Abstract
Posttranscriptional gene regulation is a rapid and efficient process to adjust the proteome of a cell to a changing environment. RNA-binding proteins (RBPs) are the master regulators of mRNA processing and translation and are often aberrantly expressed in cancer. In addition to well-studied transcription factors, RBPs are emerging as fundamental players in tumor development. RBPs and their mRNA targets form a complex network that plays a crucial role in tumorigenesis. This paper describes mechanisms by which RBPs influence the expression of well-known oncogenes, focusing on precise examples that illustrate the versatility of RBPs in posttranscriptional control of cancer development. RBPs appeared very early in evolution, and new RNA-binding domains and combinations of them were generated in more complex organisms. The identification of RBPs, their mRNA targets, and their mechanism of action have provided novel potential targets for cancer therapy.
1. Introduction
Traditionally, it has been well accepted that cancer development is dictated in part by aberrant transcriptional events and signaling pathways. More recently, it has become clear that posttranscriptional regulation of gene expression also controls cell proliferation, differentiation, invasion, metastasis, apoptosis, and angiogenesis which influence initiation and progression of cancer [1–4]. Regulation of already transcribed messenger RNAs (mRNAs) is an efficient and rapid way to alter gene expression and plays a crucial role in tumorigenesis.
After transcription, nascent mRNAs undergo several processing steps including splicing, capping, 3′ end formation, surveillance, nucleocytoplasmic transport, and, for many transcripts, localization before being translated and finally degraded [5, 6]. The mRNA does not exist alone in the cell, and its metabolism is largely defined by bound RNA-binding proteins (RBPs). RBPs, which regulate all steps of RNA biogenesis, form dynamic units with the RNA, called ribonucleoprotein complexes (RNPs) [7]. Different sets of RBPs are associated to the mRNA at different time points and in different compartments, thereby regulating the fate of their target in a time- and space-dependent way. RBPs often provide a landing platform for the recruitment of additional factors and enzymes to the mRNA. RBPs are the master regulators of post-transcriptional gene expression and, thus, are expected to play important roles in cancer development [1]. Besides RBPs, the discovery of microRNAs (miRNA) was of great inspiration for the RNA field and provided a new powerful tool to regulate gene expression. miRNAs associate with RBPs to form microRNPs (miRNP) which regulate translation and RNA stability by binding to complementary sequences in target mRNAs. miRNPs have been found to regulate expression of factors implicated in tumorigenesis, but we will not discuss this mechanism here (for recent reviews see [8, 9]).
RBPs bind to specific sequences or secondary structures typically found in the untranslated regions (UTRs) but also in the open reading frame (ORF) of target mRNAs [10, 11]. UTRs in particular have offered more flexibility to evolution, as the constraints of encoding a protein product have not been imposed upon them. As a consequence, diverse and often conserved regulatory elements are present in the UTRs [12]. In the 5′UTR, ribose methylation of the cap structure as well as 5′ terminal polypyrimidine sequences or secondary structures such as internal ribosome entry sites (IRESs) control protein expression. Sequence elements in the 3′UTR regulate the stability of the mRNA, its translational efficiency and localization. Specific binding of regulatory proteins to these elements is achieved through RNA-binding domains (RBDs). More than 40 RBDs have been identified. Among them, the most prominent are the RNA recognition motif (RRM), K-homology domain (KH), double stranded RNA-binding domain (dsRBD), zinc finger, Arginine-rich domain, cold-shock domain (CSD), and the PAZ and PIWI domains [13]. An RNA-binding protein can contain combinations of different RBDs, which allow a high flexibility for interaction with different targets. RBP purification techniques followed by high throughput proteomics will hopefully allow us in the near future to identify new RNA-binding proteins as well as new RNA-binding domains. Powerful techniques like CLIP-seq (UV cross-linking and immunoprecipitation followed by high throughput sequencing) are helping to identify new RBP targets in a genome wide scale, as well as new RBP binding sites [14–16]. The list of RBPs, RBDs and their targets is far from being complete. New technology is proving helpful to unravel the complexity of post-transcriptional gene regulation.
In cancer cells, expression of numerous oncoproteins or tumor suppressors is under the control of specific RBPs. Splicing, stability, localization as well as translation of these mRNAs are highly regulated, often in a tissue-specific manner [6]. Many RBPs are aberrantly expressed in cancer cells and have thus a cancer-specific regulatory activity [1, 17, 18]. Deregulation of RBP expression in cancer may have its origin on epigenetic events or on miRNA-dependent controls, although the detailed molecular mechanisms are often obscure [19–21]. An additional layer of regulation is provided by signaling: the phosphorylation status of some RBPs is defined by signaling pathways that are deregulated in cancer, and this phosphorylation controls RBP activity and subsequently the expression of its target mRNAs [22, 23]. Signaling pathway alterations occur in different stages of tumor formation and are often correlated with tumor grade.
In this paper, we will summarize the different functions of RBPs in post-transcriptional gene regulation and the impact of aberrant regulation on tumorigenesis. In addition, we will discuss the conservation of specific RBPs across eukaryotes, which may yield hints on how diversity has been generated.
2. RNA-binding Proteins Implicated in Cancer Development
Post-transcriptional gene regulation implies factors which act at different levels of mRNA metabolism, including alternative splicing, localization, stability of the mRNA or cap-dependent and -independent translation. In this section I will introduce a subset of RBPs involved in cancer development which play key roles in each of the steps of RNA regulation, namely, Sam68, eIF4E, La, and HuR to illustrate the powerful RBP regulatory capacity in cancer.
2.1. Sam68 Regulates Alternative Splicing of Cancer-Related mRNAs
Sam68 belongs to the evolutionarily conserved signal transduction and activation of RNA (STAR) family of RBPs [4, 24, 25]. Sam68 is predominately nuclear but has also been detected in the cytoplasm and exerts multiple activities in gene expression, from transcription and signaling to splicing regulation [4, 26]. RNA binding is achieved by a KH domain embedded in a highly conserved region called GSG (GRP/Sam68/GLD1) domain [27] (Figure 1). RNA binding is used for splicing regulation and is modulated by posttranslational modifications, such as phosphorylation or acetylation [22, 25, 28] (Figure 2).
Figure 1.
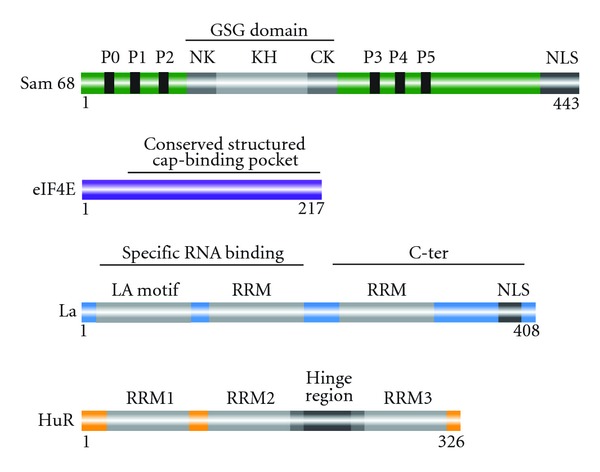
Schematic representation of the 4 RBPs discussed in this paper: Sam68, eIF4E, La, and HuR; RBDs are depicted in light gray. RRM: RNA recognition motif; GSG: GRP33/SAM68/GLD-1 domain, composed of a KH domain (KH) flanked by N-terminal (NK) and C-terminal (CK) extensions; LA: La motif. Phosphorylation sites of La are indicated in black (P0–P5). Nuclear localization signals (NLSs) are represented in dark gray. The number of amino acids of each protein is indicated.
Figure 2.
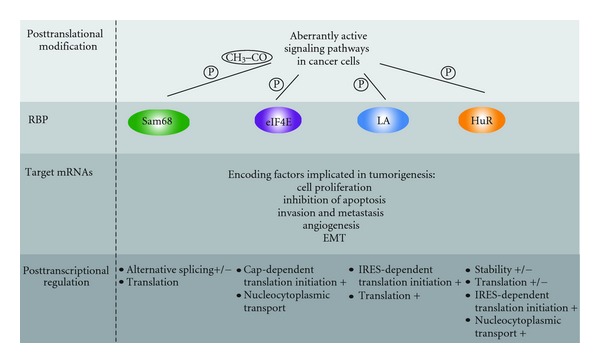
Overview of posttranscriptional gene regulation by Sam68, eIF4E, La, and HuR in tumorigenesis. In cancer cells, RBPs are posttranslationally modified by aberrantly active signaling pathways that activate their binding to targets encoding proteins implicated in tumorigenesis. The steps of mRNA metabolism regulated by RBPs are indicated. (+) and (–) specify up- or downregulation. P and CH3–CO indicate phosphorylation and acetylation of RBPs. EMT: epithelial to mesenchymal transition.
The role of Sam68 in alternative splicing seems directly related to its oncogenic properties. Alternative splicing (AS) allows the majority of human genes to encode for multiple protein isoforms, which often play different or even opposite roles [29]. In addition to the spliceosome, a set of RBPs are necessary to control alternative splicing [7]. Aberrant expression of RBPs in cancer can lead to deregulation of splicing, and subsequent changes in the proteome [30]. The splicing targets of Sam68 support its involvement in tumor progression [4, 31]. Furthermore, the function of Sam68 in AS is regulated by signaling pathways which are often deregulated in cancer cells, establishing a link between signal transduction, alternative splicing, and gene expression during tumorigenesis [22, 32, 33] (Figure 2).
Sam68 is overexpressed in breast, prostate, renal, and cervical cancer cells [26, 34–36] and is also frequently upregulated in tumors [34, 37].
The first hard evidence that Sam68 is involved in regulation of alternative splicing with an impact on tumorigenesis was provided by the demonstration that it promotes inclusion of exon v5 in the CD44 pre-mRNA [33]. CD44 encodes a cell surface molecule involved in cancer cell proliferation. CD44 transcript isoforms are alternatively generated by the inclusion of 10 variant exons, which are decisive in tumor progression [38]. Depletion of Sam68 strongly reduces the inclusion of several variable exons. Interestingly, Sam68 activity is controlled by the Ras signaling pathway, and Sam68 phosphorylation by ERK is needed to promote v5 inclusion [33].
Sam68 also regulates AS of cyclin D1, a protooncogene frequently deregulated in cancer cells [39, 40]. In addition, Sam68 promotes the generation of a stable SF2/ASF, isoform through regulation of splicing. The protooncogene SF2/ASF, also a splicing factor, is in turn responsible for processing of ΔRon pre-mRNA, which encodes a factor involved in EMT in colon cancer cells [41].
Another connection of Sam68 with cancer could be provided by the control of AS of the Bcl-x transcript. The Bcl-x gene can yield the antiapoptotic Bcl-x(L) factor or the proapoptotic Bcl-x(S) [22, 42]. Some studies have reported that Sam68 overexpression causes the accumulation of proapoptotic Bcl-x(s) in a manner that depends on the RNA-binding activity of Sam68 [22, 42]. However, the observation that Sam68 and the antiapoptotic Bcl-x(L) are upregulated in prostate cancer cells is at odds with a proposed activity of Sam68 in Bclx(S) upregulation [34, 43]. This apparent contradiction was resolved by the finding that the activity of Sam68 on Bcl-x AS depends on its phosphorylation status, which can switch Sam68 function from proapototic to antiapoptotic in cancer cells. Indeed, Src-like kinase, which is often activated in cancer, phosphorylates Sam68 and thereby promotes splicing of the antiapoptotic Bcl-x(l) variant which inhibits cell death [22].
Intriguingly, in advanced breast and renal tumors, Sam68 was found to localize in the cytoplasm [26, 35]. These observations suggest a potential function of Sam68 in translational control in advanced stages of tumorigenesis. In accordance with a potential role of Sam68 in translation, it was previously proposed to regulate the translation of selected mRNAs in male germ cells and neurons [44, 45].
Other RBPs regulating splicing in cancer cells are hnRNPs (A/B) H, SR proteins (ASF/SF2), RBM5, HuR, and PTB. The interested reader can refer to the following reviews and articles [30, 46].
2.2. eIF4E Overexpression in Cancer Enhances Translation Initiation of Specific mRNAs
Translation initiation is a critical step of protein synthesis and is highly regulated [47]. One of the most crucial regulators is the cap-binding protein eIF4E (eukaryotic initiation factor 4E) [48]. In the cytoplasm, eIF4E binds directly to the m7GTP-cap structure present at the 5′end of all mRNAs and interacts with eIF4G, which in turn recruits the 43S ribosomal complex during initiation of translation. eIF4E and eIF4G together with the RNA helicase eIF4A form the eIF4F complex, which is often targeted for translational regulation [47].
Early findings indicated that eIF4E overexpression leads to malignant transformation of fibroblasts [49, 50]. Since then, numerous studies have reported overexpression of eIF4E in different tumor types (e.g., breast, prostate, gastric colon, lung, skin, and lymphomas) [51]. Elevated expression of eIF4E often correlates with malignancy and poor prognosis [52, 53]. Surprisingly, overexpression of eIF4E does not induce a global increase in protein synthesis but augments translation of a subset of mRNAs encoding mostly prooncogenic proteins [2, 54] (Figure 2). mRNAs regulated by eIF4E overexpression include those encoding components of the cell cycle machinery (cyclin D1, CDK2, c-myc, RNR2, ODC, surviving, Mcl-1, Bcl-2) or factors implicated in angiogenesis (VEGF, FGF-2, PDGF) and invasion (MMP9) [2, 51, 55, 56].
It has been proposed that mRNAs coding for proteins upregulated in oncogenesis contain long and highly structured 5′UTRs [10]. mRNAs bearing stable secondary structures in the 5′UTR are poorly translated in normal conditions and may be particularly dependent on the eIF4F complex and the unwinding capacity of the eIF4A helicase to initiate translation. Thus eIF4E overexpression may lead to enhanced translation of otherwise inefficiently translated transcripts involved in tumorigenesis [2]. Interestingly, eIF4E seems to be implicated in nucleocytoplasmic transport of mRNAs (e.g., cyclin D) and thus may regulate expression of some genes in an initiation-independent way [57].
eIF4E activity is regulated by signaling pathways amplified in human cancers (Figure 2). The protein kinase mTor phosphorylates eIF4E-binding proteins (4E-BP). In their unphosphorylated state, 4E-BPs bind to eIF4E on the same site recognized by eIF4G, blocking the formation of the cap-binding complex. Phosphorylation of 4E-BP leads to loss of affinity for eIF4E and increases translation [2]. In addition, eIF4E phosphorylation by MAPK-integrating kinases MNK1 and MNK2 enhances cap-dependent initiation [47, 54].
Given the important role of eIF4E in tumorigenesis, reducing either eIF4E activity or levels in cancer cells has become an attractive anticancer strategy [51, 58]. Many compounds inhibiting mTor kinase activity have proven to be efficient. For example, PP242, Tonin1, and INK128 are ATP active site inhibitors of mTOR and block the phosphorylation of all mTor targets including 4E-BP [51]. Unfortunately, cells of some cancer types are insensitive to treatment with mTor inhibitors [59]. As an alternative strategy, inhibiting eIF4E expression with antisense oligonucleotides (AON) has given promising results in suppressing tumor growth in vivo [60].
2.3. La Is an ITAF Implicated in Cancer
The multifunctional RNA-binding protein La is primarily nuclear but can shuttle between the nucleus and the cytoplasm [61, 62]. According to its localization, La functions in small RNA processing [63] and in translation of mRNAs [64–66]. La can be divided into three regions: the N-terminus, which contains the conserved La motif; a less conserved RNA recognition motif (RRM); and a weakly conserved C terminus, which contains an RRM, and a nuclear localization signal (NLS) [67] (Figure 1). The La motif folds into an RRM and its high conservation suggests that it carries out a specific function [68, 69]. La interacts with cellular and viral mRNAs and regulates IRES and cap-dependent translation initiation [64, 66, 70–73]. An IRES is a nucleotide sequence folding in a specific secondary structure that recruits ribosomes independently of the cap structure [74]. During cellular stress, cap-dependent translation is downregulated, and IRES-dependent translation of many mRNAs is favored [75]. For example, under the hypoxic conditions usually found in the interior of a tumor, IRES-mediated translation of the angiogenic factor VEGF is favored leading to vascularization of the tumor [76]. Specific RNA-binding proteins termed IRES transacting factors (ITAFS) are required to regulate IRES-dependent translation in cancer development [74]. La is an ITAF that regulates the IRES-dependent translation of mRNAs involved in cell proliferation, angiogenesis and apoptosis [64, 77, 78] (Figure 2).
As an ITAF, La interacts directly with the IRES of the mRNA encoding the proapoptotic factor XIAP [64]. In addition, La regulates IRES-dependent translation of LamB1, a factor that drives invasion, angiogenesis and metastasis [79, 80]. La also binds to the IRES of cyclin D1 (CCND1) in cervical cancer tissues, and its overexpression correlates with upregulation of cyclin D1 while its depletion leads to a reduction of cyclin D1 levels and a defect in cell proliferation [77].
La is overexpressed in chronic myeloid leukemia, cervical cancer tissues, oral squamous cell carcinoma (SCC), and in a number of cancer cell lines compared to nontumorigenic cells [66, 77, 78, 81]. In SCC, La is required for expression of β-catenin and MMP-2, proteins implicated in cell-cell adhesion and cell motility, respectively [78]. In leukemia, increased levels of La correlate with upregulation of MDM2 (an oncogenic tyrosine kinase). La interacts directly with the 5′UTR of mdm2 mRNA and enhances its translation [66].
Using mouse glial progenitor cells, Brennet proposed that La functions as a translational regulator during KRas/Akt oncogenic signaling [62]. Ras and Akt pathways are aberrantly active in cancer cells and play a pivotal role in the formation and regulation of glioblastoma [82]. In this tumor type La is phosphorylated by Akt, and this changes its distribution from the nucleus to the cytoplasm leading to association of a subset of La-bound mRNAs to polysomes. Many of these mRNAs encode factors implicated in oncogenesis such as Cyclin G2, Bcl2, and PDGFA [62].
The number of known La mRNA targets is still limited and further studies are necessary to understand its function in tumorigenesis. However, La already represents a promising target for cancer therapy. As an example, La activity has been efficiently blocked by a synthetic peptide corresponding to amino acids 11 to 28 of La. By competition, the peptide inhibits IRES-driven translation of Hepatitis C without affecting cap-dependent translation of cellular mRNAs [83]. This peptide could also be used to block expression of cancer related mRNA targets of La.
Other ITAFs implicated in cancer are PTB, hnRNP A1, hnRNP E1, hnRNP E2, and YB1. The interested reader can refer to the following reviews and articles [84, 85].
2.4. HuR Regulates the Stability and Translation of Cancer-Related Transcripts
The human antigen R (HuR) is the most prominent RBP known to be implicated in tumorigenesis [3]. Overexpression of HuR has been observed in lymphomas, gastric, breast, pancreatic, prostate, oral, colon, skin, lung, ovarian, and brain cancers [86–91]. Elevated cytoplasmic accumulation of HuR correlates with high-grade malignancy and serves as a prognostic factor of poor clinical outcome in some cancer types [92–95]. Localized in the nucleus of normal cells, HuR often translocates to the cytoplasm in transformed cells [96, 97]. HuR's subcellular localization is regulated by posttranslational modifications, and the enzymes modifying HuR are all implicated in cancer [97] (Figure 2). In the cytoplasm, HuR binds to adenine- and uridine-rich elements (AU-rich elements or AREs) located in 3′UTR of target mRNAs [98]. AU-rich elements serve as binding sites for a variety of RBPs that modulate mRNA half-life [11]. An estimated 10% of all mRNAs bear AU-rich sequences [99]. The minimal functional ARE sequence is a nonamer UUAUUUAWW [100]. Most RBPs binding to AREs promote rapid deadenylation and degradation of substrate mRNAs by targeting them to the exosome (e.g., TTP, AUF1, CUGBP2) [101]. On the contrary, HuR most often enhances the stability of its target mRNAs [3]. In addition, HuR can also regulate the splicing of a certain number of targets [102].
HuR is a member of the embryonic lethal abnormal vision (ELAV) family of proteins and contains three RRMs that provide high-affinity RNA binding [103] (Figure 1). HuR target mRNAs encode products that promote proliferation, inhibit apoptosis, increase angiogenesis, and facilitate invasion and metastasis. For an extensive list of HuR targets, see [3]. Below I will give an overview of HuR targets and will summarize the different mechanisms by which HuR regulates their expression.
Upon binding to the 3′UTR, HuR stabilizes the mRNAs coding for cyclins (cyclin D1, E1, A2, B1), favoring cell cycle progression and promoting proliferation of cancer cells [104–106]. HuR also promotes cancer cell survival by stabilizing transcripts encoding antiapoptotic factors like Bcl-2, Mcl-1, SIRT1, and p21 [90, 107–110]. mRNAs coding for proteins implicated in invasion and metastasis (MMP-9) [111, 112], cell migration and adhesion (Urokinase A and uPA receptor) [113] or EMT (snail) are also stabilized by HuR [114]. Expression of the proangiogenic factors VEGF and HIF-1α is controlled by HuR. Regulation of HIF-1α mRNA is interesting, as HuR binds to both the 5′ and 3′UTRs and promotes translation and stability [115, 116]. The mechanism by which HuR stabilizes its targets is still unclear, but recent studies have proposed an interplay between HuR and miRNAs [117]. HuR is able to suppress activity of miRNAs, by inhibiting their recruitment to the mRNA or even by promoting their downregulation. Some examples of cross-talk between HuR and miRNAs will be given in the next paragraph.
ERBB-2 overexpression is associated with development and progression of prostate cancer. HuR enhances ERBB-2 expression using a miRNA-dependent mechanism. HuR binds to a uridinerich element (URE) in the 3′UTR of ERBB-2 and inhibits action of miR-331-3p to a nearby site [118]. The presence of HuR on the mRNA does not alter miR-331-3p binding, which leads to the hypothesis that HuR may rather reduce association between ERBB-2 mRNA and the RNA silencing complex [118]. In colorectal cancer, HuR overexpression and localization in the cytoplasm correlate with decreased levels of miR-16, a miRNA that binds to the 3′UTR of COX-2 mRNA and inhibits its expression by mRNA decay [119]. Intriguingly, HuR interacts with miR-16 and promotes its downregulation in an mRNA ligand-dependent manner. Thus, HuR stabilizes COX-2 mRNA by binding to the ARE and by downregulating miR-16 [119].
Interestingly, HuR is able to repress the translation of the proapoptotic factor c-Myc by recruiting the let-7 miRNP to the 3′UTR [120]. HuR is not the only RBP which assists in targeting miRNPs to the 3′UTR of mRNAs, as was shown with the example of TTP [121].
HuR also represses the translation of some of its targets by binding to the 5′UTR. This is the case for p27, which prevents cell proliferation [122].
It has been recently shown that HuR can act as an ITAF binding to the IRES of XIAP mRNA, which encodes an anti-apoptotic factor [123]. HuR stimulates the translation of XIAP mRNA by binding to XIAP IRES and enhancing its recruitment into polysomes.
Interestingly in the case of the antiapoptotic factor prothymosin alpha (ProTα), HuR binding to its 3′UTR enhances nuclear export of the mRNA followed by induced translation upon UV irradiation [124].
In summary, the majority of HuR mRNA targets are stabilized upon binding, and translation is enhanced. As an ITAF, HuR binds to IRES structures and enhances translation. HuR is also able to inhibit translation by binding to 5′UTR or by recruiting miRNPs to the 3′UTR. On the other hand, HuR also inhibits miRNA binding to the 3′UTR of its target mRNAs. Finally, HuR is increasing cytoplasmic abundance of target mRNAs probably via enhanced mRNA nulear export. These examples illustrate the complexity of HuR regulatory activity.
The large spectrum of mRNA targets regulated by HuR confirms its potential to coordinate nearly all steps of tumorigenesis. Overexpressed in a high number of cancer types, HuR provides a good candidate for therapy design. Surprisingly, however, a recent study showed that elevated levels of HuR may be advantageous for cancer therapy. In pancreatic ductal adenocarcinoma, HuR levels modulate the therapeutic activity of gemcitabine (GEM), a common chemotherapeutic agent [125]. GEM exposure to cancer cells increases the amount of cytoplasmic HuR and promotes its association with dCK mRNA, which encodes the enzyme that activates GEM, establishing a positive feedback loop that improves its therapeutic efficacy. This example shows that therapies that reduce the level of HuR have to be designed carefully, and perhaps in a tumor type-dependent manner [126].
Besides HuR, a number of other factors can regulate the stability and expression of mRNAs bearing AREs [127]. The TIS11 family of RBPs composed of Tristetraprolin (TTP) and butyrate response factors 1 and 2 (BRF-1 and-2) bind and target ARE-containing mRNAs for rapid degradation [101]. AUF1 is able to stabilize or destabilize ARE-containing mRNAs [128]. The CELF family of RNA-binding proteins is composed of 6 members, which promote either mRNA decay or translation of its target mRNAs [129, 130]. For example CUGBP2 binds COX-2 mRNA which is then stabilized but translationally repressed [131]. T-cell intracellular antigen-1 (TIA-1) and TIA-1-related (TIAR) proteins are translational silencers [132]. Some of these factors have common targets and compete for binding depending on cellular conditions.
3. Conservation of RBPs across Eukaryotes
Post-transcriptional gene regulation is a coordinated, efficient, rapid and flexible mechanism to control the proteome of the cell in response to different physiological conditions. It is thus not surprising that some organisms have become highly dependent on post-transcriptional mechanisms to regulate gene expression, like, for example, the protozoan parasite, trypanosome [133–135]. The trypanosome genome encodes very few potential regulatory transcription factors, and gene regulation relies mostly on RNA-binding proteins [136]. It has been proposed that 3–11% of the proteome in bacteria, archea and eukaryotes are putative RNA-binding proteins [137]. The large number of RBPs suggests that RNA metabolism may be a central and evolutionarily conserved contributor to cell physiology. Most of the RNA-binding domains known today are present in early stages of evolution. Interestingly, several new eukaryotic-specific RNA-binding domains have emerged, like the RRM, which suggests that post-transcriptional gene regulation became more complex with evolution [137].
The RNA-binding proteins described in this paper are widely conserved across eukaryotes (Figure 3). We could detect homologues of HuR only in metazoa and not in fungi and plants. Human HuR is the most divergent family member of the ELAV proteins. While the other members, HuD, HuC, and Hel-N1, present a neuron- and brain-specific expression, where they are mostly implicated in alternative splicing, HuR is ubiquitously expressed and fulfills numerous functions [138, 139].
Figure 3.
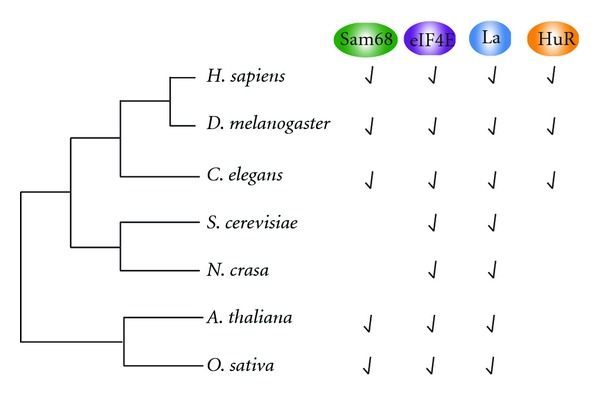
Conservation of Sam68, eIF4E, La, and HuR in different phyla. Phylogenetic tree of the RBPs described in this paper. The presence of homologues is indicated.
Sam68 homologues exist in all eukaryotes except fungi (Figure 3). In the STAR protein family, the Sam68 subfamily is composed of Sam68 (SRC-associated in mitosis, 68 kd) and the Sam68-like mammalian proteins 1 and 2 (SLM-1 and SLM-2, also named T-STAR in humans) [140–143]. As in the case of HuR, Sam68 is ubiquitously expressed, whereas SLM-1 and SLM-2 expression is restricted to few cell types or tissues [144]. In humans, Sam68 has acquired a larger spectrum of functions and plays a major role in signaling and splicing in different tissues.
Contrary to HuR and Sam68, La homologues can be identified in all three phyla: metazoa, fungi, and plants (Figure 3). La was first characterized as a human protein, and homologues have been identified in a wide variety of other eukaryotes [63]. The N-terminal part containing the La motif is highly conserved, in contrast to the C-terminal domain which varies both in size and sequence between species, ranging from 70 amino acids in the yeasts S. cerevisiae and S. pombe to more than 220 amino acids in vertebrates. Human La is phosphorylated at different sites, all located in the C terminus [63] (Figure 2). Interestingly these sites are only conserved in vertebrate La proteins. The presence of an additional C-terminal region including different functional domains and phosphorylation sites shows that La has evolved to a highly regulated and multifunctional factor in vertebrates.
The translation initiation factor eIF4E is highly conserved across eukaryotes. Sequence comparisons revealed a phylogenetically conserved 182 amino acid C-terminal region [145, 146]. In contrast, the N-terminal region is poorly conserved and is not required for cap-dependent translation [145]. Functional conservation has also been demonstrated, as mammalian eIF4E can rescue the lethality caused by disruption of the yeast eIF4E gene [147]. The crystallographic structure of eIF4E in mouse, yeast, human, and wheat has been solved [145, 148–150]. The three-dimensional structure of the C-terminal part of murine eIF4E demonstrates that the surface of the molecule resembles a cupped hand that contains a narrow cap-binding slot. The remarkable level of sequence identity across phylogeny suggests that all known eIF4Es share the same structure in their conserved C-terminal region [145]. eIF4E thus does not contain a canonical RBD but adopts a conserved three-dimensional structure which interacts with the cap.
Interestingly most eukaryotic organisms express multiple eIF4E family members, and it has been proposed that a ubiquitously expressed member of the family may be implicated in general translation initiation while others could be involved in specialized functions [151, 152]. eIF4E family members may provide an additional layer of control in translation and may regulate specific subsets of mRNAs, which could be linked to cancer development.
4. Concluding Remarks
In cancer research, the impact of post-transcriptional gene regulation has been considered only since a few years. Today, it is well established that a subset of RBPs are key regulators of processes involved in tumorigenesis. The genome wide analysis of RBPs and their RNA targets has allowed a better understanding of the complex world of mRNA metabolism and the connections existing between different RBPs. According to the “RNA operon” concept, mRNAs encoding functionally related proteins are coregulated by specific RBPs, ensuring an efficient, flexible, and coordinated response to cellular need [144, 153, 154]. RNA operons can be interconnected. HuR and eIF4E for example, share common mRNA targets like c-myc, cyclin D1 and VEGF, suggesting an orchestrated regulation of the expression of genes implicated in tumorigenesis [155, 156]. In addition, HuR regulates expression of eIF4E in cancer cells [156]. These observations show that post-transcriptional regulation events are highly linked and provide a powerful mechanism to control the fate of a cell.
RBPs are highly versatile factors that can bind to multiple RNA targets and regulate their fate by a variety of mechanisms. The fact that every step of the mRNA life cycle is narrowly controlled allows RBPs to fine tune expression in a very precise manner. The conservation of RBPs across eukaryotes and the emergence of more complexity along evolution also point to an essential role of RBPs. Post-transcriptional gene regulation is a central mechanism of emerging importance in cancer research which is expected to provide novel targets for therapy design.
Acknowledgments
The author would like to thank Fátima Gebauer for suggestions and critical reading of the paper. He also thanks Margarita Meer and Fyodor Kondrashov for performing phylogenetic analysis. L. Wurth is supported by the National Research Fund, Luxembourg, and cofounded under the Marie Curie Actions of European Commission (FP7-COFUND) and work in Fátima Gebauer's lab is supported by grants BFU2009-08243 and Consolider CSD2009-00080 from MICINN.
References
- 1.Kim MY, Hur J, Jeong S. Emerging roles of RNA and RNA-binding protein network in cancer cells. BMB Reports. 2009;42(3):125–130. doi: 10.5483/bmbrep.2009.42.3.125. [DOI] [PubMed] [Google Scholar]
- 2.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nature Reviews Cancer. 2010;10(4):254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 3.Abdelmohsen K, Gorospe M. Posttranscriptional regulation of cancer traits by HuR. Wiley Interdisciplinary Reviews. 2011;1(2):214–229. doi: 10.1002/wrna.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielli P, Busa R, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocrine-Related Cancers. 2011;18(4):R91–R102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- 5.Keene JD. Ribonucleoprotein infrastructure regulating the flow of genetic information between the genome and the proteome. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(13):7018–7024. doi: 10.1073/pnas.111145598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309(5740):1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 7.Wahl MC, Will CL, Lührmann R. The Spliceosome: design Principles of a Dynamic RNP Machine. Cell. 2009;136(4):701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 8.van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nature Reviews Cancer. 2011;11:644–656. doi: 10.1038/nrc3107. [DOI] [PubMed] [Google Scholar]
- 9.Kasinski AL, Slack FJ. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nature Reviews Cancer. 2011;11(12):849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering BM, Willis AE. The implications of structured 5′ untranslated regions on translation and disease. Seminars in Cell and Developmental Biology. 2005;16(1):39–47. doi: 10.1016/j.semcdb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Audic Y, Hartley RS. Post-transcriptional regulation in cancer. Biology of the Cell. 2004;96(7):479–498. doi: 10.1016/j.biolcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Duret L, Dorkeld F, Gautier C. Strong conservation of non-coding sequences during vertebrates evolution: potential involvement in post-transcriptional regulation of gene expression. Nucleic Acids Research. 1993;21(10):2315–2322. doi: 10.1093/nar/21.10.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunde BM, Moore C, Varani G. RNA-binding proteins: modular design for efficient function. Nature Reviews Molecular Cell Biology. 2007;8(6):479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ule J, Jensen K, Mele A, Darnell RB. CLIP: a method for identifying protein-RNA interaction sites in living cells. Methods. 2005;37(4):376–386. doi: 10.1016/j.ymeth.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Licatalosi DD, Mele A, Fak JJ, et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456(7221):464–469. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebedeva S, Jens M, Theil K, et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Molecular Cell. 2011;43(3):340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Lukong KE, Chang KW, Khandjian EW, Richard S. RNA-binding proteins in human genetic disease. Trends in Genetics. 2008;24(8):416–425. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz-Zapater E, Pineda D, Martinez-Bosch N, et al. Key contribution of CPEB4-mediated translational control to cancer progression. Nature Medicine. 2012;18:83–90. doi: 10.1038/nm.2540. [DOI] [PubMed] [Google Scholar]
- 19.Xu F, Zhang X, Lei Y, et al. Loss of repression of HuR translation by miR-16 may be responsible for the elevation of HuR in human breast carcinoma. Journal of Cellular Biochemistry. 2010;111(3):727–734. doi: 10.1002/jcb.22762. [DOI] [PubMed] [Google Scholar]
- 20.Sohn BH, Park IY, Lee JJ, et al. Functional switching of TGF-beta1 signaling in liver cancer via epigenetic modulation of a single CpG site in TTP promoter. Gastroenterology. 2010;138(5):1898–e12. doi: 10.1053/j.gastro.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 21.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Reports. 2009;10(4):400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paronetto MP, Achsel T, Massiello A, Chalfant CE, Sette C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. Journal of Cell Biology. 2007;176(7):929–939. doi: 10.1083/jcb.200701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HH, Abdelmohsen K, Lal A, et al. Nuclear HuR accumulation through phosphorylation by Cdk1. Genes and Development. 2008;22(13):1804–1815. doi: 10.1101/gad.1645808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vernet C, Artzt K. STAR, a gene family involved in signal transduction and activation of RNA. Trends in Genetics. 1997;13(12):479–484. doi: 10.1016/s0168-9525(97)01269-9. [DOI] [PubMed] [Google Scholar]
- 25.Lukong KE, Richard S. Sam68, the KH domain-containing superSTAR. Biochimica et Biophysica Acta. 2003;1653(2):73–86. doi: 10.1016/j.bbcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Li J, Zheng H, et al. Expression and cytoplasmic localization of SAM68 is a significant and independent prognostic marker for renal cell carcinoma. Cancer Epidemiology Biomarkers and Prevention. 2009;18(10):2685–2693. doi: 10.1158/1055-9965.EPI-09-0097. [DOI] [PubMed] [Google Scholar]
- 27.Lin Q, Taylor SJ, Shalloway D. Specificity and determinants of Sam68 RNA binding. Implications for the biological function of K homology domains. The Journal of Biological Chemistry. 1997;272(43):27274–27280. doi: 10.1074/jbc.272.43.27274. [DOI] [PubMed] [Google Scholar]
- 28.Babic I, Jakymiw A, Fujita DJ. The RNA binding protein Sam68 is acetylated in tumor cell lines, and its acetylation correlates with enhanced RNA binding activity. Oncogene. 2004;23(21):3781–3789. doi: 10.1038/sj.onc.1207484. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann B, Valcárcel J. Decrypting the genome’s alternative messages. Current Opinion in Cell Biology. 2009;21(3):377–386. doi: 10.1016/j.ceb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 30.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes and Development. 2010;24(21):2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukong KE, Richard S. Targeting the RNA-binding protein Sam68 as a treatment for cancer? Future Oncology. 2007;3(5):539–544. doi: 10.2217/14796694.3.5.539. [DOI] [PubMed] [Google Scholar]
- 32.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19(49):5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 33.Matter N, Herrlich P, König H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420(6916):691–695. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 34.Busà R, Paronetto MP, Farini D, et al. The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene. 2007;26(30):4372–4382. doi: 10.1038/sj.onc.1210224. [DOI] [PubMed] [Google Scholar]
- 35.Song L, Wang L, Li Y, et al. Sam68 up-regulation correlates with, and its down-regulation inhibits, proliferation and tumourigenicity of breast cancer cells. Journal of Pathology. 2010;222(3):227–237. doi: 10.1002/path.2751. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Yu CP, Zhong Y, et al. Sam68 expression and cytoplasmic localization is correlated with lymph node metastasis as well as prognosis in patients with early-stage cervical cancer. Annals of Oncology. 2012;23(3):638–646. doi: 10.1093/annonc/mdr290. [DOI] [PubMed] [Google Scholar]
- 37.Rajan P, Gaughan L, Dalgliesh C, et al. Regulation of gene expression by the RNA-binding protein Sam68 in cancer. Biochemical Society Transactions. 2008;36(3):505–507. doi: 10.1042/BST0360505. [DOI] [PubMed] [Google Scholar]
- 38.Cooper DL. Retention of CD44 introns in bladder cancer: understanding the alternative splicing of pre-mRNA opens new insights into the pathogenesis of human cancers. Journal of Pathology. 1995;177(1):1–3. doi: 10.1002/path.1711770102. [DOI] [PubMed] [Google Scholar]
- 39.Paronetto MP, Cappellari M, Busà R, et al. Alternative splicing of the cyclin D1 proto-oncogene is regulated by the RNA-binding protein Sam68. Cancer Research. 2010;70(1):229–239. doi: 10.1158/0008-5472.CAN-09-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knudsen ES, Knudsen KE. Retinoblastoma tumor suppressor: where cancer meets the cell cycle. Experimental Biology and Medicine. 2006;231(7):1271–1281. doi: 10.1177/153537020623100713. [DOI] [PubMed] [Google Scholar]
- 41.Valacca C, Bonomi S, Buratti E, et al. Sam68 regulates EMT through alternative splicing-activated nonsense-mediated mRNA decay of the SF2/ASF proto-oncogene. Journal of Cell Biology. 2010;191(1):87–99. doi: 10.1083/jcb.201001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boise LH, Gonzalez-Garcia M, Postema CE, et al. bcl-x, A bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 43.Mercatante DR, Mohler JL, Kole R. Cellular response to an antisense-mediated shift of Bcl-x pre-mRNA splicing and antineoplastic agents. The Journal of Biological Chemistry. 2002;277(51):49374–49382. doi: 10.1074/jbc.M209236200. [DOI] [PubMed] [Google Scholar]
- 44.Paronetto MP, Zalfa F, Botti F, Geremia R, Bagni C, Sette C. The nuclear RNA-binding protein Sam68 translocates to the cytoplasm and associates with the polysemes in mouse spermatocytes. Molecular Biology of the Cell. 2006;17(1):14–24. doi: 10.1091/mbc.E05-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grange J, Boyer V, Fabian-Fine R, Fredj NB, Sadoul R, Goldberg Y. Somatodendritic Localization and mRNA Association of the Splicing Regulatory Protein Sam68 in the Hippocampus and Cortex. Journal of Neuroscience Research. 2004;75(5):654–666. doi: 10.1002/jnr.20003. [DOI] [PubMed] [Google Scholar]
- 46.Izquierdo JM, Majos N, Bonnal S. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Molecular Cell. 2005;19(4):475–484. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Topisirovic I, Svitkin YV, Sonenberg N, Shatkin AJ. Cap and cap-binding proteins in the control of gene expression. Wiley Interdisciplinary Reviews. 2011;2(2):277–298. doi: 10.1002/wrna.52. [DOI] [PubMed] [Google Scholar]
- 49.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 50.Zimmer SG, Debenedetti A, Graff JR. Translational control of malignancy: the mRNA cap-binding protein, eIF-4E, as a central regulator of tumor formation, growth, invasion and metastasis. Anticancer Research. 2000;20(3):1343–1351. [PubMed] [Google Scholar]
- 51.Hsieh AC, Ruggero D. Targeting eukaryotic translation initiation factor 4E (eIF4E) in cancer. Clinical Cancer Research. 2010;16(20):4914–4920. doi: 10.1158/1078-0432.CCR-10-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graff JR, Konicek BW, Lynch RL, et al. eIF4E activation is commonly Elevated in advanced human prostate cancers and significantly related to reduced patient survival. Cancer Research. 2009;69(9):3866–3873. doi: 10.1158/0008-5472.CAN-08-3472. [DOI] [PubMed] [Google Scholar]
- 53.Coleman LJ, Peter MB, Teall TJ, et al. Combined analysis of eIF4E and 4E-binding protein expression predicts breast cancer survival and estimates eIF4E activity. British Journal of Cancer. 2009;100(9):1393–1399. doi: 10.1038/sj.bjc.6605044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wendel HG, Silva RLA, Malina A, et al. Dissecting eIF4E action in tumorigenesis. Genes and Development. 2007;21(24):3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E—from translation to transformation. Oncogene. 2004;23(18):3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 56.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23(18):3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 57.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KLB. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. Journal of Cell Biology. 2005;169(2):245–256. doi: 10.1083/jcb.200501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malina A, Cencic R, Pelletier J. Targeting translation dependence in cancer. Oncotarget. 2011;2(1-2):76–88. doi: 10.18632/oncotarget.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nature Reviews Cancer. 2004;4(5):335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 60.Graff JR, Konicek BW, Vincent TM, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. Journal of Clinical Investigation. 2007;117(9):2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rutjes SA, Utz PJ, Van Der Heijden A, Broekhuis C, Van Venrooij WJ, Pruijn GJM. The La (SS-B) autoantigen, a key protein in RNA biogenesis, is dephosphorylated and cleaved early during apoptosis. Cell Death and Differentiation. 1999;6(10):976–986. doi: 10.1038/sj.cdd.4400571. [DOI] [PubMed] [Google Scholar]
- 62.Brenet F, Socci ND, Sonenberg N, Holland EC. Akt phosphorylation of La regulates specific mRNA translation in glial progenitors. Oncogene. 2009;28(1):128–139. doi: 10.1038/onc.2008.376. [DOI] [PubMed] [Google Scholar]
- 63.Wolin SL, Cedervall T. The La protein. Annual Review of Biochemistry. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- 64.Holcik M, Korneluk RG. Functional characterization of the X-linked inhibitor of apoptosis (XIAP) internal ribosome entry site element: role of la autoantigen in XIAP translation. Molecular and Cellular Biology. 2000;20(13):4648–4657. doi: 10.1128/mcb.20.13.4648-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crosio C, Boyl PP, Loreni F, Pierandrei-Amaldi P, Amaldi F. La protein has a positive effect on the translation of TOP mRNAs in vivo. Nucleic Acids Research. 2000;28(15):2927–2934. doi: 10.1093/nar/28.15.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trotta R, Vignudelli T, Candini O, et al. BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell. 2003;3(2):145–160. doi: 10.1016/s1535-6108(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 67.Van Horn DJ, Yoo CJ, Xue D, Shi H, Wolin SL. The La protein in Schizosaccharomyces pombe: a conserved yet dispensable phosphoprotein that functions in tRNA maturation. RNA. 1997;3(12):1434–1443. [PMC free article] [PubMed] [Google Scholar]
- 68.Maraia RJ, Intine RVA. Recognition of nascent RNA by the human La antigen: conserved and divergent features of structure and function. Molecular and Cellular Biology. 2001;21(2):367–379. doi: 10.1128/MCB.21.2.367-379.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kenan DJ, Keene JD. La gets its wings. Nature Structural and Molecular Biology. 2004;11(4):303–305. doi: 10.1038/nsmb0404-303. [DOI] [PubMed] [Google Scholar]
- 70.McLaren RS, Caruccio N, Ross J. Human la protein: a stabilizer of histone mRNA. Molecular and Cellular Biology. 1997;17(6):3028–3036. doi: 10.1128/mcb.17.6.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brenet F, Dussault N, Borch J, et al. Mammalian peptidylglycine α-amidating monooxygenase mRNA expression can be modulated by the La autoantigen. Molecular and Cellular Biology. 2005;25(17):7505–7521. doi: 10.1128/MCB.25.17.7505-7521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spångberg K, Wiklund L, Schwartz S. Binding of the La autoantigen to the hepatitis C virus 3′ untranslated region protects the RNA from rapid degradation in vitro. Journal of General Virology. 2001;82(1):113–120. doi: 10.1099/0022-1317-82-1-113. [DOI] [PubMed] [Google Scholar]
- 73.Kim YK, Back SH, Rho J, Lee SH, Jang SK. La autoantigen enhances translation of Bip mRNA. Nucleic Acids Research. 2001;29(24):5009–5016. doi: 10.1093/nar/29.24.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle. 2011;10(2):229–240. doi: 10.4161/cc.10.2.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spriggs KA, Bushell M, Willis AE. Translational Regulation of Gene Expression during Conditions of Cell Stress. Molecular Cell. 2010;40(2):228–237. doi: 10.1016/j.molcel.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 76.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Molecular and Cellular Biology. 1998;18(6):3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sommer G, Rossa C, Chi AC, Neville BW, Heise T. Implication of RNA-binding protein La in proliferation, migration and invasion of lymph node-metastasized hypopharyngeal SCC cells. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025402. Article ID e25402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sommer G, Dittmann J, Kuehnert J, et al. The RNA-binding protein la contributes to cell proliferation and CCND1 expression. Oncogene. 2011;30(4):434–444. doi: 10.1038/onc.2010.425. [DOI] [PubMed] [Google Scholar]
- 79.Petz M, Them N, Huber H, Beug H, Mikulits W. La enhances IRES-mediated translation of laminin B1 during malignant epithelial to mesenchymal transition. Nucleic Acids Research. 2012;40(1):290–302. doi: 10.1093/nar/gkr717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanjuan X, Fernandez PL, Miquel R, et al. Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. The Journal of Pathology. 1996;179(4):376–380. doi: 10.1002/(SICI)1096-9896(199608)179:4<376::AID-PATH591>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 81.Al-Ejeh F, Darby JM, Brown MP. The La autoantigen is a malignancy-associated cell death target that is induced by DNA-damaging drugs. Clinical Cancer Research. 2007;13(18):5509s–5518s. doi: 10.1158/1078-0432.CCR-07-0922. [DOI] [PubMed] [Google Scholar]
- 82.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nature Genetics. 2000;25(1):55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 83.Izumi RE, Das S, Barat B, Raychaudhuri S, Dasgupta A. A peptide from autoantigen La blocks poliovirus and hepatitis C virus cap-independent translation and reveals a single tyrosine critical for La RNA binding and translation stimulation. Journal of Virology. 2004;78(7):3763–3776. doi: 10.1128/JVI.78.7.3763-3776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cobbold LC, Wilson LA, Sawicka K, et al. Upregulated c-myc expression in multiple myeloma by internal ribosome entry results from increased interactions with and expression of PTB-1 and YB-1. Oncogene. 2010;29(19):2884–2891. doi: 10.1038/onc.2010.31. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y, Frost PJ, Hoang BQ, et al. IL-6-induced stimulation of c-Myc translation in multiple myeloma cells is mediated by myc internal ribosome entry site function and the RNA-binding protein, hnRNP A1. Cancer Research. 2008;68(24):10215–10222. doi: 10.1158/0008-5472.CAN-08-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bergalet J, Fawal M, Lopez C, et al. HuR-Mediated control of C/EBPβ mRNA stability and translation in ALK-Positive anaplastic large cell lymphomas. Molecular Cancer Research. 2011;9(4):485–496. doi: 10.1158/1541-7786.MCR-10-0351. [DOI] [PubMed] [Google Scholar]
- 87.Kakuguchi W, Kitamura T, Kuroshima T, et al. HuR knockdown changes the oncogenic potential of oral cancer cells. Molecular Cancer Research. 2010;8(4):520–528. doi: 10.1158/1541-7786.MCR-09-0367. [DOI] [PubMed] [Google Scholar]
- 88.Nowotarski SL, Shantz LM. Cytoplasmic accumulation of the RNA-binding protein HuR stabilizes the ornithine decarboxylase transcript in a murine nonmelanoma skin cancer model. The Journal of Biological Chemistry. 2010;285(41):31885–31894. doi: 10.1074/jbc.M110.148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J, Wang B, Bi J, Zhang C. Cytoplasmic HuR expression correlates with angiogenesis, lymphangiogenesis, and poor outcome in lung cancer. Medical Oncology. 2010;28:577–585. doi: 10.1007/s12032-010-9734-6. [DOI] [PubMed] [Google Scholar]
- 90.Filippova N, Yang X, Wang Y, et al. The RNA-binding protein HuR promotes glioma growth and treatment resistance. Molecular Cancer Research. 2011;9(5):648–659. doi: 10.1158/1541-7786.MCR-10-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bolognani F, Gallani AI, Sokol L. mRNA stability alterations mediated by HuR are necessary to sustain the fast growth of glioma cells. Journal of Neuro-Oncology. 2012;106(3):531–542. doi: 10.1007/s11060-011-0707-1. [DOI] [PubMed] [Google Scholar]
- 92.Yoo PS, Sullivan CAW, Kiang S, et al. Tissue microarray analysis of 560 patients with colorectal adenocarcinoma: high expression of HuR predicts poor survival. Annals of Surgical Oncology. 2009;16(1):200–207. doi: 10.1245/s10434-008-0209-3. [DOI] [PubMed] [Google Scholar]
- 93.Heinonen M, Fagerholm R, Aaltonen K, et al. Prognostic role of HuR in hereditary breast cancer. Clinical Cancer Research. 2007;13(23):6959–6963. doi: 10.1158/1078-0432.CCR-07-1432. [DOI] [PubMed] [Google Scholar]
- 94.Denkert C, Weichert W, Winzer KJ, et al. Expression of the ELAV-like protein HuR is associated with higher tumor grade and increased cyclooxygenase-2 expression in human breast carcinoma. Clinical Cancer Research. 2004;10(16):5580–5586. doi: 10.1158/1078-0432.CCR-04-0070. [DOI] [PubMed] [Google Scholar]
- 95.Denkert C, Weichert W, Pest S, et al. Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression. Cancer Research. 2004;64(1):189–195. doi: 10.1158/0008-5472.can-03-1987. [DOI] [PubMed] [Google Scholar]
- 96.Hasegawa H, Kakuguchi W, Kuroshima T, et al. HuR is exported to the cytoplasm in oral cancer cells in a different manner from that of normal cells. British Journal of Cancer. 2009;100(12):1943–1948. doi: 10.1038/sj.bjc.6605084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cellular Signalling. 2008;20(12):2165–2173. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 98.Brennan CM, Steitz JA. HuR and mRNA stability. Cellular and Molecular Life Sciences. 2001;58(2):266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halees AS, El-badrawi R, Khabar KSA. ARED Organism: expansion of ARED reveals AU-rich element cluster variations between human and mouse. Nucleic Acids Research. 2008;36(1):D137–D140. doi: 10.1093/nar/gkm959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lagnado CA, Brown CY, Goodali GJ. AUUUA is not sufficient to promote poly(A) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A) Molecular and Cellular Biology. 1994;14(12):7984–7995. doi: 10.1128/mcb.14.12.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanduja S, Dixon DA. Tristetraprolin and E6-AP: killing the messenger in cervical cancer. Cell Cycle. 2010;9(16):3135–3136. doi: 10.4161/cc.9.16.12951. [DOI] [PubMed] [Google Scholar]
- 102.Izquierdo JM. Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. The Journal of Biological Chemistry. 2008;283(27):19077–19084. doi: 10.1074/jbc.M800017200. [DOI] [PubMed] [Google Scholar]
- 103.Levine TD, Gao F, King PH, Andrews LG, Keene JD. Hel-N1: an autoimmune RNA-binding protein with specificity for 3’ uridylate-rich untranslated regions of growth factor mRNAs. Molecular and Cellular Biology. 1993;13(6):3494–3504. doi: 10.1128/mcb.13.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO Journal. 2004;23(15):3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo X, Hartley RS. HuR contributes to cyclin E1 deregulation in MCF-7 breast cancer cells. Cancer Research. 2006;66(16):7948–7956. doi: 10.1158/0008-5472.CAN-05-4362. [DOI] [PubMed] [Google Scholar]
- 106.Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO Journal. 2000;19(10):2340–2350. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishimaru D, Ramalingam S, Sengupta TK, et al. Regulation of Bcl-2 expression by HuR in HL60 leukemia cells and A431 carcinoma cells. Molecular Cancer Research. 2009;7(8):1354–1366. doi: 10.1158/1541-7786.MCR-08-0476. [DOI] [PubMed] [Google Scholar]
- 108.Abdelmohsen K, Lal A, Hyeon HK, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007;6(11):1288–1292. doi: 10.4161/cc.6.11.4299. [DOI] [PubMed] [Google Scholar]
- 109.Abdelmohsen K, Pullmann R, Jr., Lal A, et al. Phosphorylation of HuR by Chk2 Regulates SIRT1 Expression. Molecular Cell. 2007;25(4):543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cho SJ, Zhang J, Chen X. RNPC1 modulates the RNA-binding activity of, and cooperates with, HuR to regulate p21 mRNA stability. Nucleic Acids Research. 2010;38(7):2256–2267. doi: 10.1093/nar/gkp1229. Article ID gkp1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akool ES, Kleinert H, Hamada FMA, et al. Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Molecular and Cellular Biology. 2003;23(14):4901–4916. doi: 10.1128/MCB.23.14.4901-4916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huwiler A, Akool ES, Aschrafi A, Hamada FMA, Pfeilschifter J, Eberhardt W. ATP Potentiates Interleukin-1β-induced MMP-9 Expression in Mesangial Cells via Recruitment of the ELAV Protein HuR. The Journal of Biological Chemistry. 2003;278(51):51758–51769. doi: 10.1074/jbc.M305722200. [DOI] [PubMed] [Google Scholar]
- 113.Tran H, Maurer F, Nagamine Y. Stabilization of urokinase and urokinase receptor mRNAs by HuR is linked to its cytoplasmic accumulation induced by activated mitogen-activated protein kinase-activated protein kinase 2. Molecular and Cellular Biology. 2003;23(20):7177–7188. doi: 10.1128/MCB.23.20.7177-7188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dong R, Lu JG, Wang Q, et al. Stabilization of Snail by HuR in the process of hydrogen peroxide induced cell migration. Biochemical and Biophysical Research Communications. 2007;356(1):318–321. doi: 10.1016/j.bbrc.2007.02.145. [DOI] [PubMed] [Google Scholar]
- 115.Galban S, Kuwano Y, Pullmann R., Jr. RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1alpha. Molecular and Cellular Biology. 2008;28(1):93–107. doi: 10.1128/MCB.00973-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sheflin LG, Zou AP, Spaulding SW. Androgens regulate the binding of endogenous HuR to the AU-rich 3′UTRs of HIF-1alpha and EGF mRNA. Biochemical and Biophysical Research Communications . 2004;322(2):644–651. doi: 10.1016/j.bbrc.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 117.Meisner NC, Filipowicz W. Properties of the regulatory RNA-binding protein HuR and its role in controlling miRNA repression. Advances in experimental medicine and biology. 2010;700:106–123. [PubMed] [Google Scholar]
- 118.Epis MR, Barker A, Giles KM. The RNA-binding protein HuR opposes the repression of ERBB-2 gene expression by microRNA miR-331-3p in prostate cancer cells. The Journal of Biological Chemistry. 2011;286(48):41442–41454. doi: 10.1074/jbc.M111.301481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Young LE, Moore AE, Sokol L, Meisner-Kober N, Dixon DA. The mRNA stability factor HuR inhibits microRNA-16 targeting of cyclooxygenase-2. Molecular Cancer Research. 2012;10(1):167–180. doi: 10.1158/1541-7786.MCR-11-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hyeon HK, Kuwano Y, Srikantan S, Eun KL, Martindale JL, Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes and Development. 2009;23(15):1743–1748. doi: 10.1101/gad.1812509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jing Q, Huang S, Guth S, et al. Involvement of microRNA in AU-Rich element-mediated mRNA instability. Cell. 2005;120(5):623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 122.Kullmann M, Göpfert U, Siewe B, Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes and Development. 2002;16(23):3087–3099. doi: 10.1101/gad.248902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Durie D, Lewis SM, Liwak U, Kisilewicz M, Gorospe M, Holcik M. RNA-binding protein HuR mediates cytoprotection through stimulation of XIAP translation. Oncogene. 2011;30(12):1460–1469. doi: 10.1038/onc.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lal A, Kawai T, Yang X, Mazan-Mamczarz K, Gorospe M. Antiapoptotic function of RNA-binding protein HuR effected through prothymosin α . EMBO Journal. 2005;24(10):1852–1862. doi: 10.1038/sj.emboj.7600661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Costantino CL, Witkiewicz AK, Kuwano Y, et al. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Research. 2009;69(11):4567–4572. doi: 10.1158/0008-5472.CAN-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brody JR, Gonye GE. HuR’s role in gemcitabine efficacy: an exception or opportunity? Wiley Interdisciplinary Reviews. 2011;2(3):435–444. doi: 10.1002/wrna.62. [DOI] [PubMed] [Google Scholar]
- 127.Lopez de Silanes I, Quesada MP, Esteller M. Aberrant regulation of messenger RNA 3′-untranslated region in human cancer. Cellular Oncology. 2007;29(1):1–17. doi: 10.1155/2007/586139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gratacos FM, Brewer G. The role of AUF1 in regulated mRNA decay. Wiley Interdisciplinary Reviews. 2011;1(3):457–473. doi: 10.1002/wrna.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barreau C, Paillard L, Méreau A, Osborne HB. Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics andbiological functions. Biochimie. 2006;88(5):515–525. doi: 10.1016/j.biochi.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 130.Vlasova IA, Bohjanen PR. Posttranscriptional regulation of gene networks by GU-rich elements and CELF proteins. RNA Biology. 2008;5(4):201–207. doi: 10.4161/rna.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mukhopadhyay D, Houchen CW, Kennedy S, Dieckgraefe BK, Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Molecular Cell. 2003;11(1):113–126. doi: 10.1016/s1097-2765(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 132.Dixon DA, Balch GC, Kedersha N, et al. Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. Journal of Experimental Medicine. 2003;198(3):475–481. doi: 10.1084/jem.20030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ouellette M, Papadopoulou B. Coordinated gene expression by post-transcriptional regulons in African trypanosomes. Journal of Biology. 2009;8(11, article 100) doi: 10.1186/jbiol203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clayton C, Shapira M. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Molecular and Biochemical Parasitology. 2007;156(2):93–101. doi: 10.1016/j.molbiopara.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 135.Haile S, Papadopoulou B. Developmental regulation of gene expression in trypanosomatid parasitic protozoa. Current Opinion in Microbiology. 2007;10(6):569–577. doi: 10.1016/j.mib.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 136.Palenchar JB, Bellofatto V. Gene transcription in trypanosomes. Molecular and Biochemical Parasitology. 2006;146(2):135–141. doi: 10.1016/j.molbiopara.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 137.Anantharaman V, Koonin EV, Aravind L. Comparative genomics and evolution of proteins involved in RNA metabolism. Nucleic Acids Research. 2002;30(7):1427–1464. doi: 10.1093/nar/30.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. The Journal of Biological Chemistry. 1996;271(14):8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 139.Antic D, Keene JD. Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. American Journal of Human Genetics. 1997;61(2):273–278. doi: 10.1086/514866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fumagalli S, Totty NF, Hsuan JJ, Courtneidge SA. A target for Src in mitosis. Nature. 1994;368(6474):871–874. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- 141.Taylor SJ, Shalloway D. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994;368(6474):867–871. doi: 10.1038/368867a0. [DOI] [PubMed] [Google Scholar]
- 142.Di Fruscio M, Chen T, Richard S. Characterization of Sam68-like mammalian proteins SLM-1 and SLM-2: SLM-1 is a Src substrate during mitosis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):2710–2715. doi: 10.1073/pnas.96.6.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Venables JP, Vernet C, Chew SL, et al. T-STAR/ETOILE: a novel relative of SAM68 that interacts with an RNA-binding protein implicated in spermatogenesis. Human Molecular Genetics. 1999;8(6):959–969. doi: 10.1093/hmg/8.6.959. [DOI] [PubMed] [Google Scholar]
- 144.Paronetto MP, Sette C. Role of RNA-binding proteins in mammalian spermatogenesis. International Journal of Andrology. 2009;33(1):2–12. doi: 10.1111/j.1365-2605.2009.00959.x. [DOI] [PubMed] [Google Scholar]
- 145.Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cocrystal structure of the messenger RNA 5′ cap-binding protein (elF4E) bound to 7-methyl-GDP. Cell. 1997;89(6):951–961. doi: 10.1016/s0092-8674(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 146.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annual Review of Biochemistry. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 147.Altmann M, Muller PP, Pelletier J, Sonenberg N, Trachsel H. A mammalian translation initiation factor can substitute for its yeast homologue in vivo. The Journal of Biological Chemistry. 1989;264(21):12145–12147. [PubMed] [Google Scholar]
- 148.Matsuo H, Li H, McGuire AM, et al. Structure of translation factor elF4E bound to m7GDP and interaction with 4E-binding protein. Nature Structural Biology. 1997;4(9):717–724. doi: 10.1038/nsb0997-717. [DOI] [PubMed] [Google Scholar]
- 149.Tomoo K, Shen X, Okabe K, et al. Crystal structures of 7-methylguanosine 5′-triphosphate (m7GTP)- and P1-7-methylguanosine-P3-adenosine-5′, 5′-triphosphate (m7GpppA)-bound human full-length eukaryotic initiation factor 4E: biological importance of the C-terminal flexible region. Biochemical Journal. 2002;362(3):539–544. doi: 10.1042/0264-6021:3620539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Monzingo AF, Dhaliwal S, Dutt-Chaudhuri A, et al. The structure of eukaryotic translation initiation factor-4E from wheat reveals a novel disulfide bond. Plant Physiology. 2007;143(4):1504–1518. doi: 10.1104/pp.106.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rhoads RE. EIF4E: new family members, new binding partners, new roles. The Journal of Biological Chemistry. 2009;284(25):16711–16715. doi: 10.1074/jbc.R900002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hernández G, Vazquez-Pianzola P. Functional diversity of the eukaryotic translation initiation factors belonging to eIF4 families. Mechanisms of Development. 2005;122(7-8):865–876. doi: 10.1016/j.mod.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 153.Morris AR, Mukherjee N, Keene JD. Systematic analysis of posttranscriptional gene expression. Wiley Interdisciplinary Reviews. 2010;2(2):162–180. doi: 10.1002/wsbm.54. [DOI] [PubMed] [Google Scholar]
- 154.Janga SC, Mittal N. Construction, structure and dynamics of post-transcriptional regulatory network directed by RNA-binding proteins. Advances in Experimental Medicine and Biology. 2011;722:103–117. doi: 10.1007/978-1-4614-0332-6_7. [DOI] [PubMed] [Google Scholar]
- 155.Topisirovic I, Siddiqui N, Borden KLB. The eukaryotic translation initiation factor 4E (eIF4E) and HuR RNA operons collaboratively regulate the expression of survival and proliferative genes. Cell Cycle. 2009;8(7):960–961. [PubMed] [Google Scholar]
- 156.Topisirovic I, Siddiqui N, Orolicki S, et al. Stability of eukaryotic translation initiation factor 4E mRNA is regulated by HuR, and this activity is dysregulated in cancer. Molecular and Cellular Biology. 2009;29(5):1152–1162. doi: 10.1128/MCB.01532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


