Abstract
The ascomycetous genus Scirrhia is presently treated as a member of Dothideomycetidae, though uncertainty remains as to which family it belongs in Capnodiales, Ascomycota. Recent collections on stems of a fern, Pteridium aquilinum (Dennstaedtiaceae) in Brazil, led to the discovery of a new species of Scirrhia, described here as S. brasiliensis. Based on DNA sequence data of the nuclear ribosomal DNA (LSU), Scirrhia is revealed to represent a member of Dothideomycetes, Capnodiales, Mycosphaerellaceae. Scirrhia is the first confirmed genus in Mycosphaerellaceae to have well developed pseudoparaphyses and a prominent hypostroma in which ascomata are arranged in parallel rows. Given the extremely slow growth rate and difficulty in obtaining cultures of S. brasiliensis on various growth media, it appears that Scirrhia represents a genus of potentially obligate plant pathogens within Mycosphaerellaceae.
Keywords: Brazil, Capnodiales, Dothideomycetes, ITS, LSU, Mycosphaerellaceae, Pteridium, systematics
INTRODUCTION
The genus Scirrhia was originally known from four species (Fuckel 1870), and later lectotypified based on S. rimosa, a species known from stems of Phragmites australis (Poaceae) collected in Europe (Saccardo 1883). Based on its ascomata arranged in linear rows in a hypostroma of pseudoparenchymatal cells, bitunicate asci, hyaline, 1-septate ascospores, and the presence of pseudoparaphyses, Müller & von Arx (1962) placed the genus in Dothiorales, Mycosphaerellaceae, listing Scirrhodothis (Theissen & Sydow 1915), Scirrhophragma (Theissen & Sydow 1915) and Metameris (Theissen & Sydow 1915) as possible generic synonyms. All three genera have bitunicate asci, hyaline, 1-septate ascospores, pseudoparaphyses, and stromata with immersed, longitudinally arranged ascomata, appearing to justify this opinion.
In a later study, von Arx & Müller (1975) again placed Scirrhia in Dothideaceae, suborder Dothideineae in Dothideales. Barr (1972) questioned the synonymy of Scirrhodothis (lectotype species S. confluens), Scirrhophragma (type species S. regalis) and Metameris (type species M. japonica) under Scirrhia, and Sivanesan (1984) also chose not to list them as such, but included species that occurred on Gymospermae, namely S. acicola and S. pini. Barr (1987) chose to reinstate these generic synonyms, and placed Scirrhia together with Mycosphaerella in Dothideaceae, Dothideales. Although little is known about the three generic synonyms listed by Müller & von Arx (1962), the species on Gymnospermae are presently treated as separate genera, namely Lecanosticta (L. acicola) and Dothistroma (D. pini) (Crous et al. 2009a). Presently, the taxonomic position of Scirrhia remains obscure.
During a recent visit to Brazil, we collected fresh material of a species of Scirrhia on stems of a fern. The aims of the present study are, therefore, to identify the species of Scirrhia, and to see if the taxonomic position of the genus could be resolved.
MATERIALS AND METHODS
Isolates
Stems bearing ascomata were soaked in water for approximately 2 h, after which they were placed in the bottom of Petri dish lids, with the top half of the dish containing 2 % malt extract agar (MEA), or potato-dextrose agar (PDA; Crous et al. 2009b). Ascospore germination patterns were examined after 24 h, and single ascospore cultures were established as described earlier (Crous et al. 1991, Crous 1998). Colonies were subcultured onto PDA and oatmeal agar (OA) (Crous et al. 2009b) and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Germinating ascospores died on MEA. Reference strains are maintained in the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands.
DNA isolation, amplification and analyses
Genomic DNA was isolated from fungal mycelium grown on MEA, using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Solana Beach, CA, USA) according to the manufacturer’s protocols. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part of the nuclear rDNA operon spanning the 3’ end of the 18S rRNA gene (SSU), ITS 1, the 5.8S rRNA gene, ITS 2 and the first 900 bases at the 5’ end of the 28S rRNA gene (LSU). The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009c) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. The PCR conditions, sequence alignment, and subsequent phylogenetic analysis followed the methods of Crous et al. (2006, 2009d). Sequences were compared with the sequences available in NCBI’s GenBank nucleotide (nr) database using a megablast search and results are discussed in the relevant species notes where applicable. Based on the Blast results, the novel sequence was added to the alignment of Crous et al. 2010 (TreeBASE study S10980). Alignment gaps were treated as new character states. Sequences derived in this study were lodged at GenBank, the alignment in TreeBASE (www.treebase.org/treebase/index.html), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
Morphology
Morphological descriptions are based on preparations made from host material in clear lactic acid, with 30 measurements determined per structure, and observations made with a Nikon SMZ1500 dissecting microscope, and with a Zeiss Axioscope 2 microscope using differential interference contrast (DIC) illumination. Colony characters and pigment production were noted after 3 mo of growth on PDA and OA (Crous et al. 2009b) incubated at 25 ºC. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970).
RESULTS
Phylogeny
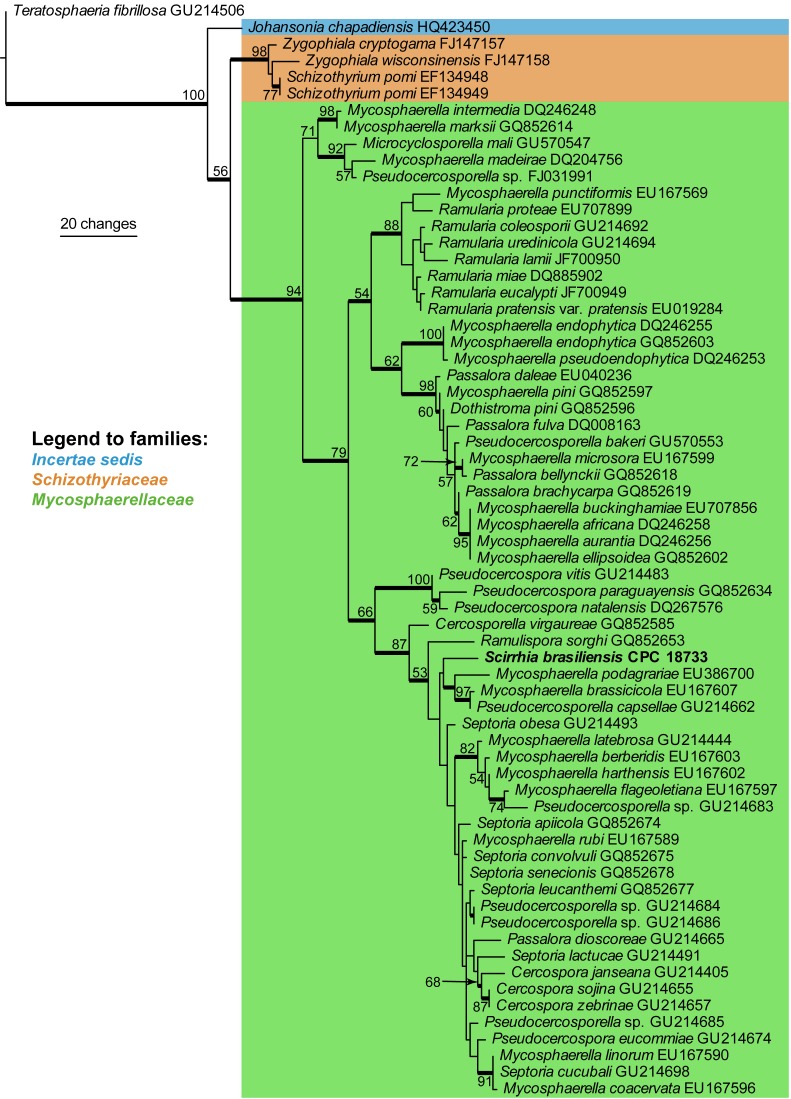
Approximately 1700 bases, spanning the ITS and LSU regions, were obtained from the sequenced culture. The LSU region was used in the phylogenetic analysis for the generic placement (Fig. 1) and ITS to determine species-rank relationships (see notes under the species description). The manually adjusted LSU alignment contained 66 taxa including the outgroup sequence and, of the 749 characters used in the phylogenetic analysis, 104 were parsimony-informative, 51 were variable and parsimony-uninformative, and 594 were constant. Only the first 1000 equally most parsimonious trees were retained from the heuristic search, the first of which is shown in Fig. 1 (TL = 415, CI = 0.494, RI = 0.851, RC = 0.421). The phylogenetic tree of the LSU region (Fig. 1) show that the obtained sequence clusters together with species belonging to Cercospora, Pseudocercospora, Pseudocercosporella and Septoria in Mycosphaerellaceae.
Fig. 1.
The first of 1000 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment. The scale bar shows 10 changes, and bootstrap support values from 1000 replicates are shown at the nodes. The novel sequence generated for this study is shown in bold. Branches present in the strict consensus tree are thickened and the tree was rooted to a sequence of Teratosphaeria fibrillosa (GenBank accession GU214506).
Taxonomy
Scirrhia rimosa (Alb. & Schwein.) Fuckel, Jb. nassau. Ver. Naturk. 23–24: 221 (1870).
Basionym: Sphaeria rimosa Alb. & Schwein., Consp. Fung.: 13 (1805).
(Fig. 2)
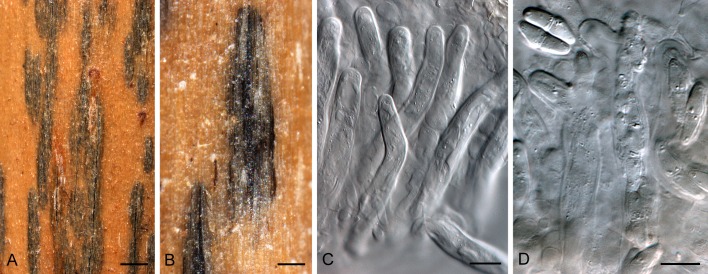
Fig. 2.
Scirrhia rimosa (BPI 1111233). A. Hypostroma on leaf of Phragmites australis. B. Horizontal section through ascomata. C–F. Asci intermingled among pseudoparaphyses. G. Ascus with basal stalk. H. Ascospores inside disintegrated ascus. Bars: A = 5 mm; B = 250 μm, all others = 10 μm.
Specimen examined: Czech Republic: Lipnik, on culms (stems) of Phragmites australis, June 1942 (Reliquiae Petrakianae Institut für Systematische Botanik Graz, exsiccati no. 1871, BPI 1111233).
Hypostroma on upper leaf surfaces, dark brown to black, immersed beneath epidermis, becoming raised, irregularly ellipsoid, to 20 mm long, 5 mm wide. Ascomata immersed in brown stroma of textura angularis; arranged in parallel rows, opening by a 10–15 μm diam central ostiole; ascomata to 250 μm diam, wall 10–20 μm wide. Asci 80–130 × 13–18 μm, hyaline, smooth, cylindrical, sessile in fascicles, short-stipitate, bitunicate with ocular chamber, 3–4 μm diam. Pseudoparaphyses hyaline, cellular, smooth, distributed among asci, septate, branched, prominently constricted at septa, 5–8 μm diam, extending above asci. Ascospores hyaline, smooth, biseriate in asci, guttulate, fusoid-ellipsoidal, widest just above septum, tapering towards both ends, but more prominently towards lower end, prominently constricted at the median septum, thin-walled, (20–)21–23(–26) × 6–7(–7.5) μm; ascospores germinating on host with germ tubes at right angles to the long axis of spore, but remaining hyaline, not distorting.
Notes: Despite several attempts, we have thus far been unable to recollect fresh material of this species on Phragmites, and thus no DNA sequence data are available. The nature of the hypostroma, ascomatal arrangement, asci, ascospores, and pseudoparaphyses, closely match that of the species described below. The specimen described and illustrated here agrees with the original description as well as locality and host of this species, occurring on stems. A later Schweinitz collection on Zizania (Poaceae) from the USA proved to be a misidentified Leptosphaeria zizaniicola (Ellis & Everhart 1892). We are unaware of any confirmed reports of Scirrhia rimosa from North America.
Scirrhia brasiliensisCrous, O.L. Pereira, Alfenas & R.F. Alfenas, sp. nov.
MycoBank MB560601
(Fig. 3)
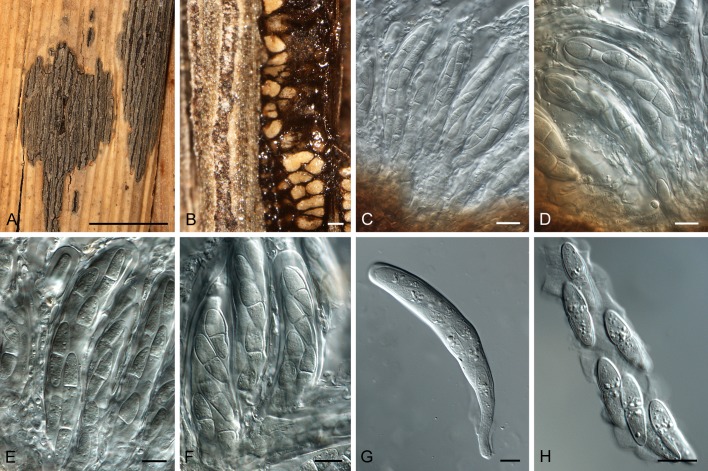
Fig. 3.
Scirrhia brasiliensis (ex-type CPC18733). A. Fronds of Pteridium aquilinum. B, C. Stems with cankers extending into inner tissue. D. Hypostroma viewed from above. E–G, K. Sections through ascomata. H–J. Ostiolar area of ascomata, showing depressed neck. L–N. Bitunicate asci (arrow denotes apical chamber). O, P. Germinating ascospores. Q. Ascospores. Bars: B, C = 5 mm; D = 1 mm; E–H = 160 μm, all others = 10 μm.
Etymology: Named after the country where the holotype was collected, Brazil.
Scirrhiae rimosae morphologice similis, sed asco saepe minus quam octosporo et ascosporis dissimiliter contractis.
Typus: Brazil: Minas Gerais: Viçosa, Serra do Brigadeiro, S20°41.487, W42°29.875, on stems of Pteridium aquilinum (Dennstaedtiaceae), 22 Aug. 2010, P.W. Crous, O.L. Pereira, A.C. Alfenas & R.F. Alfenas, (CBS H-20538 – holotypus; cultures ex-holotype CPC 18734, 18735, 18733 = CBS 128762). (GenBank accession numbers: CPC 18733, ITS JN197292, LSU JN197293).
Stem cankers narrowly fusoid, brown, at times with red-purple margin, visible on shoots and stems of host, up to 5 mm wide, 3 cm long; raised areas forming longitudinal cracks in the host epidermis through which erumpent ascomata protrude. Ascomata to 160 μm diam, 200 μm high, subepidermal, situated in rows in a brown hypostroma of textura angularis; wall consisting of to 6 layers of textura angularis, to 60 μm diam; cells brown, becoming flattened between ascomata, to 20 μm diam; ostioles ellipsoid, single, central, to 20 μm diam, situated on a dark brown, depressed neck, to 50 μm wide, 15 μm high; ostiole lined with hyaline, cylindrical periphyses with rounded ends, to 25 μm long, 3 μm wide. Asci 90–130 × 8–11 μm, fasciculate, intermingled between pseudoparaphyses, subcylindrical, with thick wall, 2–4 μm diam, inconspicuous apical chamber, 2–3 μm diam; wall appearing multi-layered with fissitunicate dehiscense, widest in middle of ascus, tapering to a long, thin, basal part and lobed base, to 9 μm wide; asci initially with 8 immature ascospores, of which some dissolve, leaving 4–6 mature ascospores per ascus. Pseudoparaphyses hyaline, cellular, smooth, septate, anastomosing, extending above asci, 3–5 μm wide. Ascospores fusoid-ellipsoidal, hyaline, smooth, guttulate, obliquely uniseriate in asci, medianly 1-septate, at times constricted at the septum, widest in the middle of apical cell, tapering towards both ends, (17–)20–23(–27) × (5–) 6–7(–8) μm; germinating from both ends, with germ tubes generally parallel to the long axis; spores neither darkening nor distorting.
Culture characteristics: Colonies after 3 mo on PDA and OA with sparse aerial mycelium and even margins, olivaceous-grey (surface and reverse), reaching to 6 mm diam, extremely slow growing, with brown, diffuse pigment visible in agar surrounding colony; colonies sterile. Most ascospores died upon germination, and only a few of the germinated spores on PDA survived to form extremely slow-growing colonies, suggesting this pathogen to be highly obligate in nature.
Notes: The only culture of a species of Scirrhia presently available in the CBS culture collection, S. aspidiorum (CBS 204.66, on Pteridium aquilinum, UK; GenBank accession number: ITS, JN197294) turned out to be related to Phoma herbarum (Didymellaceae, Pleosporales; de Gruyter et al. 2010), and thus unrelated to S. brasiliensis. Although the specimen from which this culture was derived (ETH 2662) was not examined, examination of another collection under this name (The Netherlands, Baarn, Groenevelt, Pteridium aquilinum, May 1962, J.A. von Arx, CBS H-17938), revealed a typical species of Scirrhia to be present on this fern, but no culture is presently available to confirm that it is the same taxon represented by CBS 204.66. Müller & von Arx (1962) treat S. aspidiorum in detail, citing ascospores as being 10–15 × 3–4 μm, thus being significantly smaller than those of S. brasiliensis. The ITS sequence of S. brasiliensis did not retrieve any highly identical hits with a megablast search of the NCBI’s GenBank nucleotide database; the closest hits represent species of Septoria, e.g. Septoria digitalis GenBank FJ557246; Identities = 507/534 (95 %), Gaps = 8/534 (1 %), and Cercospora, e.g. Cercospora piaropi GenBank HQ902254; Identities = 514/547 (94 %), Gaps = 9/547 (2 %) (data not shown).
DISCUSSION
Persoon (1796) was the first to use the term “stroma” to define structures on or in which ascomata of the genus Sphaeria were borne. Fruisting (1867) introduced the terms “epistroma” for the hyaline pseudoparenchymatous crust formed in the outer layers of the host cortex, in which conidia were produced, and under which a “hypostroma” was formed in which ascomata developed. Based on the concepts of Fruisting, Ruhland (1900) introduced the terms “ectostroma” and “entostroma”. Blain (1927) subsequently defined Scirrhia rimosa as having a dothideoid epistroma and a hypostroma of compressed pseudoprosenchyma. The same hypostroma anatomy observed in S. rimosa was also found in S. aspidiorum and S. brasiliensis, and appears to be typical for the genus.
Although the type species of Hadrotrichum, H. phragmitis, has been linked to S. rimosa as a possible anamorph of the genus Scirrhia (Fuckel 1870), this relationship has never been proven and has been disputed (von Arx & Müller 1975). The phylogenetic position of Hadrotrichum presently remains unknown, and this genus will have to be recollected to resolve this issue. Presently, no anamorph connections have been confirmed for Scirrhia, and none formed in culture for S. brasiliensis.
Von Arx & Müller (1975) considered Scirrhodothis, Scirrhophragma, and Metameris to be synonyms of Scirrhia. Holm & Holm (1978) mentioned that although the taxa were closely related, the synonymy was controversial given different types of centrum development in some of the species. Barr (1992) regarded Scirrhia to represent a genus in Dothideales, while members of Metameris were found to have pseudoparaphyses and 1–2-septate, Didymella-like ascospores (Pleosporales). Furthermore, based on its small ascomata, broadly cylindrical to slightly obclavate asci with a short, thick, knob-like pedicels, as well as its monocotyledonous host preference, Zhang et al. (2012) suggested Metameris to belong to Phaeosphaeriaceae, though molecular data are still lacking to resolve its final placement.
Lumbsch & Huhndorf (2010) list Scirrhia under Dothideaceae and Metameris under Phaeosphaeriaceae. The lectotype species of the genus Scirrhodothis, S. confluens (isotype CBS H-4806; Fig. 4), was examined in the present study and found to differ from Scirrhia in that the ascomata were locules formed in a stroma, not in linear rows in a hypostroma as in Scirrhia, and ascospores were Didymella-like. Although Scirrhodothis is clearly not a synonym of Scirrhia, the synonymy of these genera cannot be resolved in the absence of cultures, and they are best retained as separate entities until more data become available.
Fig. 4.
Scirrhodothis confluens (isotype specimen CBS H-4806). A, B. Superficial view of stromata with imbedded ascomata. C. Asci. D. Broken asci with Didymella-like ascospores. Bars: A, B = 2 mm; C, D = 10 μm.
Kirschner & Chen (2010) stated that the systematic position of Scirrhia was also in need of re-examination, and referred to a paper by Suetrong et al. (2009), who placed S. annulata (Juncaceae) within Mycosphaerellaceae. The clustering of S. brasiliensis in this family in the present study supports this finding and confirms Scirrhia as the first genus within Mycosphaerellaceae (Capnodiales, Dothideomycetes) with well developed pseudoparaphyses and an epi- and hypostroma. Other ascomycetous genera that have recently been shown to cluster in Mycosphaerellaceae include Phaeocryptopus and Rosenscheldiella (Winton et al. 2007, Sultan et al. 2011). Further collections are now required to resolve the questions: does S. rimosa cluster with S. brasiliensis, and what is the status of its reported synonyms, Scirrhodothis, Scirrhophragma and Metameris?
We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photo plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Arx von JA, Müller E. (1975) A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Studies in Mycology 9: 1–159 [Google Scholar]
- Barr ME. (1972) Preliminary studies on the Dothideales in temperate North America. Contributions to the University of Michigan Herbarium 9: 523–638 [Google Scholar]
- Barr ME. (1987) Prodomus to class Loculoascomycetes. Amherst, MA: M E Barr; [Google Scholar]
- Barr ME. (1992) Additions to and notes on the Phaeosphaeriaceae (Pleosporales, Loculoascomycetes). Mycotaxon 43: 371–400 [Google Scholar]
- Blain WL. (1927) Comparative morphology of dothideaceous and kindred stromata. Mycologia 19: 1–20 [Google Scholar]
- Crous PW. (1998) Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170 [Google Scholar]
- Crous PW, Barreto RW, Alfenas AC, Alfenas R, Groenewald JZ. (2010) What is Johansonia? IMA Fungus 1: 117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004) MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD. (2006) Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology 55: 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ. (2009d) Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22: 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hoog GS, Groenewald JZ. (2009c) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ. (2009a) Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009b) Fungal Biodiversity. [CBS Laboratory Manual Series1] Utrecht: Centraalbureau voor Schimmelcultures; [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991) Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632 [Google Scholar]
- Ellis JB, Everhart BM. (1892) The North American Pyrenomycetes. Published by authors, Newfield, NJ: Ellis & Everhart; [Google Scholar]
- Fruisting W. (1867) Zur entwicklungsgeschichte der Pyrenomyceten. Botanisches Zentralblatt 25: 177–311 [Google Scholar]
- Fuckel L. (1870) Symbolae mycologicae. Jahrbücher des Nassauischen Vereins für Naturkunde, Wiesbaden 23–24: 1–459 [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. (2010) Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081 [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998) Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Holm L, Holm K. (1978) Some pteridicolous ascomycetes. Botaniska Notiser 131: 97–115 [Google Scholar]
- Kirschner R, Chen C-J. (2010) Periconiella species (anamorphic Mycosphaerellaceae) from Taiwan. Fungal Diversity 44: 135–148 [Google Scholar]
- Lumbsch HT, Huhndorf SM. (2010) Myconet Volume 14. Part One. Outline of Ascomycota – 2009. Fieldiana, Life and Earth Sciences 1: 1–42 [Google Scholar]
- Müller E, von Arx JA. (1962) Die Gattungen der didymosporen Pyrenomyceten. Beitrage zur Kryptogamenflora der Schweiz 11 (2): 1–992 [Google Scholar]
- Persoon CH. (1796) Observationes Mycologicae. Vol 1 Leipzig: PP Wolf; [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Ruhland W. (1900) Untersuchungen zu einer morphologie der stromabildenen Sphaeriales. Hedwigia 39: 1–79 [Google Scholar]
- Saccardo PA. (1883) Sylloge Fungorum. Vol. 2. Pyrenomycologiae Universae. Padua: P A Saccardo; [Google Scholar]
- Sivanesan A. (1984) The Bitunicate Ascomycetes. Vaduz: J Cramer; [Google Scholar]
- Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG. (2009) Molecular systematics of the marine Dothideomycetes. Studies in Mycology 64: 155–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan A, Johnston PR, Park D, Robertson AW. (2011) Two new pathogenic ascomycetes in Guignardia and Rosenscheldiella on New Zealand’s pygmy mistletoes (Korthalsella: Viscaceae). Studies in Mycology 68: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen F, Sydow H. (1915) Die Dothideales. Kritisch-systematische Originaluntersuchungen. Annales Mycologici 13: 147–746 [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–322 San Diego: Academic Press; [Google Scholar]
- Winton LM, Stone JK, Hansen EM, Shoemaker RA. (2007) The systematic position of Phaeocryptopus gaeumannii. Mycologia 99: 240–252 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012) Pleosporales. Fungal Diversity: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]