Abstract
Aims
Instrumental activities of daily living (IADLs) are tasks that are necessary for independent community living. These tasks often require intact physical and cognitive function, the impairment of which may adversely affect health in older adults. In the current study, we examined the association between IADL impairment and incident heart failure (HF) in community-dwelling older adults.
Methods and results
Of the 5795 community-dwelling adults, aged ≥65 years, in the Cardiovascular Health Study, 5511 had data on baseline IADL and were free of prevalent HF. Of these, 1333 (24%) had baseline IADL impairment, defined as self-reported difficulty with one or more of the following tasks: using the telephone, preparing food, performing light and heavy housework, managing finances, and shopping. Propensity scores for IADL impairment, estimated for each of the 5511 participants, were used to assemble a cohort of 1038 pairs of participants with and without IADL impairment who were balanced on 42 baseline characteristics. Centrally adjudicated incident HF occurred in 26% and 21% of matched participants with and without IADL impairment, respectively, during >12 years of follow-up [matched hazard ratio (HR) 1.33; 95% confidence interval (CI) 1.11–1.59; P = 0.002]. Unadjusted and multivariable-adjusted HRs for incident HF before matching were 1.77 (95% CI 1.56–2.01; P < 0.001) and 1.33 (95% CI 1.15–1.54; P < 0.001), respectively. IADL impairment was also associated with all-cause mortality (matched HR 1.19; 95% CI 1.06–1.34; P = 0.004).
Conclusion
Among community-dwelling older adults free of baseline HF, IADL impairment is a strong and independent predictor of incident HF and mortality.
Keywords: Instrumental activities of daily living, Incident heart failure, Propensity score
Introduction
Activities of daily living (ADLs) refer to physical and cognitive activities often necessary for functional independence. Basic ADLs involves ambulation and self-feeding, activities that are fundamental to independent living within one's own residence, and their impairment may indicate a more advanced level of disability. Instrumental ADLs or IADLs, on the other hand, involve activities that require a higher level of cognitive function, such as management of money and shopping, and are necessary for a more independent living in the community. Impairments of IADLs may indicate an early decline in physical and cognitive functions, and may adversely affect health outcomes in older adults.1–5 Heart failure (HF) is common in older adults and is associated with poor outcomes.6–12 Because IADL impairment may adversely affect management of HF risk factors such as hypertension and diabetes, it is possible that older adults with IADL impairment will be at increased risk of incident HF. In the current study, we used a public-use copy of the Cardiovascular Health Study (CHS) data obtained from the National Heart, Lung, and Blood Institute (NHLBI) to examine if impairments in IADLs are associated with increased risk of incident HF in community-dwelling older adults.
Methods
Study design and participants
Funded by the NHLBI, the CHS is an epidemiological study of 5888 adults ≥65 years of age based in four US communities at Forsyth County, NC, Sacramento County, CA, Washington County, MD, and Pittsburgh, PA.13 The objective of the CHS was to understand risk factors for cardiovascular morbidity and mortality in older adults. Participants were recruited in two phases: an original cohort (1989–1990) of 5201 mostly white participants was supplemented by a second cohort (1992–1993) of 687 African American participants. Of the 5888 participants, 93 did not consent to be part of the public-use copy of the data, resulting in the final sample size of 5795 participants. Of these, 5511 had data on IADL impairment at baseline, and were also free of centrally adjudicated prevalent HF at baseline.14
Baseline instrumental activities of daily living
IADLs are defined as tasks that one must be able to perform for independent community living without assistance, supervision, or cues.1 Individual IADL tasks such as shopping, managing finances, and house cleaning often require both physical and cognitive abilities. Baseline IADL status was determined using the Assessment of Physical Function Form and involved six domains: telephone use, shopping, preparing food, performing light household work, performing heavy household work, and managing finances.15 Impairment of IADLs was defined as the need for more than a minimal amount of effort to perform the task or if the task caused symptoms.
Incident heart failure and other outcomes
Our primary outcome was new-onset HF during >12 years of mean follow-up. Incident HF was centrally adjudicated by the CHS Events Committee, and the process of adjudication has been previously described.16–18 Briefly, self-reports of physician-diagnosed HF during semi-annual visits were validated by medical record review for a constellation of symptoms, signs, and other supporting findings suggestive of HF, use of medications commonly used for HF, and follow-up surveillance assessments. Compared with the Framingham criteria for HF, the CHS central adjudication of HF is more stringent, and HF patients identified by both CHS and Framingham criteria have been shown to have similar all-cause mortality.19 Secondary outcomes included all-cause mortality, acute myocardial infarction, angina pectoris, stroke, and peripheral artery disease.
Assembly of a balanced study cohort
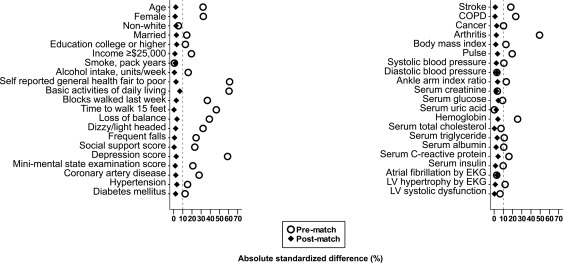
Because of the significant imbalances in baseline characteristics between those with and without IADL impairment (Figure 1, Table 1), we used propensity score matching to assemble a balanced cohort.20–22 The propensity score for IADL impairment for a participant is that individual's probability of having IADL impairment given his/her baseline characteristics. We used a non-parsimonious multivariable logistic regression model to estimate propensity scores for IADL impairment for each of the 5511 participants.17,23–25 In that model, IADL impairment was the dependent variable, and the 42 baseline characteristics (Figure 1) were used as covariates.26–28 Using a greedy matching protocol described elsewhere, we were able to match 1038 pairs of participants with and without IADL impairment who had similar propensity scores.
Figure 1.
Absolute standardized differences of baseline characteristics of older adults in the Cardiovascular Health Study with and without baseline impairment of instrumental activities of daily living, before and after propensity score matching. COPD, chronic obstructive pulmonary disease; EKG, electrocardiography; LV, left ventricular.
Table 1.
Baseline characteristics of Cardiovascular Health Study participants by impairment of instrumental activities of daily living (IADLs), before and after propensity score matching
| n (%) or mean (±SD) | Pre-match |
Post-match |
||||
|---|---|---|---|---|---|---|
| IADL impairment |
P-value | IADL impairment |
P-value | |||
| No (n = 4178) | Yes (n = 1333) | No (n = 1038) | Yes (n = 1038) | |||
| Age, years | 73 ± 5 | 75 ± 6 | <0.001 | 74 ± 6 | 74 ± 6 | 0.598 |
| Female | 2256 (54%) | 927 (70%) | <0.001 | 696 (67%) | 688 (66%) | 0.729 |
| Non-white | 644 (15%) | 231 (17%) | 0.096 | 168 (16%) | 158 (15%) | 0.589 |
| Married | 2846 (68%) | 817 (61%) | <0.001 | 653 (63%) | 668 (64%) | 0.526 |
| Education college or higher | 1861 (45%) | 510 (38%) | <0.001 | 415 (40%) | 432 (42%) | 0.479 |
| Income ≥US$25 000 | 1610 (39%) | 392 (29%) | <0.001 | 340 (33%) | 347 (33%) | 0.773 |
| Smoking, pack years | 17 ± 26 | 17 ± 28 | 0.801 | 18 ± 28 | 17 ± 28 | 0.668 |
| Alcohol intake, units/week | 3 ± 7 | 2 ± 5 | <0.001 | 2 ± 5 | 2 ± 6 | 0.861 |
| Self-reported general health fair to poor | 710 (17%) | 585 (44%) | <0.001 | 386 (37%) | 373 (36%) | 0.529 |
| Basic activities of daily living | 0.03 ± 0.21 | 0.39 ± 0.82 | <0.001 | 0.09 ± 0.40 | 0.12 ± 0.40 | 0.023 |
| Blocks walked in last week | 44 ± 58 | 25 ± 42 | <0.001 | 30 ± 43 | 29 ± 44 | 0.491 |
| Time to walk 15 feet, s | 5 ± 2 | 7 ± 3 | <0.001 | 6 ± 3 | 6 ± 2 | 0.919 |
| Loss of balance | 790 (19%) | 480 (36%) | <0.001 | 326 (31%) | 309 (30%) | 0.424 |
| Dizzy/light headed on standing | 715 (17%) | 408 (31%) | <0.001 | 281 (27%) | 271 (26%) | 0.643 |
| Frequent falls | 85 (2%) | 95 (7%) | <0.001 | 48 (5%) | 50 (5%) | 0.915 |
| Social support score | 8 ± 3 | 9 ± 3 | <0.001 | 9 ± 3 | 9 ± 3 | 0.899 |
| Depression score | 4 ± 4 | 7 ± 5 | <0.001 | 6 ± 5 | 6 ± 5 | 0.466 |
| Mini-mental state examination score, 30 points | 28 ± 3 | 27 ± 3 | <0.001 | 27 ± 3 | 27 ± 3 | 0.707 |
| Medical history | ||||||
| Coronary artery disease | 616 (15%) | 344 (26%) | <0.001 | 253 (24%) | 243 (23%) | 0.638 |
| Hypertension | 2361 (57%) | 852 (64%) | <0.001 | 620 (60%) | 638 (61%) | 0.434 |
| Diabetes mellitus | 604 (14%) | 255 (19%) | <0.001 | 171 (16%) | 179 (17%) | 0.672 |
| Stroke | 123 (3%) | 91 (7%) | <0.001 | 55 (5%) | 52 (5%) | 0.844 |
| Chronic obstructive pulmonary disease | 436 (10%) | 249 (19%) | <0.001 | 169 (16%) | 173 (17%) | 0.854 |
| Cancer | 564 (13%) | 229 (17%) | <0.001 | 181 (17%) | 170 (16%) | 0.554 |
| Arthritis | 1893 (45%) | 920 (69%) | <0.001 | 684 (66%) | 672 (65%) | 0.582 |
| Clinical examination | ||||||
| Body mass index, kg/m2 | 26 ± 4 | 27 ± 4 | <0.001 | 27 ± 4 | 27 ± 4 | 0.678 |
| Pulse rate, b.p.m. | 67 ± 11 | 70 ± 12 | <0.001 | 69 ± 11 | 69 ± 11 | 0.387 |
| Systolic blood pressure, mmHg | 136 ± 22 | 138 ± 22 | 0.001 | 137 ± 21 | 138 ± 22 | 0.587 |
| Diastolic blood pressure, mmHg | 71 ± 11 | 71 ± 11 | 0.335 | 70 ± 12 | 71 ± 11 | 0.501 |
| Ankle–arm index ratio, ≤0.9 | 478 (11%) | 213 (16%) | <0.001 | 158 (15%) | 144 (14%) | 0.391 |
| Laboratory values | ||||||
| Serum creatinine, mg/dL | 0.95 ± 0.35 | 0.97 ± 0.47 | 0.223 | 0.93 ± 0.28 | 0.94 ± 0.34 | 0.557 |
| Serum glucose, mg/dL | 110 ± 35 | 113 ± 40 | 0.003 | 111 ± 33 | 112 ± 39 | 0.306 |
| Serum uric acid, mg/dL | 5.7 ± 1.5 | 5.7 ± 1.7 | 0.922 | 6 ± 1 | 6 ± 2 | 0.568 |
| Serum total cholesterol, mg/dL | 211 ± 39 | 214 ± 40 | 0.016 | 213 ± 38 | 213 ± 40 | 0.952 |
| Serum triglyceride, mg/dL | 137 ± 73 | 146 ± 87 | <0.001 | 141 ± 71 | 143 ± 79 | 0.455 |
| Serum albumin, g/dL | 4.00 ± 0.3 | 3.97 ± 0.3 | 0.001 | 3.98 ± 0.3 | 3.99 ± 0.3 | 0.620 |
| Serum insulin, μU/mL | 16 ± 22 | 19 ± 29 | 0.001 | 18 ± 29 | 18 ± 29 | 0.731 |
| C-reactive protein, mg/dL | 4 ± 8 | 6 ± 9 | <0.001 | 5 ± 11 | 5 ± 8 | 0.330 |
| Haemoglobin, g/dL | 14.1 ± 1.3 | 13.8 ± 1.4 | <0.001 | 13.8 ± 1.3 | 13.9 ± 1.3 | 0.342 |
| Electrocardiographic findings | ||||||
| Atrial fibrillation | 83 (2%) | 32 (2%) | 0.357 | 28 (3%) | 23 (2%) | 0.568 |
| Left ventricular hypertrophy | 155 (4%) | 84 (6%) | <0.001 | 57 (5%) | 60 (6%) | 0.851 |
| Left ventricular systolic dysfunction | 294 (7%) | 117 (9%) | 0.035 | 78 (8%) | 79 (8%) | 1.000 |
Statistical analysis
For descriptive analyses, Pearson's χ2 and Wilcoxon rank-sum tests were used for the pre-match data. McNemar's test and paired sample t-test were used for post-match comparisons of means and proportions. Kaplan–Meier and Cox proportional hazard analyses were used to estimate the associations of baseline IADL impairment with outcomes. To evaluate a dose–response relationship between baseline IADL impairment and outcomes, analyses were repeated categorizing IADL impairment as one impairment or two or more impairments. A formal sensitivity analysis was performed to quantify the degree of a hidden bias due to potential imbalance of an unmeasured covariate that would need to be present to invalidate our main conclusions.29 Subgroup analyses were performed to determine homogeneity of the association of IADL impairment with incident HF. All statistical tests were two tailed with 95% confidence levels, and a P-value < 0.05 was considered significant. SPSS for Windows (Version 15) was used for all data analysis.
Results
Baseline characteristics
In the pre-matched cohort, 75% of participants reported no IADL impairments. Impairments of one, two, three, four, five, and six IADLs were reported by 18.6, 3.4, 1.3, 0.7, 0.1, and 0.1% of participants, respectively. Overall, matched participants had a mean age of 74 (±6.0) years, 67% were women, and 16% were African American. Imbalances in baseline characteristics before matching and balances achieved after matching between patients with and without IADL impairment are displayed in Table 1 and Figure 1. After matching, standardized differences for all measured covariates were <10%, suggesting substantial covariate balance across the groups (Figure 1).
Association of impairment of instrumental activities of daily living with incident heart failure and other outcomes
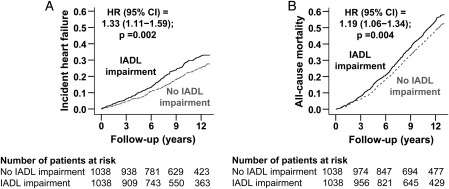
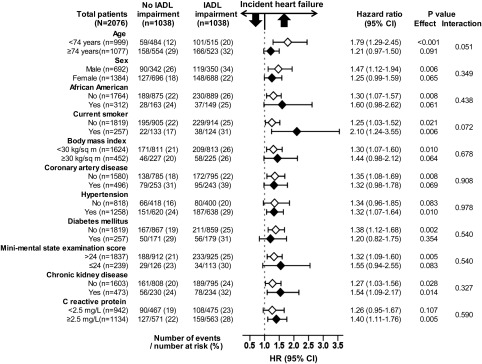
During >12 years of follow-up, incident HF developed in 26% and 21% of matched participants with and without IADL impairment [hazard ratio (HR) associated with IADL impairment 1.33; 95% confidence interval (CI) 1.11–1.59; P = 0.002; Figure 2 and Table 2]. A hidden covariate that is a near-perfect predictor of incident HF would need to increase the odds of IADL impairment by >13% before it could potentially explain away this association. The association between IADL impairment and incident HF was homogeneous across various subgroups of patients (Figure 3). Pre-match associations between IADL impairment and incident HF are displayed in Table 2. Associations of IADL impairment with other outcomes are displayed in Table 3.
Figure 2.
Kaplan–Meier plots for (A) incident heart failure and (B) all-cause mortality by baseline impairment of instrumental activities of daily living (IADLs) in a propensity-matched cohort of older adults in the Cardiovascular Health Study. CI, confidence interval; HR, hazard ratio.
Table 2.
Association of impairment of instrumental activities of daily living (IADLs) with incident heart failure in the Cardiovascular Health Study
| Event (%) |
Absolute risk difference (%) | HR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| IADL impairment | |||||
| No | Yes | ||||
| Pre-match | (n = 4178) | (n = 1333) | |||
| Unadjusted | 777 (19%) | 359 (27%) | +8% | 1.77 (1.56–2.01) | <0.001 |
| Multivariable adjusted | – | – | – | 1.33 (1.15–1.54) | <0.001 |
| Propensity adjusted | – | – | – | 1.30 (1.12–1.51) | 0.001 |
| Post-match | (n = 1038) | (n = 1038) | |||
| Propensity matched | 217 (21%) | 267 (26%) | +5% | 1.33 (1.11–1.59) | 0.002 |
CI, confidence interval; HR, hazard ratio.
Figure 3.
Association of baseline impairment of instrumental activities of daily living (IADLs) and incident heart failure in subgroups of a propensity-matched cohort of older adults in the Cardiovascular Health Study. CI, confidence interval; HR, hazard ratio.
Table 3.
Association of impairment of instrumental activities of daily living (IADLs) with other outcomes in the Cardiovascular Health Study
| Outcomes | Pre-match |
Post-match |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| All-cause mortality | 1.72 (1.58–1.87) | <0.001 | 1.19 (1.06–1.34) | 0.004 |
| Acute myocardial infarctiona | 1.48 (1.24–1.77) | <0.001 | 1.29 (0.99–1.67) | 0.056 |
| Angina pectorisa | 1.50 (1.29–1.74) | <0.001 | 1.33 (1.07–1.65) | 0.010 |
| Strokea | 1.49 (1.27–1.75) | <0.001 | 1.08 (0.87–1.34) | 0.493 |
| Peripheral artery diseasea | 1.78 (1.23–2.58) | 0.002 | 1.29 (0.77–2.17) | 0.330 |
| Transient ischaemic attacka | 1.56 (1.14–2.13) | 0.005 | 1.26 (0.81–1.97) | 0.306 |
CI, confidence interval; HR, hazard ratio.
aExcluding participants with baseline conditions; e.g. for analysis of incident stroke, those with a history of stroke at baseline were excluded.
When IADL impairment was categorized as one impairment or two or more impairments, incident HF occurred in 28, 25, and 21% of matched participants with two or more, one, and no IADL impairments, respectively (χ2 P = 0.028 and P for linear trend = 0.009). Compared with no IADL impairment, HRs for incident HF associated with one and two or more IADL impairments were 1.28 (95% CI 1.06–1.55; P = 0.010) and 1.63 (95% CI 1.19–2.22; P = 0.002), respectively (P for trend <0.001).
Discussion
Findings from the current study demonstrate that IADL impairment was associated with increased risk of incident HF in community-dwelling older adults that was independent of many traditional and non-traditional risk factors. Further, the risk of incident HF increased with increasing level of IADL impairment. Baseline IADL impairment also had unadjusted associations with mortality and other incident cardiovascular events, but only its association with all-cause mortality and incident angina pectoris was independent. To the best of our knowledge, this is the first report of an independent association of IADL impairment with incident HF. These findings suggest that impairments of tasks necessary for independent community living may adversely increase risk of incident HF in older adults.
There are several possible explanations for these findings: residual bias by measured confounders, bias by unmeasured cofounders, and a true association. Because patients in our matched cohort were well balanced on all measured baseline characteristics, bias due to imbalances on measured covariates is unlikely. Findings from our sensitivity analysis suggest that the association observed in our study was rather insensitive to bias due to unmeasured confounders. IADL measures complex function that includes both physical tasks such as heavy housework and cognitive tasks such as managing finances. It is possible that older adults with IADL impairments were not able to dispense their medications or take them at the correct time and/or at the correct dosages, leading to poor control and treatment of various HF risk factors such as hypertension or coronary artery disease among those with IADL impairments. Older adults with IADL impairment may also have difficulty keeping doctors' appointments and understanding dietary and risk factors. Reduced physical activity associated with IADL impairment may also increase the risk of incident HF. It is also possible that the IADL impairment was the early manifestation of subclinical HF in older adults. Impairment of IADLs may be a marker of frailty, which has been shown to be associated with subclinical cardiovascular disease.30
Reduced physical activity has been shown to be associated with incidence of a number of chronic diseases including cancer and cardiovascular disease.31–34 However, to the best of our knowledge, this is the first report of an association of impaired physical and cognitive function with incident HF in community-dwelling older adults free of HF at baseline. These findings are important, as both IADL impairment and HF are common in older adults, and findings from the current study suggest that IADL impairment may be used to identify older adults at increased risk for HF. These individuals may be targeted for specific interventions to improve IADL performance as well as better treatment of other risk factors such as an uncontrolled hypertension, diabetes, atrial fibrillation, or ongoing myocardial ischaemia.
Our study has several limitations. Data on IADLs were self-reported and misclassification is possible. Because data on individual IADLs were not available, an analysis to determine which IADL tasks were associated with incident HF was not possible. Also, disability is a dynamic process,35 and it is possible that some older adults without IADL impairment developed new impairments during follow-up, and vice versa. However, this regression dilution may have underestimated the true association between IADL impairment and incident HF.36 Bias due to imbalances in unmeasured confounders is possible. As discussed earlier, findings of our sensitivity analysis suggest that the association of IADL impairment with incident HF was rather insensitive to such an unmeasured confounder.
In conclusion, among community-dwelling older adults without baseline HF, the presence of IADL impairment at baseline is a novel independent risk factor for incident HF and all-cause mortality, which may be used to risk-stratify older adults for targeted interventions to improve IADL performance and address other HF risk factors. Future studies need to develop and test interventions to prevent IADL impairment in community-dwelling older adults.
Funding
C.B.B. is supported by the Birmingham/Atlanta VA Geriatric Research Education and Clinical Center, the John A. Hartford Foundation and the Southeast Center of Excellence in Geriatric Medicine. X.S. is supported by the NIH/NIDDK through grant R21 DK088195. R.M.A. is supported by the NIH/NCATS through grant 5UL1 RR025777. A.A. is supported by the NIH/NHLBI through grants R01-HL085561, R01-HL085561S and R01-HL097047, and a generous gift from Ms Jean B. Morris of Birmingham, Alabama.
Conflict of interest: none declared.
Acknowledgements
The Cardiovascular Health Study (CHS) was conducted and supported by the NHLBI in collaboration with the CHS Investigators. This manuscript was prepared using a limited access data set obtained by the NHLBI and does not necessarily reflect the opinions or views of the CHS or the NHLBI.
Footnotes
See page 565 for the editorial comment on this article (doi:10.1093/eurjhf/hfs065)
References
- 1.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 2.Ginsberg GM, Hammerman-Rozenberg R, Cohen A, Stessman J. Independence in instrumental activities of daily living and its effect on mortality. Aging (Milano) 1999;11:161–168. [PubMed] [Google Scholar]
- 3.Wedding U, Rohrig B, Klippstein A, Fricke HJ, Sayer HG, Hoffken K. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol. 2006;132:665–671. doi: 10.1007/s00432-006-0115-7. [DOI] [PubMed] [Google Scholar]
- 4.Jagger C, Matthews R, Matthews F, Robinson T, Robine JM, Brayne C Medical Research Council Cognitive Funcion andAgeing Study Investigators. The burden of diseases on disability-free life expectancy in later life. J Gerontol A Biol Sci Med Sci. 2007;62:408–414. doi: 10.1093/gerona/62.4.408. [DOI] [PubMed] [Google Scholar]
- 5.Wedding U, Rohrig B, Klippstein A, Pientka L, Hoffken K. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007;133:945–950. doi: 10.1007/s00432-007-0233-x. [DOI] [PubMed] [Google Scholar]
- 6.Najafi F, Jamrozik K, Dobson AJ. Understanding the ‘epidemic of heart failure’: a systematic review of trends in determinants of heart failure. Eur J Heart Fail. 2009;11:472–479. doi: 10.1093/eurjhf/hfp029. [DOI] [PubMed] [Google Scholar]
- 7.Filippatos G, Parissis JT. Heart failure diagnosis and prognosis in the elderly: the proof of the pudding is in the eating. Eur J Heart Fail. 2011;13:467–471. doi: 10.1093/eurjhf/hfr036. [DOI] [PubMed] [Google Scholar]
- 8.Lien CT, Gillespie ND, Struthers AD, McMurdo ME. Heart failure in frail elderly patients: diagnostic difficulties, co-morbidities, polypharmacy and treatment dilemmas. Eur J Heart Fail. 2002;4:91–98. doi: 10.1016/s1388-9842(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 9.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Witham MD, Argo IS, Johnston DW, Struthers AD, McMurdo ME. Predictors of exercise capacity and everyday activity in older heart failure patients. Eur J Heart Fail. 2006;8:203–207. doi: 10.1016/j.ejheart.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Norberg EB, Boman K, Lofgren B. Activities of daily living for old persons in primary health care with chronic heart failure. Scand J Caring Sci. 2008;22:203–210. doi: 10.1111/j.1471-6712.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–553. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary D, Pstay B, Rautaharju P, Tracy R, Weiler P. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Ettinger WH, Lind B, Newman AB, Gardin J. Physical disability in older adults: a physiological approach. Cardiovascular Health Study Research Group. J Clin Epidemiol. 1994;47:747–760. doi: 10.1016/0895-4356(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 16.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 17.Filippatos GS, Ahmed MI, Gladden JD, Mujib M, Aban IB, Love TE, Sanders PW, Pitt B, Anker SD, Ahmed A. Hyperuricaemia, chronic kidney disease, and outcomes in heart failure: potential mechanistic insights from epidemiological data. Eur Heart J. 2011;32:712–720. doi: 10.1093/eurheartj/ehq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filippatos GS, Desai RV, Ahmed MI, Fonarow GC, Love TE, Aban IB, Iskandrian AE, Konstam MA, Ahmed A. Hypoalbuminaemia and incident heart failure in older adults. Eur J Heart Fail. 2011;13:1078–1086. doi: 10.1093/eurjhf/hfr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schellenbaum GD, Rea TD, Heckbert SR, Smith NL, Lumley T, Roger VL, Kitzman DW, Taylor HA, Levy D, Psaty BM. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol. 2004;160:628–635. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.Rubin DB. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 22.Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32:1708–1708. doi: 10.1093/eurheartj/ehr031. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed MI, White M, Ekundayo OJ, Love TE, Aban I, Liu B, Aronow WS, Ahmed A. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, Pitt B. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–1343. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamopoulos C, Meyer P, Desai RV, Karatzidou K, Ovalle F, White M, Aban I, Love TE, Deedwania P, Anker SD, Ahmed A. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity-matched study. Eur J Heart Fail. 2011;13:200–206. doi: 10.1093/eurjhf/hfq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deedwania PC, Ahmed MI, Feller MA, Aban IB, Love TE, Pitt B, Ahmed A. Impact of diabetes mellitus on outcomes in patients with acute myocardial infarction and systolic heart failure. Eur J Heart Fail. 2011;13:551–559. doi: 10.1093/eurjhf/hfr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu AH, Pitt B, Anker SD, Vincent J, Mujib M, Ahmed A. Association of obesity and survival in systolic heart failure after acute myocardial infarction: potential confounding by age. Eur J Heart Fail. 2010;12:566–573. doi: 10.1093/eurjhf/hfq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. New York: Springer-Verlag; 2002. pp. p105–170. [Google Scholar]
- 30.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 31.Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15:247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- 32.Ilanne-Parikka P, Laaksonen DE, Eriksson JG, Lakka TA, Lindstr J, Peltonen M, Aunola S, Keinanen-Kiukaanniemi S, Uusitupa M, Tuomilehto J. Leisure-time physical activity and the metabolic syndrome in the Finnish diabetes prevention study. Diabetes Care. 2010;33:1610–1617. doi: 10.2337/dc09-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Borhani NO. Significance of physical activity for prevention and control of hypertension. J Hum Hypertens. 1996;10(Suppl 2):S7–S11. [PubMed] [Google Scholar]
- 35.Gill TM, Allore HG, Hardy SE, Guo Z. The dynamic nature of mobility disability in older persons. J Am Geriatr Soc. 2006;54:248–254. doi: 10.1111/j.1532-5415.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 36.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]