Abstract
Dysfunction of the γ-aminobutyric acid-ergic system in Tourette syndrome may conceivably underlie the symptoms of motor disinhibition presenting as tics and psychiatric manifestations, such as attention deficit hyperactivity disorder and obsessive–compulsive disorder. The purpose of this study was to identify a possible dysfunction of the γ-aminobutyric acid-ergic system in Tourette patients, especially involving the basal ganglia-thalamo-cortical circuits and the cerebellum. We studied 11 patients with Tourette syndrome and 11 healthy controls. Positron emission tomography procedure: after injection of 20 mCi of [11C]flumazenil, dynamic emission images of the brain were acquired. Structural magnetic resonance imaging scans were obtained to provide an anatomical framework for the positron emission tomography data analysis. Images of binding potential were created using the two-step version of the simplified reference tissue model. The binding potential images then were spatially normalized, smoothed and compared between groups using statistical parametric mapping. We found decreased binding of GABAA receptors in Tourette patients bilaterally in the ventral striatum, globus pallidus, thalamus, amygdala and right insula. In addition, the GABAA receptor binding was increased in the bilateral substantia nigra, left periaqueductal grey, right posterior cingulate cortex and bilateral cerebellum. These results are consistent with the longstanding hypothesis that circuits involving the basal ganglia and thalamus are disinhibited in Tourette syndrome patients. In addition, the abnormalities in GABAA receptor binding in the insula and cerebellum appear particularly noteworthy based upon recent evidence implicating these structures in the generation of tics.
Keywords: Tourette syndrome, tics, GABAA receptors, flumazenil, PET
Introduction
Gilles de la Tourette syndrome is a complex neuropsychiatric disorder characterized by multiple motor and vocal tics, which are associated with behavioural and emotional disturbances including symptoms of attention deficit hyperactivity disorder, obsessive–compulsive disorder, anxiety and depression. In spite of these diverse and pronounced symptoms, the causes of Tourette syndrome remain elusive. For many years the ‘dopaminergic’ theory of Tourette syndrome (Butler et al., 1979; Singer et al., 1982), which postulated that hypersensitivity of dopamine receptors and/or hyperactivity of dopaminergic neurons underlay the pathophysiology of Tourette syndrome, was prevalent. Subsequently, the majority of neuroimaging studies concentrated on evaluating striatal dopaminergic systems using PET or single-photon emission computed tomography (SPECT). However, the results obtained in these studies were inconclusive (Singer et al., 1992; Turjanski et al., 1994; Wong et al., 1997; Ernst et al., 1999; Muller-Vahl et al., 2000). Morphometric MRI studies showed abnormalities that included reduced caudate nucleus volume (Peterson et al., 2003), abnormality of the corpus callosum (Moriarty et al., 1997; Plessen et al., 2004), enlargement of the left thalamus (Lee et al., 2006), amygdala and hippocampus (Peterson et al., 2007), and increased grey matter of the left mesencephalon.
Several previous functional neuroimaging studies reported metabolic or haemodynamic abnormalities within the basal ganglia, thalamus (Braun et al., 1993; Peterson et al., 1998; Baym et al., 2008 a), insula and cerebellum (Bohlhalter et al. 2006; Lerner et al. 2007). These findings suggested that dysfunction involving striatal-pallidal-thalamic circuits could potentially contribute to the overactivity and disinhibition of the motor cortices seen in neuroimaging studies (Biswal et al., 1998; Stern et al. 2000) and neurophysiological studies of Tourette syndrome (Ziemann et al., 1997; Orth et al., 2005). Therefore, we hypothesized that an abnormality in the function of these networks could be caused by pathological changes in γ-aminobutyric acid (GABA)-ergic receptors. We designed a PET study using the ligand [11C]flumazenil to assess the involvement of the GABA-ergic system in Tourette syndrome pathology.
Materials and methods
Subjects
We studied 11 patients with Tourette syndrome and 11 normal volunteers. Patients’ ages ranged from 19 to 38 years (1 female, 10 male) (Table 3); control subjects were age- and gender-matched to the Tourette syndrome subjects (Supplementary Table 1). All patients had normal neurological examinations except for their tics. Patients were diagnosed with Tourette syndrome based on the neurological exam and Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV). All patients were also evaluated with the Structured Clinical Interview for DSM-IV (SCID) to assess for possible comorbid psychiatric disorders. Four patients had obsessive–compulsive disorder, two had subthreshold obsessive–compulsive disorder, three had current attention deficit hyperactivity disorder and two had a remote history of attention deficit hyperactivity disorder (Table 3). Tic severity was quantified using the Yale Global Tic Severity Scale (Leckman et al., 1989). The ratings and clinical evaluations were done while on usual medication. Due to constraints related to the PET scanning, only patients with mild-to-moderate tics and tics that did not interfere with the scanning procedure were included in the study. None of the patients was on any medication expected to affect the CNS for at least one week prior to imaging. During scanning some Tourette syndrome subjects had active tics; however, they were relatively mild and sporadic and did not interfere with scanning. The patients were continuously observed for occurrence of disruptive tic behaviour. The participants were instructed to relax, but were not asked specifically to suppress tics.
Table 3.
Demographic data for patients with Tourette syndrome
| Patient number | Age | Race | Sex | Socioeconomic status | YGTSS score | Y-BOCS age of onset; score | Medications: last 6 months; medication | Co-morbid disorders | Tics |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | W | M | College graduate/owns business | 29 (19/10) | In the past: Haldol; last 6 months: none | None | Motor, vocal | |
| 2 | 21 | W | M | College student | 65a (35/30) | Full remission; 7 years of age (11/9) | None for last 2 years. In the past: Prozac, Celexa, Effexor, Depakote, Risperidol, Adderal (each for a few months); last 6 months: none | MDD recurrent, OCD, ADHD, panic with agoraphobia | Motor, vocal |
| 3 | 38 | W | M | College graduate/ administrative assistant | 27 (17/10) | In the past: Haldol; last 6 months: none | None | Motor, vocal (in the past) | |
| 4 | 36 | W | M | Middle school/ unemployed | 35 (35/0) | Current; 9 years of age (10/10) | In the past for Tourette syndrome: Haldol, for ADHD/OCD: Ritalin, Clonidine, Paxil; last 6 months: none | MDD recurrent, OCD, ADHD, social phobia | Motor, vocal |
| 5 | 32 | W | M | High school/army-office work | 44 (34/10) | In the past: Clonidine; last 6 months: Guafacine stopped 1 month prior the study | Alcohol abuse (in remission) | Motor, 1 vocal | |
| 6 | 24 | W | M | High school/labourer | 43a (33/10) | In the past: Zyprexa for 2 months; last 6 months: for Tourette syndrome: Clonidine 0.3, stopped 2 weeks prior to the study | Dysthymia, social phobia | Motor, 1 vocal | |
| 7 | 31 | W | M | College graduate/ student | 100b | Current; 9 years of agec | Last 6 months: none | OCD subthreshold, social phobia, history of marijuana use, stopped 1999 | Motor, vocal |
| 8 | 24 | A | M | College graduate/ student | 7 (7/0) | None, 20 mg Ritalin for exams in college; last 6 months: none | OCD, history of ADHD | Motor, vocal | |
| 9 | 19 | W | M | College student | c | In the past: Adderal, Ritalin; last 6 months: for ADHD: Concerta 36 mg, stopped 10 days prior study | ADHD | Motor, vocal | |
| 10 | 23 | W | M | College student | 30 (20/10) | Full remission; 5 years of age (9/6) | In the past: Prozac, Zoloft; last 6 months: for OCD: Fluvoxamine 150 mg, stopped 7 days prior study | OCD, social phobia | Motor, vocal |
| 11 | 20 | W | F | College student | 48a (48/0) | Full remission; 7 years of age (2/2) | In the past: Haldol, Klonopin, Risperidol, Prolixin, Desipramine; last 6 months: for mood disorder, Prozac, discontinued 3 months prior to study | Bipolar disorder II, OCD subthreshold did not meet full criteria for OCD, ADHD childhood, substance abuse (marijuana, stopped 10 months prior to study) | Motor, vocal |
a The score for the worst week in life.
b Patient exaggerated the disorder, except for Subject 7 who submitted the score for the current symptoms.
c Forms/information not available.
Race column: W = White; A = Asian.
Values of Yale Global Tic Severity Scale are shown as a global number and then in parenthesis broken down to tic score/overall impairment score.
Values of the Y-BOCS Scale are provided for ‘lifetime worst’.
Socioeconomic status is provided as education/profession.
ADHD = attention deficit hyperactivity disorder; MDD = major depressive disorder; OCD = obsessive–compulsive disorder; Y-BOCS = Yale-Brown obsessive compulsive scale; YGTSS = Yale Global Tic Severity Scale.
The study was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke. All control subjects and patients gave written informed consent to participate.
Positron emission tomography procedure
PET scans were acquired with subjects at rest using a GE Advance scanner with septa retracted [35 contiguous slices; 4.25-mm plane separation; reconstructed 3D spatial resolution = 6–7 mm full-width at half-maximum (FWHM)]. A transmission scan was acquired to correct for attenuation. Following transmission scanning, a target dose of 20 mCi of high specific activity [11C]flumazenil was injected and 60-min dynamic emission images of the brain were acquired. Subject motion correction during the PET acquisition was performed with a mutual-information registration of each scan time-frame to a standard frame before attenuation correction (using FLIRT software, FSL 3.2, Analysis Group, FMRIB, Oxford, UK) (Andersson et al., 1995; Smith et al., 1997). Based on the calculated motion, the transmission images were resliced and projected for final reconstruction and realignment. To provide an anatomical framework for analysis of the PET images, structural MRI scans, T1-weighted pulse sequence were acquired. PET images were registered to each individual’s MRI with a mutual information algorithm.
Data analysis
Image processing and analysis were performed on a Dell 5 Linux workstation (Round Rock, Texas, USA). Binding potential images were created using the two-step version of the simplified reference tissue model (SRTM2) (Wu and Carson, 2002). The input kinetics for the reference tissue were derived from the pons (drawn on each individual's MR image), where the [11C]flumazenil binding is predominantly accounted for by free and nonspecifically bound radiotracer (Millet et al., 2002; Odano et al., 2009). The binding potential (BPND) images (already transformed to MR space) were then spatially normalized to a standard PET template based on the Montreal Neurological Institute reference brain (Ashburner and Friston, 1999) and analysed using Statistical Parametric Mapping (SPM2) (Wellcome Department of Imaging Neuroscience, UCL, London, UK) implemented in Matlab. The normalized images of 2 × 2 × 2 mm3 voxels were smoothed with a 10-mm FWHM isotropic Gaussian kernel. We performed two types of analyses, namely with global normalization using proportional scaling (Table 1) and without global normalization of BPND values (Table 2); for both of these analyses height threshold was set P = 0.05 false discovery rate (FDR) corrected for multiple comparisons. The [11C]flumazenil BPND values were compared between groups in a voxel-wise analysis using a two-sample t-test model. We performed also regression analyses using as regressors: (i) Yale Global Tic Severity Scale score; and (ii) age; the analyses were done using unscaled BPND values. Due to the small number of subjects in these analyses the height threshold was set at P = 0.001 uncorrected. The results of all analyses were converted into Talairach space (Talairach and Tournoux, 1988; Schmahmann et al., 1999).
Table 1.
Brain areas with decreased and increased binding (BPND) of GABAA receptors in patients with Tourette syndrome (analysis used global normalization of BPND)
| Cluster size | Regions (Brodmann areas) | x | y | z | Z-value |
|---|---|---|---|---|---|
| Brain areas with decreased BPND | |||||
| 5702 | Left nucleus accumbens, putamen, caudate nucleus | −8 | 10 | −7 | 6.69 |
| Left inferior frontal gyrus (BA 47) | |||||
| Right putamen, nucleus accumbens, caudate nucleus | 18 | 8 | −5 | 5.77 | |
| Right insula (anterior and posterior) | |||||
| Right transverse temporal gyrus (BA 41) | |||||
| Right inferior frontal gyrus (BA 47) | |||||
| Left and right thalamus (pulvinar, centromedian, dorsomedian nuclei) | −11 | −25 | 14 | 5.77 | |
| 131 | Right precuneus (BA 7) | 13 | −58 | 53 | 4.29 |
| 181 | Left amygdala and hippocampus | −18 | −9 | −22 | 3.97 |
| 765 | Left postcentral gyrus (BA 1, 2, 3) | −34 | −34 | 54 | 3.96 |
| 1136 | Left superior occipital gyrus (BA 19) | −31 | −75 | 29 | 3.87 |
| Right cuneus (BA 19) | 8 | −75 | 31 | 3.75 | |
| Left medial occipital gyrus (BA 19) | −38 | −75 | 12 | 3.36 | |
| 222 | Right amygdala and hippocampus | 20 | −11 | −22 | 3.85 |
| 670 | Left fusiform gyrus (BA 19) | −22 | −60 | −7 | 3.63 |
| Brain areas with increased BPND | |||||
| 1953 | Left and right SN and left periaqueductal grey | −3 | −27 | −10 | 4.83 |
| 256 | Right posterior cingulate gyrus, sulcus calloso-marginalis (BA 31) | 17 | −13 | 41 | 4.20 |
| 1656 | Left and right cerebellum, dentate nuclei | −8 | −54 | −29 | 4.10 |
| 6 | −56 | −28 |
Cluster size = number of voxels; x, y, z = stereotaxic coordinates in Talairach space; coordinates indicate the distance in millimetres from the origin (anterior commissure), with positive x indicating right, positive y indicating anterior and positive z indicating dorsal.
Table 2.
Brain areas with decreased and increased binding (BPND) of GABAA receptors in patients with Tourette syndrome (analysis used unscaled BPND)
| Cluster size | Regions (Brodmann areas) | x | y | z | Z-value |
|---|---|---|---|---|---|
| Brain areas with decreased BPND | |||||
| 607 | Left and right caudate nuclei | −18 | −20 | 19 | 5.93 |
| Right and left thalamus (dorsomedian, midline, lateral dorsal, lateral posterior, ventral anterior, ventral lateral nucleus and pulvinar) | 6 | −13 | 13 | 5.14 | |
| 59 | Left caudate nucleus | −17 | 6 | 18 | 4.67 |
| 144 | Right insula | 40 | −22 | 21 | 4.48 |
| Right transverse temporal gyrus (BA 41) | |||||
| 148 | Right caudate nucleus, nucleus accumbens | 6 | 13 | −1 | 4.35 |
| Left caudate nucleus, nucleus accumbens, putamen | −12 | 13 | −7 | 4.32 | |
| 10 | Right putamen | 20 | 12 | −4 | 3.74 |
| 2 | Right insula | 34 | 12 | 10 | 3.64 |
| Brain areas with increased BPND | |||||
| 1155 | Left pons | −12 | −23 | −29 | 4.51 |
| Left and right SN | −6 | −22 | −9 | 4.50 | |
| Left periaqueductal grey | |||||
| Left and right red nuclei | |||||
| Left and right subthalamic nuclei | |||||
| 366 | Left cerebellum, dentate nucleus, left lobules 4–5, 6, 8, 9 | −10 | −52 | −23 | 3.87 |
| 93 | Right posterior cingulate gyrus, sulcus calloso-marginalis (BA 31) | 20 | −10 | 44 | 3.86 |
| 3 | Right cerebellum, dentate nucleus | 10 | −52 | −23 | 3.35 |
Cluster size = number of voxels; x, y, z = stereotaxic coordinates in Talairach space.
Results
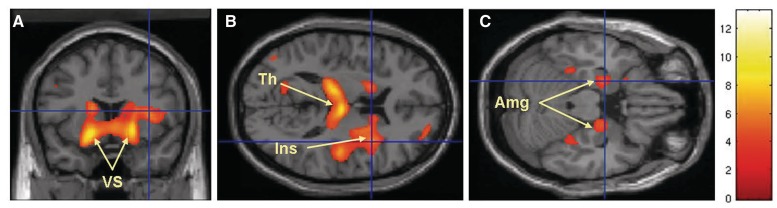
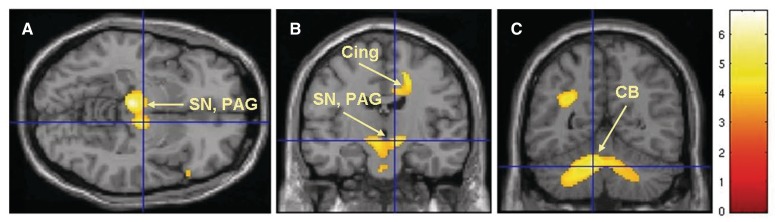
In both analyses performed using BPND values with and without global normalization we found decreased binding of GABAA receptors in Tourette syndrome patients bilaterally in the ventral portions of the caudate nuclei, putamen, accumbens nuclei and globus pallidus (Fig. 1A, Tables 1 and 2). Decreased binding was also seen in the thalamus, right insula (Fig. 1B) and amygdala (Fig. 1C, Tables 1 and 2, Supplementary Fig. 1). There was increased binding of GABAA receptors in the bilateral substantia nigra (SN), left periaqueductal grey (Fig. 2A and B), right posterior cingulate cortex (Fig. 2B) and bilateral cerebellar dentate nuclei (Fig. 2C, Tables 1 and 2, Supplementary Fig. 1). The regression analysis that used the Yale Global Tic Severity Scale score (Supplementary Table 2) showed a positive correlation with the right postcentral gyrus-sensory cortex, and a negative correlation with the left frontal eye field (BA 8), right thalamus (ventral posterior lateral nucleus and ventral posterior medial nucleus), left thalamus (dorsomedial nucleus) and bilateral prefrontal cortex (BA 9). The regression analysis that used age of Tourette syndrome patients showed only positive correlation with the right cerebellar lobule VI (Supplementary Table 3).
Figure 1.
Brain areas with decreased binding of [11C]flumazenil in Tourette syndrome patients versus control subjects: the most significant decreases were seen in the bilateral ventral striatum (VS), bilateral thalamus (Th), right insula (Ins) and bilateral amygdala (Amg).
Figure 2.
Brain areas with increased binding of [11C]flumazenil in Tourette syndrome patients versus control subjects; the highest increases were noted in the bilateral SN, left periaqueductal grey (PAG), right posterior cingulate cortex (PCC) (Cing) and bilateral cerebellum, dentate nuclei (CB). The figures are from the analysis which used non-normalized BPND values as reported in Table 1 and P < 0.05, corrected for multiple comparisons.
Both analyses with and without global normalization showed essentially the same structures; however, there was an absence of some cortical areas in the analysis performed without global normalization including precuneus, cuneus, postcentral and occipital cortices, amygdala and hippocampus (Table 2).
Discussion
This is the first PET neuroimaging study to explore GABA-ergic abnormalities in Tourette syndrome patients. The most significant finding is that relative to healthy controls, the patients with Tourette syndrome showed decreased binding of GABAA receptors in the ventral striatum, globus pallidus, thalamus, amygdala and right insula, and increased binding in the bilateral SN, left periaqueductal grey, right posterior cingulate cortex and bilateral dentate nuclei of the cerebellum. The anatomical distribution of these changes implicates regions where abnormalities of structure or function have been reported in previous neuroimaging and histopathological studies in Tourette syndrome. This study was performed using two voxel-wise analysis approaches, namely with and without global normalization of BPND values. Both methods implicated essentially the same structures confirming validity of the results. The main difference was absence of amygdala and hippocampus and some cortical areas including precuneus, cuneus, postcentral and occipital cortices (Table 1), in the analysis that used unscaled binding potential.
Structures with decreased binding of GABAA receptors
Ventral caudate, putamen, nucleus accumbens and globus pallidus
According to the ‘dopaminergic theory’ of Tourette syndrome, the pathogenesis of the disorder results from an abnormality of striatal dopaminergic neurons and/or receptors. However, the striatum, comprised of the caudate, putamen and nucleus accumbens, also contains ∼90–95% GABA-ergic neurons. The GABA-ergic neurons function as projection neurons and interneurons. The projection neurons, so-called ‘medium spiny neurons’, project from the striatum to the output nuclei of the basal ganglia (internal segment of globus pallidus and SN pars reticulata), whereas GABA-ergic interneurons form three classes distinguished by the presence of co-localizing proteins: (i) parvalbumin; (ii) calretinin and (iii) somatostatin (Kawaguchi et al., 1995). Haber et al. (1986) found a significant decrease of dynorphin-like immunoreactivity in the globus pallidus of a Tourette syndrome patient suggesting an abnormality of the GABA-ergic striatopallidal pathway, whereas recent studies by Kalanithi et al. (2005) and Kataoka et al. (2010) showed a decreased number of GABA-ergic interneurons containing parvalbumin in the caudate and putamen and globus pallidus pars externa, accompanied by an increase of these interneurons in the globus pallidus pars interna of patients with Tourette syndrome. This finding was interpreted by Kalanithi et al. as being ‘consistent with a developmental defect in tangential migration of some GABA-ergic neurons’ from the medial ganglionic eminence to the striatum, cortex and hippocampus. An abnormal function of the ventral striatum was also found in several previous neuroimaging PET studies in Tourette syndrome. The ventral striatum was shown to have decreased metabolism in Tourette syndrome (Stoetter et al., 1992) and tic-related increased activity (Baym et al., 2008b). Other studies examining involvement of the dopaminergic system found increased dopamine release (Wong et al., 2008), and increased binding of [11C] dihydrotetrabenazine (DTBZ), a marker for type 2 vesicular monoamine transporter (VMAT2) (Albin et al., 2003); this finding was interpreted as indicative of dopaminergic dysfunction.
Our study, which showed significant bilateral decrease of [11C]flumazenil binding in the ventral aspect of the striatum and globus pallidus, further emphasizes involvement of the basal ganglia in Tourette syndrome; in particular, the limbic loop of the striatum (Alexander et al., 1986; Voorn et al., 2004), which is responsible for emotional and motivational processes known to be affected in Tourette syndrome.
Thalamus
Abnormalities of the thalamus in Tourette syndrome patients were observed not only in a number of functional neuroimaging studies (Peterson et al., 1998; Stern et al., 2000; Lerner et al., 2007), but also recently in receptor binding and morphological studies. Gilbert et al. (2006) found significantly lower availability of D2 receptors in the mediodorsal nucleus of the thalamus in Tourette syndrome patients while Lee et al. (2006) observed increased left thalamic volumes in boys with Tourette syndrome.
Our study showed decreased binding of GABAA receptors in the left and right thalamus mainly in the pulvinar, centromedian and mediodorsal nuclei. The pulvinar is implicated in pain modulation, speech mechanisms and visual attention functions. The abnormality of the pulvinar conceivably may have clinical relevance and could be implicated in visuomotor integration deficits of Tourette syndrome patients (Schultz et al., 1998). Also, the pulvinar was recently found to be involved in attention deficit hyperactivity disorder (Ferreira et al., 2009), a disorder which frequently co-exists with Tourette syndrome. It is possible that the changes in the pulvinar could contribute to attention deficit hyperactivity disorder as well.
The mediodorsal nucleus has particularly prominent interconnections with the dorsolateral prefrontal cortex, which is a key area of executive functions and attentional focus. The mediodorsal nucleus is involved in planning, organization, attention, affective behaviour, memory and integration of visceral functions. Therefore, the abnormalities of two neurotransmitter systems in this nucleus, the dopaminergic D2 receptors (Gilbert et al., 2006) and GABAA receptors could potentially explain impaired mediodorsal nucleus-related functions in Tourette syndrome patients, in particular attention and affective behaviour.
Additionally, regression analysis that used the Yale Global Tic Severity Scale score (Supplementary Table 2) showed negative correlation with BPND values of the right ventral posterior medial and lateral nuclei and left dorsomedial nucleus of the thalamus. The BPND values of the right somatosensory cortex, which is reciprocally connected to ventral posterior medial and lateral nuclei, showed positive correlation with Yale Global Tic Severity Scale score, whereas BPND values of the left frontal eye field (BA 8) and prefrontal cortex (BA 9) which are reciprocally connected to the dorsomedial nucleus, showed negative correlation. Involvement of frontal eye fields conceivably may be related to the ocular tics.
The centromedian nuclei (identified only with the analysis using global normalization) belong to the group of intralaminar nuclei through which the cerebellum communicates with the striatum (Hoshi et al., 2005). Recently shown involvement of the cerebellum in Tourette syndrome (Stern et al., 2000; Bohlhalter et al., 2006; Lerner et al., 2007) could indicate dysfunction of the entire pathway involving the cerebellum, centromedian nucleus and striatum in Tourette syndrome.
The involvement of the thalamus in Tourette syndrome is further emphasized by efficacy of deep brain stimulation targeting thalamic nuclei of the centromedian–parafascicular complex used as a new treatment for tics (Visser-Vandewalle et al., 2003; Maciunas et al., 2007; Welter et al., 2008; Porta et al., 2009). The effectiveness of this approach could be explained by the interruption of major excitatory pathways from the cerebellum through the thalamus to the basal ganglia. It is possible that an abnormality of the thalamus reflects dysfunction of cerebellar and basal ganglia circuits; this dysfunction is then further projected to multiple cortical areas and detected in neuroimaging and electrophysiological studies frequently as disinhibition and overactivity.
Amygdala
Morphological abnormalities of the amygdala and hippocampus in Tourette syndrome patients have recently been reported by Peterson et al. (2007) in an MRI study and consisted of enlargement of the central and basolateral nuclei of amygdala and hippocampal dentate gyrus and sector CA3. A similar pattern of changes in the hippocampus was also observed in attention deficit hyperactivity disorder patients in an MRI study (Plessen et al., 2006) suggesting that hippocampal abnormality in the dentate gyrus may contribute to attention deficit hyperactivity disorder co-morbidity in Tourette syndrome patients. Hippocampal and amygdala volumes were also abnormal in refractory obsessive–compulsive disorder patients (Atmaca et al., 2008), implicating involvement of these structures also in obsessive–compulsive disorder co-morbidity. The decreased flumazenil binding in the amygdala and hippocampus (identified with the analysis using global normalization and unscaled BPND values at P = 0.001) in Tourette syndrome patients in our study may indicated contribution of amygdala to the attention deficit hyperactivity disorder component of Tourette syndrome and possibly also to obsessive–compulsive disorder and anxiety, as amygdala function has been implicated in both primary attention deficit hyperactivity disorder and primary obsessive–compulsive disorder (Davis, 1992; Breiter and Rauch, 1996; Szeszko et al., 1999).
Structures with increased binding of GABAA receptors
Substantia nigra and periaqueductal grey
Involvement of the midbrain dopaminergic system and periaqueductal grey in Tourette syndrome was first proposed by Devinsky (Devinsky, 1983) and then subsequently confirmed with a MRI morphometric study by Garraux et al. (2006) and functional MRI study (Baym et al., 2008b). Results of our study resemble the findings of the morphometric study by Garraux et al. (2006) with the involvement of the SN and periaqueductal grey and predominance of the changes on the left side.
The dopaminergic neurons of the SN pars compacta, which project throughout the striatum, receive potent GABA-ergic projections from the neostriatum, globus pallidus and SN pars reticulata (Paladini et al., 1999). Therefore, altered function of GABAA receptors of SN pars compacta might have a profound effect on the function of dopaminergic neurons that project into the striatum. Dysfunction involving the GABA-ergic system of the SN pars reticulata, a major output structure of basal ganglia, would also have a significant impact, especially affecting, and possibly causing, disinhibition of thalamo-cortical networks and dopaminergic neurons of the SN pars compacta.
The abnormality of the GABA-ergic system in the SN shown in our study could potentially explain the gender difference in Tourette syndrome and predominance of male subjects. The SN pars reticulata has been shown to be one of the sexually dimorphic areas of the brain (Veliskova and Moshe, 2001). This differentiation occurring during the early development and maturation of GABAA receptor signaling (the switch from depolarizing to hyperpolarizing GABA-ergic currents) follows gender-specific patterns (Galanopoulou, 2008). Peterson et al. (1992) already pointed to the role of sex hormones, in particular androgens, and their influence on dimorphic brain structures in pathogenesis of Tourette syndrome. The androgenic hormones acting on the GABAA receptors’ modulatory site could further contribute to the vulnerability of the GABA-ergic network in the SN and adversely affect its inhibitory action, causing emergence of tics. The exacerbation of Tourette syndrome by anabolic hormones has been reported by Leckman and Scahill (1990).
Cerebellum
The cerebellum has not been frequently implicated in the genesis of Tourette syndrome; however, some recent studies showed significant activation of the cerebellum during tic production (Stern et al., 2000; Bohlhalter et al., 2006; Lerner et al., 2007). In our previous article, we suggested that the overactive cerebellum in Tourette syndrome could contribute to tic generation, in particular, through its influence on the putamen and caudate via the thalamic intralaminar nuclei (Hoshi et al., 2005). The presence of an abnormality in the dentate nuclei which form the major output from the cerebellum appears to confirm cerebellar involvement in Tourette syndrome.
The regression analysis that used the age of the Tourette syndrome subjects (Supplementary Table 3) showed a positive correlation with the right cerebellar lobule VI. Cerebellar lobule VI is a part of the posterior lobe and has cognitive and affective functions as postulated by Schmahmann (2004), but it also contains some sensorimotor representation. Lobule VI was reported to be activated during orofacial movements (Dresel et al., 2005), during language-related activity (Jansen et al., 2005) and also during spatial, affective and working memory tasks (Stoodley and Schmahmann, 2010). Lobule VI was seen to be activated during tic release in a previous PET study (Lerner et al., 2007), and an MRI volumetric study (Tobe et al., 2010) showed changes in lobule VI that correlated with the severity of tics, particularly vocal tics.
In both analyses, several cortical areas showed abnormal binding of GABAA receptors, namely the limbic cortices (insula, posterior cingulate), auditory cortex, area 41, frontal cortex area 47. However, only the analysis with global normalization showed abnormal binding in the somatosensory cortex, areas 1, 2 and 3, visual cortex area 19. All these cortical areas except for the posterior cingulate cortex showed decreased binding of GABAA receptors. Many of these areas have been shown to be involved in Tourette syndrome pathology.
Insula
A functional abnormality of the insula in Tourette syndrome patients was found in a number of neuroimaging studies; in perfusion studies (Stern et al., 2000; Bohlhalter et al., 2006; Lerner et al., 2007), PET [18F]fluorodeoxyglucose (FDG) study of metabolism (Stoetter et al., 1992) and PET study of opiate binding (Weeks et al., 1996). The insula, which serves as a cortical site for integrated interception where information about all bodily sensations converges (Craig, 2002), was also proposed to be responsible for controlling and suppressing natural urges (Lerner et al., 2009). Therefore, disordered function of the insula conceivably might contribute to the premonitory urges of Tourette syndrome and difficulties with tic suppression, and probably also to the cognitive-behavioural disturbances associated with Tourette syndrome, especially, obsessive–compulsive disorder. Our study showed decreased binding of GABAA receptors in the right insula. This fact is particularly interesting, because the right insula was proposed to be involved in perception and processing of internal stimuli associated with stress responses and conveyed by the sympathetic nervous system (Craig, 2005). It is probable that in Tourette syndrome, the presence of tics and attempt to suppress them activates stress-related pathways.
Other issues
Possible reasons for altered GABAA receptor binding
The mechanism underlying the changes in flumazenil BPND in Tourette syndrome remains unclear, but conceivably may have multiple explanations. One proposed by Kalanithti et al. (2005) is a reduction of neurons arising from a ‘developmental defect in tangential migration of some GABA-ergic neurons’. However, similar developmental defects might be more pervasive in Tourette syndrome and affect other brain areas as well. If so, this could explain the widespread changes in the BPND of GABAA receptors in our study.
Another possible reason for the changes of flumazenil BPND in Tourette syndrome is alteration in affinity of GABAA receptors, which might imply some structural changes within the receptor. A mutation of one or more of GABAA receptor subunits may potentially alter pharmacological properties of the receptor. Such mutations were shown to underlie a number of epilepsy syndromes (Noebels, 2003; Benarroch, 2007) and alter cortical excitability (Fedi et al., 2008). There is also the possibility that flumazenil is sensitive to endogenous GABA levels (Frankle et al., 2009).
Limitations
The results of this study probably reflect not only differences in the BPND of GABAA receptors between Tourette syndrome patients and control subjects but also the characteristic distribution of different GABAA receptors in the brain and their variable pharmacological profiles. This constitutes one of the limitations of this study that is related to the differential affinity of various GABAA subtypes for flumazenil. Some subunit combinations do not bind flumazenil, or bind it with lower affinity, in particular: α4, α6, γ1, δ, ε, ρ, θ (Bentue-Ferrer et al., 1996; Barnard et al., 1998; Sieghart and Sperk, 2002). This fact might have affected the results of the study because some brain structures implicated in Tourette syndrome might not have been visualized, due to the presence of subunits with low affinity for flumazenil. This is in particular the case of many thalamic nuclei and parts of the caudate and putamen, globus pallidus, subthalamic nucleus, cerebellar cortex and many brainstem nuclei (Dennis et al., 1988; Zezula et al., 1988; Kultas-Ilinsky et al., 1998). The results of this PET study reflect only changes of GABAA receptors which bind flumazenil, therefore the present PET study could have missed potential abnormalities of some key structures involved in Tourette syndrome such as parts of basal ganglia, thalamus and cerebellum, which contain GABAA receptors subunits with low affinity for flumazenil such as α4 and α6. At this point, the picture of GABA-ergic involvement in Tourette syndrome is incomplete and imaging of other GABAA receptor subunits could bring important and complementary information.
Regarding potential impact of tics on radioligand uptake we do not think that tics occurring during the scanning would affect the results of the PET study as they were mild, occurring sporadically and of short duration. Also, the effect of medication, and comorbid illnesses could have influenced the findings of this study. However, the majority of Tourette syndrome patients were not taking any medication for years, and the four patients who were taking medication stopped generally 2 weeks before the study (Table 3). Regarding the influence of co-morbid illnesses, the majority of Tourette syndrome patients had attention deficit hyperactivity disorder and/or obsessive–compulsive disorder; however, these disorders form an integral component to the spectrum of phenotypic disturbances seen in the Tourette syndrome and examining the correlates of discrete symptoms would require a much larger sample size.
Conclusion
The abnormalities of GABA-ergic neurons found in so many functionally diverse structures might be responsible for motor, cognitive and affective dysregulation in Tourette syndrome and suggest a developmental pathogenesis for Tourette syndrome (Stern et al., 2008). The complex set of functional domains affected in Tourette syndrome putatively involves developmental and/or genetic factors with superimposed plastic reorganization of neuronal circuits in different brain regions due to reactive changes down- and up-stream of the initial abnormality.
In view of such a prominent abnormality in flumazenil BPND in Tourette syndrome patients, it is conceivable that dysfunction involving the GABAA receptor system may play a major role in the pathophysiology of Tourette syndrome. The GABA-ergic system is the main inhibitory system in the CNS and GABA-ergic neurons are present in every brain structure, accounting in some structures (e.g. striatum) for up to 95% of neurons. Additionally, this system plays an important role in the development of many brain structures. Recently, GABA-ergic interneurons were shown to play a key role in regulating cortical development including neuronal proliferation, migration and differentiation (Anderson et al., 1999; Di Cristo, 2007). They were also found to have a critical role in the development of the striatum, cerebellum and hippocampus (Marin et al., 2000; Pleasure et al., 2000; Takayama, 2005; Huang et al., 2007). Therefore, alteration of the GABA-ergic system can affect and alter function and morphology of many brain structures already implicated in Tourette syndrome, such as the cortex, striatum, hippocampus and cerebellum. However, in light of known abnormalities affecting other neurotransmitter systems [e.g. dopaminergic, serotonergic (Wong et al., 2008), cholinergic (Kataoka et al., 2010) and opioid], the role of GABAA receptor dysfunction in Tourette syndrome pathophysiology remains unclear, but merits further research. The alterations found in other neurotransmitter systems could represent compensatory changes due to a primary defect in the GABA-ergic system. We think that further studies of other neurotransmitter systems are necessary to delineate the final biological signature of Tourette syndrome; however, at this point we would like to propose to consider GABA-ergic morphological changes as detected by flumazenil binding as one of the biomarkers of Tourette syndrome.
Funding
The Intramural Research Programs of the NINDS and NIMH, National Institutes of Health.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank all subjects who participated in this study and the staff of the NIH PET Department for successful completion of the scanning studies in particular Shielah Conant for her help with processing and analysis of data; Dr William Theodore for his help with acquiring PET data and analysis; Allison Nugent, PhD. for the help with analysis; psychologist Lucy Justement for her help in evaluating patients and Devera Schoenberg, MSc for editorial work.
Glossary
Abbreviations
- BPND
binding potential
- GABA
γ-aminobutyric acid
- SN
substantia nigra
References
- Albin RL, Koeppe RA, Bohnen NI, Nichols TE, Meyer P, Wernette K, et al. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology. 2003;61:310–5. doi: 10.1212/01.wnl.0000076181.39162.fc. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–54. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Vagnhammar BE, Schneider H. Accurate attenuation correction despite movement during PET imaging. J Nucl Med. 1995;36:670–8. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Ozler S, Kara B, Ozler Z, et al. Hippocampus and amygdalar volumes in patients with refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1283–6. doi: 10.1016/j.pnpbp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Baym CL, Corbett BA, Wright SB, Bunge SA. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain. 2008a;131:165–79. doi: 10.1093/brain/awm278. [DOI] [PubMed] [Google Scholar]
- Baym CL, Corbett BA, Wright SB, Bunge SA. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain. 2008b;131:165–79. doi: 10.1093/brain/awm278. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology. 2007;68:612–614. doi: 10.1212/01.wnl.0000255669.83468.dd. [DOI] [PubMed] [Google Scholar]
- Bentue-Ferrer D, Bureau M, Patat A, Allain H. Flumazenil. CNS Drug Reviews. 1996;2:390–414. [Google Scholar]
- Biswal B, Ulmer JL, Krippendorf RL, Harsch HH, Daniels DL, Hyde JS, et al. Abnormal cerebral activation associated with a motor task in Tourette syndrome. AJNR Am J Neuroradiol. 1998;19:1509–12. [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–37. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Braun AR, Stoetter B, Randolph C, Hsiao JK, Vladar K, Gernert J, et al. The functional neuroanatomy of Tourette's syndrome: an FDG-PET study. I. Regional changes in cerebral glucose metabolism differentiating patients and controls. Neuropsychopharmacology. 1993;9:277–91. doi: 10.1038/npp.1993.64. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL. Functional MRI and the study of OCD: from symptom provocation to cognitive-behavioral probes of cortico-striatal systems and the amygdala. Neuroimage. 1996;4:S127–38. doi: 10.1006/nimg.1996.0063. [DOI] [PubMed] [Google Scholar]
- Butler IJ, Koslow SH, Seifert WE, Jr, Caprioli RM, Singer HS. Biogenic amine metabolism in Tourette syndrome. Ann Neurol. 1979;6:37–9. doi: 10.1002/ana.410060109. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dennis T, Dubois A, Benavides J, Scatton B. Distribution of central omega 1 (benzodiazepine1) and omega 2 (benzodiazepine2) receptor subtypes in the monkey and human brain. An autoradiographic study with [3H]flunitrazepam and the omega 1 selective ligand [3H]zolpidem. J Pharmacol Exp Ther. 1988;247:309–22. [PubMed] [Google Scholar]
- Devinsky O. Neuroanatomy of Gilles de la Tourette's syndrome. Possible midbrain involvement. Arch Neurol. 1983;40:508–14. doi: 10.1001/archneur.1983.04210070048013. [DOI] [PubMed] [Google Scholar]
- Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- Dresel C, Castrop F, Haslinger B, Wohlschlaeger AM, Hennenlotter A, Ceballos-Baumann AO. The functional neuroanatomy of coordinated orofacial movements: sparse sampling fMRI of whistling. Neuroimage. 2005;28:588–97. doi: 10.1016/j.neuroimage.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Jons PH, Matochik JA, Pascualvaca D, Cohen RM. High presynaptic dopaminergic activity in children with Tourette's disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:86–94. doi: 10.1097/00004583-199901000-00024. [DOI] [PubMed] [Google Scholar]
- Fedi M, Berkovic SF, Macdonell RA, Curatolo JM, Marini C, Reutens DC. Intracortical hyperexcitability in humans with a GABAA receptor mutation. Cereb Cortex. 2008;18:664–9. doi: 10.1093/cercor/bhm100. [DOI] [PubMed] [Google Scholar]
- Ferreira PE, Palmini A, Bau CH, Grevet EH, Hoefel JR, Rohde LA, et al. Differentiating attention-deficit/hyperactivity disorder inattentive and combined types: a (1)H-magnetic resonance spectroscopy study of fronto-striato-thalamic regions. J Neural Transm. 2009;116:623–9. doi: 10.1007/s00702-009-0191-3. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Narendran R, Mason NS, Vora S, Litschge M, et al. Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology. 2009;34:624–33. doi: 10.1038/npp.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraux G, Goldfine A, Bohlhalter S, Lerner A, Hanakawa T, Hallett M. Increased midbrain gray matter in Tourette's syndrome. Ann Neurol. 2006;59:381–5. doi: 10.1002/ana.20765. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Christian BT, Gelfand MJ, Shi B, Mantil J, Sallee FR. Altered mesolimbocortical and thalamic dopamine in Tourette syndrome. Neurology. 2006;67:1695–7. doi: 10.1212/01.wnl.0000242733.18534.2c. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kowall NW, Vonsattel JP, Bird ED, Richardson EP., Jr Gilles de la Tourette's syndrome. A postmortem neuropathological and immunohistochemical study. J Neurol Sci. 1986;75:225–41. doi: 10.1016/0022-510x(86)90097-3. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–3. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Di CG, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–86. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- Jansen A, Floel A, Van RJ, Konrad C, Rotte M, Forster AF, et al. Crossed cerebro-cerebellar language dominance. Hum Brain Mapp. 2005;24:165–72. doi: 10.1002/hbm.20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA. 2005;102:13307–12. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–91. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–35. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kultas-Ilinsky K, Leontiev V, Whiting PJ. Expression of 10 GABA(A) receptor subunit messenger RNAs in the motor-related thalamic nuclei and basal ganglia of Macaca mulatta studied with in situ hybridization histochemistry. Neuroscience. 1998;85:179–204. doi: 10.1016/s0306-4522(97)00634-9. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–73. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Scahill L. Possible exacerbation of tics by androgenic steroids. N Engl J Med. 1990;322:1674. doi: 10.1056/nejm199006073222314. [DOI] [PubMed] [Google Scholar]
- Lee JS, Yoo SS, Cho SY, Ock SM, Lim MK, Panych LP. Abnormal thalamic volume in treatment-naive boys with Tourette syndrome. Acta Psychiatr Scand. 2006;113:64–7. doi: 10.1111/j.1600-0447.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Boudreau EA, Hanakawa T, Pagan F, Mari Z, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68:1979–87. doi: 10.1212/01.wnl.0000264417.18604.12. [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Hanakawa T, Boudreau EA, Pagan F, Mari Z, et al. Involvement of insula and cingulate cortices in control and suppression of natural urges. Cereb Cortex. 2009;19:218–23. doi: 10.1093/cercor/bhn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107:1004–14. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–76. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet P, Graf C, Buck A, Walder B, Ibanez V. Evaluation of the reference tissue models for PET and SPECT benzodiazepine binding parameters. Neuroimage. 2002;17:928–42. [PubMed] [Google Scholar]
- Moriarty J, Varma AR, Stevens J, Fish M, Trimble MR, Robertson MM. A volumetric MRI study of Gilles de la Tourette's syndrome. Neurology. 1997;49:410–5. doi: 10.1212/wnl.49.2.410. [DOI] [PubMed] [Google Scholar]
- Muller-Vahl KR, Berding G, Kolbe H, Meyer GJ, Hundeshagen H, Dengler R, et al. Dopamine D2 receptor imaging in Gilles de la Tourette syndrome. Acta Neurol Scand. 2000;101:165–71. doi: 10.1034/j.1600-0404.2000.101003165.x. [DOI] [PubMed] [Google Scholar]
- Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- Odano I, Halldin C, Karlsson P, Varrone A, Airaksinen AJ, Krasikova RN, et al. [18F]flumazenil binding to central benzodiazepine receptor studies by PET–quantitative analysis and comparisons with [11C]flumazenil. Neuroimage. 2009;45:891–902. doi: 10.1016/j.neuroimage.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Orth M, Amann B, Robertson MM, Rothwell JC. Excitability of motor cortex inhibitory circuits in Tourette syndrome before and after single dose nicotine. Brain. 2005;128:1292–300. doi: 10.1093/brain/awh473. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Celada P, Tepper JM. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience. 1999;89:799–812. doi: 10.1016/s0306-4522(98)00355-8. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, et al. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry. 2007;64:1281–91. doi: 10.1001/archpsyc.64.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Scahill L, Naftolin F, Keefe D, Charest NJ, et al. Steroid hormones and CNS sexual dimorphisms modulate symptom expression in Tourette's syndrome. Psychoneuroendocrinology. 1992;17:553–63. doi: 10.1016/0306-4530(92)90015-y. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–33. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–24. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, et al. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–40. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Wentzel-Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, et al. Altered interhemispheric connectivity in individuals with Tourette's disorder. Am J Psychiatry. 2004;161:2028–37. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375–80. doi: 10.1212/WNL.0b013e3181bd809b. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–60. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Carter AS, Gladstone M, Scahill L, Leckman JF, Peterson BS, et al. Visual-motor integration functioning in children with Tourette syndrome. Neuropsychology. 1998;12:134–45. doi: 10.1037//0894-4105.12.1.134. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Singer HS, Butler IJ, Tune LE, Seifert WE, Jr, Coyle JT. Dopaminergic dsyfunction in Tourette syndrome. Ann Neurol. 1982;12:361–6. doi: 10.1002/ana.410120408. [DOI] [PubMed] [Google Scholar]
- Singer HS, Wong DF, Brown JE, Brandt J, Krafft L, Shaya E, et al. Positron emission tomography evaluation of dopamine D-2 receptors in adults with Tourette syndrome. Adv Neurol. 1992;58:233–9. [PubMed] [Google Scholar]
- Smith AM, Bruckbauer T, Wienhard K, Pietrzyk U, Byars LG. Spatial transformation during 3D reconstruction in positron emission tomography. Eur J Nucl Med. 1997;24:1413–7. doi: 10.1007/s002590050168. [DOI] [PubMed] [Google Scholar]
- Stern ER, Blair C, Peterson BS. Inhibitory deficits in Tourette's syndrome. Dev Psychobiol. 2008;50:9–18. doi: 10.1002/dev.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E, Silbersweig DA, Chee KY, Holmes A, Robertson MM, Trimble M, et al. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000;57:741–8. doi: 10.1001/archpsyc.57.8.741. [DOI] [PubMed] [Google Scholar]
- Stoetter B, Braun AR, Randolph C, Gernert J, Carson RE, Herscovitch P, et al. Functional neuroanatomy of Tourette syndrome. Limbic-motor interactions studied with FDG PET. Adv Neurol. 1992;58:213–26. [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–44. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, et al. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:913–9. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Takayama C. GABAergic signaling in the developing cerebellum. Int Rev Neurobiol. 2005;71:63–94. doi: 10.1016/s0074-7742(05)71003-5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. New York: Thieme Medical Publishers, Inc.; 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Tobe RH, Bansal R, Xu D, Hao X, Liu J, Sanchez J, et al. Cerebellar morphology in Tourette syndrome and obsessive-compulsive disorder. Ann Neurol. 2010;67:479–87. doi: 10.1002/ana.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski N, Sawle GV, Playford ED, Weeks R, Lammerstma AA, Lees AJ, et al. PET studies of the presynaptic and postsynaptic dopaminergic system in Tourette's syndrome. J Neurol Neurosurg Psychiatry. 1994;57:688–92. doi: 10.1136/jnnp.57.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veliskova J, Moshe SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Visser-Vandewalle V, Temel Y, Boon P, Vreeling F, Colle H, Hoogland G, et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99:1094–100. doi: 10.3171/jns.2003.99.6.1094. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–74. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Weeks RA, Turjanski N, Brooks DJ. Tourette's syndrome: a disorder of cingulate and orbitofrontal function? QJM. 1996;89:401–8. doi: 10.1093/qjmed/89.6.401. [DOI] [PubMed] [Google Scholar]
- Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65:952–7. doi: 10.1001/archneur.65.7.952. [DOI] [PubMed] [Google Scholar]
- Wong DF, Brasic JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239–51. doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Singer HS, Brandt J, Shaya E, Chen C, Brown J, et al. D2-like dopamine receptor density in Tourette syndrome measured by PET. J Nucl Med. 1997;38:1243–7. [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–52. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- Zezula J, Cortes R, Probst A, Palacios JM. Benzodiazepine receptor sites in the human brain: autoradiographic mapping. Neuroscience. 1988;25:771–95. doi: 10.1016/0306-4522(88)90036-x. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette's disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154:1277–84. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.