Abstract
Control of expression of molecular receptors for chemical messengers and modulation of these receptors’ activity are now established as ways to alter cellular reaction. This paper extends these mechanisms to the arena of pathological pain by presenting the hypothesis that increased expression of α-adrenergic receptors in primary afferent neurons is part of the etiology of pain in classical causalgia. It is argued that partial denervation by lesion of peripheral nerve or by tissue destruction induces a change in peripheral nociceptors, making them excitable by sympathetic activity and adrenergic substances. This excitation is mediated by α-adrenergic receptors and has a time course reminiscent of experimental denervation supersensitivity. The change in neuronal phenotype is demonstrable after lesions of mixed nerves or of the sympathetic postganglionic supply. Similar partial denervations also produce a substantial increase in the number of dorsal root ganglion neurons evidencing the presence of α-adrenergic receptors. The hypothesis proposes the increased presence of α-adrenergic receptors in primary afferent neurons to result from an altered gene expression triggered by cytokines/growth factors produced by disconnection of peripheral nerve fibers from their cell bodies. These additional adrenergic receptors are suggested to make nociceptors and other primary afferent neurons excitable by local or circulating norepinephrine and epinephrine. For central pathways, the adrenergic excitation would be equivalent to that produced by noxious events and would consequently evoke pain. In support, evidence is cited for a form of denervation supersensitivity in causalgia and for increased expression of human α-adrenergic receptors after loss of sympathetic activity.
This essay is an outgrowth of a Colloquium session in which new evidence was presented on how molecular receptors for chemical synaptic mediators can specify and regulate neuronal responses in systems associated with pain mechanisms. These data build on the concept that not only the nature, but also the magnitude, of the transfer of information between cells is at least as much a function of receptive molecules as of the chemical messengers. Therefore, selective regulation of receptor expression and quantitative control of receptor activity are factors defining or modulating synaptic function. Importantly, such concepts, when applied to disease, open novel possibilities of pharmaceutical manipulation and treatment. My purpose is to extend such considerations of receptor regulation to a pathological process involving pain.
There now is considerable agreement that in mammals, the detection and signaling of tissue damage or pathology, that is, nociception, is a normal somatosensory function. In this view, pain, one of the reactions to tissue injury, represents the sensory concomitant of nociception. By logical extension, pain in the absence of peripheral tissue damage is abnormal, in other words, pathological.
Causalgia and Postsympathectomy Pain.
S. Weir Mitchell (1) named a syndrome causalgia after its most prominent symptom, a burning pain referred to a particular body region appearing spontaneously or after innocuous stimulation. As classically described, causalgia appears after partial disruption of the innervation to a limb, typically after injury to a large mixed nerve. It probably is relevant that the full-blown syndrome is not usually reported after lesions of smaller, purely cutaneous nerves. Some years after the original descriptions, Rene Leriche (2) pointed out that the syndrome of causalgia had features suggesting abnormal sympathetic nervous system functioning and proposed sympathectomy as a treatment. Subsequently, the list of disorders in which pain was presumably related to sympathetic nervous activity expanded beyond the original descriptions of “classical” causalgia and acquired other terminologies. It is not clear that all of these later additions to the category of sympathetically related pain disorders share a common etiology and pathology to the classical causalgic syndrome. For the purpose of focusing our consideration on a disorder with a common causative process, the following starts from classical causalgia without implying extension to either more general or to more specific terminologies and classifications: e.g., reflex sympathetic dystrophy (3), complex regional pain syndrome (4), and various others (5).
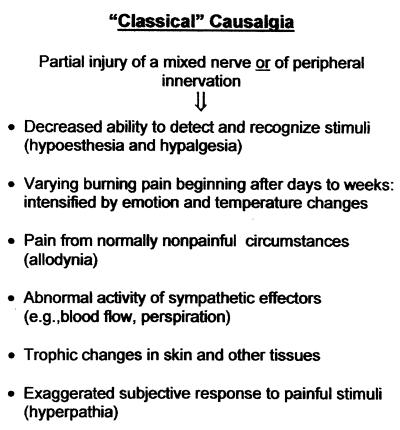
As outlined in Fig. 1, the classical syndrome of causalgia includes the following features. (i) It follows partial denervation of a region, usually by traumatic injury of a large mixed nerve. (ii) The partially denervated area is hypalgesic. (iii) Days to weeks after the disturbance of innervation, spontaneous pain appears, typically burning in nature, referred to the partially denervated and nearby regions. (iv) Pain is produced or increased by normally nonpainful stimuli, e.g., skin cooling or light touch (allodynia). (v) Abnormal sympathetic function is evident in the region (e.g., vasomotion, perspiration). (vi) The pain is aggravated by emotional upset. (vii) Trophic changes appear in the partially denervated tissues and nearby regions including abnormal coloration and turgor of the skin, unusual growth of hair, and changes in bone and other subcutaneous tissues. Certain of the physical signs are suggestive of chronic inflammation.
Figure 1.
Features of causalgia as classically described.
After Leriche’s suggestion, regional sympathectomy or regional sympathetic block has been used as a therapy for this syndrome with, in many cases, at least temporary success (6, 7). In cases with successful outcome, the abnormal pain is reduced or abolished, and there is amelioration of trophic changes (2, 6, 7). During remission of the signs and symptoms after sympathectomy or sympathetic block, local injection of norepinephrine into the skin of the previously painful region has been reported to recreate the former causalgic pain (ref. 8; see also ref. 9). These observations suggest that sympathetic activity and adrenergic mediators have a part in the aberrant pain and other features of causalgia.
The connection between adrenergic receptors and the pathological pain of causalgia proposed herein is circumstantial. The general concepts have been addressed previously (10, 11). Evidence is drawn from experimental studies in animal models and observations on clinical cases. Admittedly, the choice of material from the voluminous literature on sympathetically related sensory phenomena is selective. In part, the selection attempts to avoid mixing disparate material or possibly different clinical or experimental situations and partially represents the writer’s view of relevance.
It is important to our argument that in addition to classical causalgia, there are clinical reports of a painful dystrophy after regional sympathectomy, usually performed for vascular problems (12–14). The postsympathectomy painful dystrophies differ from classical causalgia in that there often is a deep rather than cutaneous reference for the pain (15). Furthermore, postsympathetic pain is usually described as spontaneously remitting, whereas the symptoms of classical causalgia persist in the absence of a remission produced by sympathectomy.
Adrenergic Responsiveness of Primary Afferent Neurons.
The logic for the proposal that an increased expression of adrenergic receptors underlies the syndrome of causalgia begins with observations on the effects of peripherally applied adrenergic substances on cutaneous nociceptors in vivo. Norepinephrine injected into the skin of normal human beings does not evoke pain. Prior work had shown that some afferent fibers terminating in a neuroma at the central stump of a transected nerve, therefore injured, are excitable by norepinephrine, an effect with α-adrenergic features (16, 17). In experimental animals, norepinephrine or epinephrine applied peripherally does not excite nociceptors (18–20). On the other hand, after injury to part of a mixed peripheral nerve, some of the nociceptors in the injured nerve become excitable by sympathetic stimulation and adrenergic substances (20–22). However, in these experiments the primary afferent elements exhibiting the adrenergic excitation are not those whose peripheral fibers had been transected. Furthermore, the adrenergically excitable nociceptors are otherwise functionally equivalent to those found in normal nerve. In rabbit, the pharmacology of this adrenergic excitation proved consistent with mediation by α2-adrenergic receptors (20, 21); however, in primate, other α-adrenergic receptors may be involved (23). The novel adrenergic excitation of nociceptors is manifest shortly after the time of nerve injury and persists for months. Whether other classes of primary afferent neurons also participate in the changed response to sympathetic stimuli and adrenergic substances has not been established. Importantly, though, regional sympathectomy also has been found to induce an adrenergically mediated excitation of C-fiber nociceptors, although features of the adrenergically induced responses after sympathectomy appear to differ from those seen after mixed peripheral nerve damage (24).
Adrenergic Receptors and Primary Afferent Neurons.
Thus, the events unleashed by a partial denervation, consisting of interruption of some peripheral sensory fibers and/or postganglionic sympathetic fibers, alter the phenotype of nociceptors that otherwise remain functionally intact in the injured or another nerve supplying the region. A clue that this change is possibly related to an increase in receptor population comes from the observations that the excitatory effect has a pharmacological profile of a specific adrenergic α-receptor. Moreover, the time course of the development of the adrenergic excitation suggested that although it was manifest within a few days, the peak of excitatory effects is reached 2–3 weeks after the nerve injury (21). This time course is reminiscent of that for denervation supersensitivity in which sympathetically denervated organs become much more responsive to adrenergic agents (25). Sympathetic supersensitivity has been related to increased numbers of adrenergic receptors (26–28). Moreover, it is significant that the adrenergic, sympathetic excitation of nociceptors appears to occur in the region of the peripheral receptive terminals (21).
We explored the possibility that the appearance of the excitatory response by cutaneous nociceptors to sympathetic stimulation and to adrenergic agents is associated with alterations in adrenergic receptors in primary afferent neurons. The number of dorsal root ganglion (DRG) cells labeled by an antibody putatively recognizing α2A- adrenergic receptors was found to markedly increase after both partial and complete transections of the rat sciatic nerve (29). The principal increase in the population of DRG neurons expressing this immunoreactivity appears in neurons with somata of medium-to-medium-large diameters (20–40 μm). Double labeling with markers for injury and growth (c-jun protein) (30, 31) or for transected fibers (fluorogold) (32) indicated that the increased immunoreactivity to the α2A-directed antibody occurs both in injured neurons and in those without evidence of damage. The latter predominate. The increase in the number of DRG neurons expressing α2A-adrenergic receptor immunoreactivity following nerve injury is selective. Increased immunoreactivity to the α2A-adrenergic receptor antibody does not appear after localized artificial inflammation produced by injection of formalin or Freund’s complete adjuvant, and immunoreactivity to a α2C antibody is not increased after sciatic nerve injury (29). An earlier autoradiographic study with the partial α2 agonist, p-iodoclonidine (125I-labeled) had indicated increased binding in ipsilateral DRG after partial or complete nerve transection; however, the diameter spectrum of the p-iodoclonidine-labeled DRG neurons (mainly small-diameter) partially differs from that with the α2A-adrenergic receptor antibody (refs. 10 and 29; K. Nishiyama and E.R.P., unpublished data).
An Hypothesis.
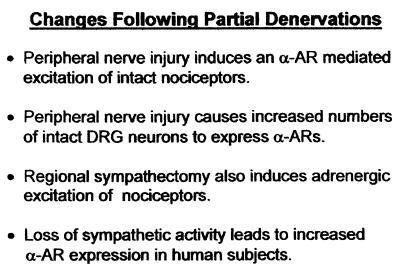
The effects of experimental nerve injury on the responsiveness of cutaneous nociceptors to adrenergic substances and on α-adrenergic receptor expression in dorsal root ganglia suggest a possible relationship to the etiology and symptoms of causalgia. The most salient factors are outlined in Fig. 2. This leads to the hypothesis summarized by Fig. 3. It is proposed that injury to part of the innervation to a bodily region, e.g., partial transection of a mixed nerve supplying part of a limb, induces production of cytokines and/or growth factors by the injured neurons and associated cells (e.g., Schwann cells). These chemical factors, among other effects, mediate responses to injury or are associated with regrowth and lead to altered gene expression in uninjured neurons of the nerve and region. Disconnection of sympathetic postganglionic fibers from their targets, by itself and in conjunction with injury of primary afferent fibers, contributes to these signals, leading to altered expression of α-adrenergic receptors. The fact that classical causalgia usually follows injury to large mixed nerves raises the possibility that the required lesion for the full syndrome is the combined interruption of primary afferent fibers and sympathetic postganglionic fibers. Possibly the loss of the presence of sympathetic mediators in the vicinity of primary afferent terminals because of interruption of sympathetic postganglionic fibers is part of the pathophysiological process. The net result after such partial loss of innervation is that afferent neurons, normally expressing few α-adrenergic receptors capable of producing an excitatory response, develop them. As a result, sensory neurons, particularly nociceptors, become excitable through these newly formed receptors. The afferent neuron excitation would occur by norepinephrine locally released by the remaining sympathetic supply to the vasculature or norepinephrine and epinephrine circulating from other parts of the body. For central mechanisms, signals produced in nociceptors by adrenergic agents are equivalent to those evoked by noxious events and lead to the sensation of pain. Signals interpreted as the result of noxious stimuli can result from activation by α-adrenergic receptors in DRG neurons by trace quantities of norepinephrine and epinephrine. The outcome is spontaneous pain or sensitization of nociceptors.
Figure 2.
Prominent adrenergic consequences of partial denervations.
Figure 3.
Factors in the hypothesis connecting increased expression of α-adrenergic receptors to the pain of causalgia.
Observations on human subjects offer circumstantial support for facets of this concept. Loss or decrease of sympathetic activity and the consequent decrease in circulating sympathetic postganglionic mediators has been shown to increase expression of α-adrenergic receptors (27, 28, 33). Those observations were made on blood platelets. By inference, one can argue that the same process could affect DRG neurons. Regional sympathectomy in experimental animals does increase binding of α-adrenergic agonists in DRG neurons of the innervated region (ref. 10 and K. Nishiyama and E.R.P., unpublished data). Thus, one manipulation that leads to induction of adrenergic excitation of nociceptors, loss of sympathetic innervation, increases α-adrenergic receptor expression in some tissues.
It is pertinent that the affected limb in persons suffering from pain disorders fitting the criteria of classical causalgia exhibits lower concentrations of norepinephrine and a degradation product (3,4-dihydroxphenylethyleneglycol) in its venous return than contralaterally. This could imply that the affected limb has less functioning sympathetic innervation. At the same time, the affected limb exhibits increased activity by sympathetically innervated tissues (34, 35). These human data suggest that a process akin to denervation supersensitivity may operate in classical causalgia and possibly other varieties of sympathetically related pain disorders.
To repeat, my suggestion here is that primary afferent neuron excitation by adrenergic agents in classical causalgia results from novel α-adrenergic receptor production in dorsal root ganglia neurons evoked by direct and indirect effects of injury to peripheral innervation. The increased adrenergic receptor expression, in part, involves primary afferent neurons, particularly nociceptors, with intact connections to the periphery and the central nervous system. As a consequence of the novel α-adrenergic receptor production, some of these afferent neurons develop an excitatory response to trace amounts of adrenergic substances in peripheral tissues. Such excitation would be the start of abnormal signals activating pain pathways and central pain-related mechanisms.
The concept just outlined, like most hypotheses, has difficulties. First, it cannot explain all parts of a complex syndrome. In particular, it does not account for the trophic changes, allodynia, and psychological alterations. The hypothesis suggests only that nerve injury and partial denervation unleash a set of circumstances leading to an abnormal production of α-adrenergic receptors in sensory neurons. Experimental studies suggest that the adrenergic receptor type may be of the α2 (possibly also α1) type (28, 29, 23). These adrenergic receptors become part of a messenger system whereby adrenergic substances excite or sensitize peripheral sensory neurons related to nociception and pain, which represents a step in the process leading to spontaneous pain and to activation of central pathways. Subsequently, the abnormally initiated central activity can lead to sensitization and other plastic changes in central neuronal mechanisms. Although not an explanation of all signs and symptoms of causalgia, this proposal provides a possible etiology of the pathological process and some insight into factors that could operate to maintain the process. Second, there is the issue of the effects mediated by α2-adrenergic receptors that usually are presumed in neurons to mediate inhibitory actions. In this context, it should be remembered that α2-adrenergic receptors are intermediate arteriolar smooth muscle constriction. Therefore, this class of receptors is capable of being part of an excitatory signal-transduction process (36); furthermore, the signaling system induced by nerve injury may not be identical to that occurring in neurons normally.
Does the idea of a change in cellular phenotype by the enhanced production of membrane receptors possibly apply to other situations? A similar process could operate in other versions of sympathetically related pain. It could also relate to Raynaud’s disease, another pathological process which, in part, appears to represent overreaction to sympathetic mediators and could possibly result from an increased expression of adrenergic receptors (37). Furthermore, enhanced reactions to adrenergic mediators by the vasculature have also been postulated for certain forms of hypertension (38, 39). To conclude, the concept of increased expression of molecular receptors as a mechanism of disease, and in particular of pathological pain, deserves serious consideration and further exploration.
Acknowledgments
I thank Ms. S. Derr for her assistance. Preparation of this paper was aided by grants NS 10321 and NS 14899 of the National Institute of Neurological Disorders and Stroke.
ABBREVIATION
- DRG
dorsal root ganglion
References
- 1.Mitchell W. Injuries of Nerves and Their Consequences. Philadelphia: Lippincott; 1872. [Google Scholar]
- 2.Leriche R. Presse Méd. 1916;24:178–180. [Google Scholar]
- 3.Evans J A. Surg Clin North Am. 1946;26:435–448. [PubMed] [Google Scholar]
- 4.Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definition of Terms. Seattle: IASP Press; 1995. [Google Scholar]
- 5.Kozin F, McCarty D J, Sims J, Genant H. Am J Med. 1976;60:321–331. doi: 10.1016/0002-9343(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 6.Schumacker H B, Speigel I J, Upjohn R H. Surg Gynecol Obstet. 1948;86:76–86. [PubMed] [Google Scholar]
- 7.Richards R L. Arch Neurol. 1967;16:339–350. doi: 10.1001/archneur.1967.00470220003001. [DOI] [PubMed] [Google Scholar]
- 8.Wallin E, Torebjörk E, Hallin R. In: Sensory Functions of the Skin in Primates, with Special Reference to Man. Zotterman Y, editor. Oxford: Pergamon; 1976. pp. 489–502. [Google Scholar]
- 9.Torebjörk E, Wahren L, Wallin G, Hallin R, Koltzenburg M. Pain. 1995;63:11–20. doi: 10.1016/0304-3959(95)00140-N. [DOI] [PubMed] [Google Scholar]
- 10.Perl E R. In: Progress in Pain Research and Management. Fields H L, Liebeskind J C, editors. Seattle: IASP Press; 1994. pp. 129–150. [Google Scholar]
- 11.Perl E R. In: Touch, Temperature, and Pain in Health and Disease: Mechanisms and Assessments, Progress in Pain Research and Management. Boivie J, Hansson P, Lindblom U, editors. Vol. 3. Seattle: IASP Press; 1994. pp. 231–248. [Google Scholar]
- 12.Tracy G D, Cockett F B. Lancet i . 1957;272:12–14. doi: 10.1016/s0140-6736(57)92433-9. [DOI] [PubMed] [Google Scholar]
- 13.Litwin M S. Arch Surg. 1962;84:591–595. [Google Scholar]
- 14.Raskin N H, Levinson S A, Hoffman P M, Pickett J B E, Fields H L. Am J Surg. 1974;128:75–78. doi: 10.1016/0002-9610(74)90238-4. [DOI] [PubMed] [Google Scholar]
- 15.Churcher M D. Lancetii. 1984;8395:131–133. doi: 10.1016/s0140-6736(84)91048-1. [DOI] [PubMed] [Google Scholar]
- 16.Devor M, Jänig W. Neurosci Lett. 1981;24:43–47. doi: 10.1016/0304-3940(81)90356-6. [DOI] [PubMed] [Google Scholar]
- 17.Devor M. J Auton Nerv Syst. 1983;7:371–384. doi: 10.1016/0165-1838(83)90090-5. [DOI] [PubMed] [Google Scholar]
- 18.Shea V, Perl E R. J Neurophysiol. 1985;54:491–501. doi: 10.1152/jn.1985.54.3.491. [DOI] [PubMed] [Google Scholar]
- 19.Barasi S, Lynn B. Brain Res. 1986;378:21–27. doi: 10.1016/0006-8993(86)90282-9. [DOI] [PubMed] [Google Scholar]
- 20.O’Halloran K D, Perl E R. Brain Res. 1997;759:233–240. doi: 10.1016/s0006-8993(97)00261-8. [DOI] [PubMed] [Google Scholar]
- 21.Sato J, Perl E R. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 22.Bossut D F, Perl E R. J Neurophysiol. 1995;73:1721–1723. doi: 10.1152/jn.1995.73.4.1721. [DOI] [PubMed] [Google Scholar]
- 23.Ali Z, Ringkamp M, Hartke T V, Chien H F, Flavahan N A, Campbell J N, Meyer R A. J Neurophysiol. 1999;81:455–466. doi: 10.1152/jn.1999.81.2.455. [DOI] [PubMed] [Google Scholar]
- 24.Bossut D F, Shea V, Perl E R. J Neurophysiol. 1995;75:514–517. doi: 10.1152/jn.1996.75.1.514. [DOI] [PubMed] [Google Scholar]
- 25.Cannon W B, Rosenblueth . The Supersensitivity of Denervated Structures: A Law of Denervation. New York: McMillan; 1949. [Google Scholar]
- 26.Arnett C O, Davis J A. J Pharmacol Exp Ther. 1979;211:394–400. [PubMed] [Google Scholar]
- 27.Davies I B, Sudera D, Sagnella G, Marchesi-Saviotti E, Mathias C, Bannister R, Sever P S. J Clin Invest. 1982;69:779–784. doi: 10.1172/JCI110516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan B, Neubig R, Julius S. Clin Pharmacol Ther. 1985;38:519–524. doi: 10.1038/clpt.1985.217. [DOI] [PubMed] [Google Scholar]
- 29.Birder L A, Perl E R. J Physiol (London) 1999;515:533–542. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins R, Hunt S P. Neurosci Lett. 1991;129:107–110. doi: 10.1016/0304-3940(91)90731-8. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins R, McMahon S B, Bond A B, Hunt S P. Eur J Neurosci. 1993;5:751–759. doi: 10.1111/j.1460-9568.1993.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 32.Baranowski A P, Anand U, McMahon S B. Neurosci Lett. 1992;141:53–56. doi: 10.1016/0304-3940(92)90332-2. [DOI] [PubMed] [Google Scholar]
- 33.Davies I B, Sever P S. In: Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. Bannister R, editor. Oxford: Oxford Univ. Press; 1988. pp. 348–366. [Google Scholar]
- 34.Drummond P D, Finch P M, Smythe G A. Brain. 1991;114:2025–2036. doi: 10.1093/brain/114.5.2025. [DOI] [PubMed] [Google Scholar]
- 35.Drummond P D, Finch P M, Edvinsson L, Goadsby P J. Clin Auto Res. 1994;4:113–116. doi: 10.1007/BF01845774. [DOI] [PubMed] [Google Scholar]
- 36.Nichols A J, Ruffolo R R., Jr . In: Progress in Basic and Clinical Pharmacology. Ruffolo R R Jr, editor. Vol. 8. Basel: Karger; 1991. pp. 115–179. [Google Scholar]
- 37.Edwards J M, Phinney E S, Taylor L M, Jr, Keenan E J, Porter J M. J Vasc Surg. 1987;5:38–45. [PubMed] [Google Scholar]
- 38.Michel M C, Insel P A, Brodde O. FASEB J. 1989;3:139–144. doi: 10.1096/fasebj.3.2.2536629. [DOI] [PubMed] [Google Scholar]
- 39.De Champlain, J. (1989) J. Hypertension8, Suppl. 7, S77–S85.