Abstract
Underexpression of the transcriptional coactivator PGC-1α is causally linked to certain neurodegenerative disorders, including Huntington's Disease (HD). HD pathoprogression is also associated with aberrant NMDAR activity, in particular an imbalance between synaptic versus extrasynaptic (NMDAREX) activity. Here we show that PGC-1α controls NMDAREX activity in neurons and that its suppression contributes to mutant Huntingtin (mHtt)-induced increases in NMDAREX activity and vulnerability to excitotoxic insults.
We found that knock-down of endogenous PGC-1α increased NMDAREX activity and vulnerability to excitotoxic insults in rat cortical neurons. In contrast, exogenous expression of PGC-1α resulted in a neuroprotective reduction of NMDAREX currents without affecting synaptic NMDAR activity. Since HD models are associated with mHtt-mediated suppression of PGC-1α expression, as well as increased NMDAREX activity, we investigated whether these two events were linked. Expression of mHtt (148Q) resulted in a selective increase in NMDAREX activity, compared with wild-type Htt (18Q), and increased vulnerability to NMDA excitotoxicity. Importantly, we observed that the effects of mHtt and PGC-1α knockdown on NMDAREX activity and vulnerability to excitotoxicity were nonadditive and occluded each other, consistent with a common mechanism. Moreover, exogenous expression of PGC-1α reversed mtHtt-mediated increases in NMDAREX activity and protected neurons against excitotoxic cell death. The link between mHtt, PGC-1α, and NMDAR activity was also confirmed in rat striatal neurons. Thus, targeting levels of PGC-1α expression may help reduce aberrant NMDAREX activity in disorders where PGC-1α is underexpressed.
Introduction
The transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator 1a (PGC-1α) controls important physiological processes in many tissues, including glucose metabolism, energy homeostasis, adaptive thermogenesis, and mitochondrial biogenesis (Handschin, 2009). In neurons, PGC-1α regulates mitochondrial density and antioxidant defenses (St-Pierre et al., 2006; Wareski et al., 2009). Moreover, exogenous PGC-1α expression has demonstrable protective/ameliorating effects in models of Huntington's, Parkinson's, and Alzheimer's diseases (HD, PD, and AD, respectively), as well as in ALS and following ischemic/excitotoxic insults (Cui et al., 2006; Luo et al., 2009; Okamoto et al., 2009; Qin et al., 2009; Chen et al., 2010; Shin et al., 2011; Soriano et al., 2011; Zhao et al., 2011; Mudò et al., 2012).
Importantly, underexpression of PGC-1α has been causally linked to disease progression in models of HD and PD, as well as being associated with AD (Cui et al., 2006; Weydt et al., 2006; Okamoto et al., 2009; Qin et al., 2009; Shin et al., 2011). In HD, PGC-1α underexpression is attributable to mutant Huntingtin (mHtt)-mediated transcriptional repression of the PGC-1α promoter (Cui et al., 2006; Weydt et al., 2006). HD is a disorder linked to aberrant NMDAR activity and excitotoxicity, associated with neuronal loss in the cortex and striatum (Fan and Raymond, 2007). Recent studies showed that in an HD mouse model, early elevation of extrasynaptic NMDAR (NMDAREX) activity in the cortex and striatum contributes to phenotype onset (Milnerwood et al., 2010), although the mechanism is unclear. Excessive activation of NMDAREXs is known to trigger several prodeath pathways, in contrast to the neuroprotective effects of the transsynaptic activation of synaptic NMDARs (NMDARSYNs) (Hardingham and Bading, 2010). In HD, the synaptic/extrasynaptic NMDAR balance plays a key role in regulating mHtt toxicity in cortical and striatal neurons (Okamoto et al., 2009). Furthermore, selective blockade of NMDAREX activity, achieved by low doses of memantine (Xia et al., 2010), ameliorates HD symptoms (Okamoto et al., 2009; Milnerwood et al., 2010).
Here we report that PGC-1α is a negative regulator of NMDAREX activity, offering an explanation for the potent antiexcitotoxic effects of PGC-1α. We also provide evidence that links mHtt's suppression of PGC-1α expression to the deleterious increase in NMDAREX activity. Together with previous studies (Okamoto et al., 2009; Milnerwood et al., 2010), our study suggests a model whereby mtHtt toxicity, NMDAREX activity, and PGC-1α repression all positively feed back on each other to promote neuronal dysfunction.
Materials and Methods
Neuronal culture, transfection, luciferase assays, immunofluorescence.
Cortical and striatal neurons from E21 Sprague Dawley rats of either sex were cultured as described previously (Papadia et al., 2008); experiments were performed at DIV10. Neurons were transfected in trophic transfection medium with plasmids (2 μg/ml total) and/or siRNA (100 nm) using Lipofectamine 2000. Experiments were performed 48 h posttransfection. For luciferase assays, firefly PGC-1α-Luc was transfected plus pTK-RL renilla control and plasmid of interest (e.g., mHtt) with a ratio of 2:1:2. Assays were performed using the Dual-Glo assay kit (Promega). Immunofluorescence was performed as described previously (Papadia et al., 2008). Antibodies used were DARPP-32 (1:100; Abcam) and PGC-1α (1:50; Millipore).
Plasmids.
pcDNA-PGC-1α was a gift from P. Puigserver (Dana Farber Cancer Institute, Boston, MA). pEF-PGC-1α was made by excising the ORF from the pcDNA vector from EcoRI/AgeI sites, blunting and inserting to the EvorV site of pEF/V5-His A vector. Myc-wtHtt-N63-18Q (wtHtt(18Q)) and Myc-mtHtt-N63-148Q (mtHtt-(148Q)) were a gift from Chris Ross (John Hopkins Medical School, Baltimore, MD). PGC-1α-luc was a gift from A. Fukamizu (University of Tsukuba, Tsukuba, Japan).
Electrophysiological recording and analysis.
NMDA-evoked whole-cell steady-state currents (normalized to cell capacitance) were measured 48 h after transfection, as described previously (Papadia et al., 2008). NMDA (100 μm) was applied for 30 s, repeated twice for each cell. Data were filtered at 1 kHz and digitized at 5 kHz for subsequent off-line analysis. Miniature EPSCs (mEPSC) recordings were performed and analyzed as described previously (Baxter and Wyllie, 2006). Recordings of NMDAREX currents were performed 48 h posttransfection. Neurons were placed in Mg2+-free aCSF supplemented with picrotoxin (50 μm), TTX (300 nm), and MK-801 (10 μm). Under these conditions, spontaneous presynaptic release of single quanta of glutamate activate NMDARSYNs, which are then blocked by MK-801 (Papadia et al., 2008). After 10 min incubation (sufficient to block NMDARSYNs, Fig. 1A), neurons were washed with MK-801-free aCSF and placed in a recording chamber. Steady-state NMDAR currents were then measured from three to four cells per coverslip and the mean current density was calculated and treated as a single replicate. To measure both whole-cell and NMDAREX currents in the same cell, NMDA-evoked currents were measured before and after NMDARSYN blockade via the aforementioned 10 min incubation with TTX/MK-801-supplemented Mg2+-free aCSF (Papadia et al., 2008).
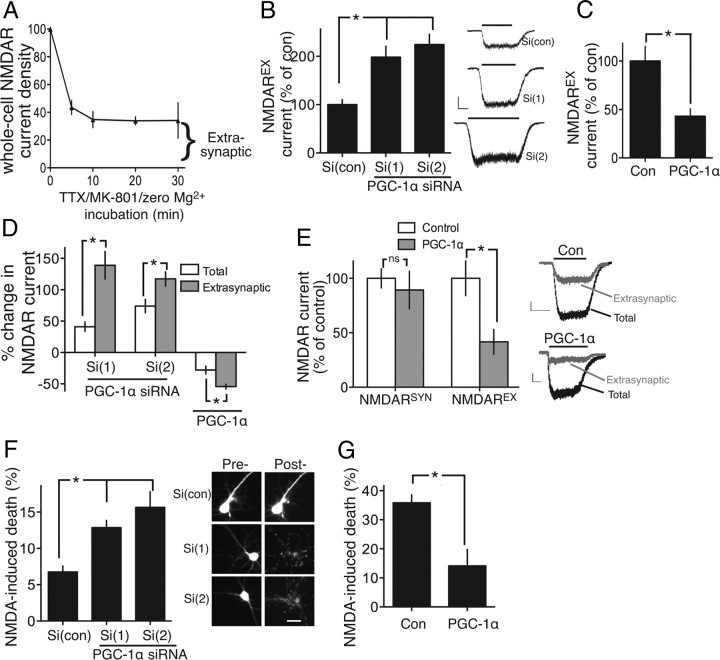
Figure 1.
PGC-1α regulates NMDAREX activity in cortical neurons. A, Time course of the quantal block of NMDARSYNs by MK-801, leaving NMDAREXs unblocked. Neurons were exposed to Mg2+-free aCSF (+ MK-801 + TTX) for the indicated times. NMDA (100 μm)-induced currents were recorded, normalized to capacitance, and expressed relative to total NMDAR currents with no synaptic blockade. Incubation for 10 min was sufficient for maximal blockade of NMDARSYNs; the remaining current represents the extrasynaptic pool (20 cells analyzed). B, Neurons transfected as indicated, plus eGFP marker. NMDAREX currents were recorded 48 h posttransfection, normalized to capacitance, and expressed as a percentage relative to control (con) here and throughout. *p < 0.05; n = 5, 7, 7 independent experiments. Each replicate (i.e., n) represents the mean of three to five cells analyzed here and throughout the manuscript. Scale bars, 200 pA,10 s. C, NMDAREX currents measured in β-globin versus PGC-1α-expressing cortical neurons (normalized to β-globin control). *p < 0.05; n = 5, 4. D, Effect of PGC-1α siRNA and PGC-1α expression on total whole-cell NMDAR currents was measured and expressed as a percentage change compared with control. This percentage change was compared with the percentage change observed in NMDAREX currents.*p < 0.05; n = 4–5. E, Total and NMDAREX currents were sequentially measured in neurons expressing β-globin (Con) or PGC-1α. NMDARSYN currents were calculated as total-extrasynaptic currents. *p < 0.05, n = 11 (Con); n = 5 (PGC-1α). Inset shows example traces; scale bar, 2 s, 100 pA. F, Vulnerability of neurons transfected with the indicated siRNAs to 10 μm NMDA exposure (1 h). Cell death assessed at 24 h here and throughout (see Materials and Methods). *p < 0.05; 150–300 cells analyzed per condition here and in all cell death experiments (n = 3; scale bar, 20 μm). Pre-, Pretransfection; Post-, posttransfection; Si, siRNA. G, Vulnerability of neurons transfected with the indicated plasmids to 20 μm NMDA exposure. *p < 0.05; n = 3.
Following the fate of transfected neurons following excitotoxic insult.
This procedure was performed as described previously (Papadia et al., 2008; Soriano et al., 2011). Neurons were transfected with the plasmid of interest plus eGFP marker, and pictures taken using a Leica AF6000LX system (DFC350 FX camera) before 10–20 μm NMDA treatment for 1 h, after which 10 μm MK-801 was added. Using cell-finder software, images of the same neurons were taken 24 h postinsult. Cell death was determined by counting the number of surviving GFP-expressing cells postinsult. Death was indentified by the absence of healthy GFP-expressing neurons: in >90% of cases, fluorescent cell debris and fragmented nuclei was observed. For each condition, the fate of 200–300 neurons was monitored over three or four experiments done on independent cultures.
Statistical analysis and controls.
Mean ± SEM is shown. All experiments were performed on three to five independent culture batches. In all cases, treatments/interventions were compared with their respective controls within that culture batch (and recorded/measured on the same day), i.e., PGC1α, PGC1α siRNA, and mHtt-expressing/treated neurons were always paired with their respective controls (β-globin, control siRNA, and wild-type Htt (wHtt), respectively) within each culture batch. Statistical testing involved a two-tailed paired or unpaired t test. For studies using multiple testing (e.g., the use of two pairs of siRNA), a one-way ANOVA was used. For studies involving two variables (e.g., siRNAs ± mHtt/wHtt), a two-way ANOVA was used. ANOVAs were followed by Fisher's least squares difference post hoc test.
Results
PGC-1α controls NMDAREX activity and excitotoxicity
Exogenous PGC-1α expression protects neurons against excitotoxic insults (Luo et al., 2009; Chen et al., 2010; Soriano et al., 2011). Because excessive activation of NMDAREXs is an important mediator of excitotoxicity, we investigated the influence of PGC-1α on NMDAREX currents.
To measure NMDAREX currents, we used the established quantal block technique of blocking NMDARSYNs by incubating neurons with the open-channel blocker MK-801, plus TTX in Mg2+-free aCSF (Papadia et al., 2008). Under these conditions, spontaneous presynaptic release of single quanta of glutamate give rise to mEPSCs that activate NMDARSYNs, which are then immediately/irreversibly blocked by MK-801. Subsequent to this open-channel blockade of NMDARSYNs, NMDA-evoked currents are recorded under voltage clamp, and are now only mediated by NMDAREXs that were not activated during the quantal block protocol. Time course experiments revealed that 10 min of MK-801/TTX/zero Mg2+ treatment is sufficient to achieve blockade of NMDARSYNs; longer treatments have no further effect (Fig. 1A).
We studied the effect of knocking down PGC-1α in cortical neurons using two previously validated siRNA sequences (Soriano et al., 2011). We first confirmed that PGC-1α knockdown did not affect the frequency or amplitude of mEPSCs (p = 0.76 or 0.60, respectively; one-way ANOVA, n = 5–6), which (if different) could have affected the rate of MK-801-mediated NMDARSYN blockade. We also confirmed that 10 min of MK-801/TTX/zero Mg2+ treatment was sufficient for NMDA-induced currents to bottom out in PGC-1α siRNA-transfected neurons (data not shown), reassuring us that the remaining currents were mediated by NMDAREXs, out of reach of presynaptically released quanta of glutamate. PGC-1α knockdown led to a large increase in NMDAREX currents (>100%; Fig. 1B). Total whole-cell currents were also increased but by a far lower proportion (∼40%; Fig. 1D). Assessment of NMDAR currents before and after NMDARSYN blockade revealed that NMDAREX currents represent 34.1 ± 6.5% of whole-cell currents (n = 6). Thus, the PGC-1α siRNA-mediated increase in whole-cell currents is largely attributable to the increase in NMDAREX currents.
In contrast to knock-down, exogenous PGC-1α expression reduced NMDAREX currents (Fig. 1C). Again, the effect was proportionally greater than the effect on total whole-cell currents, indicative of a selective effect on NMDAREXs (Fig, 1D). To test this selectivity directly, we recorded (in control vs PGC-1α-expressing neurons) total NMDAR currents and NMDAREX currents in the same cell (see Materials and Methods, above), and from this, calculated the NMDARSYN current (i.e., total NMDAR current minus NMDAREX current). We found that, while (as expected) PGC-1α expression reduced NMDAREX currents, it had no effect on NMDARSYN currents (Fig. 1E), confirming the selectivity of PGC-1α's influence.
Consistent with a role for NMDAREX in excitotoxicity (Hardingham and Bading, 2010), we found that PGC-1α siRNA exacerbated neuronal death in response to a modest (10 μm) dose of NMDA (Fig. 1F), while exogenous expression protected neurons against a higher, more toxic dose (20 μm; Fig. 1G).
mHtt-mediated control of NMDAREX activity via PGC-1α repression
PGC-1α is underexpressed in HD patients, HD mouse models, and mHtt-expressing cells due to mHtt-mediated repression of the PGC-1α promoter (Cui et al., 2006; Weydt et al., 2006; Okamoto et al., 2009; Chaturvedi et al., 2010; McConoughey et al., 2010), which we confirmed by studying a PGC-1α reporter and endogenous protein expression (Fig. 2A). Increased cortical/striatal NMDAREX activity is observed in the YAC128 HD mouse, which contributes to phenotype onset (Milnerwood et al., 2010). Given our observations in Figure 1, we investigated the possibility that HD mouse model-associated increases in NMDAREX activity could be due at least in part to the suppression of PGC-1α by mHtt.
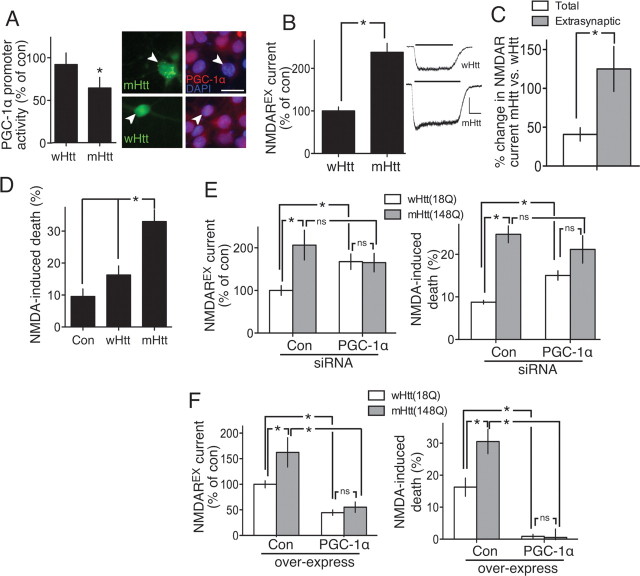
Figure 2.
Mutant Huntingtin-mediated increases in NMDAREXs activity via PGC-1α repression. A, Left, Assay of a luciferase-based reporter of the PGC-1α promoter (Daitoku et al., 2003) in cortical neurons cotransfected with N-terminal wHtt or mHtt. *p < 0.05; n = 5. Right, Transfection of mHtt lowers levels of endogenous PGC-1α, confirming earlier studies (eGFP marker identifies transfected cells; scale bar, 20 μm). B, NMDAREX currents recorded in wHtt- and mHtt-expressing neurons, normalized to wHtt level. *p < 0.05; n = 4. Scale bar, 300 pA, 5 s. C, Total NMDAR currents were measured in mHtt(148Q)-expressing neurons and expressed as a percentage change compared with wHtt(18Q)-expressing neurons. This percentage change was then compared with the percentage change observed in NMDAREX currents. *p < 0.05; n = 4–6. D, Vulnerability of neurons transfected with the indicated expression constructs [control (Con; β-globin), wHtt, or mHtt], to 10 μm NMDA exposure. *p < 0.05; n = 4–6. E, Left, NMDAREX currents were measured in neurons transfected with control versus PGC-1α siRNA, plus either wHtt or mHtt, normalized to control/wHtt condition *p < 0.05; n = 4. (Right, Vulnerability of neurons transfected as indicated to 10 μm NMDA exposure. *p < 0.05; n = 3. F, Left, NMDAREX currents were measured in neurons transfected with control (β-globin) versis PGC-1α-expressing vectors, plus either wHtt or mHtt, normalized to control/wHtt condition. *p < 0.05; n = 4–7. Right, Vulnerability of neurons transfected as indicated to 20 μm NMDA exposure. *p < 0.05; n = 4–6.
We first investigated whether acute expression of N-terminal mHtt exon 1 (148Q) had any effect on whole-cell or NMDAREX currents compared with N-terminal wHtt (18Q) control. Our studies focused initially on cortical neurons (Fig. 2) and subsequently striatal neurons (Fig. 3), both of which are affected in HD. As with PGC-1α knockdown experiments, we confirmed that mEPSC frequencies and amplitudes were unaffected (mHtt vs wHtt; p = 0.26 vs 0.31; t test, n = 6). Following the quantal block protocol (described earlier), we verified that 10 min of MK-801/TTX/zero Mg2+ treatment was sufficient for NMDA-induced currents to bottom-out (data not shown). MHtt expression led to a striking increase in NMDAREX currents compared with wHtt (Fig. 2B), consistent with the YAC128 mouse findings (Milnerwood et al., 2010). Moreover, the mHtt-induced effect on extrasynaptic currents was far more pronounced than its effect on total currents, again indicative of a preferential effect on NMDAREXs over NMDARSYNs (Fig. 2C). In line with previous studies, and consistent with an upregulation of NMDAREX currents, mHtt (but not wHtt) increased neuronal vulnerability to low doses of NMDA (Fig. 2D).
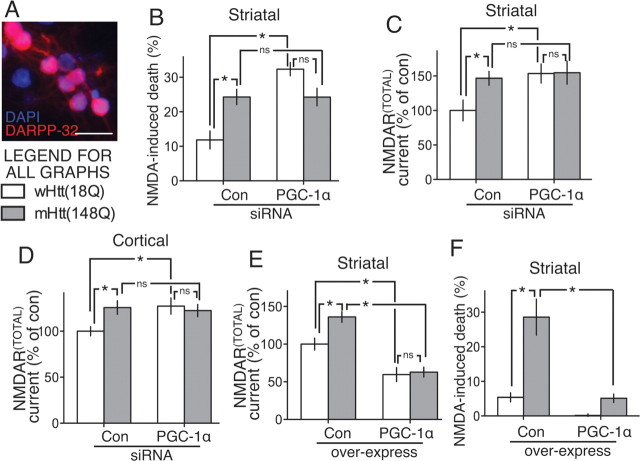
Figure 3.
Link between NMDAR currents, mHtt, and PGC-1α in striatal neurons. A, Darpp-32 immunofluorescence of striatal cultures; scale bar, 20 μm. B, Vulnerability of striatal neurons transfected as indicated to 10 μm NMDA exposure. *p < 0.05; n = 3. C, Total whole-cell NMDAR currents were measured in striatal neurons transfected with control (con) versus PGC-1α siRNA, plus either wHtt or mHtt, normalized to control/wHtt condition *p < 0.05; n = 6–8. D, As in C but in cortical neurons. *p < 0.05; n = 18–29. E, Total whole-cell NMDAR currents were measured in striatal neurons transfected with control versus PGC-1α, plus either wHtt or mHtt, normalized to control/wHtt condition. *p < 0.05; n = 5–7. F, Vulnerability of neurons transfected as indicated to 20 μm NMDA exposure. *p < 0.05; n = 3.
We next investigated the possibility that the effects of mHtt on NMDAREX currents could be due to repression of PGC-1α expression. We tested two predictions of this model: (1) the effects of PGC-1α knockdown and mHtt expression should be nonadditive and occlude each other, and (2) exogenous PGC-1α expression should rescue the effects of mHtt. We studied the effects of combinations of wHtt or mHtt with control or PGC-1α-directed siRNA on NMDAREX currents and vulnerability to excitotoxicity. In neurons transfected with PGC-1α siRNA, mHtt had no additional effect on NMDAREX activity compared with wHtt (Fig. 2E, left). Similarly, in neurons expressing mHtt, PGC-1α siRNA had no additional effect on NMDAREX activity compared with control siRNA (Fig. 2E, left). Moreover the exacerbating effects of PGC-1α siRNA and mHtt on NMDA excitotoxicity were similarly nonadditive and occluded each other (Fig. 2E, right). These observations support the hypothesis that mHtt triggers increases in NMDAREX activity (at least in part) via repression of PGC-1α.
We next performed rescue experiments by driving exogenous PGC-1α expression in mHtt-expressing neurons. This completely reversed the effect of mHtt on NMDAREX activity: against a background of PGC-1α overexpression, mHtt had no effect on NMDAREX currents (Fig. 2F, left). Exogenous PGC-1α also protected neurons against mHtt-induced vulnerability to excitotoxicity (Fig. 2F, right), confirming results from other laboratories. Collectively, these data support a model whereby mHtt suppresses PGC-1α expression, leading to enhanced NMDAREX currents, and rescue of mHtt-mediated vulnerability against excitotoxicity can be achieved by restoring PGC-1α expression.
The electrophysiological assessment of synaptic versus extrasynaptic NMDAR currents in striatal cultures is problematic since glutamatergic inputs onto striatal neurons originate from outside the striatum. Our quantal block method could not be used as there would be little spontaneous release of glutamate in such cultures. Nevertheless, we wanted to determine whether mHtt expression and PGC-1α knockdown has nonadditive effects on NMDAR currents and vulnerability to excitotoxicity in striatal cultures (Fig. 3A). As with cortical neurons, both PGC-1α knockdown and mHtt expression increased vulnerability to subtoxic NMDA doses, and the effect of either intervention was just as strong as that of both combined (Fig. 3B). Analysis of NMDAR currents was restricted to total whole-cell currents (i.e., mediated by both NMDARSYN and NMDAREX) and revealed that the effects of PGC-1α knockdown and mHtt expression mirrored those observed in cortical neurons (i.e., increasing currents in a nonadditive manner; Fig. 3C,D). Furthermore, exogenous expression of PGC-1α lowered NMDAR currents, prevented mHtt-mediated increases in NMDAR currents (Fig. 3E), and protected striatal neurons against excitotoxic NMDA exposure (Fig. 3F). These data support the notion that the link between mHtt, PGC-1α, and NMDAR currents applies to both striatal and cortical neurons, the major sites of neurodegeneration in HD.
Discussion
This study reveals an unexpected role for PGC-1α in regulating NMDAREX activity and, together with recent studies, illuminates the complex interdependence of PGC-1α, synaptic/extrasynaptic NMDAR activity, and mHtt toxicity.
Reciprocal links between NMDAREX activity, mHtt, and PGC-1α
Recent work from Okamoto and coworkers (2009) showed that the balance between NMDARSYN and NMDAREX activity controls mHtt toxicity. NMDARSYN activity, by inducing expression of the chaperonin subunit TCP-1, suppresses mHtt toxicity by promoting the formation of nontoxic inclusions (Okamoto et al., 2009). In contrast, NMDAREX activity promotes disaggregation of mHtt, enhancing its toxicity associated with Rhes expression, which mediates mHtt sumoylation, disaggregation, and toxicity (Subramaniam et al., 2009). Our study and that of Milnerwood et al. (2010) demonstrates that there is also a reciprocal link: mHtt itself upsets the NMDAR balance in favor of NMDAREX activity.
Another known effect of mHtt is suppression of PGC-1α expression, both directly and by increasing NMDAREX activity (Cui et al., 2006; Weydt et al., 2006; Okamoto et al., 2009; Chaturvedi et al., 2010; McConoughey et al., 2010). PGC-1α is a CREB target gene, and enhanced NMDAREX activity is known to trigger CREB shut-off by dephosphorylation (Hardingham et al., 2002), something observed in the HD mouse model and rescued by antagonizing NMDAREXs with low-dose memantine (Milnerwood et al., 2010). Our study shows that the reciprocal relationship also exists: PGC-1α itself controls NMDAREX activity and that mHtt-induced changes in NMDAREX activity are due, at least in part, to its effect on PGC-1α expression.
The consequence of these interdependencies may be a series of positive feedback loops promoting pathoprogression. Any increase in mHtt levels, NMDAREX activity, or PGC-1α promoter repression may become amplified because all three events have the capacity to positively reinforce the other two. For example, aberrant NMDAREX activity is known to suppress CREB-dependent PGC-1α expression and enhance mHtt toxicity. MHtt in turn leads to PGC-1α repression even further, and underexpression of PGC-1α leads to further enhancement of NMDAREX activity, and so on. A prediction of this model would be that therapies aimed at supporting PGC-1α expression (McConoughey et al., 2010) could break the cycle of pathoprogression.
Mechanistically, how PGC-1α lowers NMDAREX expression is unclear. Indeed, mechanisms that control the NMDARSYN:NMDAREX balance in general are poorly understood. One potential influence is GluN2 subunit composition: GluN2A may be enriched at synaptic locations, and GluN2B at extrasynaptic locations, although this is controversial (Hardingham and Bading, 2010). However, we found that the sensitivity of NMDAR whole-cell currents to the GluN2B-specific antagonist was not affected by PGC-1α overexpression (C. Puddifoot, G. Hardingham, unpublished observations), ruling out changes in GluN2 subunit composition as a mechanism. Since PGC-1α can control metabolic pathways, energy homeostasis, mitochondrial biogenesis, and antioxidant defenses (St-Pierre et al., 2006; Handschin, 2009; Wareski et al., 2009), it could be that perturbation to these processes leads to redistribution of NMDARs; for example, through alterations to Ca2+ levels, energy levels, or redox status. These issues await further investigation.
PGC-1α repression in other neurodegenerative disorders
The effects of PGC-1α underexpression are not limited to HD, since PGC-1α repression is observed in AD (Qin et al., 2009) and PD (Shin et al., 2011). In AD, PGC-1α expression was found to be negatively correlated with dementia (Qin et al., 2009). Moreover, overexpression of PGC-1α in Tg2576 AD mouse neurons suppressed amyloidogenic processing of Aβ, promoting α-secretase nonamyloidogenic processing (Qin et al., 2009). Of note, the NMDARSYN:NMDAREX balance also influences Aβ production: NMDARSYN activity promotes α-secretase nonamyloidogenic processing and downregulates APP expression, while NMDAREX activity fails to do this and in fact promotes expression of amyloidogenic isoforms of APP (Hoey et al., 2009; Bordji et al., 2010). Our study suggests the possibility of PGC-1α–NMDAR feedback loops controlling APP processing.
Our study does not question the importance of non-NMDAR-dependent effects of PGC-1α underexpression in neuronal degeneration/dysfunction in neurodegenerative disease. As a regulator of mitochondrial biogenesis and antioxidant defenses, the effects of PGC-1α underexpression are likely to be many. For example, in a recent landmark paper, PGC-1α repression by Parkin-interacting substrate was identified as a causal factor in dopaminergic neuronal loss in models of PD (Shin et al., 2011), a disorder not generally associated with excitotoxicity or aberrant NMDAREX activity. That notwithstanding, the effects of PGC-1α on NMDAR currents, and particularly NMDAREXs, may contribute to the consequences of PGC-1α underexpression in neurological disorders.
Footnotes
This work was supported by the Medical Research Council, The Wellcome Trust, The Royal Society, and the Biotechnology and Biological Research Council. We thank Pere Puigserver, Chris Ross, Stuart Lipton, Akiyoshi Fukamizu, and Guang Bai for plasmids.
References
- Baxter AW, Wyllie DJ. Phosphatidylinositol 3 kinase activation and AMPA receptor subunit trafficking underlie the potentiation of miniature EPSC amplitudes triggered by the activation of L-type calcium channels. J Neurosci. 2006;26:5456–5469. doi: 10.1523/JNEUROSCI.4101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordji K, Becerril-Ortega J, Nicole O, Buisson A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ss production. J Neurosci. 2010;30:15927–15942. doi: 10.1523/JNEUROSCI.3021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi RK, Calingasan NY, Yang L, Hennessey T, Johri A, Beal MF. Impairment of PGC-1alpha expression, neuropathology and hepatic steatosis in a transgenic mouse model of Huntington's disease following chronic energy deprivation. Hum Mol Genet. 2010;19:3190–3205. doi: 10.1093/hmg/ddq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SD, Lin TK, Yang DI, Lee SY, Shaw FZ, Liou CW, Chuang YC. Protective effects of peroxisome proliferator-activated receptors gamma coactivator-1alpha against neuronal cell death in the hippocampal CA1 subfield after transient global ischemia. J Neurosci Res. 2010;88:605–613. doi: 10.1002/jnr.22225. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes. 2003;52:642–649. doi: 10.2337/diabetes.52.3.642. [DOI] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Handschin C. The biology of PGC-1alpha and its therapeutic potential. Trends Pharmacol Sci. 2009;30:322–329. doi: 10.1016/j.tips.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J Neurosci. 2009;29:4442–4460. doi: 10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhu W, Jia J, Zhang C, Xu Y. NMDA receptor dependent PGC-1alpha up-regulation protects the cortical neuron against oxygen-glucose deprivation/reperfusion injury. J Mol Neurosci. 2009;39:262–268. doi: 10.1007/s12031-009-9196-5. [DOI] [PubMed] [Google Scholar]
- McConoughey SJ, Basso M, Niatsetskaya ZV, Sleiman SF, Smirnova NA, Langley BC, Mahishi L, Cooper AJ, Antonyak MA, Cerione RA, Li B, Starkov A, Chaturvedi RK, Beal MF, Coppola G, Geschwind DH, Ryu H, Xia L, Iismaa SE, Pallos J, et al. Inhibition of transglutaminase 2 mitigates transcriptional dysregulation in models of Huntington disease. EMBO Mol Med. 2010;2:349–370. doi: 10.1002/emmm.201000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signalling and expression contributes to phenotype onset in Huntington's disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Mudò G, Mäkelä J, Liberto VD, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Mälkiä A, Bonomo A, Kairisalo M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell Mol Life Sci. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, Léveillé F, Papadia S, Bell KF, Puddifoot C, Hardingham GE. Neuronal activity controls the antagonistic balance between PGC-1α and SMRT in regulating antioxidant defences. Antioxid Redox Signal. 2011;14:1425–1436. doi: 10.1089/ars.2010.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A. PGC-alpha and PGC-1beta regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–21385. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ, Bammler TK, Strand AD, Cui L, Beyer RP, Easley CN, Smith AC, Krainc D, Luquet S, Sweet IR, Schwartz MW, La Spada AR. Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1alpha in Huntington's disease neurodegeneration. Cell Metab. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Varghese M, Yemul S, Pan Y, Cheng A, Marano P, Hassan S, Vempati P, Chen F, Qian X, Pasinetti GM. Peroxisome proliferator activator receptor gamma coactivator-1alpha (PGC-1alpha) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2011;6:51. doi: 10.1186/1750-1326-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]