Abstract
Macular oedema typically results from blood–retinal barrier disruption. It has recently been reported that patients with multiple sclerosis treated with FTY-720 (fingolimod) may exhibit macular oedema. Multiple sclerosis is not otherwise thought to be associated with macular oedema except in the context of comorbid clinical uveitis. Despite a lack of myelin, the retina is a site of inflammation and microglial activation in multiple sclerosis and demonstrates significant neuronal and axonal loss. We unexpectedly observed microcystic macular oedema using spectral domain optical coherence tomography in patients with multiple sclerosis who did not have another reason for macular oedema. We therefore evaluated spectral domain optical coherence tomography images in consecutive patients with multiple sclerosis for microcystic macular oedema and examined correlations between macular oedema and visual and ambulatory disability in a cross-sectional analysis. Participants were excluded if there was a comorbidity that could account for the presence of macular oedema, such as uveitis, diabetes or other retinal disease. A microcystic pattern of macular oedema was observed on optical coherence tomography in 15 of 318 (4.7%) patients with multiple sclerosis. No macular oedema was identified in 52 healthy controls assessed over the same period. The microcystic oedema predominantly involved the inner nuclear layer of the retina and tended to occur in small, discrete patches. Patients with multiple sclerosis with microcystic macular oedema had significantly worse disability [median Expanded Disability Score Scale 4 (interquartile range 3–6)] than patients without macular oedema [median Expanded Disability Score Scale 2 (interquartile range 1.5–3.5)], P = 0.0002. Patients with multiple sclerosis with microcystic macular oedema also had higher Multiple Sclerosis Severity Scores, a measure of disease progression, than those without oedema [median of 6.47 (interquartile range 4.96–7.98) versus 3.65 (interquartile range 1.92–5.87), P = 0.0009]. Microcystic macular oedema occurred more commonly in eyes with prior optic neuritis than eyes without prior optic neuritis (50 versus 27%) and was associated with lower visual acuity (median logMAR acuity of 0.17 versus −0.1) and a thinner retinal nerve fibre layer. The presence of microcystic macular oedema in multiple sclerosis suggests that there may be breakdown of the blood–retinal barrier and tight junction integrity in a part of the nervous system that lacks myelin. Microcystic macular oedema may also contribute to visual dysfunction beyond that explained by nerve fibre layer loss. Microcystic changes need to be assessed, and potentially adjusted for, in clinical trials that evaluate macular volume as a marker of retinal ganglion cell survival. These findings also have implications for clinical monitoring in patients with multiple sclerosis on sphingosine 1-phosphate receptor modulating agents.
Keywords: multiple sclerosis, optical coherence tomography, retina, macular oedema
Introduction
Multiple sclerosis is a disease of the CNS characterized by immune-mediated injury, demyelination and neuroaxonal loss (Lassmann et al., 2007). Despite a lack of myelin, which is thought to contain the principal target(s) of immune activation in multiple sclerosis, the retina is a site of neuronal and axonal loss in multiple sclerosis (Trip et al., 2005; Costello et al., 2006; Fisher et al., 2006; Gordon-Lipkin et al., 2007; Pulicken et al., 2007; Green et al., 2010; Talman et al., 2010; Saidha et al., 2011b). The retina is also a site of inflammation and blood–retinal barrier disruption in multiple sclerosis (Rucker, 1944; Lightman et al., 1987; Kerrison et al., 1994, Green et al., 2010). As in the brain, retinal inflammation in multiple sclerosis is most prominent in the perivascular space and can sometimes be identified on clinical examination in the form of retinal periphlebitis (ter Braak and van Herwaarden, 1933; Rucker, 1944; Lightman et al., 1987), a finding associated with greater overall multiple sclerosis disease activity (Sepulcre et al., 2007). Inflammation and microglial activation are also evident within the parenchyma of the inner retina in multiple sclerosis (Green et al., 2010), but this pathological finding has not yet been confirmed in a clinical setting. Given that the retina is unmyelinated, exploring how retinal inflammation arises in multiple sclerosis may yield insights about the relationship between inflammation and neurodegeneration in the disease.
Optical coherence tomography (OCT) is a non-invasive technique in which the backscatter of infrared light directed against a target tissue is used to generate cross-sectional images (Frohman et al., 2006). Advances in spectral domain OCT afford faster scan times, automation of data collection and superior structural resolution. OCT in multiple sclerosis has traditionally been used as a measure of axonal loss, with numerous studies demonstrating thinning of the peripapillary retinal nerve fibre and a reduction in total macular volume (Trip et al., 2005; Costello et al., 2006, 2009; Fisher et al., 2006; Pulicken et al., 2007; Burkholder et al., 2009; Talman et al., 2010; Saidha et al., 2011a). Recent work using OCT also demonstrates that there is thinning of the retinal ganglion cell, inner and outer nuclear layers in multiple sclerosis (Saidha et al., 2011b).
Macular oedema, the collection of fluid within the retina, has a number of potential causes, such as diabetes, uveitis, retinal vein occlusion and age-related macular degeneration (Early Treatment Diabetic Retinopathy Study Research Group, 1985; Marmor, 1999; Tran et al., 2008; Scholl et al., 2010; Sugar et al., 2011). In multiple sclerosis, cystoid macular oedema can occur in patients being treated with fingolimod, an oral sphingosine 1-phosphate receptor modulator (Cohen et al., 2010; Kappos et al., 2010). Cystoid macular oedema is also a known complication of uveitis and pars planitis in multiple sclerosis (Malinowski et al., 1993). In the absence of uveitis, fingolimod treatment or another comorbidity, however, macular oedema is not thought to be associated with multiple sclerosis.
OCT has been shown to be a robust technique for identifying macular oedema (Mackenzie et al., 2011; Sugar et al., 2011). Microcystic macular oedema on spectral domain OCT was clinically observed in a subset of patients with multiple sclerosis imaged in our laboratory who did not have another reason to have macular oedema, such as symptomatic uveitis, diabetes or fingolimod exposure. Macular oedema was not observed on histopathology at autopsy of patients who died with multiple sclerosis, but this approach may have been limited by fixation, sectioning and time of sampling (Green et al., 2010). We therefore analysed retinal spectral domain OCT images in consecutive patients with multiple sclerosis for the presence of microcystic macular oedema and examined correlations between microcystic macular oedema and both visual and ambulatory disability in a cross-sectional analysis.
Materials and methods
Study population
The neurodiagnostics laboratory at the University of California, San Francisco Multiple Sclerosis Centre provides a spectral domain OCT service for patients with neurological disease. The study base for this cross-sectional analysis was consecutive patients with multiple sclerosis referred for spectral domain OCT imaging at the University of California, San Francisco Multiple Sclerosis Centre as a standard evaluation for multiple sclerosis in our clinic between January 2010 and August 2011. Spectral domain OCT images of 52 healthy controls without known neurological or ophthalmological disease, some of who were friends or spouses of patients with multiple sclerosis, were also examined for evidence of microcystic macular oedema. All participants provided written informed consent, and the University of California, San Francisco Committee on Human Research approved the study protocol.
Participants classified as having multiple sclerosis satisfied 2005 International Panel diagnostic criteria (Polman et al., 2005). Participants were excluded if there was any history of a comorbidity that could plausibly account for the presence of macular oedema, including a prior history of uveitis (n = 8), diabetes (n = 4), retinal vein occlusion, age-related macular degeneration (n = 1), retinal infection (n = 1), retinal surgery (n = 1), retinitis pigmentosa (n = 2), cataract surgery (within 1 year) or intraocular malignancy (n = 1). Participants with a history of glaucoma were also excluded to minimize possible confounding for correlations with retinal nerve fibre layer (RNFL) thickness and visual function. Subtype and stage of multiple sclerosis were assigned by the treating multiple sclerosis specialists and confirmed by the study investigators through medical record review. A history of acute demyelinating optic neuritis, defined clinically as a subacute episode of visual blurring or loss associated with eye pain, was determined by the treating multiple sclerosis specialist and confirmed by study investigators through subject interview and medical record review. Disease duration was defined as the time from the first clinical symptom attributable to multiple sclerosis to the date of OCT evaluation. The Expanded Disability Score Scale (EDSS) (Kurtzke, 1983) was assigned by the treating multiple sclerosis specialist and confirmed by the study investigators through medical record review (and not calculated during the time of an acute relapse). The Multiple Sclerosis Severity Score, a predictor of future disability in multiple sclerosis, was calculated from the EDSS and clinical disease duration (Roxburgh et al., 2005).
Visual evaluations
High contrast visual acuity was measured using a computerized Early Treatment Diabetic Retinopathy Study chart (ProVideo system, INNOVA Systems). For statistical analysis, visual acuity was converted from the Snellen to the LogMAR scale, which was calculated as the negative log (base 10) of the decimal value of the Snellen acuity. A LogMAR value of 0 is equivalent to 20/20 vision, with positive values indicating less than 20/20 vision and negative values indicating better than 20/20 vision. Slit-lamp examinations and dilated retinal ophthalmoscopy were not routinely performed.
Spectral domain optical coherence tomography
Spectral domain OCT was performed using the Spectralis OCT system (Heidelberg Engineering). For evaluation of the macula and macular volume measures, raster scans of the macula (20 × 15°) consisting of 19 line scans were obtained. For circumpapillary measurements of RNFL, the peripapillary RNFL was measured at a distance of 3.4 mm from the centre of the papilla. Prior to analysis, RNFL thickness was calculated using the provided software, with quality control measures including confirmation of accurate inner limiting membrane identification and absence of other significant retinal pathology. Furthermore, scans with insufficient signal to noise ratio or edge detection/retinal thickness algorithm failure were excluded and measurements were repeated until a good quality image was achieved. Automatic real-time is a method for maintaining OCT B-scan alignment and registration during image acquisition on the Spectralis OCT. The automatic real-time number is the number of B-scans averaged to produce the final image. In Spectralis OCT the quality number is a measure of signal strength. Our laboratory standard includes a target automatic real-time number of 48 for raster scans of the macula and a quality number of 20.
Microcystic macular oedema was defined as cystic, lacunar areas of hyporeflectivity with clear boundaries (Brar et al., 2010) on spectral domain OCT macular volume images, excluding lesions due to speckling artefact. Scans reported as demonstrating microcycstic macular oedema were additionally confirmed by a retinal specialist (D.M.S.), blinded to neurological history. For confirmation we required that microcystic abnormalities be unequivocally identified on two adjacent B-scans and on two separate acquisitions.
Statistical analyses
Differences in demographic variables between patients with multiple sclerosis with and without macular oedema were analysed using the student's t-test for age, the χ2 test for sex and the Wilcoxon Rank Sum test for EDSS, Multiple Sclerosis Severity Score and disease duration (non-parametric analysis). Differences in optic neuritis history between eyes with and without macular oedema were analysed using the χ2 test. Multiple linear regression was used to examine differences in visual acuity, RNFL and macular volume between the groups of eyes with and without microcystic macular oedema, adjusting for age, sex, disease duration and optic neuritis. To account for within-patient inter-eye correlations, the standard error was adjusted for possible clustering using the clustered sandwich estimator ordinary least squares method. A P-value of <0.05 was interpreted as significant. Statistical analyses were performed using Stata 12.0 (Statacorp).
Results
Microcystic macular oedema was observed in 15 of 318 (4.7%) unique, consecutive patients with multiple sclerosis over the 20-month study period. The microcystic oedema was bilateral in five patients. Six additional patients with multiple sclerosis exhibited various manifestations of macular oedema on spectral domain OCT, but were excluded from analysis due to a history of diabetes, viral retinitis, retinitis pigmentosa and prior ocular radiation. None of the patients with microcystic macular oedema were taking fingolimod at the time of baseline OCT scanning or had taken fingolimod prior to the baseline scan. None of 52 unaffected controls [mean age 36.9 years (standard deviation, SD 11.8), 56% female, logMAR acuity median −0.12 (interquartile range −0.3 to −0.12)] had evidence of microcystic macular oedema on spectral domain OCT.
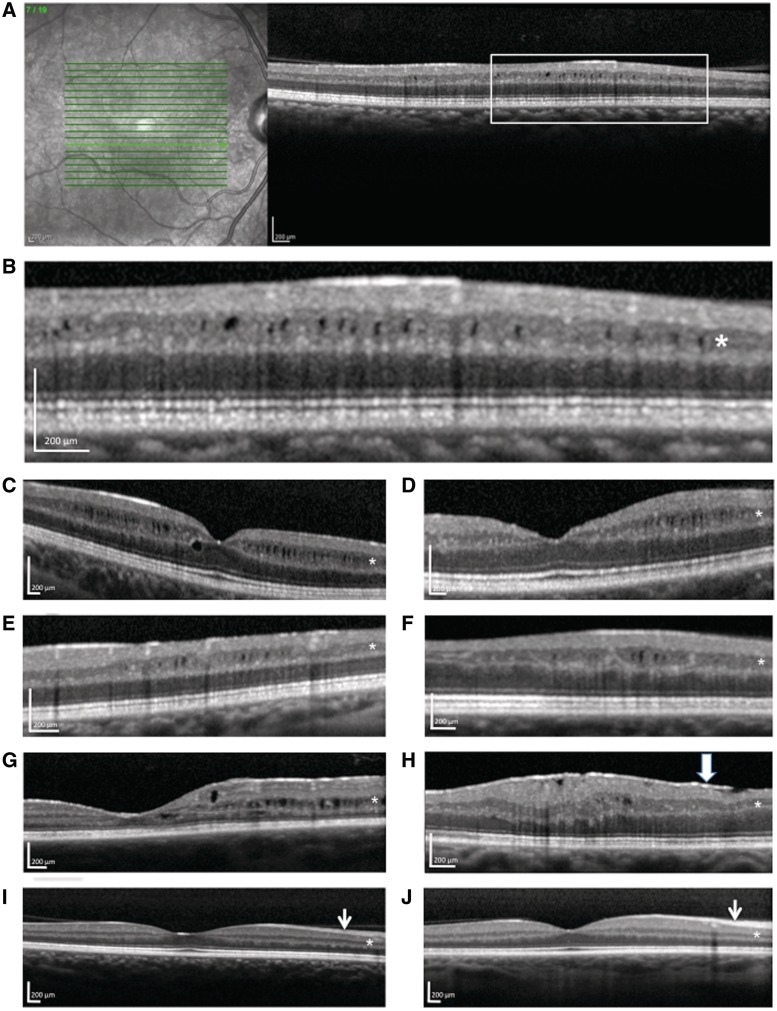
As illustrated in Fig. 1, the microcystic oedema predominantly involved the inner nuclear layer of the retina and tended to occur in small, discrete patches. The microcysts ranged in size from ∼20 × 30 µm to ∼70 × 90 µm along the x–y axis. Below the microcysts, lower reflectance (darker colour on spectral domain OCT) was observed in deeper retinal layers, which is indicative of shadowing and supportive of the presence of fluid in the inner nuclear layer. The location of microcystic oedema detected ranged from the foveal centre to as far as 2300 µm from the fovea, but most of the oedema occurred ∼500–1800 µm from the foveal centre and variably affected all quadrants. In three eyes with microcystic changes in the inner nuclear layer, there was concomitant involvement of the outer nuclear layer (Fig. 1G), which was directly foveal (140–300 µm from the foveal centre) in one patient and 800–900 µm from the fovea in the others. Two eyes with microcystic oedema in the inner nuclear layer also exhibited mild involvement in the ganglion cell layer. An epiretinal membrane was noted in 2 of the 15 patients with multiple sclerosis with microcystic oedema, one of which demonstrated minimal retina traction (not thought to be significant enough to cause oedema). None of the patients with microcystic macular oedema had evidence of hyaloid membrane traction. None of the patients with microcystic macular oedema had evidence of retinal periphlebitis.
Figure 1.
Microcystic macular oedema on spectral domain optical coherence tomography in multiple sclerosis. (A–H) Seven different patients with multiple sclerosis; B is a magnified view of A. The microcysts predominantly involved the inner nuclear layer of the retina (asterisks). For comparison purposes, I is a representative scan without macular oedema in a patient with relapsing-remitting multiple sclerosis and J is a representative scan from an unaffected control. Note that the retinal nerve fibre layer (short arrow) is thinner in the patient with multiple sclerosis (I) than in the control participant (J). An epiretinal membrane is noted in H (wide arrow).
Patients with multiple sclerosis with microcystic macular oedema (Table 1) tended to be slightly older and have longer disease durations than patients with multiple sclerosis without macular oedema, but these differences were not statistically significant.
Table 1.
Patients with multiple sclerosis with and without microcystic macular oedema
| Patients with multiple sclerosis without oedema (n = 303) | Patients with multiple sclerosis with microcystic macular oedema (n = 15) | P-value | |
|---|---|---|---|
| Age (years), mean (SD) | 41.5 (13.2) | 44.9 (13) | 0.33a |
| Female (%) | 203 (67) | 7 (47) | 0.1b |
| Disease duration (years), median (IQR) | 6.2 (2.3–11.7) | 9.2 (4.7–22.2) | 0.1c |
| EDSS, median (IQR) | 2 (1.5–3.5) | 4 (3.0–6.25) | 0.0002c |
| Multiple Sclerosis Severity Score, median (IQR) | 3.65 (1.92–5.87) | 6.47 (4.96–7.98) | 0.0009c |
| Multiple sclerosis subtype, n (%) | |||
| Clinically isolated syndrome | 21 (7) | 0 (0) | 0.23d |
| Relapsing-remitting multiple sclerosis | 230 (76) | 12 (80) | |
| Secondary-progressive multiple sclerosis | 30 (10) | 1 (6) | |
| Primary-progressive multiple sclerosis | 17 (6) | 2 (13) | |
| Progressive-relapsing multiple sclerosis | 5 (2) | 0 (0) | |
Percentages do not add up to 100 due to rounding.
a Student’s t-test.
b χ2 test.
c Wilcoxon Rank Sum test.
d ANOVA.
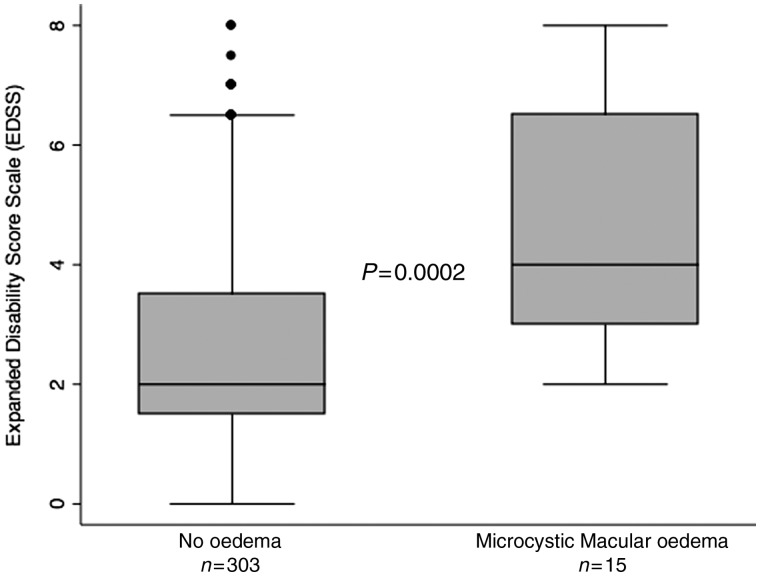
Patients with multiple sclerosis with microcystic macular oedema had significantly worse disability (as measured by median EDSS) than patients with multiple sclerosis without macular oedema [4 (interquartile range, IQR 3–6) versus 2 (IQR 1.5–3.5), P = 0.0002; Table 1 and Fig. 2]. Adjustment for age and disease duration using a logistic regression model did not change the results. Patients with multiple sclerosis with microcystic macular oedema also had higher Multiple Sclerosis Severity Scores than patients without oedema [6.47 (IQR 4.96–7.98) versus 3.65 (IQR 1.92–5.87), P = 0.0009; Table 1], indicating a higher risk of future disability.
Figure 2.
Microcystic macular oedema in multiple sclerosis is associated with greater disability (EDSS). The P-value was calculated by the Wilcoxon Rank Sum test. The line in the centre of the box indicates the median and the shaded box denotes the IQR. The whiskers denote minimum and maximum values, excluding outliers (defined as >1.5 times the lower and upper quartile values).
Visual acuity was more impaired in eyes with microcystic macular oedema (median logMAR acuity of 0.17, which is equivalent to a Snellen acuity of about 20/30) than multiple sclerosis eyes without oedema (median logMAR acuity of −0.1). Even so, one-third of multiple sclerosis eyes with microcystic macular oedema had a visual acuity better than 20/25. Visual acuity impairment remained associated with microcystic macular oedema after adjustment for optic neuritis history and age, sex, disease duration and RNFL thickness in a linear regression model (P = 0.02), and in a model adjusting for RNFL thickness as a categorical variable (75 µm and below versus >75 µm) given possible threshold effects of RNFL loss on visual dysfunction (Costello et al., 2006).
A history of optic neuritis was more common in eyes with microcystic macular oedema than eyes without oedema (Table 2). The optic neuritis was acute (within 14 days) in a single patient with microcystic oedema and in the others occurred a median of 3 years prior to scanning (IQR 1–11 years, range 0.5–31 years). The patient with a recent acute optic neuritis had just completed a course of corticosteroids; a second patient with microcystic oedema received a dose of monthly pulse steroids 2 months prior to OCT scanning; a third patient received a course of intravenous pulse steroids 5 months prior; and a fourth patient received steroids 7 months prior; none of the other 11 patients with microcystic macular oedema had received corticosteroids for more than a year prior to OCT scanning.
Table 2.
Ocular parameters in patients with multiple sclerosis with and without microcystic macular oedema
| Eyes without macular oedema (n = 606) | Eyes with microcystic macular oedema (n = 20)a | P-value | |
|---|---|---|---|
| Prior symptomatic optic neuritis in that eye, n (%) | 161 (27) | 10 (50) | 0.02b |
| Total RNFL thickness (µm), mean (SD) | 87 (15) | 66.7 (15.4) | <0.001c |
| Macular volume (mm3), mean (SD) | 3.01 (0.22) | 2.9 (0.15) | 0.12c |
| Foveal thickness (µm), mean (SD) | 271.2 (21.9) | 276.4 (28.8) | 0.50c |
| Visual acuity (logMAR), median (IQR) | −0.1 (−0.1 to 0) | 0.17 (0 to 0.4) | 0.001c |
| 0.03d |
a Excluding fellow eyes in patients with unilateral microcystic macular oedema.
b χ2 test.
c Linear regression, with the standard error adjusted for possible clustering at the patient level (within-patient inter-eye correlations).
d Linear regression of differences in visual acuity between groups, adjusting for RNFL thickness, optic neuritis history in that eye, age, sex and disease duration, with the standard error adjusted for possible clustering at the patient level (within-patient inter-eye correlations).
The total RNFL was thinner in eyes with microcystic macular oedema (median 66 µm) than multiple sclerosis eyes without oedema (median 87 µm) (Table 1). The total RNFL was also thinner in eyes with microcystic macular oedema after adjustment for age, sex, disease duration and history of symptomatic optic neuritis in that eye (P < 0.001). Macular volume trended lower in eyes with microcystic oedema than multiple sclerosis eyes without oedema, but after adjustment for history of optic neuritis, this was not significant (P = 0.3).
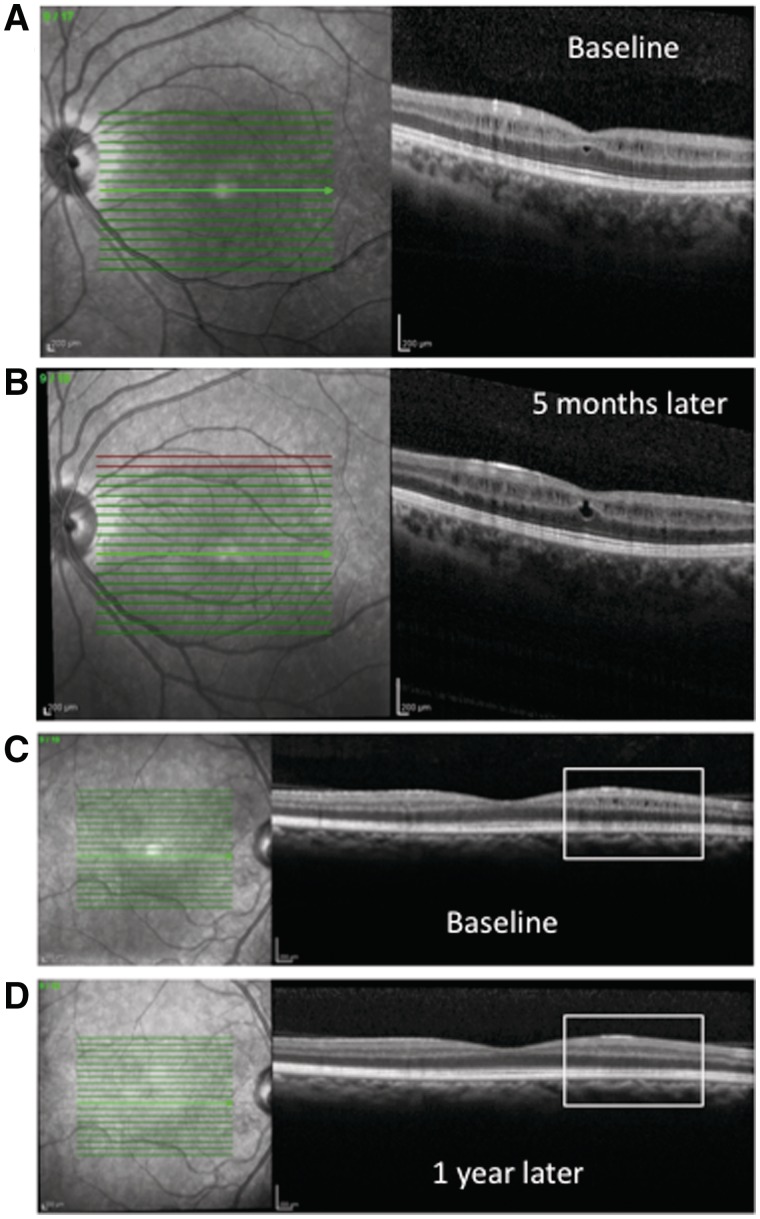
Longitudinal spectral domain OCT scanning was available on a convenience sample of six of the 15 patients with microcystic macular oedema. Microcystic changes appeared to be dynamic over time, improving in size, appearance and area of involvement in some patients and becoming more prominent in others (Fig. 3). The microcystic oedema improved in two of the patients (completely resolving in one), was relatively stable in one and worsened in the three others (median follow-up time 9.5 months, range 3–12 months). Two of the six patients with longitudinal follow-up of microcystic macular oedema elected to proceed with fingolimod therapy—the macular oedema was stable in one patient at 9 months of follow-up and slightly more prominent in the other patient at 3 months of follow-up, with no significant change in visual acuity.
Figure 3.
Microcystic macular oedema worsened at 5 months of follow-up in one patient (A and B), with an increase in macular volume from 3.14 to 3.19 mm3, while improving at 12 months of follow-up in another patient (C and D), with a decrease in macular volume from 3.01 to 2.99 mm3. In A and B, the automatic real-time was 70 and quality was 26 and 22, respectively. In C and D, the automatic real-time was 30 and the quality was 29 and 22, respectively.
Discussion
Microcystic macular oedema, predominantly involving the inner nuclear layer of the retina, was observed in a subset of patients with multiple sclerosis with no other identifiable cause. The presence of microcystic macular oedema was associated with greater overall disability (EDSS), disease severity (Multiple Sclerosis Severity Score), reduced visual acuity and occurred more commonly in eyes with prior optic neuritis.
In a large series of patients with macular oedema evaluated in a subspecialty retina clinic, microcysts involving the inner nuclear layer were observed on spectral domain OCT in a quarter of eyes demonstrating diffuse fluorescein leakage (a marker of blood–retinal barrier breakdown) (Brar et al., 2010). Microcysts were not detected on fluorescein angiography in any of those cases, possibly due to the lower spatial resolution of fluorescein angiography compared with spectral domain OCT, even when performed using a scanning laser ophthalmoscope (Brar et al., 2010). These observations further illustrate the importance of source image analysis of retinal OCT in multiple sclerosis and not just a review of automated quantitative algorithms. Increases in macular volume are sometimes used as a marker for macular oedema (Sugar et al., 2011), but macular volumes tended to be lower in eyes with microcystic macular oedema than eyes without oedema in our cross-sectional analysis. This is likely to be attributable to microcystic oedema occurring more frequently in eyes with prior optic neuritis, a well-established cause of macular volume loss (Trip et al., 2005; Pulicken et al., 2007; Burkholder et al., 2009).
In Phase III trials of fingolimod, a sphingosine 1-phosphate receptor modulator used as disease modifying therapy in multiple sclerosis, cystoid macular oedema was observed in 0–0.5% of patients randomized to the 0.5 mg dose and 0.7–1% of patients randomized to the 1.2 mg dose (Cohen et al., 2010; Kappos et al., 2010; Khatri et al., 2011). Macular oedema was also observed in renal transplant patients taking fingolimod (Salvadori et al., 2006; FDA, 2010). Screening and follow-up testing in those studies, however, were performed using older generation time-domain OCT platforms that lack the resolution to readily identify the kinds of microcysts described here. Fingolimod-associated macular oedema is thought to be reversible with treatment cessation (Saab et al., 2008), but at least one patient with multiple sclerosis with cystoid macular oedema on fingolimod therapy required surgery to repair a full-thickness macular hole (FDA, 2010). Whether the initiation of fingolimod augments or accelerates pre-existing microcystic macular oedema in multiple sclerosis is unknown. Spectral domain OCT screening of patients with multiple sclerosis prior to fingolimod initiation may be helpful to detect baseline microcystic macular oedema and track associations with therapy.
Macular oedema typically results from breakdown of the blood–retinal barrier (Marmor, 1999). The blood–retinal barrier is analogous to the blood–brain barrier, with shared biochemical and molecular mechanisms for maintenance of endothelial tight junctions (Cunha-Vaz et al., 1966; Runkle and Antonetti, 2011). Disruption of the blood–brain barrier is a pathological and radiological hallmark of acute multiple sclerosis plaques (Gay and Esiri, 1991; Gaitan et al., 2011). Histopathological evidence of blood–brain barrier leakage is also evident in normal appearing white matter in multiple sclerosis (Kirk et al., 2003; Padden et al., 2007). The cause of microcystic macular oedema in multiple sclerosis is unknown, but if it relates to blood–retinal barrier disruption, possibly due to subclinical uveitis or retinitis (Donaldson et al., 2007; Vidovic-Valentincic et al., 2009), it would suggest that breakdown of tight junction integrity in multiple sclerosis may also occur in an area of the nervous system without myelin. One possible explanation for how this may occur is that retinal neuronal and axonal injury could lead to release of cellular distress signals, which, in turn, could lead to focal intraretinal microglial activation and inflammation. This could cause local blood–retinal barrier disruption and fluid accumulation with cystic changes within the retina. Another possible mechanism is that blood–retinal barrier breakdown may occur concurrently with blood–brain barrier breakdown, as evidenced by the association of multifocal sites of leakage on fluorescein angiography in some patients with acute optic neuritis (Lightman et al., 1987).
The inner nuclear layer is a prominent site of retinal inflammation and microglial activation in multiple sclerosis (Green et al., 2010). Inner nuclear layer thinning in multiple sclerosis is also associated with greater disease severity (Multiple Sclerosis Severity Score) (Saidha et al., 2011b). A mixed retinal phenotype with inner and outer nuclear layer thinning associated with RNFL and ganglion cell layer and inner plexiform layer thinning was also recently described in multiple sclerosis (Saidha et al., 2011a). The predominance of microcystic changes within the inner nuclear layer raises the hypothesis that microcystic macular oedema may be a clinical correlate of inner nuclear inflammation and microglial activation in multiple sclerosis. Microglia in the retina are concentrated within two parallel networks in the inner and outer plexiform layers (Hume et al., 1983), which are the layers that lie immediately adjacent to the inner nuclear layer. Longitudinal studies will be needed to determine if macular oedema is associated with neuronal loss in the inner nuclear layer or possibly transsynaptic degeneration. If such an association exists, it may help to account for the recently described macular thinning phenotype of retinal neuronal loss in a subset of patients with aggressive multiple sclerosis (Saidha et al., 2011b).
One of the most significant advances in retinal OCT imaging in recent years is the combination of the spectral domain technique with automated image registration. This enables dramatically increased imaging speed, which provides the capability to repeatedly sample individual B-scans and average these multiple snapshots into a crisper composite image by reducing signal to noise (which is the concept of the automatic real-time measure on the Heidelberg spectral domain OCT platform used in this study). While the use of high automatic real-time sequences improves accuracy of retinal thickness measures, too high an automatic real-time can have the paradoxical effect of over-averaging underlying retinal pathology, potentially leading to obscuration of microcystic changes. Averaging B-scans without real-time tracking would also be likely to make microcystic changes more difficult to resolve. Incorporation of macular volume scans with less averaging into the standard OCT protocol for evaluation of patients with multiple sclerosis may help to improve detection of macular pathology.
This study has some important limitations. Fluorescein angiography was not performed as part of the study protocol. Future studies examining this phenotype of microcystic macular oedema in multiple sclerosis would benefit from the inclusion of fluorescein angiography, as leakage of fluorescein would be direct evidence of breakdown of the blood–retinal barrier. As spectral domain OCT examines only a limited area of retina (with poor coverage of the retinal periphery), our methods are also likely to underestimate the prevalence of microcystic retinal oedema in multiple sclerosis. The inclusion of imaging at greater eccentricity from the fovea in future studies would also provide added coverage to screen for microcystic changes in retinal regions not included in macular OCT scans. The dynamic nature of microcystic retinal oedema in multiple sclerosis could also bias the results towards under-sampling as this was a cross-sectional analysis. As patients with a prior history of uveitis were excluded from this analysis, the prevalence of microcystic macular oedema in multiple sclerosis overall may also be underestimated. It is also possible that unrecognized comorbidities not detected through routine clinical evaluation contributed to the formation of microcystic macular oedema in these patients.
If this finding is confirmed in other studies, microcystic macular oedema may prove to be a clinically and mechanistically meaningful marker of disease activity in multiple sclerosis. Further research is needed to understand why microcystic macular oedema in multiple sclerosis occurs and whether microcystic oedema could also be a potentially reversible cause of visual dysfunction in multiple sclerosis.
Funding
American Academy of Neurology Clinical Research Training Fellowship (to J.M.G.); National Institutes of Health (KL2 RR-024130), Howard Hughes Medical Institute Physician Scientist Early Career Award (57006497) and Debbie and Andy Rachleff Distinguished Professorship of Neurology (to A.J.G.).
Glossary
Abbreviations
- EDSS
Expanded Disability Score Scale
- OCT
optical coherence tomography
- RNFL
retinal nerve fibre layer
References
- Brar M, Yuson R, Kozak I, Mojana F, Cheng L, Bartsch DU, et al. Correlation between morphologic features on spectral-domain optical coherence tomography and angiographic leakage patterns in macular edema. Retina. 2010;30:383–9. doi: 10.1097/IAE.0b013e3181cd4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder BM, Osborne B, Loguidice MJ, Bisker E, Frohman TC, Conger A, et al. Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol. 2009;66:1366–72. doi: 10.1001/archneurol.2009.230. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–9. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci. 2009;281:74–9. doi: 10.1016/j.jns.2009.02.354. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG, Shakib M, Ashton N. Studies on the permeability of the blood-retinal barrier. I. On the existence, development, and site of a blood-retinal barrier. Br J Ophthalmol. 1966;50:441–53. doi: 10.1136/bjo.50.8.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson MJ, Pulido JS, Herman DC, Diehl N, Hodge D. Pars planitis: a 20-year study of incidence, clinical features, and outcomes. Am J Ophthalmol. 2007;144:812–7. doi: 10.1016/j.ajo.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- FDA. Center for Drug Evaluation and Research (CDER) Peripheral and Central Nervous System Drugs Advisory Committee Meeting Report. Fingolimod (NDA 22-527) Background Package, 20 June 2010. [Google Scholar]
- Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Frohman E, Costello F, Zivadinov R, Stuve O, Conger A, Winslow H, et al. Optical coherence tomography in multiple sclerosis. Lancet Neurol. 2006;5:853–63. doi: 10.1016/S1474-4422(06)70573-7. [DOI] [PubMed] [Google Scholar]
- Gaitan MI, Shea CD, Evangelou IE, Stone RD, Fenton KM, Bielekova B, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70:22–9. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Esiri M. Blood-brain barrier damage in acute multiple sclerosis plaques. An immunocytological study. Brain. 1991;114:557–72. doi: 10.1093/brain/114.1.557. [DOI] [PubMed] [Google Scholar]
- Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69:1603–9. doi: 10.1212/01.wnl.0000295995.46586.ae. [DOI] [PubMed] [Google Scholar]
- Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133(Pt 6):1591–601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA, Perry VH, Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983;97:253–7. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Kerrison JB, Flynn T, Green WR. Retinal pathologic changes in multiple sclerosis. Retina. 1994;14:445–51. doi: 10.1097/00006982-199414050-00010. [DOI] [PubMed] [Google Scholar]
- Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10:520–9. doi: 10.1016/S1474-4422(11)70099-0. [DOI] [PubMed] [Google Scholar]
- Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol. 2003;201:319–27. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–8. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S, McDonald WI, Bird AC, Francis DA, Hoskins A, Batchelor JR, et al. Retinal venous sheathing in optic neuritis. Its significance for the pathogenesis of multiple sclerosis. Brain. 1987;110(Pt 2):405–14. doi: 10.1093/brain/110.2.405. [DOI] [PubMed] [Google Scholar]
- Mackenzie S, Schmermer C, Charnley A, Sim D, Vikas T, Dumkyj M, et al. SDOCT imaging to identify macular pathology in patients diagnosed with diabetic maculopathy by a digital photographic retinal screening programme. PLoS One. 2011;6:e14811. doi: 10.1371/journal.pone.0014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski SM, Pulido JS, Folk JC. Long-term visual outcome and complications associated with pars planitis. Ophthalmology. 1993;100:818–25. doi: 10.1016/s0161-6420(93)31567-8. [DOI] [PubMed] [Google Scholar]
- Marmor MF. Mechanismultiple sclerosis of fluid accumulation in retinal edema. Doc Ophthalmol. 1999;97:239–49. doi: 10.1023/a:1002192829817. [DOI] [PubMed] [Google Scholar]
- Padden M, Leech S, Craig B, Kirk J, Brankin B, McQuaid S. Differences in expression of junctional adhesion molecule-A and beta-catenin in multiple sclerosis brain tissue: increasing evidence for the role of tight junction pathology. Acta Neuropathol. 2007;113:177–86. doi: 10.1007/s00401-006-0145-x. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–92. doi: 10.1212/01.wnl.0000294876.49861.dc. [DOI] [PubMed] [Google Scholar]
- Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005;64:1144–51. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- Rucker C. Sheathing of the retinal veins in multiple sclerosis. Proceedings of the Staff Meetings of the Mayo Clinic1944; 19: 176–8. [Google Scholar]
- Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011;686:133–48. doi: 10.1007/978-1-60761-938-3_5. [DOI] [PubMed] [Google Scholar]
- Saab G, Almony A, Blinder KJ, Schuessler R, Brennan DC. Reversible cystoid macular edema secondary to fingolimod in a renal transplant recipient. Arch Ophthalmol. 2008;126:140–1. doi: 10.1001/archophthalmol.2007.23. [DOI] [PubMed] [Google Scholar]
- Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011a;17:1449–63. doi: 10.1177/1352458511418630. [DOI] [PubMed] [Google Scholar]
- Saidha S, Syc SB, Ibrahim MA, Eckstein C, Warner CV, Farrell SK, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011b;134(Pt 2):518–33. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- Salvadori M, Budde K, Charpentier B, Klempnauer J, Nashan B, Pallardo LM, et al. FTY720 versus MMF with cyclosporine in de novo renal transplantation: a 1-year, randomized controlled trial in Europe and Australasia. Am J Transplant. 2006;6:2912–21. doi: 10.1111/j.1600-6143.2006.01552.x. [DOI] [PubMed] [Google Scholar]
- Scholl S, Kirchhof J, Augustin AJ. Pathophysiology of macular edema. Ophthalmologica. 2010;224(Suppl. 1):8–15. doi: 10.1159/000315155. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488–94. doi: 10.1212/01.wnl.0000260612.51849.ed. [DOI] [PubMed] [Google Scholar]
- Sugar EA, Jabs DA, Altaweel MM, Lightman S, Acharya N, Vitale AT, et al. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol. 2011;152:1044–52. doi: 10.1016/j.ajo.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman LS, Bisker ER, Sackel DJ, Long DA, Jr, Galetta KM, Ratchford JN, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–60. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Braak J, van Herwaarden A. Ophthalmo-encephalomyelitis (Clinical Journal of Ophthalmology) Klinische Monatsblätter für Augenheilkunde. 1933;91:316–43. [Google Scholar]
- Tran TH, de Smet MD, Bodaghi B, Fardeau C, Cassoux N, Lehoang P. Uveitic macular oedema: correlation between optical coherence tomography patterns with visual acuity and fluorescein angiography. Br J Ophthalmol. 2008;92:922–7. doi: 10.1136/bjo.2007.136846. [DOI] [PubMed] [Google Scholar]
- Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–91. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- Vidovic-Valentincic N, Kraut A, Hawlina M, Stunf S, Rothova A. Intermediate uveitis: long-term course and visual outcome. Br J Ophthalmol. 2009;93:477–80. doi: 10.1136/bjo.2008.149039. [DOI] [PubMed] [Google Scholar]