille R, Remy M, Bellance S, Chabassier P, Fricain JC, Amedee J. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010;6:2494–500. doi: 10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Hsu S, Thakar R, Li S. Haptotaxis of endothelial cell migration under flow. Methods Mol Med. 2007;139:237–50. doi: 10.1007/978-1-59745-571-8_15. [DOI] [PubMed] [Google Scholar]
- 31.Hsu S, Thakar R, Liepmann D, Li S. Effects of shear stress on endothelial cell haptotaxis on micropatterned surfaces. Biochem Biophys Res Commun. 2005;337:401–9. doi: 10.1016/j.bbrc.2005.08.272. [DOI] [PubMed] [Google Scholar]
- 32.Huang NF, Patlolla B, Abilez O, Sharma H, Rajadas J, Beygui RE, Zarins CK, Cooke JP. A matrix micropatterning platform for cell localization and stem cell fate determination. Acta Biomater. 2010;6:4614–21. doi: 10.1016/j.actbio.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito Y, Hasuda H, Terai H, Kitajima T. Culture of human umbilical vein endothelial cells on immobilized vascular endothelial growth factor. J Biomed Mater Res A. 2005;74:659–65. doi: 10.1002/jbm.a.30360. [DOI] [PubMed] [Google Scholar]
- 34.Itoga K, Kobayashi J, Tsuda Y, Yamato M, Okano T. Second-generation maskless photolithography device for surface micropatterning and microfluidic channel fabrication. Anal Chem. 2008;80:1323–7. doi: 10.1021/ac702208d. [DOI] [PubMed] [Google Scholar]
- 35.Itoga K, Kobayashi J, Yamato M, Kikuchi A, Okano T. Maskless liquid-crystal-display projection photolithography for improved design flexibility of cellular micropatterns. Biomaterials. 2006;27:3005–9. doi: 10.1016/j.biomaterials.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Itoga K, Yamato M, Kobayashi J, Kikuchi A, Okano T. Cell micropatterning using photopolymerization with a liquid crystal device commercial projector. Biomaterials. 2004;25:2047–53. doi: 10.1016/j.biomaterials.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 37.Itoga K, Yamato M, Kobayashi J, Kikuchi A, Okano T. Micropatterned surfaces prepared using a liquid crystal projector-modified photopolymerization device and microfluidics. J Biomed Mater Res A. 2004;69:391–7. doi: 10.1002/jbm.a.30010. [DOI] [PubMed] [Google Scholar]
- 38.Iwanaga S, Akiyama Y, Kikuchi A, Yamato M, Sakai K, Okano T. Fabrication of a cell array on ultrathin hydrophilic polymer gels utilising electron beam irradiation and UV excimer laser ablation. Biomaterials. 2005;26:5395–404. doi: 10.1016/j.biomaterials.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 39.Janakiraman V, Kienitz BL, Baskaran H. Lithography Technique for Topographical Micropatterning of Collagen-Glycosaminoglycan Membranes for Tissue Engineering Applications. J Med Device. 2007;1:233–237. doi: 10.1115/1.2775937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang K, Sato K, Tanaka Y, Xu Y, Sato M, Nakajima T, Mawatari K, Konno T, Ishihara K, Kitamori T. An efficient surface modification using 2-methacryloyloxyethyl phosphorylcholine to control cell attachment via photochemical reaction in a microchannel. Lab Chip. 2010;10:1937–45. doi: 10.1039/c002239j. [DOI] [PubMed] [Google Scholar]
- 41.Jiang X, Takayama S, Qian X, Ostuni E, Wu H, Bowden N, LeDuc P, Ingber DE, Whitesides GM. Controlling Mammalian Cell Spreading and Cytoskeletal Arrangement with Conveniently Fabricated Continuous Wavy Features on Poly(dimethylsiloxane). Langmuir. 2002;18:3273–80. [Google Scholar]
- 42.Kam L, Boxer SG. Cell adhesion to protein-micropatterned-supported lipid bilayer membranes. J Biomed Mater Res. 2001;55:487–95. doi: 10.1002/1097-4636(20010615)55:4<487::aid-jbm1041>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 43.Kato S, Ando J, Matsuda T. MRNA expression on shape-engineered endothelial cells: adhesion molecules ICAM-1 and VCAM-1. J Biomed Mater Res. 2001;54:366–72. doi: 10.1002/1097-4636(20010305)54:3<366::aid-jbm80>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 44.Kidoaki S, Matsuda T. Shape-engineered vascular endothelial cells: nitric oxide production, cell elasticity, and actin cytoskeletal features. J Biomed Mater Res A. 2007;81:728–35. doi: 10.1002/jbm.a.31112. [DOI] [PubMed] [Google Scholar]
- 45.Kofron CM, Hoffman-Kim D. Optimization by Response Surface Methodology of Confluent and Aligned Cellular Monolayers for Nerve Guidance. Cell Mol Bioeng. 2009;2:554–572. doi: 10.1007/s12195-009-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni SS, Orth R, Ferrari M, Moldovan NI. Micropatterning of endothelial cells by guided stimulation with angiogenic factors. Biosens Bioelectron. 2004;19:1401–7. doi: 10.1016/j.bios.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Lamponi S, Di Canio C, Forbicioni M, Barbucci R. Heterotypic interaction of fibroblasts and endothelial cells on restricted area. J Biomed Mater Res A. 2010;92:733–45. doi: 10.1002/jbm.a.32364. [DOI] [PubMed] [Google Scholar]
- 48.Lamponi S, Forbicioni M, Barbucci R. The role of fibronectin in cell adhesion to spiral patterned TiO2 nanoparticles. J Appl Biomater Biomech. 2009;7:104–10. [PubMed] [Google Scholar]
- 49.Lawson ND, Weinstein BM. Arteries and veins: making a difference with zebrafish. Nat Rev Genet. 2002;3:674–82. doi: 10.1038/nrg888. [DOI] [PubMed] [Google Scholar]
- 50.Leslie-Barbick JE, Shen C, Chen C, West JL. Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses. Tissue Eng Part A. 2011;17:221–9. doi: 10.1089/ten.tea.2010.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Bhatia S, Hu YL, Shiu YT, Li YS, Usami S, Chien S. Effects of morphological patterning on endothelial cell migration. Biorheology. 2001;38:101–8. [PubMed] [Google Scholar]
- 52.Lidington EA, Moyes DL, McCormack AM, Rose ML. A comparison of primary endothelial cells and endothelial cell lines for studies of immune interactions. Transpl Immunol. 1999;7:239–46. doi: 10.1016/s0966-3274(99)80008-2. [DOI] [PubMed] [Google Scholar]
- 53.Lin X, Helmke BP. Micropatterned structural control suppresses mechanotaxis of endothelial cells. Biophys J. 2008;95:3066–78. doi: 10.1529/biophysj.107.127761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res. 2007;101:e44–52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]
- 55.Matsuda T, Inoue K, Sugawara T. Development of micropatterning technology for cultured cells. ASAIO Trans. 1990;36:M559–62. [PubMed] [Google Scholar]
- 56.Matsuda T, Sugawara T. Development of surface photochemical modification method for micropatterning of cultured cells. J Biomed Mater Res. 1995;29:749–56. doi: 10.1002/jbm.820290611. [DOI] [PubMed] [Google Scholar]
- 57.Moon JJ, Hahn MS, Kim I, Nsiah BA, West JL. Micropatterning of poly(ethylene glycol) diacrylate hydrogels with biomolecules to regulate and guide endothelial morphogenesis. Tissue Eng Part A. 2009;15:579–85. doi: 10.1089/ten.tea.2008.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nahmias Y, Odde DJ. Micropatterning of living cells by laser-guided direct writing: application to fabrication of hepatic-endothelial sinusoid-like structures. Nat Protoc. 2006;1:2288–96. doi: 10.1038/nprot.2006.386. [DOI] [PubMed] [Google Scholar]
- 59.Nahmias YK, Gao BZ, Odde DJ. Dimensionless parameters for the design of optical traps and laser guidance systems. Appl Opt. 2004;43:3999–4006. doi: 10.1364/ao.43.003999. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, Ohuchi K, Iwasaki Y, Horie M, Morita I, Takatani S. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005;11:1658–66. doi: 10.1089/ten.2005.11.1658. [DOI] [PubMed] [Google Scholar]
- 61.Nakayama Y, Anderson JM, Matsuda T. Laboratory-scale mass production of a multi-micropatterned grafted surface with different polymer regions. J Biomed Mater Res. 2000;53:584–91. doi: 10.1002/1097-4636(200009)53:5<584::aid-jbm19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 62.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell. 2004;15:2943–53. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishiyama Y, Nakamura M, Henmi C, Yamaguchi K, Mochizuki S, Nakagawa H, Takiura K. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng. 2009;131:035001. doi: 10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- 64.Okochi N, Okazaki T, Hattori H. Encouraging effect of cadherin-mediated cell-cell junctions on transfer printing of micropatterned vascular endothelial cells. Langmuir. 2009;25:6947–53. doi: 10.1021/la9006668. [DOI] [PubMed] [Google Scholar]
- 65.Ouyang M, Sun J, Chien S, Wang Y. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc Natl Acad Sci U S A. 2008;105:14353–8. doi: 10.1073/pnas.0807537105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papenburg BJ, Vogelaar L, Bolhuis-Versteeg LA, Lammertink RG, Stamatialis D, Wessling M. One-step fabrication of porous micropatterned scaffolds to control cell behavior. Biomaterials. 2007;28:1998–2009. doi: 10.1016/j.biomaterials.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 67.Pompe T, Zschoche S, Herold N, Salchert K, Gouzy MF, Sperling C, Werner C. Maleic anhydride copolymers--a versatile platform for molecular biosurface engineering. Biomacromolecules. 2003;4:1072–9. doi: 10.1021/bm034071c. [DOI] [PubMed] [Google Scholar]
- 68.Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng Part A. 2010;16:2255–63. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhee SW, Taylor AM, Tu CH, Cribbs DH, Cotman CW, Jeon NL. Patterned cell culture inside microfluidic devices. Lab Chip. 2005;5:102–7. doi: 10.1039/b403091e. [DOI] [PubMed] [Google Scholar]
- 70.Roca-Cusachs P, Alcaraz J, Sunyer R, Samitier J, Farre R, Navajas D. Micropatterning of single endothelial cell shape reveals a tight coupling between nuclear volume in G1 and proliferation. Biophys J. 2008;94:4984–95. doi: 10.1529/biophysj.107.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sato M, Levesque MJ, Nerem RM. Micropipette aspiration of cultured bovine aortic endothelial cells exposed to shear stress. Arteriosclerosis. 1987;7:276–86. doi: 10.1161/01.atv.7.3.276. [DOI] [PubMed] [Google Scholar]
- 73.Satomi T, Nagasaki Y, Kobayashi H, Otsuka H, Kataoka K. Density control of poly(ethylene glycol) layer to regulate cellular attachment. Langmuir. 2007;23:6698–703. doi: 10.1021/la0624384. [DOI] [PubMed] [Google Scholar]
- 74.Sung HJ, Yee A, Eskin SG, McIntire LV. Cyclic strain and motion control produce opposite oxidative responses in two human endothelial cell types. Am J Physiol Cell Physiol. 2007;293:C87–94. doi: 10.1152/ajpcell.00585.2006. [DOI] [PubMed] [Google Scholar]
- 75.Takano H, Sul JY, Mazzanti ML, Doyle RT, Haydon PG, Porter MD. Micropatterned substrates: approach to probing intercellular communication pathways. Anal Chem. 2002;74:4640–6. doi: 10.1021/ac0257400. [DOI] [PubMed] [Google Scholar]
- 76.Tan JL, Liu W, Nelson CM, Raghavan S, Chen CS. Simple approach to micropattern cells on common culture substrates by tuning substrate wettability. Tissue Eng. 2004;10:865–72. doi: 10.1089/1076327041348365. [DOI] [PubMed] [Google Scholar]
- 77.Trkov S, Eng G, Di Liddo R, Parnigotto PP, Vunjak-Novakovic G. Micropatterned three-dimensional hydrogel system to study human endothelial-mesenchymal stem cell interactions. J Tissue Eng Regen Med. 2010;4:205–15. doi: 10.1002/term.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uttayarat P, Chen M, Li M, Allen FD, Composto RJ, Lelkes PI. Microtopography and flow modulate the direction of endothelial cell migration. Am J Physiol Heart Circ Physiol. 2008;294:H1027–35. doi: 10.1152/ajpheart.00816.2007. [DOI] [PubMed] [Google Scholar]
- 79.Uttayarat P, Perets A, Li M, Pimton P, Stachelek SJ, Alferiev I, Composto RJ, Levy RJ, Lelkes PI. Micropatterning of three-dimensional electrospun polyurethane vascular grafts. Acta Biomater. 2010;6:4229–37. doi: 10.1016/j.actbio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 80.Uttayarat P, Toworfe GK, Dietrich F, Lelkes PI, Composto RJ. Topographic guidance of endothelial cells on silicone surfaces with micro- to nanogrooves: orientation of actin filaments and focal adhesions. J Biomed Mater Res A. 2005;75:668–80. doi: 10.1002/jbm.a.30478. [DOI] [PubMed] [Google Scholar]
- 81.van Kooten TG, von Recum AF. Cell adhesion to textured silicone surfaces: the influence of time of adhesion and texture on focal contact and fibronectin fibril formation. Tissue Eng. 1999;5:223–40. doi: 10.1089/ten.1999.5.223. [DOI] [PubMed] [Google Scholar]
- 82.Vartanian KB, Berny MA, McCarty OJ, Hanson SR, Hinds MT. Cytoskeletal structure regulates endothelial cell immunogenicity independent of fluid shear stress. Am J Physiol Cell Physiol. 2010;298:C333–41. doi: 10.1152/ajpcell.00340.2009. [DOI] [PubMed] [Google Scholar]
- 83.Vartanian KB, Kirkpatrick SJ, Hanson SR, Hinds MT. Endothelial cell cytoskeletal alignment independent of fluid shear stress on micropatterned surfaces. Biochem Biophys Res Commun. 2008;371:787–92. doi: 10.1016/j.bbrc.2008.04.167. [DOI] [PubMed] [Google Scholar]
- 84.Vartanian KB, Kirkpatrick SJ, McCarty OJ, Vu TQ, Hanson SR, Hinds MT. Distinct extracellular matrix microenvironments of progenitor and carotid endothelial cells. J Biomed Mater Res A. 2009;91:528–39. doi: 10.1002/jbm.a.32225. [DOI] [PubMed] [Google Scholar]
- 85.Wang YC, Ho CC. Micropatterning of proteins and mammalian cells on biomaterials. Faseb J. 2004;18:525–7. doi: 10.1096/fj.03-0490fje. [DOI] [PubMed] [Google Scholar]
- 86.Woodrow KA, Wood MJ, Saucier-Sawyer JK, Solbrig C, Saltzman WM. Biodegradable meshes printed with extracellular matrix proteins support micropatterned hepatocyte cultures. Tissue Eng Part A. 2009;15:1169–79. doi: 10.1089/ten.tea.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu CC, Li YS, Haga JH, Kaunas R, Chiu JJ, Su FC, Usami S, Chien S. Directional shear flow and Rho activation prevent the endothelial cell apoptosis induced by micropatterned anisotropic geometry. Proc Natl Acad Sci U S A. 2007;104:1254–9. doi: 10.1073/pnas.0609806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xia N, Thodeti CK, Hunt TP, Xu Q, Ho M, Whitesides GM, Westervelt R, Ingber DE. Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation. Faseb J. 2008;22:1649–59. doi: 10.1096/fj.07-090571. [DOI] [PubMed] [Google Scholar]
- 89.Xu F, Sun Y, Chen Y, Sun Y, Li R, Liu C, Zhang C, Wang R, Zhang Y. Endothelial cell apoptosis is responsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku J Exp Med. 2009;218:25–33. doi: 10.1620/tjem.218.25. [DOI] [PubMed] [Google Scholar]
- 90.Xu T, Rohozinski J, Zhao W, Moorefield EC, Atala A, Yoo JJ. Inkjet-mediated gene transfection into living cells combined with targeted delivery. Tissue Eng Part A. 2009;15:95–101. doi: 10.1089/ten.tea.2008.0095. [DOI] [PubMed] [Google Scholar]
- 91.Yoshimoto K, Ichino M, Nagasaki Y. Inverted pattern formation of cell microarrays on poly(ethylene glycol) (PEG) gel patterned surface and construction of hepatocyte spheroids on unmodified PEG gel microdomains. Lab Chip. 2009;9:1286–9. doi: 10.1039/b818610n. [DOI] [PubMed] [Google Scholar]
- 92.Yung YC, Chae J, Buehler MJ, Hunter CP, Mooney DJ. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci U S A. 2009;106:15279–84. doi: 10.1073/pnas.0905891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zinchenko YS, Culberson CR, Coger RN. Contribution of non-parenchymal cells to the performance of micropatterned hepatocytes. Tissue Eng. 2006;12:2241–1251. doi: 10.1089/ten.2006.12.2241. [DOI] [PubMed] [Google Scholar]