Abstract
Micro-RNAs (miRNAs) are non-coding RNAs of 18–24 nucleotides that are involved in post-transcriptional regulation of protein expression. Their role in ischemic preconditioning (IPC) is currently unknown. We hypothesized that miRNAs induced after IPC in the heart may create a preconditioned phenotype through up-regulating proteins including eNOS/iNOS and HSP70 which are implicated in the late phase protection of IPC. miRNAs were extracted from hearts of ICR mice following IPC. The purified miRNAs were injected in vivo into the left ventricle wall of mice and, 48 h later, the hearts were subjected to regional ischemia/reperfusion (I/R) injury by LAD ligation for 30 min followed by reperfusion for 24 h. IPC caused no changes in miRNA-23b and miRNA-483 whereas miRNA-1, miRNA-21 and miRNA-24 were significantly increased. The IPC-miRNA treatment caused an increase in eNOS mRNA and protein, whereas iNOS was not changed. Heat shock transcription factor 1 (HSF-1) and HSP-70 were also increased with IPC-miRNA treatment versus control. Moreover, injection of IPC-miRNA protected the hearts against I/R injury as shown by a reduction of infarct size as compared with saline or non-IPC miRNA-treated control. We conclude that IPC-induced miRNAs trigger cardioprotection similar to the delayed phase of IPC, possibly through up-regulating eNOS, HSP70 and its transcription factor HSF-1.
Keywords: miRNA, eNOS, HSP-70, ischemia-reperfusion, preconditioning
Introduction
Ischemic preconditioning (IPC) is a powerful cardioprotective phenomenon whereby repeated brief episodes of ischemia protect the heart against future myocardial infarction (1). Genetic reprogramming emerging during or following IPC which simulates angina in the clinical setting can be characterized as protective in nature. Several mechanisms for IPC have been proposed, which broadly include the release of endogenous mediators including adenosine, activation of G-coupled receptors, protein kinase C and synthesis of cytoprotective proteins including eNOS, iNOS, Cox-2 and HSP70, which either individually or in concert lead to protection against ischemia/reperfusion (I/R) injury (2,3).
In recent years, micro-RNAs (miRNAs) have emerged as novel regulators of gene expression. The miRNAs are a family of small RNA with average length of 24 nucleotides which is too short to code for any proteins. For a long time, miRNAs were considered as by-products of mRNA transcription, or even as “evolutionary transcriptional debris”. However, recent studies suggest that miRNAs participate in many cellular processes, such as apoptosis, fat metabolism, cell differentiation, tumorogenesis and cardiogenesis (4). miRNAs are also critically involved in the pathological process of adult hearts, including cardiac hypertrophy (5), angiogenesis, arrhythmogenesis and heart failure. We recently observed that whole body heat shock leads to synthesis of several miRNAs, which lead to protection against I/R injury (6). However, the potential role of endogenous miRNAs in IPC has never been investigated.
In the current study, we tested the hypothesis that miRNAs induced by IPC play an important role in protection against myocardial I/R injury. We induced miRNAs by short bursts of global ischemia and reperfusion in the isolated-perfused hearts. The induced miRNAs (IPC-miRNAs) were then injected directly into the myocardium in vivo 48 h prior to I/R injury. For the first time, our results show that miRNAs cause significant reduction in infarct size which is associated with the upregulation of protective proteins including eNOS, heat shock transcription factor-1 (HSF-1) and HSP-70 that are implicated in the delayed phase of IPC in the heart.
Materials and Methods
Details of the IPC/infarction protocol, miRNA isolation, their verification and injection as well as measurement of infarct size are provided in the online data supplement.
Results and Discussion
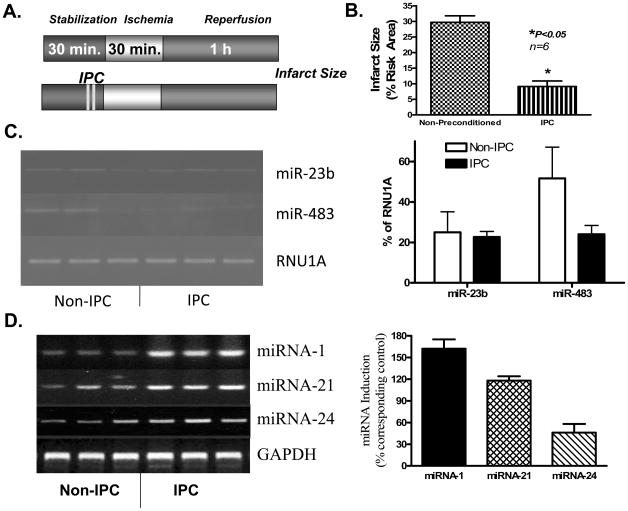
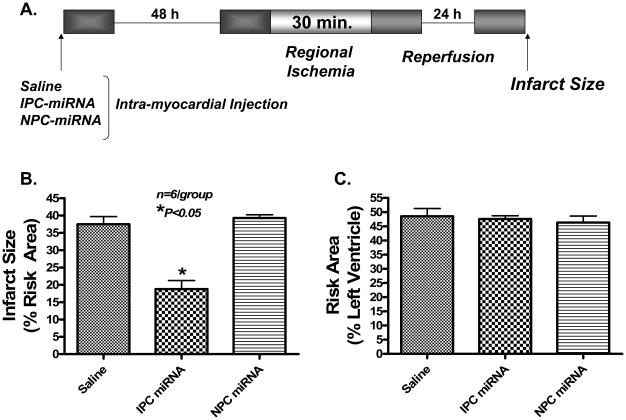
In the Langendorff model, IPC stimulus (2 bursts of 30 sec global ischemia followed by 90 sec reperfusion, Figure 1A) reduced infarct size from 29.7±2.1% in control-group to 9.1±1.8 % in the IPC-group (69.3% reduction, mean±SEM, P<0.05, Figure 1B). The IPC protocol caused no changes in miRNA-23b and miRNA-483 (Figure 1C), but caused a significant induction of miRNA-1 (162±13%), miRNA-21 (118 ± 6%), and miRNA-24 (46 ± 12%) as compared to control (Figure 1D). To determine the cause-and-effect relationship between IPC-induced endogenous miRNAs and cardioprotection, we injected the pool of extracted miRNAs from non-IPC and IPC hearts directly into the left ventricular wall in situ in a separate set of mice (miRNA-injected group). As shown in Figure 3A, a significant uptake of miRNA-21 was evident after 1 h of injection in the risk zone. Forty-eight h later, the mice were subjected to I/R injury in vivo by ligation of left coronary artery for 30 min followed by reperfusion for 24 h. Our results show that miRNAs derived from IPC hearts produced a protective phenotype with significantly lower infarction (18.8±2.5%) as compared to saline-injected controls (37.5±2.2%) or miRNAs prepared from non-IPC hearts (39.3±2.3%). There was no difference in infarct size between saline-injected controls versus non-IPC miRNA-treated hearts. Also, there were no significant differences in risk areas between the groups (Figure 2B).
Figure 1. Induction of miRNA by ischemic preconditioning (IPC) in the Langendorff isolated perfused heart.
(A) Experimental protocol. (B) Infarct size reduction following IPC (n=6/group). (C) Left panel- Gel electrophoresis image of RT-PCR products of miRNAs; Right panel- average normalized results showing no change in miRNA-23b and miRNA-483 following IPC. Endogenous U1A small nuclear RNA (RNU1A) was used as control for miRNA-23b and miRNA-483. The results are means±SEM from 3 independent hearts. (D) The left panel: gel electrophoresis image of RT-PCR products of miRNAs; Right panel- average normalized changes in miRNA-1, miRNA-21 and miRNA-24. The results are means±SEM from 3 independent hearts. *P<0.05 versus non-preconditioned controls.
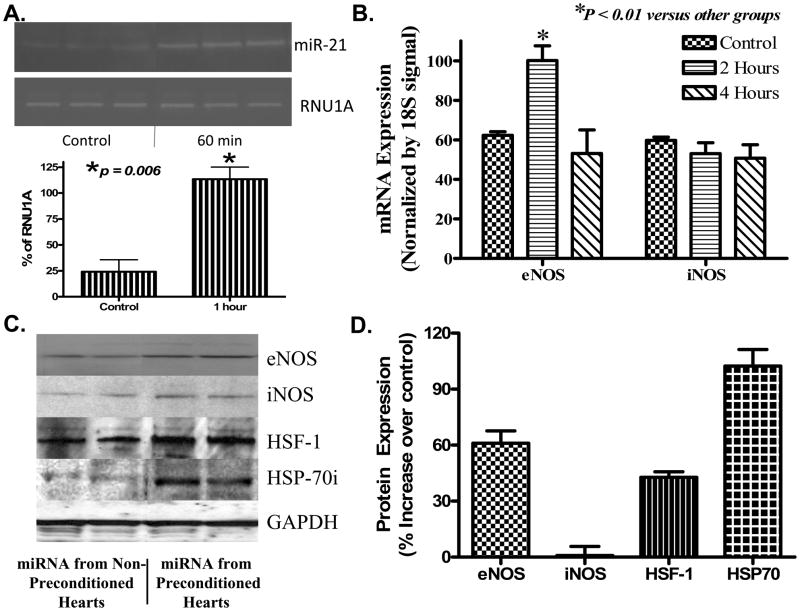
Figure 3. Effect of miRNA on induction of cardioprotective proteins.
(A)Uptake of miRNA-21 following injection in LV wall. Top panel: gel electrophoresis image of the RT-PCR for miRNA-21; Bottom panel: average normalized changes in miRNA-21. Endogenous U1A small nuclear RNA (RNU1A) was used as control. (B) R-T PCR showing eNOS and iNOS mRNA levels. A significant increase in eNOS was noted 2 h following treatment with IPC-miRNA. (C) Representative western blot showing expression of eNOS, iNOS, HSF-1 at 4 h and HSP 70 at 48 h following treatment with IPC-miRNA or non-IPC-miRNA control. (D) Bar diagram showing average % increase in proteins shown in ‘B’. Results are means±SEM from 3 independent hearts per group.
Figure 2. Effect of miRNA on myocardial infarct size following ischemia/reperfusion.
(A) Experimental protocol for in vivo studies. (B) Reduction of myocardial infarct size (% of risk area) following direct delivery of IPC-miRNA into the heart. (C) Risk area between three groups. *P<0.05 versus saline control and non-IPC miRNA treated hearts.
To gain further insight into the mechanisms underlying miRNA-induced protection, we probed several target protective proteins that are implicated in IPC, including eNOS, iNOS, HSP 70 and its transcription factor, HSF-1. As shown in Figure 3A, induction of eNOS mRNA (61±6.7%) was detected in the IPC-miRNA group 2 h following treatment. However, no changes in iNOS mRNA were observed. Western blot analysis confirmed a significant up-regulation in eNOS protein (62.0±6.7%) and HSF-1 (42.7±3.0%) 4 h after IPC-miRNA treatment. HSP70 was also significantly increased (102.3±8.9%) 48 h after IPC-miRNA treatment. Again, similar to mRNA, iNOS protein was not significantly changed (Figure 3C and 3D).
Despite potential species differences in cardioprotection (7), it is widely known that the protective effects of IPC occur in an early phase that develops rapidly after the initial stimulus but dissipates within 2–3 h, and a late phase that becomes apparent 12–24 h later and persists for approximately 72 h. The role of NO derived from eNOS in the late phase of IPC has been suggested previously (8). Although the critical role of iNOS has also been well documented in the late phase of IPC (9) and pharmacological preconditioning (10,11), we did not observe upregulation of this protein in the IPC-miRNA-treated hearts. This is possibly due to the strength of the IPC stimulus, which may not be sufficiently potent to trigger miRNA changes for synthesis of iNOS. A previous study has shown that six cycles of 4-min coronary occlusion/4-min reperfusion cause significant increase in myocardial iNOS (9). In this study, we utilized a less stringent IPC protocol i.e., 2 bursts of 30 sec ischemia and 90 sec reperfusion.
The induction of heat shock proteins by stressful stimuli such as elevated temperature or ischemia (12) is mediated by heat shock transcription factor (HSF-1). It is known that the cytoplasmic HSF-1 monomer forms a trimer and moves to the nucleus where it binds to its target sites (known as heat shock elements) in the regulatory regions of the HSP genes. Following its phosphorylation, HSF-1 induces expression of HSP70 that could protect hearts against ischemic injuries during the late phase of IPC or direct gene transfer of protective proteins as reported previously. Our results show that IPC-miRNAs can induce HSF-1 and HSP 70 which may play a role in ischemic tolerance observed in the heart. However, we do not know which particular miRNA is responsible for the increased synthesis of HSF-1/HSP70 or eNOS in the present study. miRNA-1 has been linked in post-transcriptional repression of HSP60 and HSP70 in H9c2 cells which is in contrast to the upregulation of HSP70 synthesis observed in the present study. Also, eNOS introns contain miRNAs which regulate eNOS expression (13). Although we observed a significant upregulation of miRNA-1, miRNA-21 and miRNA-24, it is possible that other miRNAs may have caused increased expression of the cytoprotective proteins as well. It is known that miRNAs generally function as inhibitory mechanisms of gene expression and therefore it is possible that suppression of genes participating in injurious processes during I/R injury may underlie miRNA-induced protection in the heart. At the same time, it is possible that the suppression of injurious genes may lead to upregulation of protective proteins including eNOS and HSP 70 as shown in the current study. Identification of such injurious genes is critical for validation of this hypothesis. Nevertheless, the protection observed against I/R injury in this study clearly suggests that concerted action of one or perhaps several miRNAs, induced following IPC, may have been responsible for the increased expression of eNOS and HSP70. Further studies are needed to identify the miRNA(s) from IPC that could increase the cytoprotective proteins without adverse side effects. The delivery of such miRNA(s) in the heart would have immense therapeutic potential in reducing myocardial infarction in patients with heart disease.
Supplementary Material
Acknowledgments
This study is supported by NIH grants HL51045, HL59469, HL79424 and HL093685 to Dr. R.C. Kukreja.
Footnotes
Disclosures: None.
Reference List
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–83. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 3.Heusch G, Boengler K, Schulz R. Cardioprotection: Nitric Oxide, Protein Kinases, and Mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436(7048):214–20. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 5.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100(3):416–24. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 6.Yin C, Wang X, Kukreja RC. Endogenously synthesized microRNA induce protective effect against ischemia/reperfusion injury. FEBS Letters. 2008;582:4137–4142. doi: 10.1016/j.febslet.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–8. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 8.Xuan YT, Tang XL, Qiu Y, Banerjee S, Takano H, Han H, Bolli R. Biphasic response of cardiac NO synthase isoforms to ischemic preconditioning in conscious rabbits. Am J Physiol Heart Circ Physiol. 2000;279:H2360–H2371. doi: 10.1152/ajpheart.2000.279.5.H2360. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi L, Jarret N, Hess ML, Kukreja RC. Essential role of inducible nitric oxide in monophosphoryl lipid A induced delayed ischemic protection: evidence from pharmacological inhibition and gene knockout mice. Circulation. 1999;99:2157–63. doi: 10.1161/01.cir.99.16.2157. [DOI] [PubMed] [Google Scholar]
- 11.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil (Viagra) induces delayed preconditioning through iNOS-dependent pathway in mouse heart. Circ Res. 2003;92:595–7. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 12.Nishizawa J, Nakai A, Matsuda K, et al. Reactive oxygen species play an important role in the activation of heat shock factor 1 in ischemic-reperfused heart. Circulation. 1999;99:934–941. doi: 10.1161/01.cir.99.7.934. [DOI] [PubMed] [Google Scholar]
- 13.Zhang MX, Ou H, Shen YH, Wang J, Wang J, Coselli J, Wang XL. Regulation of endothelial nitric oxide synthase by small RNA. Proc Natl Acad Sci U S A. 2005;102:16967–72. doi: 10.1073/pnas.0503853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.