Abstract
In radical behaviorism, the difference between overt and covert responses does not depend on properties of the behavior but on the sensitivity of the measurement tools employed by the experimenter. Current neuroscientific research utilizes technologies that allow measurement of variables that are undetected by the tools typically used by behavior analysts. Data from a specific neuroscientific technique, event-related potential (ERP), suggest that emission of otherwise covert responses can be indexed and that such covert responses are sensitive to stimulus control and selection by consequences. The P3 ERP effect is proposed as indicative of emission. Moreover, ERP results in semantic priming experiments suggest that operants are sensitive to changes in stimulus control even when they are not emitted (latent responses). Changes in response strength of latent responses as a function of stimulus control can in fact be measured by reaction time data and an ERP dependent variable called the N400 effect. If the interpretations provided in this paper are accurate, an index of covertly emitted operants (P3 effect) constitutes experimental evidence suggesting the validity of a Skinnerian radical behaviorist perspective on behavior. Moreover, in a Skinnerian paradigm, measured fluctuations in the response strength of latent operants as a function of environmental changes (N400 effect) would validate Palmer's (2009) concept of the repertoire.
Keywords: covert behavior, electroencephalography, event-related potential, N400, neuroscience, P3, radical behaviorism, response strength
A radical behaviorist approach to human behavior assumes that covert responses differ in their accessibility to an observer but are not qualitatively different from overt responses. Because the difference between covert and overt behavior is assumed to be only quantitative in this sense, radical behaviorists infer that overt and covert behaviors obey the same laws (Skinner, 1945). Ergo, the distinction between overt and covert behavior is exclusively a function of available technology (Palmer, 2003). In the current paper, results from experiments that have employed neuroscientific technologies are described, with the goal of assessing the empirical validity of radical behaviorist interpretations (Donahoe & Palmer, 1994; Palmer, 1991, 2003, 2009; Skinner, 1953, 1957).
If the ability to detect responses and study functional relations among discriminative stimuli, responses, and reinforcers depends on the sensitivity of our measurement tools, increasing that sensitivity may lead to detection of functional relations previously unnoticed. For example, a lever, a button, or a microphone would be inadequate measurement tools for detecting tiny facial responses. But electrodes placed on facial muscles would allow measurement of otherwise unnoticeable responses. When these responses are measured and consequences provided, patients with motor disabilities can gain operant control of external devices (Barreto, Scargle, & Adjouadi, 2000; Huang, Chen, & Chung, 2006).
Any measured change in behavior that enters into a meaningful functional relation with discriminative stimuli and reinforcers becomes part of a three-term contingency. Electrodes placed over the heads of disabled patients allow the measurement of electroencephalography (EEG) waves that are sensitive to antecedents and consequences. For example, some patients with amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, have entirely lost operant control of their muscles. These patients are able to acquire operant control of their EEG waves and, using a computerized language support program, learn to choose pictograms or words in that manner; thus, they regain their ability to communicate with the outside world (Birbaumer, 1999; Birbaumer et al., 2000).
The dependent variable in these settings—movement of the facial muscles or a change over time in the amplitude of EEG waves—could not be measured with the tools that have been historically employed to keep track of what we call overt responses (e.g., switch closure caused by pushing a button). Yet after improved technology allows measurement of that variable, by definition it becomes overt. In other words, the definitions of overt and covert do not depend on structural properties of the response; all measured variables are to be considered overt. As Skinner (1953) put it,
The line between public and private is not fixed. The boundary shifts with every discovery of a technique for making private events public. Behavior which is of such small magnitude that it is not ordinarily observed may be amplified. Covert verbal behavior may be detected in slight movements of the speech apparatus. Deaf-mutes who speak with their fingers behave covertly with their fingers, and the movements may be suitably amplified. … The problem of privacy may, therefore, eventually be solved by technical advances. (p. 287)
Neuroscientific research has introduced measurement tools that can be relevant for behavior analysts both in answering important questions in basic research and in developing useful technologies in applied settings. Behavior analysts' ability to predict and control behavior can benefit from tools that can measure responses that have been previously hidden because of their low magnitude or because they are too brief to be captured by older technologies. Moreover, radical behaviorist interpretations of complex human behavior describe precisely a picture in which many responses occur but go unnoticed by an external observer (e.g., Palmer, 1991) because they do so covertly (i.e., they are emitted at a strength too low to be measured by the current technology). More sensitive measurement tools will allow us to test those interpretations and to expand behavior-analytic research into domains claimed so far by groups of researchers who tend to share an essentialistic perspective on behavior.
RESPONSE STRENGTH
Neuroscientific research abounds with data that have not typically been interpreted within a behavior-analytic framework. When conducting a Skinnerian analysis of existent neuroscientific research, one promising tool is the concept of response strength. The concept is used in behavior analysis to describe multiple behavioral dimensions that tend to covary with one another (Killeen & Hall, 2001; Palmer, 2009). Palmer (2009) described response strength in the following way:
The shouting of the football fan at the game-saving goal will be immediate, loud, repetitive, persistent, and will crowd out any tendency to comment on the weather. That is to say, behavior tends to hang together in its latency, intensity, frequency, resistance to extinction, and tendency to occur in competition with other responses. These response measures can sometimes be differentiated by conflicting contingencies: … a high frequency, short latency response might be uttered at low intensity in the presence of a sleeping baby, a grouchy librarian, or a crowded restaurant, ill-bred cell phone users notwithstanding. However, the exceptions prove the rule: All other things being equal, the measures tend to vary together. (p. 49)
Palmer states that as the strength of a response decreases, it will become less likely that the response will be detected, at some point dropping below the threshold of observability and becoming a covert response. Donahoe and Palmer (1994; Palmer, 2009) also described experimental evidence that supports the idea that the strength of latent responses continuously changes as a function of ambient stimulation. Additional evidence for the interpretations given by Donahoe and Palmer comes from experiments that involve event-related potential (ERP) as a neuroscientific dependent variable. The early attempts presented herein to build a theoretical bridge between radical behaviorism and neuroscience, although tightly constrained by established behavior-analytic principles, should be taken as a first step in a promising direction.
EVENT-RELATED POTENTIALS
Within the vast neuroscientific literature, EEG plays an important role because it permits measurement of moment-to-moment changes in brain activity via electrodes placed on the scalp. Activity from a single neuron would be too small to be detected using scalp electrodes, but simultaneous activity of a large number of neurons (thousands to millions) can summate to a degree measurable at the scalp level (Luck, 2005). In addition to simultaneous activation, measurement of postsynaptic potentials requires that the neurons in question be aligned with each other. Each neuron constitutes a dipole with a specific orientation; many neurons with the same orientation will functionally become one dipole whose voltage affects scalp electrodes. If neurons in the same area are not oriented in the same direction, dipoles may cancel each other. Also, as the distance of the neurons from the scalp electrodes increases, it becomes less likely that their activity will be measured. Thus, an EEG generally measures well-aligned cortical pyramidal neurons, and changes in postsynaptic potentials from deep structures are less likely to be captured by scalp electrodes (Luck, 2005).
A specific kind of EEG technique called ERP measures brain activity that consistently follows or precedes presentation of stimuli. Although EEG is a continuous measure of brain activity, ERP involves only the portion of EEG that is time locked to a stimulus presentation or to the emission of a response. An ERP experimenter generally analyzes brain activity in the 2,000 ms following stimulation. From the continuous EEG, short sections of time-locked brain activity, called epochs, are extracted to form groups of epochs relative to the different experimental conditions. Trials relative to each experimental condition are usually averaged together. Averaging over many trials allows what is considered “noise” to be averaged out so that only brain activation that reliably follows a stimulus in a specific experimental condition is considered. The experimenter is typically interested in differences across conditions (i.e., differences in brain activation following stimuli that belong to different experimental conditions).
ERP data consist of a sequence of positive and negative peaks relative to an electrically neutral reference level (see Figures 1 and 2). ERP researchers label these peaks and the relative experimental effects they constitute (usually differences between peak amplitudes or mean amplitudes within a time window) according to the polarity (positive or negative) and timing of the peaks. For example, the P3 is typically the third positive peak following stimulus presentation. Sometimes the peak occurs consistently at a specific timing after stimulus onset, as in the case of the N400. In this case, N stands for negative peak, and 400 stands for its occurrence 400 ms after stimulus presentation.
Figure 1.
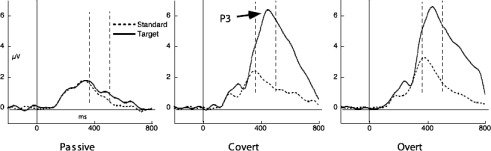
Event-related potentials showing differential brain activity to SDs (targets) and SΔs (standards) during a passive condition (in which participants were passively looking at the stimuli), a covert condition (in which participants were covertly counting SDs), and an overt condition (in which participants were pressing a button after SDs). Data were measured from electrode CP, a centro-parietal electrode located in the midline. Data reflect the grand average of 26 participants; for each participant there was a range of 40 to 80 trials in the SD condition and 160 to 320 trials in the SΔ condition. (Figure reproduced by permission of Elsevier, from Potts, 2004.)
Figure 2.
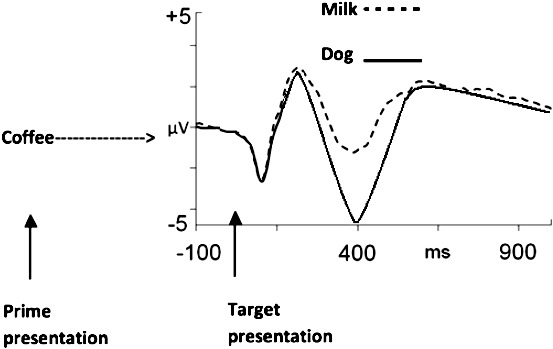
Example of an N400 effect elicited during a semantic priming procedure. The prime is presented followed by either a related or an unrelated target. Amplitude (measured in microvolts) of brain activity following a related target is more positive compared to an unrelated one. Similar results can be seen with ∼40 trials per condition, depending on how clean the initial EEG is.
ERPs can integrate well within a behavior-analytic paradigm because several components and effects can be seen and manipulated at the single-subject level. Although ERP data are generally averaged across participants, this form of averaging is not necessary, at least in the case of the P3 and the N400, to assess the presence of the effect. Given a clean EEG recording, both effects are large and can be seen clearly at the level of the single participant and the P3 at the level of a single trial (e.g., Jung et al., 2001; Karniski & Blair, 1989; Kutas & Hillyard, 1980). The effects on a single subject can be discerned visually, and those data can be analyzed without reference to a population.
Despite their promise, several important limitations of EEG and ERPs should be noted. First, although the temporal precision of ERP preparations is advantageous, the poor spatial resolution constitutes a disadvantage. It is not possible to calculate which parts of the brain are generating a specific electrical distribution over the participant's scalp. Determination of what parts of the brain are responsible for elicitation of ERP components is generally achieved through intracranial recordings and through simultaneous EEG/fMRI recordings. The inability to detect which parts of the brain are responsible for a specific ERP effect precludes mapping behavior-analytic functions onto brain structures. Thus, if variations in response strength originated uniquely from a specific brain structure, it would not be possible to detect this using ERP measures. Also, augmented ERP amplitude does not reflect an increase in brain activity, and diminished amplitude does not reflect a decrease in brain activity. What is considered to be meaningful is the detected difference in brain activity across experimental conditions. In an ERP experiment, if a difference across conditions is detected, it is possible to conclude that the brain is reacting differently across experimental conditions, but it is not possible to infer that the brain is more active in one condition than another. In spite of these limitations, EEG/ERP measurements constitute a promising tool to measure brain activation that is indicative of the presence of behavior, allowing detection of responses that are usually not captured by the conventional measurement tools used by behavior analysts.
THE P3 EFFECT: AN INDEX OF COVERT EMISSION?
The P3, a widely studied ERP effect (e.g., Polich & Kok, 1995), reflects brain activation in response to evocative stimuli relative to activation in response to nonevocative ones.1 A large positive peak occurs typically 300 to 400 ms after the discriminative stimulus (SD; usually called the target stimulus in the P3 literature) but not after the nondiscriminative stimulus (SΔ; called standard stimulus). The amplitude of the P3 component increases as the probability of the SD decreases (e.g., Duncan-Johnson & Donchin, 1977; Gonsalvez & Polich, 2002). In one experiment (Potts, 2004), participants were instructed to press a button when seeing a specific number on the screen (e.g., 2)—the SD—and not to press when seeing another number (e.g., 4)—the SΔ. A large positive peak, called the P3, was observed only following the evocative stimulus. It is important to note that this difference in brain activity following presentations of SDs and SΔs was preserved in a task that involved a covert response. In this latter case, participants were asked to count presentations of SDs but not SΔs silently. The same pattern of results was observed: The P3 peak followed the SD but not the SΔ. If no contingency was arranged by the experimenter and the participant was passively looking at the stimuli, the P3 effect was not observed (see Figure 1). These data suggest that the P3 ERP response is sensitive to stimulus control but do not demonstrate conclusively that what is measured is the counting response. Other variables that are correlated with the counting response could be the ones measured by the ERP effect. For instance, a component common to both overt and covert responses (e.g., an orienting response) could be the one detected by the electrophysiological measure (on the relation between the P3 and the orienting response, see Nieuwenhuis, de Geus, & Aston-Jones, 2011).
Other work has revealed that the P3 is sensitive to consequences (e.g., Miltner, Larbig, & Braun, 1986; Roger & Galand, 1981; Sommer, 1987; Sommer & Schweinberger, 1992). Sommer and Schweinberger (1992) delivered reinforcement contingent on P3 peaks with large amplitudes, but not to the peaks with small amplitudes, to see if amplitudes are sensitive to their consequences. In a yoked control condition, consequences were delivered randomly. Only in the condition in which reinforcement was contingent on large amplitudes did the amplitudes increase, thereby suggesting a reinforcement effect. Moreover, Miltner et al. (1986) reported that behavioral reaction times were significantly shorter in the condition in which large-amplitude P3 waves were reinforced compared to those in the condition in which small-amplitude P3 waves were reinforced. As the amplitude of the ERP response increased, reaction times decreased (see also Bahramali et al., 1998; Begleiter, Porjesz, Chou, & Aunon, 1983; Donchin & Lindsley, 1966; Gonsalvez & Polich, 2002; Holm, Rantaaho, Sallinen, Karjalainen, & Müller, 2006; cf. Gonsalvez, Barry, Rushby, & Polich, 2007).
If the P3 occurs as a function of discriminative stimuli (and reinforcers), then such procedures should yield similar results with nonhuman animals, assuming there is continuity across species in the way the central nervous system reacts to similar contingencies. Jodo, Takeuchi, and Kayama (1995) used a simple auditory discrimination and discovered that 400 to 500 ms after presentation of the SD, but not after the SΔ, a large positive peak morphologically similar to the human P3 appeared in rats. These results indicate consistency of the P3 effect across humans and nonhumans.
To summarize, the P3 effect is measured after evocative, but not after nonevocative, stimuli, suggesting it could be an index of both overt and covert responses. Measuring the P3 may be an effective way to lower what Palmer (2003) calls the “threshold of observability” of responses. In addition, the P3 peak amplitude has been shown to be sensitive to consequences; increases in amplitude have been correlated with shorter reaction times, implying that amplitude may constitute a measure of response strength. In fact, if response strength is defined as the tendency of measurements along different behavioral dimensions to covary, and if reaction times can be considered a measure of response strength, then the covariation between reaction times with the amplitude of the ERP suggests that both measures might be useful in assessing response strength. Moreover, the effect measured in humans is consistent with the effect measured in other species. Finally, although the P3 should be considered a promising tool for future behavior-analytic research, it is important to be cautious about what the P3 is really measuring, because the P3 may be measuring an orienting response or some other behavioral event that is correlated with the response.
SEMANTIC PRIMING AND RESPONSE-STRENGTH FLUCTUATIONS IN THE REPERTOIRE
Operants can be assumed to vary in strength as a function of the history of reinforcement and current stimulus control. Only when response strength is sufficiently high relative to competing behavior is the operant emitted, overtly or covertly (Palmer, 2009). The interpretation that operants vary in strength even when not emitted is supported by data from semantic priming experiments in which the dependent variables of interest are reaction times and brain activity measured by the ERP.
In a semantic priming procedure, two stimuli (usually words) are presented sequentially. When the first stimulus (called prime) is presented, a textual response is emitted (e.g., the participant reads the word coffee), and after a delay another word is presented (the target) that evokes another textual response (e.g., the participant reads the word milk). The experiment consists of contrasting behavioral and electrophysiological effects of related primes and targets (e.g., coffee and milk) to unrelated ones (e.g., coffee and dog). Using the taxonomy described by Skinner (1957), it can be said that the semantic priming procedure compares responses to words that are related intraverbally to words that are not related intraverbally. Typically, the participant is asked to perform a task after presentation of the target. One of the tasks widely employed is the pronunciation task, in which the participant reads aloud the target word. Reaction times to target textual responses in the related-word condition are consistently faster than ones in the unrelated-word condition; participants are quicker to read a word when it is preceded by a related one (Lupker, 1984; Neely, 1991; Schreuder, Flares d'Arcais, & Glazenborg, 1984; Seidenberg, Waters, Sanders, & Langer, 1984).
According to the behavior-analytic interpretation of semantic priming formulated by Donahoe and Palmer (1994; Palmer, 2009), the presentation of the prime (e.g., coffee) in the related-word condition simultaneously increases the response strength of many responses (e.g., sugar, cup, espresso, latte, etc.), including milk, which is eventually emitted textually when the target stimulus is presented. Conversely, the presentation of the word coffee is unlikely to increase the strength of the response dog in most individuals. Consistent with a response-strength interpretation of priming, the presentation of a prime that increases the strength of a specific response will decrease reaction times of the relative textual response. As discussed earlier, reaction time of responses is considered to be one measure of response strength. An important point is that the increase in strength, as measured by a decrease in reaction time, of the response milk following coffee is independent of the textual emission controlled by the target stimulus. Because any emitted response can prime (i.e., decrease reaction times to) a large number of responses, it is safe to assume that those responses are all primed at the same time (Palmer, 2009). Any emitted response alters the strength of a large number of other latent responses. Presenting the target stimulus is a convenient way to probe the strength of responses as a function of preceding stimulation.
In ERP experiments on semantic priming (e.g., Nobre & McCarthy, 1994), electrophysiological responses are measured at the onset of the target stimulus. Four hundred milliseconds after the presentation of the target stimulus, a negative peak is typically observed: the N400 peak (see Figure 2). N400 peaks following related targets that evoked responses strengthened by the prime (e.g., coffee strengthens milk) are more positive than ones that had not been strengthened (coffee does not strengthen dog). The difference in amplitude is called the N400 effect (see Figure 2) and is typically measured between 300 and 500 ms poststimulus. As mentioned earlier, reaction times to an overt task that follows the related target are faster than the ones that follow the unrelated target. In other words, the relative positive amplitude of the ERP is correlated with shorter reaction times in a manner similar to the P3 results discussed earlier (Miltner et al., 1986).
The N400 effect is also observed using mathematical operations. For example, the stimulus 7 × 4 can be assumed to strengthen the response 28, and the N400 peak following 28 is more positive than the one following 26 (Niedeggen & Rösler, 1999). The effect is also present 400 ms after the final word in a sentence with an anomalous ending of presumably low response strength such as “I drink my coffee with cream and dog” (Kutas & Hillyard, 1980), compared to a sentence with a high response strength ending such as “I drink my coffee with cream and sugar.” Presentation of each word in the sentence progressively increases the strength of the response sugar, and the N400 effect indexes the difference between the emission of a response that had been strengthened by the preceding words (sugar) and the emission of a response that had not been strengthened (dog). The N400 peak after dog will therefore be more negative than the peak after sugar. Interestingly, one presentation of the sentence with the low response-strength ending is enough to strengthen the response in a second presentation, as shown by attenuated N400 effects. Repeated presentations of a sentence with an anomalous ending yield progressively more positive ERPs to the final word (Besson & Kutas, 1993; Besson, Kutas, & Van Petten, 1992). By implication, temporally contiguous textual emissions modify the strength of the intraverbal relations that occur among words within the sentence. If in an individual's experience the words A and B have never been presented sequentially, presentation of A should not increase the strength of B. But when presentation of A has often been followed by B, presentation of A will increase the strength of B (i.e., the strength of the A–B intraverbal relation has increased).
The apparent sensitivity of the N400 effect in indexing moment-to-moment fluctuations in strength of latent behavior makes it a potentially useful tool in investigating experimental questions that involve verbal behavior. For example, research has shown that variation in strength of responses is not constrained by conventional definitions of units of analysis in language, and other experiments have shown that the verbal community to which an individual belongs determines how that person will respond to a contingency.
An experiment conducted by Nieuwland and Van Berkum (2006) suggests sensitivity of the N400 effect to extended verbal stimuli that do not conform to the sentence as classically defined by linguists. For example, the ending of the sentence “The peanut was salted” can be made to have low response strength given appropriate preceding stimulation. At the same time, in the sentence “The peanut was in love,” the phrase “in love” can be brought to have high response strength after “the peanut was.” Consider the following story:
A woman saw a dancing peanut who had a big smile on his face. The peanut was singing about a girl he had just met. And judging from the song, the peanut was totally crazy about her. The woman thought it was really cute to see the peanut singing and dancing like that. The peanut was salted/in love, and by the sound of it, this was definitively mutual. He was seeing a little almond. (Nieuwland & Van Berkum, 2006, p. 1106)
ERPs measured while experimental participants were listening to “the peanut was salted” and “the peanut was in love” in the above short story yielded an N400 effect with a more positive peak measured after “in love” compared to the one measured after “salted”; that is, the response strength of “in love,” given the preceding stimulation, was higher than that of “salted.” The sentence in itself , “The peanut was salted” or “The peanut was in love,” is not sufficient to determine the strength of the ending response if there is a preceding context that itself has altered the response strength of a large number of responses. The sentence cannot be isolated from the context just because it constitutes a structural unit.
The N400 effect has also been used to illustrate how effects of stimulation on the repertoire are directly dependent on the verbal community to which the individual belongs. Among Dutch people it is well known that trains in Holland are yellow. An experiment with Dutch participants by Hagoort, Hald, Bastiaansen, and Peterson (2004) measured the ERP during the presentation of the following sentences:
The Dutch trains are yellow and very crowded.
The Dutch trains are white and very crowded.
The Dutch trains are sour and very crowded.
Results indicated that yellow elicited a more positive N400 peak, indicating higher response strength, than white and sour, whose relatively negative N400s indicated lower response strength. These results underscore the role of individual experience by showing that rapid changes in the repertoire are dependent on the degree to which the external stimulation is consistent with one's learning history. Moreover these findings suggest that changes in response strength in the repertoire are partly a function of the specific verbal community to which the individual belongs.
To summarize, the N400 effect may reflect fluctuations of response strength of latent responses as a function of differential stimulus control. In a semantic priming experiment, the effects of the priming stimuli might be viewed as the differential strengthening of responses; presentation of targets allows the experimenter to measure the electrophysiological contrast between responses that have been strengthened and responses that have not been strengthened, but changes in strength following the priming stimuli are independent of target presentation. This proposed electrophysiological measure of response strength correlates with overt task reaction-time measures. Specifically, N400 peaks following responses that have been strengthened are more positive than ones that have not been strengthened, and (consistent with the P3 experiments described earlier) more positive peaks are correlated with shorter behavioral reaction times.
Unlike the P3 effect, the N400 effect has not been investigated in the context of a selectionist paradigm that involves operant procedures, nor has it been investigated in nonhuman animals (with the possible exception of Pineda & Nava, 1993). The lack of nonhuman research seems to be due to the dominant interpretation of the N400 effect. Probably because it was discovered in a series of experiments involving linguistic stimuli (Kutas & Hillyard, 1980), the N400 effect is generally considered to be a language-specific ERP component (Kutas & Van Petten, 1988) reflecting the role of semantic memory.2 Because language is often considered to be something typically human, research on other species has not been initiated, and operant procedures have not been employed to investigate, among other things, sensitivity of the N400 to consequences. Behavior analysts are in the perfect position to study this effect within a paradigm consistent with selectionist principles.
IS AMPLITUDE OF THE ERP A MEASURE OF RESPONSE STRENGTH?
ERPs appear to be a useful tool in indexing the emission of responses below the threshold of observability (P3) and the variation in response strength below the threshold of emission (N400). As discussed earlier, in both cases relatively positive peaks in ERP amplitudes between 300 and 800 ms poststimulus are correlated with shorter reaction times, leading to the inference that amplitude of ERPs may be considered to be a measure of response strength. Another ERP effect, named the old-new repetition effect, shows a similar pattern.
The old-new repetition effect occurs between 300 and 800 ms poststimulus and is obtained by comparing brain activity following the first presentation of a stimulus to activity following the second presentation, usually with other items presented in the interim (Rugg, 1985; Rugg & Doyle, 1994; Rugg & Nagy, 1987). ERPs following the second presentation are more positive than those following the first presentation, and there is a decrease in task reaction times between the first and second presentations of the stimulus (Rugg, 1985). The effect might index an increase in response strength due to repetition. Interestingly, repetition effects were found with words (Rugg, 1985) and with pronounceable nonwords such as blint or fict but were not found with unpronounceable nonwords such as tjrkde (Rugg & Nagy, 1987). These results suggest that the stimulus has to evoke a textual response in order to show a repetition effect, presumably because the response must occur in order to increase in strength. These results are consistent with the interpretation presented herein regarding the P3 and N400 results in relation to the concept of response strength. It is plausible that the first presentation of a stimulus increases the strength of the corresponding textual response in the behavioral repertoire; this increase may be indexed by decreased reaction times and a relative ERP positivity following the second presentation of the stimulus.
The consistency of the three ERP effects discussed here suggests that amplitude of ERPs, correlated with reaction times, may constitute an index of response strength. This hypothesis could be evaluated by experiments that employ a wider range of dependent variables such as rate and intensity of responses, together with the variables described in this paper. One final consideration about the relations among the P3, the N400, and the old-new effect is that if the P3 indexes generic emission of a response, then it should be seen also in the N400 and old-new effects (in which textual emission occurs). Indeed, a positive peak often follows the N400 (see, e.g., Curran, Tucker, Kutas, & Posner, 1993), and the old-new effect may just be a modulation of the P3 due to stimulus repetition (Friedman, 1990).
CONCLUSION
The measurement of behavioral dependent variables that have until now been below the threshold of observability has many potential applications. Interpretations of complex human behavior would benefit greatly from an experimental analysis of behavior that could, for example, include covert speech. At present it may not be possible to accurately measure the specific topography of the covert response: If the interpretations provided in this paper are correct, we may be able to index a lever press and its intensity (as a P3 with its specific amplitude), but we would not be able to tell if it involved a finger, a toe, or a nose. Yet as measurement tools improve, topography of covert responses may become clearer, and the P3 may play an important role in interpreting such phenomena in the future. Moreover, if it is true that the P3 is indicative of the emission of a response, regardless of whether the response is overt or covert by traditional standards, then it constitutes one more piece of evidence in favor of the radical behaviorist interpretation of behavior within the skin sketched by Skinner (1953, 1957).
Palmer (2009) elaborated on Skinner's interpretations by developing the concept of the behavioral repertoire in which the strength of both emitted and nonemitted operants continuously fluctuates as a function of ambient stimulation. As discussed in this paper, this idea is supported by N400 data that clearly parallel the typical reaction-time results of a classical semantic priming paradigm. Together with the P3 and N400 results, the old-new repetition effect supports the interpretation that, in general, more positive peaks and amplitudes in the ERP correlate with shorter reaction times. Therefore, brain activity as measured by the ERP could potentially index response strength. These interpretations need to be validated and expanded in experimental designs and methods that put more emphasis on the single subject and, as much as possible given the current sensitivity of our measurement tools, on single trials.
Radical behaviorism, and specifically the concept of response strength, has provided a useful framework for interpreting the specific subset of neuroscientific data described in this paper. The experimental effects, typically considered separately in the neurosciences, were discussed together here in light of radical behaviorist descriptions of the concept of response strength in the behavior-analytic literature. However, the benefits are bidirectional: Not only does radical behaviorism facilitate interpretation of neuroscientific data, but neuroscientific data also provide additional evidence that radical behavioral interpretations of human behavior are valid. Most important, within a radical behaviorist framework, neuroscientific data can indicate the occurrence of behavior as long as the neuroscientific dependent variable of interest enters into a meaningful functional relation with the other terms of the three-term contingency.
As tentative as the interpretations given in this paper are, they constitute a starting point from which behavior analysts can explore covert behavior and how it relates to complex human behavior in future experiments. For instance, if the P3 indexes emission of a response, it could be useful to assess if the listener behaves verbally by emitting covert echoics (Schlinger, 2008) and if the mnemonic behavior of remembering constitutes a form of problem solving (Palmer, 1991). In general, selectionist ideas that constitute the philosophical foundations of behavior analysis and shape its methods are likely to aid investigation of the adaptive nature of neuroscientific dependent variables, just as they did for traditional, overt variables. The experimental approach derived from the study of environment–behavior relations changing over time, and the emphasis on single-subject data, are likely to yield more interpretable results than the approach that currently dominates neuroscience, which employs inferential statistics as a tool to infer phylogenetically selected structures. Behavior analysts have indeed started to explore neuroscientific dependent variables (Barnes-Holmes et al., 2005; Haimson, Wilkinson, Rosenquist, Ouimet, & McIlvane, 2009; Schlund & Ortu, 2010; Schlund, Rosales-Ruiz,Vaidya, Glenn, & Staff, 2008). As behavior-analytic methods are employed to investigate a range of variables that have been out of reach until recently, it is entirely possible that the line of demarcation between behavior analysis and neuroscience will become fuzzy and then entirely disappear.
Acknowledgments
This research was funded by SINAPSE (www.sinapse.ac.uk).
Footnotes
The P3 effect is typically described in terms of context updating and resource allocation: “After initial sensory processing, an attention-driven comparison process evaluates the representation of the previous event in working memory. … If no stimulus attribute change is detected, the current mental model or ‘schema’ of the stimulus context is maintained. … If a new stimulus is detected, attentional processes govern a change or ‘updating’ of the stimulus representation that is concomitant with P300. … Discriminating the target from the standard stimulus produces a robust P300 that increases in amplitude as the target's global and local sequence probability decreases. … Target stimulus probability effects served as the basis for the suggestion that P300 originates from task conditions involving working memory … and that conscious awareness may be related to stimulus sequence effects.” (Polich, 2007, pp. 2129–2130)
“The N400's broad sensitivity to meaningful stimuli and semantic manipulations meant that it could be used to ask questions about how meaning-related information is stored in the brain in what is often called semantic memory” (Kutas & Federmeier, 2011, p. 627).
REFERENCES
- Bahramali H, Gordon E, Li W.M, Rennie C, Wright J, Meares R. Fast and slow reaction times and associated ERPs in patients with schizophrenia and controls. International Journal of Neuroscience. 1998;95:155–165. doi: 10.3109/00207459809003337. [DOI] [PubMed] [Google Scholar]
- Barnes-Holmes D, Staunton C, Whelan R, Barnes-Holmes Y, Commins S, Walsh D, et al. Derived stimulus relations, semantic priming, and event-related potentials: Testing a behavioral theory of semantic networks. Journal of the Experimental Analysis of Behavior. 2005;84:417–433. doi: 10.1901/jeab.2005.78-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto A.B, Scargle S.D, Adjouadi M. A practical EMG-based human–computer interface for users with motor disabilities. Journal of Rehabilitation Research and Development. 2000;37:53–63. [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Chou C.L, Aunon J.I. P3 and stimulus incentive values. Psychophysiology. 1983;20:95–101. doi: 10.1111/j.1469-8986.1983.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Besson M, Kutas M. The many facets of repetition: A cued-recall and event-related potential analysis of repeating words in same versus different sentence contexts. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:1115–1133. doi: 10.1037//0278-7393.19.5.1115. [DOI] [PubMed] [Google Scholar]
- Besson M, Kutas M, Van Petten C. An event-related potential (ERP) analysis of semantic congruity and repetition effects in sentences. Journal of Cognitive Neuroscience. 1992;4:132–149. doi: 10.1162/jocn.1992.4.2.132. [DOI] [PubMed] [Google Scholar]
- Birbaumer N. Slow cortical potentials: Plasticity, operant control, and behavioral effects. Neuroscientist. 1999;5:74–78. [Google Scholar]
- Birbaumer N, Kubler A, Ghanayim N, Hinterberger T, Perelmouter J, Kaiser J, et al. The thought translation device (TTD) for completely paralyzed patients. Rehabilitation Engineering, IEEE Transactions. 2000;8:190–193. doi: 10.1109/86.847812. [DOI] [PubMed] [Google Scholar]
- Curran T, Tucker D.M, Kutas M, Posner M.I. Topography of the N400: Brain electrical activity reflecting semantic expectation. Electroencephalography and Clinical Neurophysiology. 1993;88:188–209. doi: 10.1016/0168-5597(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Donahoe J.W, Palmer D.C. Learning and complex behavior. 1994. Allyn and Bacon. [Google Scholar]
- Donchin E, Lindsley D.B. Average evoked potentials and reaction times to visual stimuli. Electroencephalography and Clinical Neurophysiology. 1966;20:217–223. doi: 10.1016/0013-4694(66)90086-1. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson C.C, Donchin E. On quantifying surprise: The variation of event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Friedman D. ERPs during continuous recognition memory for words. Biological Psychology. 1990;30:61–87. doi: 10.1016/0301-0511(90)90091-a. [DOI] [PubMed] [Google Scholar]
- Gonsalvez C.J, Barry R.J, Rushby J.A, Polich J. Target-to-target interval, intensity, and P300 from an auditory single-stimulus task. Psychophysiology. 2007;44:245–250. doi: 10.1111/j.1469-8986.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- Gonsalvez C.J, Polich J. P300 amplitude is determined by target-to-target interval. Psychophysiology. 2002;39:388–396. doi: 10.1017/s0048577201393137. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Hald L, Bastiaansen M, Peterson K.M. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304:438–441. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- Haimson B, Wilkinson K.M, Rosenquist C, Ouimet C, McIlvane W.J. Electrophysiological correlates of stimulus equivalence processes. Journal of the Experimental Analysis of Behavior. 2009;92:245–256. doi: 10.1901/jeab.2009.92-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm A, Rantaaho P.O, Sallinen M, Karjalainen P.A, Müller K. Relationship of P300 single-trial responses with reaction time and preceding stimulus sequence. International Journal of Psychophysiology. 2006;61:244–252. doi: 10.1016/j.ijpsycho.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Huang C.N, Chen C.H, Chung H.Y. Application of facial electromyography in computer mouse access for people with disabilities. Disability and Rehabilitation. 2006;19:231–237. doi: 10.1080/09638280500158349. [DOI] [PubMed] [Google Scholar]
- Jodo E, Takeuchi S, Kayama Y. P3b-like potential of rats recorded in an active discrimination task. Electroencephalography and Clinical Neurophysiology. 1995;96:555–560. doi: 10.1016/0013-4694(95)00067-9. [DOI] [PubMed] [Google Scholar]
- Jung T.P, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski T.J. Analysis and visualization of single-trial event-related potentials. Human Brain Mapping. 2001;14:166–185. doi: 10.1002/hbm.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniski W, Blair R.C. Topographical and temporal stability of the P300. Electroencephalography and Clinical Neurophysiology. 1989;72:373–383. doi: 10.1016/0013-4694(89)90043-6. [DOI] [PubMed] [Google Scholar]
- Killeen P.R, Hall S.S. The principal components of response strength. Journal of the Experimental Analysis of Behavior. 2001;75:111–134. doi: 10.1901/jeab.2001.75-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier K.D. Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annual Review of Psychology. 2011;62:621–627. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard S.A. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C. Event-related brain potential studies of language. In: Ackles P.K, Jennings J.R, Colcs M.G.H, editors. Advances in psychophysiology. Greenwich, CT: JAI Press; 1988. pp. 139–187. (Eds.) [Google Scholar]
- Luck S.J. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Lupker S.J. Semantic priming without association: A second look. Journal of Verbal Learning and Verbal Behavior. 1984;23:709–733. [Google Scholar]
- Miltner W, Larbig W, Braun C. Biofeedback of visual evoked potentials. International Journal of Neuroscience. 1986;29:291–303. doi: 10.3109/00207458608986158. [DOI] [PubMed] [Google Scholar]
- Neely J. Semantic priming effects in visual word recognition: A selective review of current findings and theories. In: Besner D, Humphreys G, editors. Basic processes in reading: Visual word recognition. Hillsdale, NJ: Erlbaum; 1991. pp. 264–336. (Eds.) [Google Scholar]
- Niedeggen M, Rösler F. N400 effects reflect activation spread during retrieval of arithmetic facts. Psychological Science. 1999;10:271–276. [Google Scholar]
- Nieuwenhuis S, de Geus E.J, Aston-Jones G. The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology. 2011;48:162–175. doi: 10.1111/j.1469-8986.2010.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland M.S, Van Berkum J.J.A. When peanuts fall in love: N400 evidence for the power of discourse. Journal of Cognitive Neuroscience. 2006;18:1098–1111. doi: 10.1162/jocn.2006.18.7.1098. [DOI] [PubMed] [Google Scholar]
- Nobre A.C, McCarthy G. Language-related ERPs: Scalp distributions and modulation by word type and semantic priming. Journal of Cognitive Neuroscience. 1994;6:233–255. doi: 10.1162/jocn.1994.6.3.233. [DOI] [PubMed] [Google Scholar]
- Palmer D.C. A behavioral interpretation of memory. In: Hayes L.J, Chase P.N, editors. Dialogues on verbal behavior. Reno, NV: Context Press; 1991. pp. 261–279. (Eds.) [Google Scholar]
- Palmer D.C. Cognition. In: Lattal K.A, Chase P.N, editors. Behavior theory and philosophy. New York: Kluwer Academic/Plenum; 2003. pp. 167–185. (Eds.) [Google Scholar]
- Palmer D.C. Response strength and the concept of the repertoire. European Journal of Behavior Analysis. 2009;10:49–60. [Google Scholar]
- Pineda J.A, Nava C. Event-related potentials in macaque monkey during passive and attentional processing of faces in a priming paradigm. Behavioral Brain Research. 1993;53:177–187. doi: 10.1016/s0166-4328(05)80277-3. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: An integrative review. Biological Psychology. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Potts G.F. An ERP index of task relevance evaluation of visual stimuli. Brain and Cognition. 2004;56:5–31. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Roger M, Galand G. Operant conditioning of visual evoked potentials in man. Psychophysiology. 1981;18:477–482. doi: 10.1111/j.1469-8986.1981.tb02485.x. [DOI] [PubMed] [Google Scholar]
- Rugg M.D. The effects of semantic priming and word repetition on event-related potentials. Psychophysiology. 1985;22:642–647. doi: 10.1111/j.1469-8986.1985.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Rugg M.D, Doyle M.C. Event-related potentials and stimulus repetition in direct and indirect tests of memory. In: Heinze H.J, Munte T, Mangun G.R, editors. Cognitive electrophysiology. Boston: Birkhauser; 1994. pp. 124–148. (Eds.) [Google Scholar]
- Rugg M.D, Nagy M.E. Lexical contribution to nonword-repetition effects: Evidence from event-related potentials. Memory & Cognition. 1987;15:473–481. doi: 10.3758/bf03198381. [DOI] [PubMed] [Google Scholar]
- Schlinger H.D. Listening is behaving verbally. The Behavior Analyst. 2008;31:145–161. doi: 10.1007/BF03392168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund M.W, Ortu D. Experience-dependent changes in human brain activation during contingency learning. Neuroscience. 2010;165:151–158. doi: 10.1016/j.neuroscience.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Schlund M.W, Rosales-Ruiz J, Vaidya M, Glenn S.S, Staff D. Experience-dependent plasticity: Differential changes in activation associated with repeated reinforcement. Neuroscience. 2008;155:17–23. doi: 10.1016/j.neuroscience.2008.04.076. [DOI] [PubMed] [Google Scholar]
- Schreuder R, Flares d'Arcais G.B, Glazenborg G. Effects of perceptual and conceptual similarity in semantic priming. Psychological Research. 1984;45:339–354. [Google Scholar]
- Seidenberg M, Waters G, Sanders M, Langer P. Pre- and postlexical loci of contextual effects on word recognition. Memory and Cognition. 1984;12:315–327. doi: 10.3758/bf03198291. [DOI] [PubMed] [Google Scholar]
- Skinner B.F. The operational analysis of psychological terms. Psychological Review. 1945;52:270–277. [Google Scholar]
- Skinner B.F. Science and human behavior. New York: Macmillan; 1953. [Google Scholar]
- Skinner B.F. Verbal behavior. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Sommer W. Operant conditioning of P300. Psychophysiology. 1987;24:612. [Google Scholar]
- Sommer W, Schweinberger S. Operant conditioning of P300. Biological Psychology. 1992;33:37–49. doi: 10.1016/0301-0511(92)90004-e. [DOI] [PubMed] [Google Scholar]


