Abstract
Infection with Leishmania parasites results in a range of clinical manifestations and outcomes. Control of Leishmania parasite transmission is extremely difficult due to the large number of vectors and potential reservoirs, and none of the current treatments are ideal. Vaccination could be an effective strategy to provide sustained control. In this review, the current global situation with regard to leishmaniasis, the immunology of Leishmania infection and various efforts to identify second generation vaccine candidates are briefly discussed. The variety of clinical trials conducted using the only current second generation vaccine approved for clinical use, LEISH-F1 + MPL-SE, are described. Given that epidemiological evidence suggests that reducing the canine reservoir also positively impacts human incidence, efforts at providing a vaccine for leishmaniasis in dogs are highlighted. Finally, potential refinements and surrogate markers that could expedite the introduction of a vaccine that can limit the severity and incidence of leishmaniasis are discussed.
Keywords: Leishmania, T cell, TLR, protozoa, vaccine
1. Leishmaniasis – The current situation
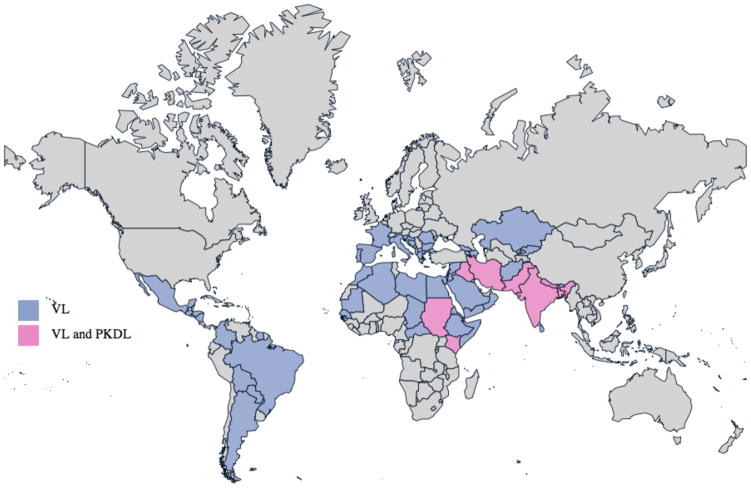
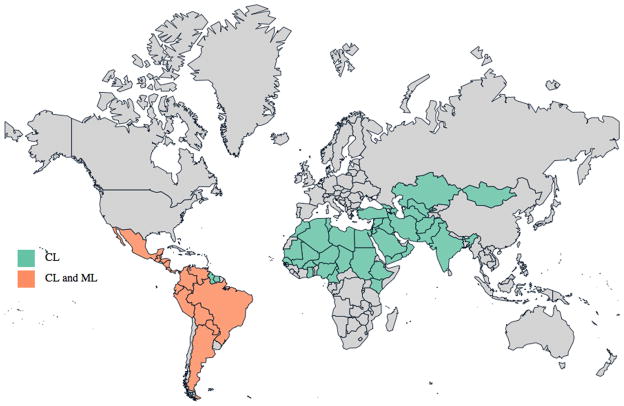
Leishmaniasis causes human suffering on a global scale, with an estimated 12 million current cases, 2 million additional cases annually and threatening approximately 350 million people in endemic areas. The epidemiology is extremely diverse, with 20 protozoan parasite species of the genus Leishmania known to be pathogenic for humans [1]. The geographic distribution of each Leishmania species affects the type of disease that occurs in each region, as well as its severity. Visceral leishmaniasis (VL; also known as kala-azar) is caused by L. donovani in South Asia and Africa, while L. infantum causes VL in the Mediterranean, the Middle East, Latin America and parts of Asia (Table 1 and Figure 1)[2]. Among the many other mammals that can be infected with Leishmania spp., dogs develop canine visceral leishmaniasis (CaVL) and they serve as an important parasitic reservoir in these regions. Cutaneous leishmaniasis (CL) is caused by L. major in Africa, the Middle East and parts of Asia, by L. tropica in the Middle East, the Mediterranean and parts of Asia, and by L. aethiopica in parts of Africa. Many different species may be involved in the Americas, where CL can be found throughout South America and as far north as Mexico (Table 1 and Figure 2)[2]. Limited foci of infection have been reported in Canada and the US, and CaVL caused by L. infantum has been reported within foxhound kennels in various US states. Australia appeared to be free of Leishmania spp. but infection among captive kangaroos, wallabies and other marsupials began to be reported in 2004 [3, 4].
Table 1.
Disease phenotype and geographical burden attributed to various Leishmania species [2]. Depending on the strain, Leishmania infection may cause lesions on skin (cutaneous leishmaniasis; CL) or mucosa (mucosal leishmaniasis; ML), or disseminate to internal organs, including the spleen and liver (visceral leishmaniasis; VL). Post-kalaazar dermal leishmaniasis (PKDL) is caused by persistence of parasites in the skin following apparently successful treatment of VL.
| Disease | Leishmania sp. [3] | Geographical Burden |
|---|---|---|
| Cutaneous Leishmaniasis (CL) | L. mexicana complex (ZCL) | Argentina, Belize, Bolivia, Brazil. Colombia, Costa Rica, Ecuador, French Guiana, Guatemala, Mexico, Peru, Suriname, USA, and Venezuela |
| L.tropica complex (ACL) | Afghanistan, Azerbaijan, India, Iran, Iraq, Israel, Morocco, Pakistan, Syria, Turkey, and Uzbekistan | |
| L.major complex (ZCL) | Afghanistan, Algeria, Azerbaijan, Burkina Faso, Cameroon, Chad, Egypt, Ethiopia, Gambia, Georgia, Ghana, Guinea Bissau, India, Iran, Iraq, Israel, Jordan, Kazakhstan, Kenya, Kuwait, Libya, Mali, Mauritania, Mongolia, Morocco, Niger, Nigeria, Oman, Pakistan, Saudi Arabia, Senegal, the Sudan, Syria, Tunisia, Turkey, Turkmenistan, Uzbekistan, and Yemen | |
| L.aethiopica complex (ZCL) | Ethiopia, Kenya, and Uganda | |
| L.brazilensis complex (ZCL) | Argentina, Belize, Bolivia, Brazil. Colombia, Costa Rica, Ecuador, French Guiana, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, and Venezuela | |
| L.guyanensis complex (ZCL) | Argentina, Belize, Bolivia, Brazil. Colombia, Costa Rica, Ecuador, French Guiana, Guatemala, Guyana, Honduras, Nicaragua, Panama, Peru, Suriname, and Venezuela | |
| Mucosal/mucocutaneous Leishmaniasis (ML) | L.braziliensis complex | Argentina, Belize, Bolivia, Brazil. Colombia, Costa Rica, Ecuador, French Guiana, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, and Venezuela |
| L.guyanensis complex | Colombia, Costa Rica, Ecuador, Guatemala, Honduras, Nicaragua, and Panama | |
| Visceral Leishmaniasis (VL; Kala-Azar) | L.donovani complex (AVL, ZVL) | Afghanistan, Albania, Algeria, Argentina, Armenia, Azerbaijan, Bangladesh, Bhutan, Bolivia, Bosnia & Herzegovina, Brazil, Bulgaria, Chad, Central African Republic, China, Colombia, Croatia, Cyprus, Djibouti, Egypt, El Salvador, Eritrea, Ethiopia, France, Gambia, Georgia, Greece, Guatemala, Honduras, India, Iran, Iraq, Israel. Italy, Jordan, Kazakhstan, Kenya, Kyrgyzstan, Lebanon, Libya, Macedonia, Malta, Mauriatania, Mexico, Monaco, Montenegro, Morocco, Nepal, Nicaragua, Oman, Pakistan, Paraguay, Portugal, Romania, Saudi arabia, Senegal. Slovenia, Somalia, Spain, Srilanka, the Sudan, Syria, and Yemen |
| Post-Kala –Azar Dermal Leishmaniasis (PKDL) | L.donovani complex | Bangladesh, China, Nepal, India, Iran, Iraq, Kenya, Pakistan, the Sudan |
Figure 1.
Worldwide distribution of VL and PKDL cases. Information used to generate this map was extracted from WHO Technical Report Series; Control of the Leishmaiases [2].
Figure 2.
Worldwide distribution of CL and ML cases. Information used to generate this map was extracted from WHO Technical Report Series; Control of the Leishmaiases [2].
Leishmania parasites are transmitted by the bite of infected female sandflies (phlebotomine), of which 30 species are proven vectors. Biting an animal or a human affected by the disease first infects the sand fly, then the parasites multiply in the gut and become infective 1–3 weeks later. Parasites are passed on when the sandfly takes a subsequent blood meal. There are two main epidemiological entities: (1) zoonotic, which includes animal reservoir hosts in the transmission cycle and (2) anthroponotic, in which man is the sole source of infection for the vector. Humans and dogs can thus not only represent the primary affected population in many areas but can also be the main reservoir for Leishmania parasites. Indeed, where L. donovani predominates, humans are a VL reservoir. Elevated incidence of L. donovani infection in dogs can be linked with increased infection rates in humans [5]. In VL-endemic regions of the Mediterranean and Latin America, dogs are the most important reservoir of L. infantum, and thereby represent a target for epidemiological intervention of transmission to humans.
Human leishmanial infections may cause diverse clinical manifestations, ranging from disfiguring and potentially disabling lesions that manifest in CL, a mutilating mucosal leishmaniasis (ML), to potentially fatal VL. Other presentations include diffuse cutaneous leishmaniasis (DCL), which is a long-lasting disease due to a deficient cellular-mediated immune response, and post-kala-azar dermal leishmaniasis (PKDL), which is a skin manifestation subsequent to apparently successful treatment of VL.
2. The need for vaccines for leishmaniasis
2.1. Current treatment issues
Treatment of leishmaniasis currently relies on chemotherapy, and various largely effective chemotherapies are available. Until recently, chemotherapy was severely limited by factors such as high cost, toxicity or route of administration (such as intravenous infusion) [6]. The pentavalent antimonials such as sodium stibogluconate and meglumine antimoniate have been widely used to treat leishmaniasis for over 70 years, and it is perhaps not surprising that resistance to these drugs is increasing. In some endemic areas their use is limited due to a lack of efficacy. Second line drugs and novel regimens are being developed that reduce side effects. Recently, rather than treating with 30 days of injections with pentavalent antimony compounds, 15 of the most endemic districts in Bihar, India have been effectively treating VL with 28 days of oral miltefosine. Liposomal amphotericin B (AmBisome®) has proven to be highly effective and safe as a monotherapy and as a single-dose treatment [7–9]. A key development that now allows AmBisome to be considered for use as a first-line drug in countries where VL is endemic was the preferential pricing agreement secured by WHO in 2007, reducing the cost of treatment to 10% of its original price in developing countries [10]. It has also been indicated that a single intravenous dose of 10 mg/kg AmBisome results in a 95.7% cure rate at 6 months of follow-up, with a lower frequency of adverse events than observed with other available VL treatments [11]. Although improved drug regimens are becoming available, elimination can likely only be achieved through widespread vaccination. Vaccination could provide long term reductions in potential reservoirs that can limit transmission.
2.2. Leishmania infection and protective immunity
Understanding the protective immune response against leishmania is important for the rationale design of vaccines and an improved capacity to direct vaccine-induced responses. Immunity against Leishmania parasites is mediated through a complex array of immune parameters, including both innate (dendritic cells, macrophages, neutrophils) and adaptive (B cells and T cells) immunity. Leishmania parasites reside mainly within macrophages and these cells play a pivotal role in immunity. A subversive activity of Leishmania parasites in the infectious process is the inhibition of interleukin (IL)-12 production, a cytokine necessary for the leishmanicidal activity of macrophages through its ability to upregulate inducible nitric oxide synthase, nitric oxide and interferon gamma (IFNγ). Cytokine secretion promotes the recruitment of other proinflammatory cells (neutrophils, mast cells and macrophages) to the site of infection. Amastigote uptake by dendritic cells at the infection site stimulates IL-12 production and the presentation of antigens through the MHC I and MHC II pathways, leading to generation of Leishmania-specific CD4+ and CD8+ T cell responses that are essential for acquired resistance against Leishmania.
Vaccine studies against VL are hampered by the lack of small animal models that accurately reflect all aspects of the human disease, and the best animal models of VL are L. donovani in golden hamsters and the natural infection of dogs by L. infantum or L. chagasi [16]. On the other hand, mouse models of CL are relative simple and the majority of experimental vaccines have been tested against the cutaneous form of the disease. The discovery of the Th1/Th2 paradigm of CD4 T cell response was aided largely by studies using CL-resistant and CL-susceptible inbred mouse strains [12–14]. Given the broad relevance of Leishmania models to understanding the development of T cell responses and vaccines against intracellular organisms, basic research has provided a greater understanding of how to induce protective immune responses against Leishmania [15]. Protection studies against CL, particularly in mice, have demonstrated the Th1-dependence of effective immunity against Leishmania. Importantly, appropriate Th1 cell responses also correlate with protection against CL in humans. Although there are more complexities in the mechanisms responsible for acquired immunity, the ideal vaccine against Leishmania species would induce strong, long-lasting Th1 cell responses directed against the parasites, both preventing disease and reducing transmission.
2.3. First generation leishmaniasis vaccines
Vaccination with live virulent parasites, termed leishmanization, was practiced from ancient times until recently in many endemic areas [17]. Inoculation of live L. major parasites into the skin of children (typically in hidden areas such as the buttocks) induced mild infection and subsequent immunity. The practice has now largely been abandoned as unsafe.
Vaccine trials involving whole, killed parasites were conducted in the 1970s and 1980s. More recent trials were conducted with inactivated whole parasite vaccines in Ecuador (composed of three strains of locally obtained parasites), Colombia (Biobras single strain L. amazonensis vaccine), Iran and Sudan (autoclaved L. major with BCG included as an adjuvant: ALM + BCG) [18–24]. With the exception of the Ecuadorian trial, in which a locally prepared vaccine was used, none of these trials demonstrated protection [25]. Similar vaccines have been used for immunotherapy of human CL, with success reported in Brazil and Venezuela [26, 27]. Based on these results, a first generation vaccine has been registered in Brazil as an adjunct to antimony therapy [26].
The use of crude antigens with appropriate adjuvants has provided protection against leishmaniasis in various animals, with protection against CaVL reported in Iran but not in Brazil [28, 29]. Partial efficacy, primarily against CL, has been obtained in the clinic using more refined first generation vaccines. Probably because this strategy involves the use of crude preparations that can neither be standardized nor optimally formulated to induce effective immune responses in the absence of undesirable ones, clinical results have been inconsistent. Furthermore, given vaccine regulations and release criteria, this does not represent a sustainable, viable option for widespread use.
3. Advances toward defined second generation vaccines
Second generation vaccines with further refined products, such as recombinant proteins with adjuvant or expression in heterologous microbial vectors, represent a more feasible option for mass vaccination campaigns. The recombinant nature of the product means it is accessible to large scale, reproducible and cost-effective approaches, and responses elicited upon vaccination can be potentiated and refined by appropriate formulation with adjuvant [30, 31]. Although the stability of second generation vaccines can pose difficulties in field conditions, this problem is common across all vaccine platforms. Defined antigens, delivered as plasmid DNA, vectored DNA, or as recombinant protein in adjuvant, have often proven to be effective in animal models. Of these platform technologies, while both protein- and DNA-based vaccines have advanced as veterinary products, to date only recombinant proteins have advanced to licensure in human vaccines.
A variety of recombinant proteins have been investigated as Leishmania vaccine antigen candidates in animal models [32–41]. Among these, the surface expressed glycoprotein leishmaniolysin (gp63) generated promising findings from animal models following delivery by numerous immunization regimens; those results were, however, overshadowed by mostly negative T cell responses in humans [42, 43]. Immunization with native Parasite Surface Antigen (PSA)-2 polypeptides, but not other forms, protected mice against infection [44–48]. Although protective efficacy of Leishmania homologue for receptors of activated C kinase (LACK) has been demonstrated mainly in the L. major model, LACK failed to protect against experimental VL [49, 50]. To date, very few antigens have advanced to clinical or veterinary trials.
One caveat regarding the use of recombinant proteins is that, alone, they generally induce only weak T cell responses. Effective and durable T cell responses can however be generated by the addition of certain adjuvants. The development of adjuvants that can be used to design safe and effective vaccines, along with the development of defined vaccine candidates for leishmaniases, could have a major impact on the control of disease. The discovery that properly formulated Toll-like receptor (TLR) agonists can stimulate Th1 immune responses has profoundly impacted vaccine development against intracellular pathogens like Leishmania. In particular, the extensive experience with monophosphoryl lipid A (MPL), a TLR4 agonist obtained from the cell wall of Salmonella, and MPL’s approval in vaccines for hepatitis B and human papilloma virus, have demonstrated the safety and efficacy of engaging TLR4. MPL is the only defined TLR agonist currently in approved vaccines, and has an extensive history of safety and efficacy [51]. Our first defined vaccine against leishmaniasis came with the use of recombinant fusion protein Leish-111f (L111f; LEISH-F1; comprising the L. major homologue of eukaryotic thiol-specific antioxidant (TSA), the L. major stress-inducible protein-1 (LmSTI1) and the L. braziliensis elongation and initiation factor (LeIF)), together with MPL formulated in an oil-in-water emulsion (MPL-SE). This vaccine candidate protects mice, hamsters, and rhesus macaques, and LEISH-F1+ MPL-SE was the first defined Leishmania vaccine to enter clinical trials [52–55].
3.1. Clinical trials of second generation vaccines
3.1.1. Vaccination of humans
In initial trials targeting prevention of CL, healthy Colombian adult volunteers with no history of leishmaniasis received three injections of the LEISH-F1 + MPL-SE vaccine (consisting of 10 μg LEISH-F1 antigen + 25 μg MPL-SE adjuvant) [55]. During screening, volunteers were evaluated for evidence of previous subclinical infection with L. braziliensis/L. panamensis based on the Montenegro skin test (MST). Twelve MST-positive subjects were enrolled in an open-label, uncontrolled clinical trial and sixty-eight MST-negative subjects were enrolled in a randomized, double-blind, controlled trial in which individuals were randomly assigned to receive LEISH-F1 + MPL-SE (n=34), LEISH-F1 alone (n=17), or a saline placebo (n=17). An anti-LEISH-F1 IgG antibody response was induced in all vaccine recipients. In both trials, LEISH-F1-specific IFNγ responses were observed in more than half, and a LEISH-F1-specific delayed-type hypersensitivity (DTH) was observed in most, of the vaccine recipients. The LEISH-F1 + MPL-SE vaccine was safe and well tolerated in subjects with and without evidence of previous subclinical infection.
In a subsequent trial targeting prevention of VL, healthy Indian adult volunteers were evaluated for evidence of previous infection with L. donovani based on the direct agglutination test (DAT) [56]. Three cohorts of 6 DAT-negative and 6 DAT-positive subjects were enrolled in an open-label, dose-escalating, uncontrolled clinical trial and received three injections of the LEISH-F1 + MPL-SE vaccine. The vaccine was safe and well-tolerated in DAT-negative and DAT-positive subjects and induced T cell production of IFNγ and other cytokines in response to stimulation with the LEISH-F1 antigen.
The LEISH-F1 + MPL-SE vaccine has also been evaluated as an adjunct immunotherapy with standard chemotherapy in CL. Adult CL patients were randomly assigned to receive three injections of either the LEISH-F1 + MPL-SE vaccine (consisting of escalating protein doses with a set adjuvant dose), adjuvant alone, or saline placebo, in conjunction with chemotherapy. The injections were given subcutaneously on days 0, 28, and 56, with follow up for safety and immunological endpoints. Forty-four CL patients were enrolled in this trial, with all receiving chemotherapy with meglumine antimoniate starting on the first day of vaccination [57]. Nearly all vaccine recipients and no adjuvant-alone or placebo recipients demonstrated an IgG antibody response to LEISH-F1 at Day 84. At this timepoint, an increased proportion of vaccine recipients were clinically cured (80%), compared to adjuvant-alone and placebo recipients (50% and 38% respectively). The LEISH-F1 + MPL-SE vaccine was safe and immunogenic in CL patients and appeared to shorten their time to cure when used in combination with meglumine antimoniate chemotherapy.
Several years ago an observational open-label study was conducted to evaluate the utility of vaccination with a mixture of several antigens with GM-CSF on drug-refractory ML [58]. Five of six patients, all of whom had failed previous drug therapy, were in complete clinical remission 9 months after their final vaccine dose and all 6 were asymptomatic 5 years after treatment. In a more recent double-blind trial conducted with ML as the target indication, LEISH-F1 + MPL-SE vaccine or saline were provided in conjunction with sodium stibogluconate [59]. The vaccine was again safe and well tolerated, and induced both humoral and cell-mediated immune responses. Furthermore, an increase in the proportion of memory LEISH-F1-specific IL-2-producing CD4 T cells was observed after vaccination, which was associated with clinical cure.
Together, several clinical trials demonstrate that the LEISH-F1 + MPL-SE vaccine is safe and immunogenic in healthy subjects with and without history of previous infection with Leishmania as well as in patients with CL and ML.
3.1.2. Vaccination of dogs
Among the domesticated animals affected by Leishmania infection, dogs are the most commonly affected species. Treatment options for CaVL are limited and infected dogs are commonly euthanized [60]. Chemotherapy is not widely recommended because it is only marginally effective and in addition, canine treatment is performed with the same drugs as those used to treat human infection raising the risk of the emergence of drug resistant parasites [61]. A canine vaccine for either prevention or treatment could be extremely beneficial because dogs harboring L. infantum serve as a reservoir closely linked with human infection (South America and southern Europe) [5]. The use of a canine vaccine to reduce this reservoir of infection and interrupt transmission of Leishmania parasites to humans could eliminate the need for euthanasia. Several studies have demonstrated varying degrees of efficacy in both prophylactic and therapeutic vaccine approaches in CaVL [62–67].
Prophylactic use of vaccines against CaVL has been investigated in naturally exposed dogs. L111f + MPL-SE did not protect dogs in an extremely high L. infantum endemic region in southern Italy. Of 39 dogs enrolled in the study, 37 had been infected and developed disease by the end of the 2 year study [62]. These results contrast with those achieved using the fucose mannose ligand (FML)-vaccine of L. donovani in Brazil. Only 8% of dogs vaccinated with the FML-vaccine showed mild signs of CaVL after 2 years of monitoring, whereas 33% of non-vaccinated animals developed clinical or fatal disease associated with L. donovani infection [63]. In a follow-up study, only 5% of dogs receiving FML-QuilA vaccine, versus 25% of the controls, developed clinical and fatal disease [64]. The FML-QuilA vaccine induced a significant, long lasting and strong protective effect against CaVL in the field. The FML-vaccine was industrialized and licensed for commercialization in Brazil in 2004 under the name of Leishmune®.
Leishmune is also used as an immunotherapeutic with a double saponin adjuvant concentration. The Leishmune therapeutic vaccine was assessed as either a stand-alone immunotherapy or as an immunochemotherapy in combination with allopurinol or amphotericinB/allopurinol, in infected dogs [65]. After 3 months of treatment, compared to infected untreated dogs, immunotherapy and immunochemotherapy similarly increased the proportion of dogs demonstrating response to Leishmania antigen and reduced the proportion of symptomatic cases, the proportion of parasite evidence in lymph nodes by PCR and the proportion of deaths. After 8 months, negative lymph node PCR results were obtained in 80% of the immunochemotherapy-treated dogs, but only in 33% of the immunotherapy group, and protection against death was lost 4 years after immunotherapy but not immunochemotherapy.
Both the L111f + MPL-SE vaccine and the non His-tag form of L111f (L110f), that has been successful as a prophylactic vaccine in mouse models of CL and VL [68], have been tested in similar canine immunotherapy trials. Both an open and a blinded trial were performed in Salvador, Brazil to evaluate its therapeutic efficacy in cases of CaVL caused by natural infection [67]. In the open trial, dogs received L111f + MPL-SE vaccine; Glucantime chemotherapy; vaccine plus Glucantime; or no treatment. The vaccine was given weekly for four weeks, while the drug was administered daily. At the 6-month assessment, while all 12 untreated dogs either died or showed no clinical improvement, 39 of 43 dogs across all treatment groups improved. The therapeutic efficacy observed 3 years after treatment with L111f + MPL-SE vaccine (improvement in 9 of 12 for vaccine; 7 of 11 for Glucantime; 5 of 10 for combined treatment) was confirmed in a subsequent blinded trial wherein vaccine was again given weekly [67]. L110f + MPL-SE demonstrated efficacy during evaluation within an immunochemotherapy protocol to treat symptomatic dogs naturally infected with L. chagasi in Belo Horizonte, Brazil [69]. Dogs responded immunologically to weekly vaccination and even though most dogs remained parasite-positive and many had one or more symptoms after the 6-month study period, vaccinated dogs had a better survival rate than dogs receiving either Glucantime or no treatment. Similar to the results obtained in human trials, these results support the notion of using a well-characterized recombinant vaccine as an adjunct to improve the current chemotherapy of canine leishmaniasis.
The potential interruption of the transmission by the Leishmune vaccine has been assayed by monitoring dogs within an epidemic area [70]. Leishmune-vaccinated dogs demonstrated a complete absence of clinical signs and of parasites in skin, lymph node and blood samples (by PCR for Leishmania DNA) nearly a year after vaccination. In contrast, one quarter of the untreated controls became symptomatic cases, with 56.7% and 15.7% having positive PCR reactions in lymph node and blood, respectively. The absence of Leishmania DNA and parasites in Leishmune-vaccinated animals indicates the non-infectious condition of the vaccinated dogs, suggesting they are no longer capable of transmitting parasites during blood meals. Between 2004 and 2006, Palatnik-de-Sousa and colleagues analyzed the possible additive effect of Leishmune® vaccination over dog culling, on the decrease of the incidence of CaVL and VL in two Brazilian endemic areas [66]. The decreases in the incidence of both canine and human disease correlated to the increase of the number of vaccinated dogs. In Aracatuba, a 25% of decline was seen in CaVL with a 61% decline in human cases, indicating the additive effect of Leishmune vaccination of the healthy dogs on regular dog culling. In Belo Horizonte, rising incidence of canine and human VL were observed in some districts, while the incidence started to decrease or stabilized after Leishmune vaccination. Among the districts showing a percent decrease of human incidence (−36.5%), Centro Sul and Pampulha showed the highest percentage of vaccinated dogs (63.27% and 27.27%, respectively) and the lowest dog incidence (−3.36% and 1.89%, respectively). Much lower proportions of vaccinated dogs were found in regions that exhibited pronounced increases in both canine and human incidence. Together, the data suggest an additive control effect of vaccination over dog culling, reducing the parasite reservoir and protecting dogs, and coincidentally becoming an effective control tool capable of reducing the risk of transmission of VL to humans.
3.2.3. Vaccine refinement at IDRI
As part of our vaccine development pipeline we continue to optimize both antigen and adjuvant preparations. We have initiated studies with our novel synthetic TLR4-based adjuvant, GLA-SE [68]. Our data reveal GLA-SE and MPL-SE adjuvanted responses to be dose-dependent, skewing towards a Th1-like response in mice that receive either of these TLR4 agonists. Furthermore, GLA-SE appears to be more potent than MPL-SE in terms of stimulating antigen-presenting cell activation and inducing greater IFNγ secretion by antigen-specific T cells [68]. We have also observed that low dose GLA-SE induces a high frequency of multi-functional effector T cells. This is important because such T cells have been hypothesized to serve as a correlate of protection against parasite challenge [60]. We recently investigated if either of these TLR4 agonists (MPL or GLA) could synergize with a TLR9 agonist to produce IL-12, and if this synergy would be useful in the context of L110f as a therapeutic Leishmania vaccine [71]. While L110f formulated with a single TLR agonist was protective against L. major challenge in a prophylactic setting, only the combination-agonists vaccine reduced parasite burden where there was pre-established disease [53, 68, 71, 72]. Our data also indicated that the ratio of terminal effector T cells to multi-functional T cells was higher in mice treated with the combination-agonist vaccine, further demonstrating that adjuvant use can manipulate the subsequent responses.
We have also identified additional vaccine antigen candidates that could serve as back-ups, if required, to the L111f/L110f antigen. Whereas Leish-111f was developed primarily as a therapeutic candidate, recent efforts have focused on antigens that meet more stringent criteria for prophylactic use. These criteria include sequence conservation among Leishmania spp., the ability to protect in CL and VL models and a lack of sequence identity with human genes, along with practical considerations such as the ability to express at high levels and to be purified. Forty-three genes/antigens relevant to infection were identified through screening of a L. infantum genomic expression library with sera from VL patients and experimentally infected hamsters [73]. A fraction of these proteins was subsequently evaluated in murine CL and VL prophylaxis models. Sterol 24-c-methyltransferase (SMT) induced protection against both CL and VL challenge [74, 75]. We also cloned and purified recombinant proteins of amastigote-expressed genes shown to confer protection as DNA vaccines against L. major challenge. These recombinant antigens, however, failed to protect when used in combination with adjuvants as vaccines in our models of VL prophylaxis and CL immunotherapy.
Additionally, we have produced a new fusion protein antigen termed KSAC. KSAC is comprised of KMP-11, SMT, A2 and CPB, and each component of this fusion protein has been demonstrated to individually confer protection in different disease models of VL [32, 34, 74–78]. Two of the individual antigens, SMT and CPB, provided the majority of the protection provided by KSAC when delivered as protein/adjuvant in our VL model. However, because KMP-11 was shown to be an effective antigen when delivered as DNA and because A2 has shown some degree of efficacy in dogs, these antigens were retained in the KSAC fusion [34, 79]. KSAC induced protection equal to or better than L110f in our mouse protection models of CL [80]. Moreover, we have shown that the KSAC components SMT, A2 and CPB were strongly recognized by T cells from individuals cured of VL. Preliminary data indicate that KSAC is effective in protecting mice against L. major using a sand fly challenge (manuscript in preparation).
4. How to interpret protective vaccination?
Among the greatest challenges for vaccine development is expedient evaluation of candidate molecules, particularly for prophylactic vaccine trials when long periods of time are required to generate clinical outcome data. In the screening of vaccine candidates it is important to select candidates that evoke immune responses associated with protection, but valid surrogate markers of protection against human leishmaniasis are still lacking. With regard to leishmaniasis, a study of individuals provided leishmanization (a controlled injection of live Leishmania) was conducted to identify parameters relevant to protection of humans. Lesions developed approximately 2 months after leishmanization, then resolved by 7–8 months and imparted protection against subsequent lesion development [81]. Nylen and colleagues used leishmanization to evaluate the efficacy of an alum-precipitated autoclaved L. major with BCG vaccine in order to more accurately define surrogate markers of immunity to leishmaniasis in humans [82]. Cellular immune responses to this artificial infection were monitored and comparisons were made between those volunteers who developed a lesion after infection and those who did not. No significant differences in leishmanin skin test (LST), IFNγ production, or source of IFNγ between those who developed a lesion and those who did not after leishmanization were detected, with the exception that ulcer development was associated with an enhanced number of IFNγ secreting CD4+ CD45RA- (memory) T cells. The IFNγ responses in the enrolled subjects were, however, significantly lower compared to volunteers with previous history of CL, raising the possibility that the selection of LST-negative volunteers in an endemic area may have biased the study towards potentially non/low L. major-reactive volunteers.
The most widely used measure of vaccine potential has been to monitor anti-Leishmania immune responses over time. Antibody (DAT) and cellular (MST) responses have previously been used at study enrolment to evaluate volunteers for evidence of previous subclinical infection [55, 56]. Various relatively simple serological parameters, such as DAT, fast agglutination screening test (FAST) or detection of antibodies against the FML, k26, k28 and k39 antigens, that are all indicative of exposure or infection, have been or can be used to regulate entry into clinical trials [83–93]. Analysis of individuals successfully treated with chemotherapy indicates persistence of antibodies against k39 whereas a latex agglutination test (KAtex; which detects a stable, non-protein) showed no positive reaction, indicating the differential use of these tests could be beneficial in an immunotherapeutic setting [94–96].
It is also becoming more feasible to detect and measure antigens in body fluids. KAtex is available for the detection of leishmanial antigen in the urine of VL patients, although the low sensitivity (~75%) somewhat limits its utility in assessing a prophylactic vaccine [97]. Nucleic acid detection is very sensitive and real-time PCR is an established method for detection of Leishmania parasite in blood. A urine PCR assay, as well as antigen detection, has been demonstrated to be an effective tool for diagnosis of VL and monitoring of treatment efficacy [98].
Dogs provide an excellent VL disease model but still require extended periods of follow-up and invasive procedures such as lymph node biopsy or bone marrow aspiration to explore parasite burden. The relatively constrained array of reagents available for immunological assessment of dogs is a further limitation. Analysis of samples from Leishmune-vaccinated dogs indicated that the vaccine not only conferred humoral responses against both vaccine and crude Leishmania antigens, and peripheral blood mononuclear cell IFNγ responses to FML and total antigen in the majority of animals, but also reduced CD4+CD25+ T cell presence compared to that observed before vaccination [99]. Comprehensive immunological evaluation of mice before and after Leishmania challenge could help establish an immune signature for efficacy of various vaccines but this can only be accomplished paradoxically with the availability of an effective vaccine candidate. Further characterization of protective vaccines with additional markers would be greatly facilitated by the availability of defined antigens against which to measure immune responses, particularly when discriminating vaccine-from infection-induced responses.
5. Summary
Vaccination holds promise for sustained control of leishmaniasis. Data suggest that prophylactic vaccination of both humans and dogs could generate long term protection and interrupt transmission, ultimately reducing disease incidence. Vaccination in a therapeutic setting as an adjunct with various chemotherapies has demonstrated safety and efficacy against various manifestations of Leishmania infection. These data indicate not only the promise of a therapeutic vaccination to improve response to treatment and perhaps minimize relapse, but also suggest that the use of a vaccine in human VL carriers could reduce transmission and prevent disease. We continue to refine both antigen and adjuvant components of our vaccines, as well as identify parameters that can be used to expedite clinical trials, to provide the best range of vaccines aimed at controlling disease incidence and severity.
Highlights.
A general background to leishmaniasis
-
Brief background on first- and second-generation vaccines
Summary of our own human clinical trials
Summary of trials within the dog reservoir
Summary of potential biomarkers to support vaccine trials
Acknowledgments
IDRI’s leishmaniasis vaccine program is funded by grants from the Bill and Melinda Gates Foundation (631 and 39129) and the National Institutes of Health (AI25038). We would like to thank Krishna Janakiraman for generating the maps depicted in this review and the many preclinical and clinical researchers, along with patients and volunteers, who have contributed in numerous ways to this program.
Abbreviations
- ALM
autoclaved L. major
- CaVL
canine visceral leishmaniasis
- CL
cutaneous leishmaniasis
- DAT
direct agglutination test
- DCL
diffuse cutaneous leishmaniasis
- FAST
fast agglutination screening test
- FML
fucose mannose ligand
- IFNγ
interferon gamma
- IL-12
interleukin-12
- KMP-11
kinetoplastid membrane protein-11
- LACK
Leishmania homologue for receptors of activated C kinase
- LeIF
L. braziliensis elongation and initiation factor
- LmSTI1
L. major stress-inducible protein-1
- LST
leishmanin skin test
- ML
mucosal leishmaniasis
- MPL
monophosphoryl lipid A
- MST
Montenegro skin test
- PKDL
post kala-azar dermal leishmaniasis
- PSA-2
Parasite Surface Antigen 2
- TLR
Toll-like receptor
- TSA
thiol-specific antioxidant
- VL
visceral leishmaniasis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scientific working group on Leishmaniasis Meeting report. Geneva, Switzerland: Feb 2–4, 2004. http://apps.who.int/tdr/publications/tdr-research-publications/swg-report-leishmaniasis/pdf/swg_leish.pdf, 2004. [Google Scholar]
- 2.WHO. WHO Technical Report Series. World Health Organization; Geneva: 2010. Control of the Leishmanises. [Google Scholar]
- 3.The Center for Food Security and Public Health, I.S.U. Animal Disease Resource Index Factsheets. 2009. Leishmaniasis (cutaneous & visceral) [Google Scholar]
- 4.Gelanew T, et al. Inference of population structure of Leishmania donovani strains isolated from different Ethiopian visceral leishmaniasis endemic areas. PLoS Negl Trop Dis. 2010;4(11):e889. doi: 10.1371/journal.pntd.0000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zerpa O, et al. Canine visceral leishmaniasis on Margarita Island (Nueva Esparta, Venezuela) Trans R Soc Trop Med Hyg. 2000;94(5):484–7. doi: 10.1016/s0035-9203(00)90059-2. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira LF, et al. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta tropica. 2011;118(2):87–96. doi: 10.1016/j.actatropica.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Sundar S, et al. Treatment of Indian visceral leishmaniasis with single or daily infusions of low dose liposomal amphotericin B: randomised trial. Bmj. 2001;323(7310):419–22. doi: 10.1136/bmj.323.7310.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundar S, et al. Low-dose liposomal amphotericin B in refractory Indian visceral leishmaniasis: a multicenter study. The American journal of tropical medicine and hygiene. 2002;66(2):143–6. doi: 10.4269/ajtmh.2002.66.143. [DOI] [PubMed] [Google Scholar]
- 9.Sundar S, et al. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;38(3):377–83. doi: 10.1086/380971. [DOI] [PubMed] [Google Scholar]
- 10.Matlashewski G, et al. Visceral leishmaniasis: elimination with existing interventions. The Lancet infectious diseases. 2011;11(4):322–5. doi: 10.1016/S1473-3099(10)70320-0. [DOI] [PubMed] [Google Scholar]
- 11.Sundar S, et al. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. The New England journal of medicine. 2010;362(6):504–12. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 12.Scott P, et al. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–82. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 13.Sypek JP, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177(6):1797–802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinzel FP, et al. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177(5):1505–9. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol. 2001;Chapter 19(Unit 19):2. doi: 10.1002/0471142735.im1902s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melby PC, et al. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol. 2001;166(3):1912–20. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- 17.Badaro R, et al. Successful use of a defined antigen/GM-CSF adjuvant vaccine to treat mucosal Leishmaniasis refractory to antimony: A case report. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2001;5(4):223–32. doi: 10.1590/s1413-86702001000400008. [DOI] [PubMed] [Google Scholar]
- 18.Khalil EA, et al. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet. 2000;356(9241):1565–9. doi: 10.1016/s0140-6736(00)03128-7. [DOI] [PubMed] [Google Scholar]
- 19.Momeni AZ, et al. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1999;17(5):466–72. doi: 10.1016/s0264-410x(98)00220-5. [DOI] [PubMed] [Google Scholar]
- 20.Armijos RX, et al. Field trial of a vaccine against New World cutaneous leishmaniasis in an at-risk child population: safety, immunogenicity, and efficacy during the first 12 months of follow-up. J Infect Dis. 1998;177(5):1352–7. doi: 10.1086/515265. [DOI] [PubMed] [Google Scholar]
- 21.Bahar K, et al. Comparative safety and immunogenicity trial of two killed Leishmania major vaccines with or without BCG in human volunteers. Clin Dermatol. 1996;14(5):489–95. doi: 10.1016/0738-081x(96)00071-5. [DOI] [PubMed] [Google Scholar]
- 22.Sharifi I, et al. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998;351(9115):1540–3. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- 23.Velez ID, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg. 2005;99(8):593–8. doi: 10.1016/j.trstmh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Armijos RX, et al. Field trial of a vaccine against new world cutaneous leishmaniasis in an at-risk child population: how long does protection last? The Journal of Infectious Diseases. 2003;187(12):1959–61. doi: 10.1086/375399. [DOI] [PubMed] [Google Scholar]
- 25.Noazin S, et al. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis: a meta-analysis. Vaccine. 2009;27(35):4747–53. doi: 10.1016/j.vaccine.2009.05.084. [DOI] [PubMed] [Google Scholar]
- 26.Mayrink W, et al. Immunotherapy, immunochemotherapy and chemotherapy for American cutaneous leishmaniasis treatment. Rev Soc Bras Med Trop. 2006;39(1):14–21. doi: 10.1590/s0037-86822006000100003. [DOI] [PubMed] [Google Scholar]
- 27.Convit J, et al. Immunotherapy versus chemotherapy in localised cutaneous leishmaniasis. Lancet. 1987;1(8530):401–5. doi: 10.1016/s0140-6736(87)90116-4. [DOI] [PubMed] [Google Scholar]
- 28.Genaro O, et al. Vaccine for prophylaxis and immunotherapy, Brazil. Clin Dermatol. 1996;14(5):503–12. doi: 10.1016/0738-081x(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 29.Mohebali M, et al. Double-blind randomized efficacy field trial of alum precipitated autoclaved Leishmania major vaccine mixed with BCG against canine visceral leishmaniasis in Meshkin-Shahr district, I.R. Iran. Vaccine. 2004;22(29–30):4097–100. doi: 10.1016/j.vaccine.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 30.Reed SG, et al. New horizons in adjuvants for vaccine development. Trends in Immunology. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Duthie MS, et al. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239(1):178–96. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafati S, et al. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine. 2005;23(28):3716–25. doi: 10.1016/j.vaccine.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Soong L, et al. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63(9):3559–66. doi: 10.1128/iai.63.9.3559-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu R, et al. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J Immunol. 2005;174(11):7160–71. doi: 10.4049/jimmunol.174.11.7160. [DOI] [PubMed] [Google Scholar]
- 35.Streit JA, et al. BCG expressing LCR1 of Leishmania chagasi induces protective immunity in susceptible mice. Exp Parasitol. 2000;94(1):33–41. doi: 10.1006/expr.1999.4459. [DOI] [PubMed] [Google Scholar]
- 36.Stager S, Smith DF, Kaye PM. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000;165(12):7064–71. doi: 10.4049/jimmunol.165.12.7064. [DOI] [PubMed] [Google Scholar]
- 37.Tewary P, et al. A heterologous prime-boost vaccination regimen using ORFF DNA and recombinant ORFF protein confers protective immunity against experimental visceral leishmaniasis. J Infect Dis. 2005;191(12):2130–7. doi: 10.1086/430348. [DOI] [PubMed] [Google Scholar]
- 38.Iborra S, et al. The Leishmania infantum acidic ribosomal protein P0 administered as a DNA vaccine confers protective immunity to Leishmania major infection in BALB/c mice. Infect Immun. 2003;71(11):6562–72. doi: 10.1128/IAI.71.11.6562-6572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saravia NG, et al. Protective immunogenicity of the paraflagellar rod protein 2 of Leishmania mexicana. Vaccine. 2005;23(8):984–95. doi: 10.1016/j.vaccine.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 40.Aguilar-Be I, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005;73(2):812–9. doi: 10.1128/IAI.73.2.812-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samant M, et al. Immunization with the DNA-encoding N-terminal domain of proteophosphoglycan of Leishmania donovani generates Th1-type immunoprotective response against experimental visceral leishmaniasis. J Immunol. 2009;183(1):470–9. doi: 10.4049/jimmunol.0900265. [DOI] [PubMed] [Google Scholar]
- 42.Russo DM, et al. Human T cell responses to gp63, a surface antigen of Leishmania. J Immunol. 1991;147(10):3575–80. [PubMed] [Google Scholar]
- 43.Burns JM, Jr, et al. Characterization of a membrane antigen of Leishmania amazonensis that stimulates human immune responses. J Immunol. 1991;146(2):742–8. [PubMed] [Google Scholar]
- 44.McMahon-Pratt D, et al. Loss of the GP46/M-2 surface membrane glycoprotein gene family in the Leishmania braziliensis complex. Mol Biochem Parasitol. 1992;50(1):151–60. doi: 10.1016/0166-6851(92)90252-f. [DOI] [PubMed] [Google Scholar]
- 45.Handman E, et al. Protective vaccination with promastigote surface antigen 2 from Leishmania major is mediated by a TH1 type of immune response. Infect Immun. 1995;63(11):4261–7. doi: 10.1128/iai.63.11.4261-4267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sjolander A, et al. Vaccination with recombinant Parasite Surface Antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection. Vaccine. 1998;16(20):2077–84. doi: 10.1016/s0264-410x(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 47.Sjolander A, et al. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J Immunol. 1998;160(8):3949–57. [PubMed] [Google Scholar]
- 48.Handman E, et al. Therapy of murine cutaneous leishmaniasis by DNA vaccination. Vaccine. 2000;18(26):3011–7. doi: 10.1016/s0264-410x(00)00109-2. [DOI] [PubMed] [Google Scholar]
- 49.Mougneau E, et al. Expression cloning of a protective Leishmania antigen. Science. 1995;268(5210):563–6. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 50.Melby PC, et al. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect Immun. 2001;69(8):4719–25. doi: 10.1128/IAI.69.8.4719-4725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert review of vaccines. 2011;10(4):471–86. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 52.Skeiky YA, et al. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine. 2002;20(27–28):3292–303. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 53.Coler RN, et al. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect Immun. 2007;75(9):4648–54. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campos-Neto A, et al. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infection and immunity. 2001;69(6):4103–8. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Velez ID, et al. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28(2):329–37. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 56.Chakravarty J, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1 + MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine. 2011;29(19):3531–7. doi: 10.1016/j.vaccine.2011.02.096. [DOI] [PubMed] [Google Scholar]
- 57.Nascimento E, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1 + MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine. 2010;28(40):6581–7. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 58.Badaro R, et al. Immunotherapy for drug-refractory mucosal leishmaniasis. J Infect Dis. 2006;194(8):1151–9. doi: 10.1086/507708. [DOI] [PubMed] [Google Scholar]
- 59.Llanos-Cuentas A, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1 + MPL-SE vaccine when used in combination with sodium stibogluconate for the treatment of mucosal leishmaniasis. Vaccine. 2010;28(46):7427–35. doi: 10.1016/j.vaccine.2010.08.092. [DOI] [PubMed] [Google Scholar]
- 60.Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18(9):399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- 61.Noli C, Auxilia ST. Treatment of canine Old World visceral leishmaniasis: a systematic review. Vet Dermatol. 2005;16(4):213–32. doi: 10.1111/j.1365-3164.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 62.Gradoni L, et al. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine. 2005;23(45):5245–51. doi: 10.1016/j.vaccine.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 63.da Silva VO, et al. A phase III trial of efficacy of the FML-vaccine against canine kala-azar in an endemic area of Brazil (Sao Goncalo do Amaranto, RN) Vaccine. 2000;19(9–10):1082–92. doi: 10.1016/s0264-410x(00)00339-x. [DOI] [PubMed] [Google Scholar]
- 64.Borja-Cabrera GP, et al. Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (Sao Goncalo do Amarante, RN) Vaccine. 2002;20(27–28):3277–84. doi: 10.1016/s0264-410x(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 65.Borja-Cabrera GP, et al. Immunotherapy with the saponin enriched-Leishmune vaccine versus immunochemotherapy in dogs with natural canine visceral leishmaniasis. Vaccine. 2010;28(3):597–603. doi: 10.1016/j.vaccine.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 66.Palatnik-de-Sousa CB, et al. Decrease of the incidence of human and canine visceral leishmaniasis after dog vaccination with Leishmune in Brazilian endemic areas. Vaccine. 2009;27(27):3505–12. doi: 10.1016/j.vaccine.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 67.Trigo J, et al. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine. 2010;28(19):3333–40. doi: 10.1016/j.vaccine.2010.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bertholet S, et al. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27(50):7036–45. doi: 10.1016/j.vaccine.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miret J, et al. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine. 2008;26(12):1585–94. doi: 10.1016/j.vaccine.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nogueira FS, et al. Leishmune vaccine blocks the transmission of canine visceral leishmaniasis: absence of Leishmania parasites in blood, skin and lymph nodes of vaccinated exposed dogs. Vaccine. 2005;23(40):4805–10. doi: 10.1016/j.vaccine.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Raman VS, et al. Applying TLR synergy in immunotherapy: Implications in Cutaneous Leishmaniasis. J Immunol. 2010 doi: 10.4049/jimmunol.1000238. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 73.Goto Y, et al. Cloning, characterization, and serodiagnostic evaluation of Leishmania infantum tandem repeat proteins. Infect Immun. 2006;74(7):3939–45. doi: 10.1128/IAI.00101-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goto Y, et al. Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine. 2007;25(42):7450–8. doi: 10.1016/j.vaccine.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goto Y, et al. Leishmania infantum sterol 24-c-methyltransferase formulated with MPL-SE induces cross-protection against L. major infection. Vaccine. 2009;27(21):2884–90. doi: 10.1016/j.vaccine.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukhopadhyay S, et al. Immunoprophylaxis and immunotherapy against experimental visceral leishmaniasis. Vaccine. 1999;17(3):291–300. doi: 10.1016/s0264-410x(98)90017-2. [DOI] [PubMed] [Google Scholar]
- 77.Ghosh A, Zhang WW, Matlashewski G. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine. 2001;20(1–2):59–66. doi: 10.1016/s0264-410x(01)00322-x. [DOI] [PubMed] [Google Scholar]
- 78.Rafati S, Zahedifard F, Nazgouee F. Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine. 2006;24(12):2169–75. doi: 10.1016/j.vaccine.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 79.Fernandes AP, et al. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine. 2008;26(46):5888–95. doi: 10.1016/j.vaccine.2008.05.095. [DOI] [PubMed] [Google Scholar]
- 80.Goto Y, et al. KSAC, the first defined polyprotein vaccine candidate for visceral leishmaniasis. Clinical and vaccine immunology: CVI. 2011;18(7):1118–24. doi: 10.1128/CVI.05024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khamesipour A, et al. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;23(28):3642–8. doi: 10.1016/j.vaccine.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 82.Nylen S, et al. Surrogate markers of immunity to Leishmania major in leishmanin skin test negative individuals from an endemic area re-visited. Vaccine. 2006;24(47–48):6944–54. doi: 10.1016/j.vaccine.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 83.Singh S, et al. Diagnostic and prognostic value of K39 recombinant antigen in Indian leishmaniasis. J Parasitol. 1995;81(6):1000–3. [PubMed] [Google Scholar]
- 84.Kumar R, et al. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin Diagn Lab Immunol. 2001;8(6):1220–4. doi: 10.1128/CDLI.8.6.1220-1224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qu JQ, et al. Serodiagnosis of Asian leishmaniasis with a recombinant antigen from the repetitive domain of a Leishmania kinesin. Trans R Soc Trop Med Hyg. 1994;88(5):543–5. doi: 10.1016/0035-9203(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 86.Sundar S, et al. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet. 1998;351(9102):563–5. doi: 10.1016/S0140-6736(97)04350-X. [DOI] [PubMed] [Google Scholar]
- 87.Luz KG, et al. Prevalence of anti-Leishmania donovani antibody among Brazilian blood donors and multiply transfused hemodialysis patients. Am J Trop Med Hyg. 1997;57(2):168–71. doi: 10.4269/ajtmh.1997.57.168. [DOI] [PubMed] [Google Scholar]
- 88.Otero AC, et al. Short report: occurrence of Leishmania donovani DNA in donated blood from seroreactive Brazilian blood donors. Am J Trop Med Hyg. 2000;62(1):128–31. doi: 10.4269/ajtmh.2000.62.128. [DOI] [PubMed] [Google Scholar]
- 89.Farajnia S, et al. Development and evaluation of Leishmania infantum rK26 ELISA for serodiagnosis of visceral leishmaniasis in Iran. Parasitology. 2008;135(9):1035–41. doi: 10.1017/S003118200800454X. [DOI] [PubMed] [Google Scholar]
- 90.Mandal J, et al. Evaluation of direct agglutination test, rk39 Test, and ELISA for the diagnosis of visceral leishmaniasis. Am J Trop Med Hyg. 2008;79(1):76–8. [PubMed] [Google Scholar]
- 91.Babakhan L, et al. Rapid detection of Leishmania infantum infection in dogs: a comparative study using fast agglutination screening test (FAST) and direct agglutination test (DAT) in Iran. Parasitol Res. 2009;105(3):717–20. doi: 10.1007/s00436-009-1456-3. [DOI] [PubMed] [Google Scholar]
- 92.Palatnik-de-Sousa CB, et al. Leishmania donovani: titration of antibodies to the fucose-mannose ligand as an aid in diagnosis and prognosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1995;89(4):390–3. doi: 10.1016/0035-9203(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 93.Pattabhi S, et al. Design, development and evaluation of rK28-based point-of-care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl Trop Dis. 2010;4(9) doi: 10.1371/journal.pntd.0000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zijlstra EE, et al. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin Diagn Lab Immunol. 1998;5(5):717–20. doi: 10.1128/cdli.5.5.717-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gavgani AS, Vatan SK, Ghazanchaei A. KAtex antigen-detection test as a diagnostic tool for latent visceral leishmaniasis cases. African Journal of Biotechnology. 2008;7(7):852–859. [Google Scholar]
- 96.Singh DP, et al. In search of an ideal test for diagnosis and prognosis of kala-azar. J Health Popul Nutr. 2010;28(3):281–5. doi: 10.3329/jhpn.v28i3.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Attar ZJCM, el-Safi S, Carney J, Azazy A, El-Hadi M, Dourado C, Hommel M. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop. 2001;78(1):11–6. doi: 10.1016/s0001-706x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 98.Fisa R, et al. Leishmania infantum DNA detection in urine from patients with visceral leishmaniasis and after treatment control. Am J Trop Med Hyg. 2008;78(5):741–4. [PubMed] [Google Scholar]
- 99.de Lima VM, et al. Diminished CD4+/CD25+ T cell and increased IFN-gamma levels occur in dogs vaccinated with Leishmune in an endemic area for visceral leishmaniasis. Vet Immunol Immunopathol. 2010;135(3–4):296–302. doi: 10.1016/j.vetimm.2009.12.008. [DOI] [PubMed] [Google Scholar]