Abstract
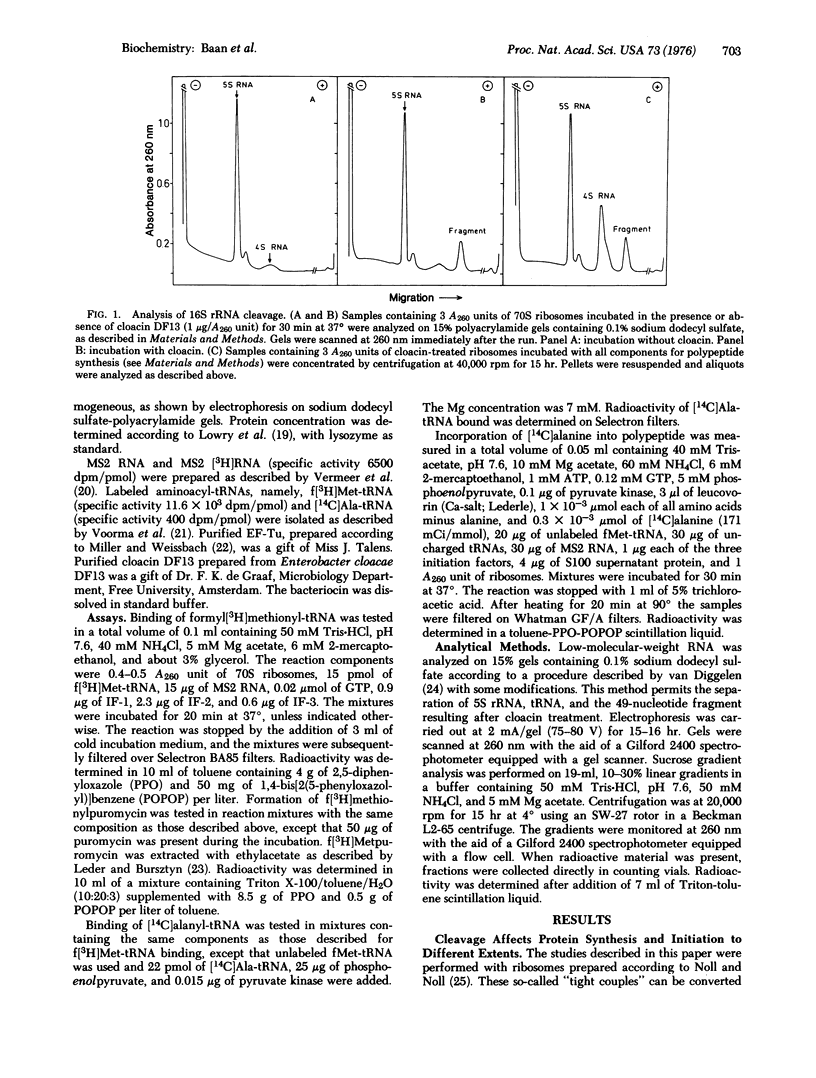
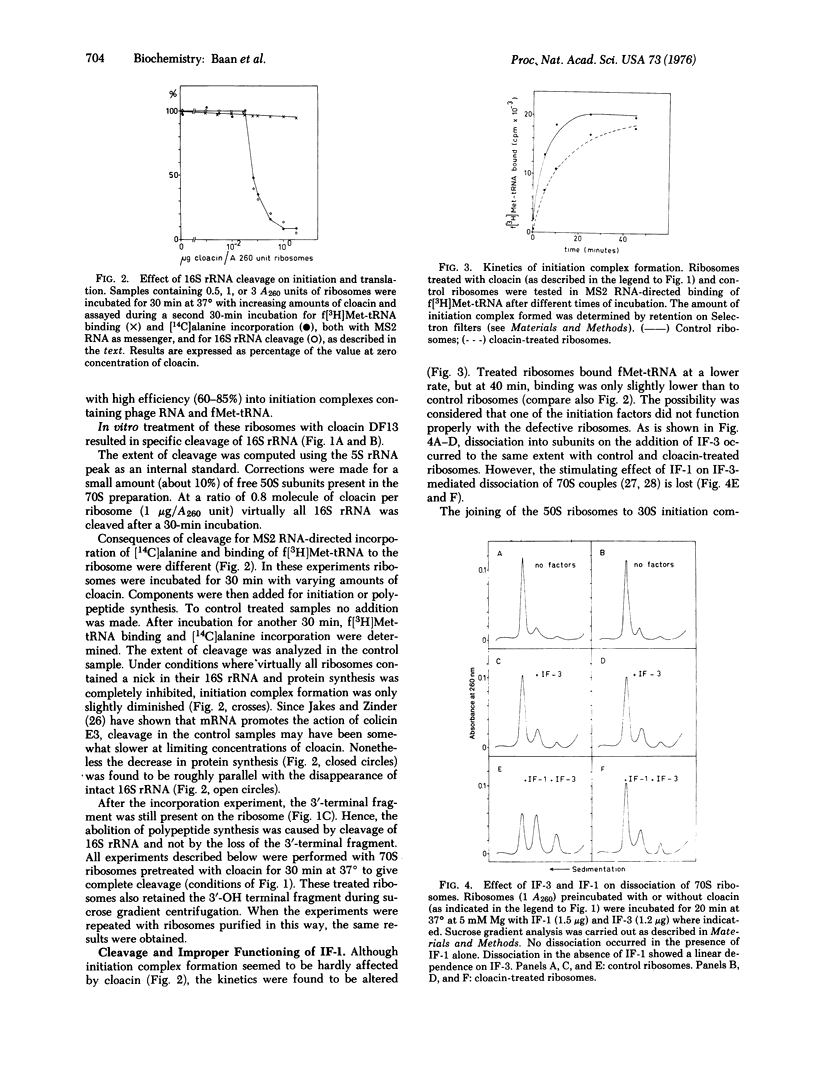
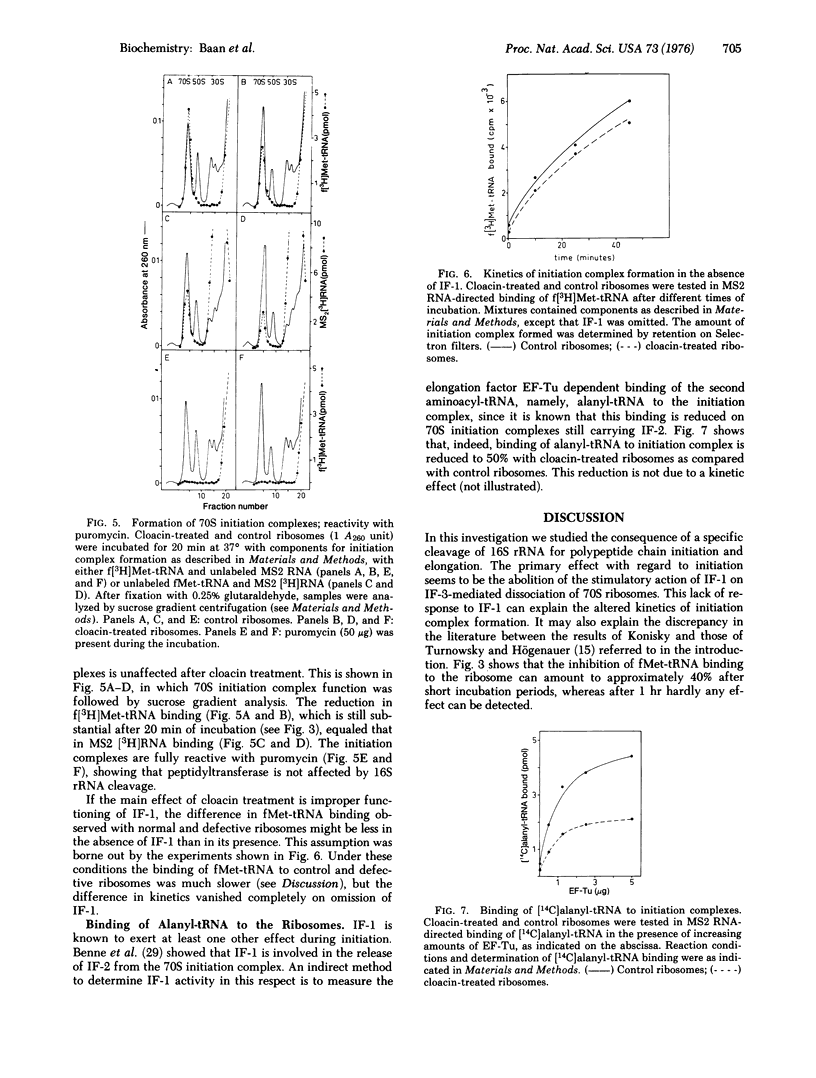
Specific in situ cleavage of 16S rRNA of E. coli has been accomplished by in vitro treatment of 70S ribosomes ("tight couples") with the bacteriocin cloacin DF13. The defective ribosomes, which have fully lost their ability to sustain polypeptide synthesis, are still able to form initiation on complexes with MS2 RNA, but the kinetics are altered. This is apparently due to an improper functioning of initiation factor IF-1, for the defective ribosomal couples respond normally to dissociation by IF-3 but the dissociation is not stimulated by IF-1. The initiation complexes formed with defective ribosomes are fully reactive with puromycin. Their ability to bind alanyl-tRNA is reduced by about 50% at all concentrations of elongation factor Tu studied. Cleavage of the 16S rRNA, not the release of the terminal fragment from the ribosome, causes the block of protein synthesis and the aberrations observed during initiation and elongation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Arentzen R., Voorma H. O. The mechanism of action of inititation factor F1 from Escherichia coli. Biochim Biophys Acta. 1972 May 10;269(2):304–310. doi: 10.1016/0005-2787(72)90440-6. [DOI] [PubMed] [Google Scholar]
- Benne R., Naaktgeboren N., Gubbens J., Voorma H. O. Recycling of initiation factors IF-1, IF-2 and IF-3. Eur J Biochem. 1973 Jan 15;32(2):372–380. doi: 10.1111/j.1432-1033.1973.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnoff J. S., Maitra U. Protein factors involved in polypeptide chain initiation in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1969;34:301–306. doi: 10.1101/sqb.1969.034.01.036. [DOI] [PubMed] [Google Scholar]
- Held W. A., Nomura M., Hershey J. W. Ribosomal protein S21 is required for full activity in the initiation of protein synthesis. Mol Gen Genet. 1974;128(1):11–22. doi: 10.1007/BF00267291. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Jakes K., Zinder N. D. Stimulation of in vitro colicin E3 action by messenger ribonucleic acid and message analogs. J Biol Chem. 1974 Sep 10;249(17):5507–5512. [PubMed] [Google Scholar]
- Kenner R. A. A protein-nucleic acid crosslink in 30S ribosomes. Biochem Biophys Res Commun. 1973 Apr 16;51(4):932–938. doi: 10.1016/0006-291x(73)90016-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Lockwood A. H., Chakraborty P. R., Maitra U. A complex between initiation factor IF2, guanosine triphosphate, and fMet-tRNA: an intermediate in initiation complex formation. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3122–3126. doi: 10.1073/pnas.68.12.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall S. H., Tamaoki T. Dissociation of Escherichia coli ribosomes. Role of initiation factors. Biochemistry. 1972 Dec 5;11(25):4826–4830. doi: 10.1021/bi00775a028. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- Noll M., Noll H. Structural dynamics of bacterial ribosomes. III. Quantitative conversion of vacant ribosome couples into an initiation complex with R17 RNA as messenger. J Mol Biol. 1974 Dec 5;90(2):237–251. doi: 10.1016/0022-2836(74)90370-2. [DOI] [PubMed] [Google Scholar]
- Phang-Cheng-Tai, Davis B. D. Activity of colicin E3-treated ribosomes in initiation and in chain elongation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1021–1025. doi: 10.1073/pnas.71.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Lelong J. C., Brawerman G., Gros F. Function of three protein factors and ribosomal subunits in the initiation of protein synthesis in E. coli. Nature. 1968 Sep 7;219(5158):1016–1021. doi: 10.1038/2191016a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Leffler S. Ribosomal protein S1 and polypeptide chain initiation in bacteria. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2325–2329. doi: 10.1073/pnas.72.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnowsky F., Högenauer G. Colicin E 3, an inactivating agent of the ribosomal A-site. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1246–1254. doi: 10.1016/s0006-291x(73)80028-2. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Vermeer C., Boon J., Talens A., Bosch L. Binding of the initiation factor IF-3 to Escherichia coli ribosomes and MS2 RNA. Eur J Biochem. 1973 Dec 3;40(1):283–293. doi: 10.1111/j.1432-1033.1973.tb03196.x. [DOI] [PubMed] [Google Scholar]
- Voorma H. O., Benne R., den Hertog T. J. Binding of aminoacyl-tRNA to ribosomes programmed with bacteriophage MS2-RNA. Eur J Biochem. 1971 Feb;18(4):451–462. doi: 10.1111/j.1432-1033.1971.tb01263.x. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]
- van Duin J., Kurland C. G., Dondon J., Grunberg-Manago M. Near neighbors of IF3 bound to 30S ribosomal subunits. FEBS Lett. 1975 Nov 15;59(2):287–290. doi: 10.1016/0014-5793(75)80394-2. [DOI] [PubMed] [Google Scholar]



