Abstract
Several recent studies have explored what Michael (e.g., 1982) termed the value-altering effect and the behavior-altering effect of motivating operations. One aspect of the behavior-altering effect that has garnered no recent attention involves changes in stimulus control produced by motivating operations. To call attention to this aspect of the behavior-altering effect, we herein review 11 studies that are concerned with the influence of varying levels of food or water deprivation on stimulus generalization. These studies suggest that motivating operations influence stimulus control (a) by changing the evocative strength of not just an established discriminative stimulus, but also of stimuli that are physically similar to it; (b) by changing the range of stimuli that evoke the operant in question; and (c) by exerting these effects in a graded fashion. These findings are potentially of conceptual and applied significance, and it appears that further research examining how motivating operations alter stimulus control, including some studies suggested herein, is warranted.
Keywords: motivation, motivating operations, stimulus control, stimulus generalization, deprivation
Although Skinner recognized the importance of variables that affect what is commonly termed motivation (e.g., Skinner, 1938, 1953, 1957), relatively little attention was paid to such variables until Michael (1982, 1993) provided an insightful analysis of what he initially termed establishing operations (EOs) and later called motivating operations (MOs; Laraway, Snycerski, Michael, & Poling, 2003). Michael distinguished subtypes of conditioned and unconditioned MOs and discussed how they are established, but he did not provide detailed coverage of the effects of these variables, except to differentiate between value-altering and behavior-altering effects.
Laraway et al. (2003) pointed out that an aspect of the behavior-altering effect is a change in the evocative strength of relevant discriminative stimuli (SDs). Specifically, the probability that such stimuli will evoke operant responses that historically produced a particular kind of reinforcer increases when an EO for that kind of reinforcer is present and decreases when an abolishing operation (AO) for that kind of reinforcer is present. Skinner used response rate as a measure of response probability; hence, the general notion is that rate of responding in the presence of a given SD should vary reliably with changes in MOs. Of course, through the process of generalization, untrained stimuli similar to an established SD also evoke responding; it stands to reason that rate of responding to untrained stimuli (i.e., generalization gradients) changes systematically as a function of motivation. To obtain a generalization gradient, responding typically is established in the presence of a particular SD and then the level of responding is determined during brief periods of extinction during which a spectrum of stimuli physically similar to the SD are presented (e.g., Guttman & Kalish, 1956). Increasing motivation may increase the range of stimuli to which responding generalizes or increase the relative, as well as the absolute, rate of responding across a range of untrained test stimuli. The former effect would be evident if the breadth of the generalization gradient increases with increasing motivation, and the latter would be evident if the generalization gradient grows flatter with increasing motivation.
With respect to conceptual analyses, it is perhaps worth noting that, if altering an MO changes the level, but not the shape, of the generalization gradient, the behavioral effect of the MO could be construed as a simple direct effect on rate of responding. If, however, the shape as well as the level of the generalization change, the behavioral effect could be construed as being due to a joint effect of the MO on rate of responding and on stimulus control. In the former case, the MO and stimuli physically similar to the SD would both affect the operant of interest, but their effects would not interact. In the latter case, the SD and the MO would interact. Such an interaction would constitute an interesting behavioral phenomenon of potential applied significance. Although Laraway et al. (2003) proposed that such an interaction would regularly occur, to our knowledge no review of the relevant literature has appeared. The purpose of the present article is to provide one.
SELECTION AND CATEGORIZATION OF ARTICLES
An initial literature review was conducted using the Scopus and PsycINFO databases. The search terms generalization, discrimination, and discriminative were each paired with the terms devaluation, body weight, prefeeding, satiation, satiated, deprivation, deprived, hunger, drive, and motivation to yield 30 combinations. English-language articles that contained these combinations in the title or abstracts were selected, and abstracts of these articles were examined to determine whether the results of generalization tests in which stimuli that varied along a specified dimension from an established exteroceptive discriminative stimulus were conducted at different deprivation levels. To facilitate comparison across studies, only studies in which food or water reinforcement was used were considered. A different person evaluated articles located by each of the databases and made a list of the articles he found appropriate for inclusion. All four authors examined those lists individually and as a collective; when appropriate, we perused abstracts and complete articles, then reached a consensus regarding the articles appropriate for inclusion. A total of 1,773 and 2,504 articles from the Scopus and PsycINFO databases were evaluated. Ten of these articles, each located by both databases, met our criteria for inclusion in the review.
LITERATURE REVIEW
Table 1 summarizes the 11 studies reported in the 10 articles with respect to the kind and number of subjects used, the MOs manipulated, the dependent variables measured, and the effects of manipulating the MOs on the dependent variables. These studies are organized and reviewed as (a) studies that altered duration of food or water deprivation, (b) studies that maintained animals at varying percentages of their free-feeding weights, and (c) studies in categories (a) or (b) that used drug-discrimination procedures.
Table 1.
Summary of All Studies
Duration of Food or Water Deprivation as an MO
In an early study, Brown (1942) compared the effects of 46 hr and 1 hr of food deprivation on generalization of the approach response in 36 rats. Rats were placed on one end of a runway opposite a light where they had previously obtained food. Prediscrimination generalization testing1 was conducted using three different light intensities, the darkest or lightest being the training stimulus. The dependent variables of interest were (a) pull force (in grams) on a harness which restrained the rats for 5 s halfway down the runway, and (b) starting time (time taken to reach the point on the runway at which the pull force apparatus restricted forward movement). The two levels of deprivation resulted in parallel reductions in mean pull strength as the test stimuli departed from the training stimulus. Mean starting time under the lower level of deprivation increased much more rapidly than under the higher level of deprivation as the test stimuli departed from the training stimulus, resulting in a flatter generalization gradient for the 46-hr deprivation (high-deprivation) group. This outcome, however, may have been due to a limit on the speed with which the subjects could reach the midpoint of the runway (i.e., a floor effect). Regarding the effect of the level of deprivation on the range of stimuli with an evocative function, given that the higher deprivation level produced considerably faster start times and greater pull strength in the presence of the stimulus most dissimilar to the training stimulus, it is reasonable to assume that the range of stimuli with an evocative function would be broader under the higher level of deprivation. That is, if the generalization gradients were extended to untested values, it appears that they would reach zero, or a minimum nonzero level, faster when the deprivation level was lower.
Newman and Grice (1965) compared the effects of 48 hr and 12 hr of food deprivation on generalization in 120 rats. During training, rats obtained food at the end of a 24-in. alley by nosing open a hinged panel in the center of a white disk. During prediscrimination generalization testing, the original disk and three progressively smaller disks served as test stimuli. The dependent variables were (a) number of times the panel was pushed, a measure of resistance to extinction, and (b) speed of the first panel push (1,000 divided by latency). In contrast to Brown's (1942) results, as the test stimuli departed from the training stimulus, both response measures dropped more rapidly for the rats in the 48-hr deprivation group, resulting in a steeper generalization gradient for the higher level of deprivation. However, both response measures for the low-deprivation group approached zero at the extreme end of the range of stimuli (i.e., the smallest disk), whereas the values for the higher deprivation group were relatively high, indicating that the range of stimuli that would evoke responding may have been broader for the high-deprivation group.
Healey (1965) examined the effects of 12 hr and 23 hr of food deprivation on generalization in 48 rats. Rats were trained to obtain food by running to the end of a 24-in. runway and nosing open a hinged panel in the center of a 79-cm2 white square. All training sessions were conducted in the presence of a 400-Hz tone. Four groups of 12 rats were tested in the presence of both the 400-Hz tone and a 200-Hz tone. Half of the rats were tested with a 32-cm2 (small) white square instead of the 79-cm2 (large) square. The group conditions were as follows: (a) small square and 12-hr food deprivation, (b) small square and 23-hr deprivation, (c) large square and 12-hr deprivation, and (d) large square and 23-hr deprivation. Running speed was higher in the presence of the training stimuli under the higher level of deprivation and lower in the presence of the stimuli that were not present during training under the lower level of deprivation. Because behavior was controlled by a compound SD (400-Hz tone and 79-cm2 square) in this study and test stimuli were varied along both dimensions, results cannot be adequately represented by a conventional generalization gradient. They are nonetheless interesting in demonstrating that the effects of level of motivation on stimulus control extend to situations that involve compound SDs.
Coate (1964) compared the effects of 5, 12, 40, and 48 hr of water deprivation on generalized responding in four groups of eight rats. In training, lever pressing was maintained under a variable-interval (VI) 1.5-min schedule of reinforcement in the presence of a pair of lights separated by either 0.5 in. or 5.5 in. and extinguished in the presence of the opposite separation value. During postdiscrimination generalization testing, the original stimulus and four other pairs of lights separated by progressively larger or smaller intervals were presented. The dependent variable was the number of lever presses in the presence of each separation interval (excluding the SΔ). Coate compared the generalization gradients obtained from the mean number of lever presses for each level of deprivation and found steeper gradients for the higher levels of deprivation. He then compared the generalization gradients obtained from the number of responses in the presence of each stimulus relative the total number of responses. In general, with both absolute and relative response measures, level of responding was directly related to level of deprivation. However, at some values, the 40-hr deprivation group responded more than the 48-hr deprivation group. Visual analysis of the generalization gradients for both the absolute and relative number of responses suggests that the range of stimuli that would have evoked responding when rats were deprived of water for relatively long periods would have been broader than when they were deprived for relatively short periods.
Percentage of Free-Feeding Weight as an MO
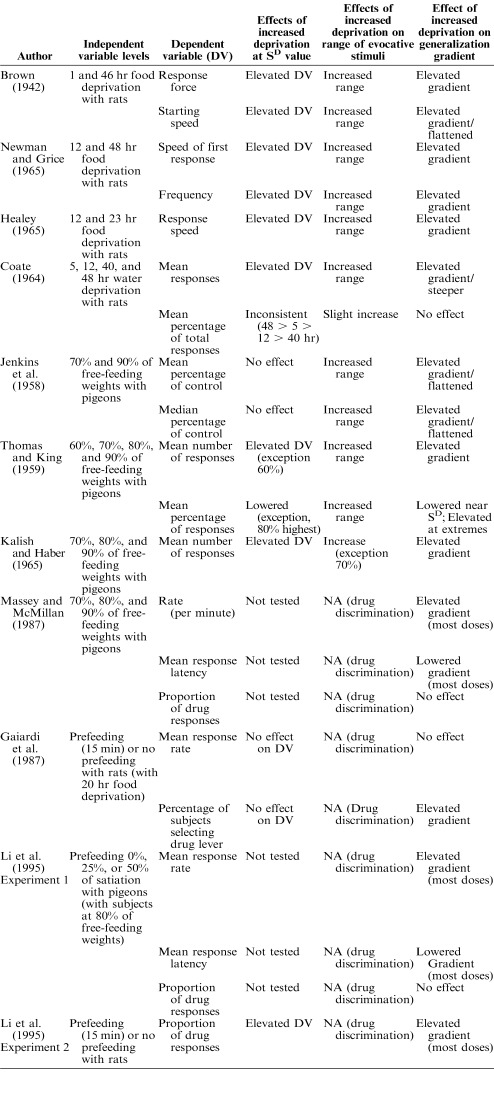
Jenkins, Pascal, and Walker (1958), in Experiment 2, assigned pigeons to groups maintained at 70% and 90% of their free-feeding weights and trained the pigeons to key peck for food in the presence of an illuminated spot (1.4-cm diameter; the SD). Prediscrimination generalization tests occurred across a range of six test stimuli (0.2-, 0.6-, 1.0-, 1.8-, 2.2-, and 2.6-cm spots). The dependent variables in the study were mean and median percentage of responses during testing emitted in the presence of each stimulus relative to the number of responses emitted in the presence of the SD prior to each test session. An equal percentage of responses occurred to the SD for both groups (Figure 1). As test stimuli departed from the training stimulus, mean and median percentage of responses decreased progressively but were relatively higher under every test stimulus value when the subjects were more food deprived. As test stimuli moved away from the SD, the reduction of responding was more rapid for the low-deprivation group than for the high-deprivation group. It is noteworthy that the smallest test stimulus (0.2 cm) engendered substantial responding in the birds deprived to 70% of their free-feeding weights, but not in the birds deprived to 90%.
Figure 1.
Median percentage of responses at various test stimulus sizes as a function of deprivation level. In this study, pigeons were trained to respond to a 1.4-cm spot and were tested with the other untrained sizes when maintained at 70% (high deprivation) and 90% (low deprivation) of free-feeding weights. This figure is based on data reported by Jenkins et al. (1958, Figure 1).
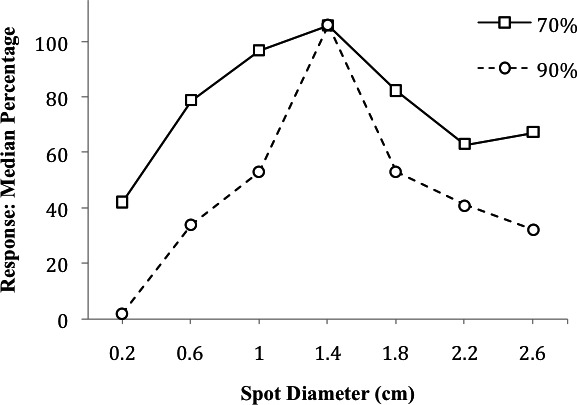
Thomas and King (1959; Experiment 1) used a procedure similar to that of Jenkins et al. (1958). During training, all of their pigeons were maintained at 80% of free-feeding weights and trained to peck a 550-nm light under a VI 1-min schedule of food delivery. During testing, four separate groups were maintained at 60%, 70%, 80%, or 90% of free-feeding weights. Prediscrimination generalization tests were conducted with 11 test stimuli (light illumination between 490 and 610 nm). Dependent variables were mean number of responses, mean responses per minute, and mean percentage of total responses at each test stimulus. At the boundary values of test stimuli, low-deprivation groups were at near-zero levels of responding, whereas high-deprivation groups were at substantially higher levels (Figure 2). Interestingly, the group deprived to 60% of free-feeding weights did not have the highest level of responding to the SD. This level of deprivation is extreme and may have limited the speed at which the birds could respond.
Figure 2.
Mean responses at each test stimulus value as a function of deprivation level. In this study, pigeons maintained and tested at 60%, 70%, 80%, and 90% of free-feeding weights were trained with a 550-nm SD and tested with the other listed values. This figure is based on data reported by Thomas and King (1959, Figure 2).
Kalish and Haber (1965, Phase 1) obtained results similar to those of Jenkins et al. (1958) and Thomas and King (1959) when groups of pigeons were maintained and tested at 70%, 80%, or 90% of their free-feeding weights. Prediscrimination generalization tests were carried out across six color stimuli (wavelengths from 490 to 550 nm). The training wavelength was 550 nm. The primary dependent variable was mean number of responses emitted to each stimulus under extinction conditions. The group maintained at 70% of free-feeding weight responded more to all stimuli that engendered responding than the group maintained at 80%, which in turn responded more than the 90% group. Although level of responding was directly related to level of deprivation observed in the presence of all stimuli that evoked responding, for all groups, the mean number of responses decreased rapidly as stimulus values departed from the SD. Responding was at near-zero levels for all groups at 490-nm and 510-nm wavelengths, thus making it difficult to determine the precise limits of the range of stimuli that would have evoked responding for each deprivation group.
Food Deprivation as an MO in Drug-Discrimination Research
In drug-discrimination research, a particular response is differentially reinforced after administration of a specific drug (and dose). During separate training sessions, a different response is differentially reinforced after administration of vehicle (e.g., saline). Subsequently, different doses of the drug or other related drugs are administered in order to examine generalization of the drug response to different drugs or doses of the training drug. This procedure is similar to a postdiscrimination stimulus generalization test, in which the training dose is the SD for the drug response (e.g., pressing the left lever) and the SΔ for the vehicle response (e.g., pressing the right lever), and the vehicle is the SD for the vehicle response and the SΔ for the drug response.
Massey and McMillan (1987) examined generalization to varying doses of phencyclidine (PCP) in pigeons maintained at 70%, 80%, and 90% of their free-feeding weights. The birds were trained to peck a particular key color when injected with PCP (1.5 mg/kg) and a different color when injected with saline. During training, a center key was illuminated, and a response on the center key initiated a trial by illuminating two different-colored side keys and darkening the center key. Responding according to a fixed-ratio (FR) 5 schedule on the injection-appropriate key color constituted a trial. After completion of 10 FR 5 trials on the correct key color, food was presented. Generalization curves were obtained for four doses of PCP (0.30, 0.56, 1.00, and 1.70 mg/kg). The dependent variables were percentage of responses on the PCP-appropriate key during each test session, response rate, and mean latency to respond on the center key. With respect to their primary dependent variable (percentage of responses on the PCP key), body weight did not systematically influence generalization gradients. In contrast, as body weights decreased, response latencies decreased and mean response rates increased across most doses.
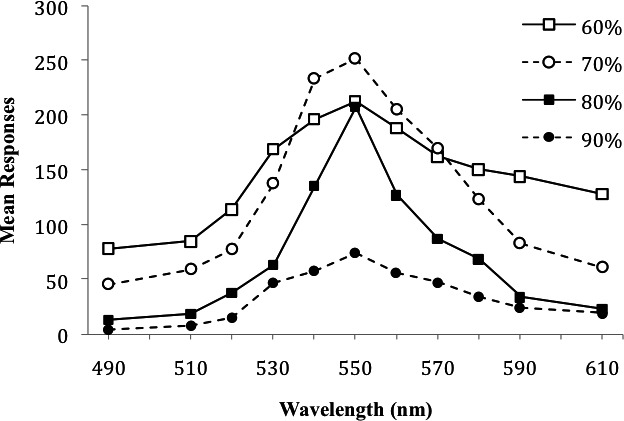
Gaiardi, Bartoletti, Bacchi, Gubellini, and Babbini (1987) examined generalization to varying doses of morphine as a function of prefeeding versus no prefeeding test conditions. Six rats food deprived for 20 hr were trained to discriminate injections of morphine (10 mg/kg) from saline injections using a two-lever operant procedure. Responding on the lever deemed appropriate for the type of injection given (e.g., the left lever after morphine, the right after saline) was reinforced with food under a tandem VI 60 FR 10 schedule. The dependent variables were (a) percentage of rats that selected the morphine lever first (i.e., the first lever on which a total of 10 responses were emitted) and (b) mean response rate. Generalization tests were conducted with four morphine doses (2.5, 5.0, 7.5, and 10.0 mg/kg) after 15 min of prefeeding or no prefeeding. When the rats were not prefed, a higher percentage of them selected the morphine lever at each untrained stimulus value than when they were not prefed (Figure 3). Prefeeding did not systematically affect choice at the training dose (i.e., 10 mg/kg). There was no systematic relation between response rate and the presence or absence of prefeeding.
Figure 3.
Percentage of animals selecting the morphine lever (emitting a total of 10 responses first on the morphine-appropriate lever prior to emitting 10 on the saline-appropriate lever) at each morphine test dose as a function of prefeeding versus no prefeeding. In this study, rats were trained to discriminate 5 mg/kg pentobarbital from saline when maintained at 80% of free-feeding weights and were tested when prefed 0%, 25%, or 50% of the amount of food required to produce satiation. This figure is based on data reported by Gaiardi et al. (1987, Figure 2).
To reexamine the discrepant results in the two studies just described, Li, Garner, Wessinger, and McMillan (1995) systematically replicated Massey and McMillan (1987) in Experiment 1 and directly replicated Gaiardi et al. (1987) in Experiment 2. In Experiment 1 pigeons were trained to discriminate pentobarbitol (5 mg/kg) from saline while being maintained at 80% of their free-feeding weights. During test conditions, the animals were prefed 0%, 25%, or 50% of the amount of food that was consumed until satiated (defined as not eating for at least 5 min). The animals were subsequently tested across four doses of pentobarbitol (1.0, 3.0, 5.6, and 10.0 mg/kg). All other reported experimental procedures were similar to those reported by Massey and McMillan. Results showed that prefeeding did not systematically influence the proportion of responses on the drug lever across test doses. Mean response rates, however, were lower across most test doses when the birds were 50% satiated than when they were 25% or 0% satiated. Also, mean response latencies were progressively longer in the 50% and 25% satiation conditions than they were in the 0% satiation condition.
In Experiment 2 (Li et al., 1995), all reported experimental procedures were the same as those arranged by Gaiardi et al. (1987) except for the following: (a) Reinforcement occurred under an FR 15 schedule of food delivery; (b) the dependent variable of interest was percentage of responses on the drug lever during testing; (c) the test doses were 0.3, 1.0, 3.2, 10.0 (SD), and 13.0 mg/kg of morphine; and (d) during prefeeding and no-prefeeding conditions, the animals were maintained at 85% of their free-feeding weights. When the animals were more food deprived, they responded proportionately more on the drug lever at all test doses, with the exception of the highest test dose (13 mg/kg; Figure 4). The difference between the ED50 values (the doses that produced 50% morphine-appropriate responding) in the prefeeding and no-prefeeding conditions was not statistically significant, but statistical tests for score differences at each test dose were not provided.
Figure 4.
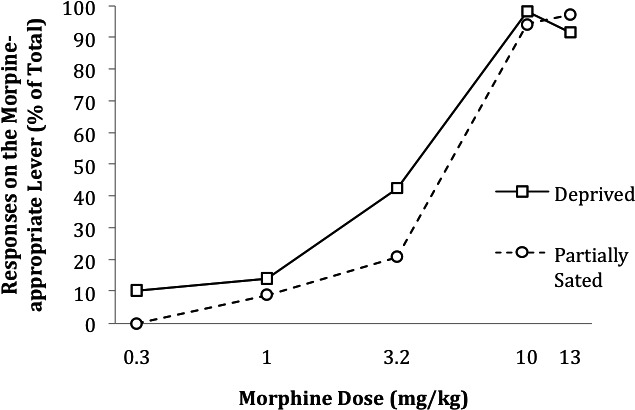
Percentage of responses on the morphine-appropriate lever (prior to first reinforcement opporunity) at various morphine test doses as a function of prefeeding (partially sated) versus no prefeeding (deprived). In this study, rats food deprived by 20 hr were trained to discriminate 10 mg/kg morphine from saline and were tested with the indicated doses when prefed for 15 min (partially sated) or not prefed (food deprived). This figure is based on data reported by Li et al. (1995, Figure 2).
DISCUSSION
With the exception of the drug-discrimination studies, in every study we reviewed the fastest or most forceful responding occurred in the presence of the SD (Table 1). Such an effect has been reliably produced in prior studies in which generalization gradients were not determined (e.g., Clark, 1958; Horner, Day, & Day, 1997; Skinner, 1938, p. 393). Moreover, in most studies we reviewed there was a direct relation between the level of deprivation and the measure of response strength in the presence of the SD and in the presence of untrained test stimuli physically similar to the SD. One exception is that the birds deprived to 60% of free-feeding weights in the Thomas and King (1959) study did not consistently respond faster than less deprived birds. As noted, the failure of birds deprived to 60% of free-feeding weights than heavier birds may have occurred because this level of deprivation is extreme and limits response rate. Another minor exception is that, in the Coate (1964) study, rats in the 40-hr water deprivation group responded more than those in the 48-hr deprivation group. This difference in deprivation level is small, and the difference in responding was not analyzed statistically, which is the reason that we designate the exception as “minor.” Based on the current review, the behavior-altering effect of MOs, that is, the change in the “response strength” (Killeen & Hall, 2001) of behavior relevant to specific consequences produced by manipulating MOs relevant to those consequences, appears to be graded.
In the drug-discrimination studies discussed, varying levels of food deprivation influenced at least one dependent measure in each study. Gaiardi et al. (1987) and Li et al. (1995, Experiment 2) demonstrated that changes in food deprivation systematically influenced the proportion of drug-appropriate responses in rats. Massey and McMillan (1987) showed that varying levels of food deprivation influenced response rate and response latency in pigeons. However, Gaiardi et al. and Li et al. (Experiment 2) did not find a systematic relation between levels of food deprivation and response rate, and Massey and Mcmillan and Li et al. (Experiment 1) did not find MO effects on the proportion of drug-appropriate responses. These discrepant results may be due in part to procedural differences or differences that were specific to the species used in each study. The drug-discrimination assay differs in some significant regards from other procedures commonly used to examine stimulus control (e.g., with drug discrimination, each training condition serves as both an SD and SΔ, training drugs can have MO effects with respect to the scheduled reinforcer, and training drugs directly alter response rate), and further research is needed to determine the extent to which MOs similarly affect discrimination of drugs and other stimuli. Attempts to determine why the drug-discrimination studies we reviewed yielded results somewhat inconsistent with one another and with those of studies that involved nondrug stimuli may also be merited.
Although most of the studies we reviewed did not include a sufficient range of untrained test stimuli for responding to fall to very low levels, visual analysis of the obtained generalization gradients suggests that the range of test stimuli that evokes responding is wider when deprivation is high than when it is lower, and studies that used a sufficiently wide range of test stimuli confirmed this relation (Gaiardi et al., 1987; Jenkins et al., 1958; Li et al., 1995; Thomas & King, 1959, Experiment 1). Such an effect has been recognized previously. For example, Keller and Schoenfeld (1950) in their chapter on motivation stated, “A point may be reached, in fact, where drive [motivation] is so strong that no external SD at all may be required for the response to appear: a starving man may ‘see’ his favorite dishes before him” (pp. 290–291). Similarly, in discussing mands Skinner (1957) wrote,
The lone man dying of thirst gasps Water! An unattended king calls A horse, a horse, my kingdom for a horse! These responses are “unreasonable” in the sense that they can have no possible effect upon the momentary environment, but the underlying process is lawful. Through a process of stimulus induction situations which are similar to earlier situations come to control the behavior, and in the extreme case a very strong response is emitted when no comparable stimulus can be detected. (pp. 46–47)
Skinner (1957) also alluded to the effects of motivation on stimulus control in his discussion of “impure tacts.” He wrote,
Insofar as the [tacting] response is likely to have a special effect upon the listener, it varies in strength with the states of deprivation or aversive stimulation associated with that effect. Stimulus control is reduced, as we have seen, and in pure fiction may be altogether lacking. Between these two extremes we are necessarily dealing with multiple variables. (p. 234)
These variables include those we would now classify as SDs and MOs.
Prominent ethologists, notably Lorenz (e.g., 1981) and Tinbergen (e.g., 1948), also emphasized that when animals were highly motivated, responses normally emitted in the presence of particular stimuli (releasers) would occur in the presence of stimuli that differed substantially from those releasers, or even in the absence of any relevant antecedents. As an example, Lorenz (p. 127) described a starling deprived of access to natural food sources that engaged in behaviors characteristic of catching, disabling, and eating insects even though no insects were present. These responses were termed vacuum activities.
Although it seems that concern with the effects of motivation on what we behavior analysts would consider to be stimulus control was widespread not so long ago, Michael's (1982, 1993) reconceptualization of motivation did not mention such an effect, and to our knowledge the effect of motivation on stimulus control has garnered no recent conceptual or empirical attention, except for being mentioned by Laraway et al. (2003). In any case, all of the studies that we reviewed appeared more than 20 years ago. Although interesting, those studies certainly do not exhaust research possibilities in the area. For example, although there is a substantial and growing literature concerning therapeutic manipulations of MOs (e.g., Iwata, Smith, & Michael, 2000), no one has examined whether MOs can be manipulated to produce desired levels of stimulus control, or whether unintended changes in MOs are sometimes responsible for the failure of trained responses to generalize, or for the breakdown of seemingly well-established discriminations.
As Skinner (1957) emphasized, motivation plays a very important role in verbal behavior. A decade ago, Sundberg and Michael (2001) noted that appropriate manipulations of MOs appear to facilitate language acquisition, although little relevant research had appeared and more was needed. More is still needed. Would, for example, children with autism learn to tact particular stimuli (e.g., a drinking straw and a spoon) more readily if motivation (and reinforcement) relevant to those specific stimuli (e.g., fluid deprivation and a juice box, food deprivation and a bowl of pudding) were arranged than when no such MO manipulation was in place? As another example, do appropriate manding responses established under conditions of relatively high motivation generalize more readily than similar responses established when motivation is weaker?
Significant basic research also is easy to envision. For example, all of the studies we reviewed utilized unconditioned MOs that pertained to food or water as reinforcers. Similar effects may occur with other primary reinforcers and with conditioned reinforcers, and these possibilities merit investigation, as do the effects of conditioned MOs on stimulus control. None of the reviewed studies used human participants, and it is certainly worthwhile to examine how motivation affects various aspects of stimulus control, in particular aspects that are of practical importance, in our own species. It is also worth conducting studies that examine generalization across a wide range of stimuli, which is necessary to ascertain the range of stimuli that affect a given operant at different MO values. As noted, most of the studies we reviewed did not examine sufficient stimulus values to make this determination.
It is interesting to note that Powell (1971) reported that, under some conditions, stimulus control in pigeons' responding under multiple schedules with an extinction component decreased (as evidenced by lower discrimination ratios, calculated as the number of responses emitted in the reinforcement component divided by the number of responses extinction component) as the birds' body weights were progressively reduced from approximately 95% to 70% of free-feeding levels. That is, the relative rate of responding in the presence of the SΔ increased as relative body weight decreased and the EO for food increased in intensity. It would be interesting to examine further the conditions under which such an effect occurs. It would also be interesting to train animals with the SD defined along different dimensions (e.g., the SD as a 550-nm light and the SΔ as a 10,000-Hz tone), and then to compare the effects of MO manipulations on generalization gradients and on relative levels of SΔ responding. Although it is reasonable to assume that manipulations that increase SΔ responding would also flatten and broaden generalization gradients, such an outcome is not foregone.
MOs appear to have a wide range of behavioral effects, but further research is needed to delineate those effects fully and to ascertain their conceptual and applied significance. Be that as it may, our purpose in writing this article is to encourage colleagues to include the full range of behavioral effects of MOs in their conceptual analyses and to conduct further research to explore these effects and their practical significance.
Footnotes
Prediscrimination generalization testing refers to testing after a history in which only the training stimulus (SD), but no SΔ, has been presented prior to generalization tests (Hearst, 1962). Conversely, postdiscrimination generalization testing refers to testing after a history in which both the SD and SΔ have been presented prior to generalization tests.
REFERENCES
- Brown J.S. The generalization of approach responses as a function of stimulus intensity and strength of motivation. Journal of Comparative Psychology. 1942;33:209–226. [Google Scholar]
- Clark F.C. The effect of deprivation and frequency of reinforcement on variable-interval responding. Journal of the Experimental Analysis of Behavior. 1958;1:221–228. doi: 10.1901/jeab.1958.1-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate W.B. Effects of deprivation on postdiscrimination stimulus generalization in the rat. Journal of Comparative and Physiological Psychology. 1964;57:134–138. doi: 10.1037/h0045387. [DOI] [PubMed] [Google Scholar]
- Gaiardi M, Bartoletti M, Bacchi A, Gubellini C, Babbini M. Increased sensitivity to the stimulus properties of morphine in food deprived rats. Pharmacology Biochemistry & Behavior. 1987;26:719–723. doi: 10.1016/0091-3057(87)90603-4. [DOI] [PubMed] [Google Scholar]
- Guttman N, Kalish H. Discriminability and stimulus generalization. Journal of Experimental Psychology. 1956;51:79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- Healey A.F. Compound stimuli, drive strength, and primary stimulus generalization. Journal of the Experimental Analysis of Behavior. 1965;5:536–538. doi: 10.1037/h0021837. [DOI] [PubMed] [Google Scholar]
- Hearst E. Concurrent generalization gradients for food-controlled and shock-controlled behavior. Journal of the Experimental Analysis of Behavior. 1962;5:19–31. doi: 10.1901/jeab.1962.5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner R.H, Day H.M, Day J.R. Using neutralizing routines to reduce problem behaviors. Journal of Applied Behavior Analysis. 1997;30:601–604. doi: 10.1901/jaba.1997.30-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata B.A, Smith R.G, Michael J. Current research on the influence of establishing operations on behavior in applied settings. Journal of Applied Behavior Analysis. 2000;33:411–418. doi: 10.1901/jaba.2000.33-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins W.O, Pascal G.R, Walker R.W. Deprivation and generalization. Journal of Experimental Psychology. 1958;56:274–277. doi: 10.1037/h0043850. [DOI] [PubMed] [Google Scholar]
- Kalish H.L, Haber A. Prediction of discrimination from generalization following variations in deprivation level. Journal of Comparative and Physiological Psychology. 1965;60:125–128. doi: 10.1037/h0022345. [DOI] [PubMed] [Google Scholar]
- Keller F.S, Schoenfeld W.N. Principles of psychology. New York: Appleton-Century-Crofts; 1950. [Google Scholar]
- Killeen P.R, Hall S.S. The principal components of response strength. Journal of the Experimental Analysis of Behavior. 2001;75:111–134. doi: 10.1901/jeab.2001.75-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraway S, Snycerski S, Michael J, Poling A. Motivating operations and terms to describe them: Some further refinements. Journal of Applied Behavior Analysis. 2003;36:407–414. doi: 10.1901/jaba.2003.36-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Garner H.R, Wessinger W.D, McMillan D.E. Effects of food deprivation and satiation on sensitivity to the discriminative-stimulus effects of pentobarbital in pigeons and morphine in rats. Behavioural Pharmacology. 1995;6:724–731. [PubMed] [Google Scholar]
- Lorenz K. The foundations of ethology. New York: Springer-Verlag; 1981. [Google Scholar]
- Massey B.W, McMillan D.E. Effects of body weight on discriminative-stimulus control by phencyclidine in the pigeon. Journal of the Experimental Analysis of Behavior. 1987;47:233–239. doi: 10.1901/jeab.1987.47-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J. Distinguishing between discriminative and motivational functions of stimuli. Journal of the Experimental Analysis of Behavior. 1982;37:149–155. doi: 10.1901/jeab.1982.37-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J. Establishing operations. The Behavior Analyst. 1993;16:191–206. doi: 10.1007/BF03392623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J.R, Grice G.R. Stimulus generalization as a function of drive level, and the relation between two measures of response strength. Journal of Experimental Psychology. 1965;69:357–362. doi: 10.1037/h0021856. [DOI] [PubMed] [Google Scholar]
- Powell R.W. Evidence of interaction between deprivation effects and stimulus control. Journal of the Experimental Analysis of Behavior. 1971;16:95–104. doi: 10.1901/jeab.1971.16-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner B.F. The behavior of organisms. New York: Appleton-Century-Crofts; 1938. [Google Scholar]
- Skinner B.F. Science and human behavior. New York: MacMillan; 1953. [Google Scholar]
- Skinner B.F. Verbal behavior. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Sundberg M.L, Michael J. The benefits of Skinner's analysis of verbal behavior for children with autism. Behavior Modification. 2001;25:698–724. doi: 10.1177/0145445501255003. [DOI] [PubMed] [Google Scholar]
- Thomas D.R, King K.A. Stimulus generalization as a function of level of motivation. Journal of Experimental Psychology. 1959;57:323–328. doi: 10.1037/h0042183. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. Social releasers and the experimental method required for their study. Wilson Bulletin. 1948;60:6–51. [Google Scholar]