Abstract
We are interested in the role of neural activity mediated through regulated vesicular release in the stopping and early branching of the thalamic projections in the cortex. Axon outgrowth, arrival to cortical subplate, side branch formation during the waiting period and cortical plate innervation of embryonic thalamocortical projections occurs without major abnormalities in the absence of regulated release in Snap25−/− mice (Washbourne et al., 2002; Molnár et al., 2002). The fact that Snap25−/− mice die at birth limited our previous experiments to the prenatal period. We therefore investigated the behaviour of thalamic projections in co-culture paradigms using heterochronic thalamic (E16/18) and cortical (P0/3) explants where the stopping and branching behaviour has been previously documented. Our current co-culture experiments established that thalamic projections from E16–18 Snap25+/+ or Snap25−/− explants behaved in identical fashion in P0/3 Snap25+/+ CTX explants after seven days in vitro. Thalamic projections from Snap25−/− explants developed similar fibre ingrowth pattern to the cortex, stopped and formed branches at similar depth of the Snap25+/+ cortical slice as in control cultures. These results imply that thalamic projections can reach their ultimate target cells in layer 4, stop and start to develop branches in the absence of regulated vesicular transmitter release from their own terminals.
Introduction
Development of neural connections relies on multiple mechanisms of pathfinding, target selection, and activity-dependent plasticity (Shatz and Stryker, 1978; Katz and Shatz, 1996; Ming et al., 2001; Sur and Rubenstein, 2005; Hanson and Landmesser, 2004; Nicol et al., 2007). In particular, several studies have highlighted an instructive role for early neural activity in area-specific targeting, invasion of the cortical plate and lamina-specific termination of thalamocortical axons (Anderson and Price, 2002; Lee et al, 2005a,b; Uesaka et al., 2006; 2007). However, the nature of this neuronal activity and its precise role in these early events remains unclear.
Disruption of activity patterns with the sodium channel antagonist tetrodoxin (TTX), which blocks the propagation of action potentials, has been demonstrated to result in the incorrect targeting of visual thalamic fibres into the auditory cortex of embryonic cat brain, a region that fibres usually bypass en route to the visual cortex (Catalano and Shatz, 1998). It has been suggested, moreover, that during the initial targeting of layer 4 of cortex by TCAs, activity-based interactions lead to the functional arrangement and refinement of these thalamic inputs (Friauf et al., 1990; Krug et al., 1998; Arber, 2004).
There is evidence to suggest that the requirement for neural activity in establishing appropriate thalamocortical circuitry may stem from its role in regulating the lamina-specific targeting of thalamic projections. For example, infusion of TTX into the developing embryonic cat brain in vivo during the period when TCAs reside in the intermediate zone prior to their invasion of the cortex, resulted in the fibres growing through layer 4 to the pial surface of the brain (Herrmann and Shatz, 1995). Similar results have been reported using ex vivo explant co-culture systems, where addition of TTX during axonal outgrowth prevented the proper termination of axons in layer 4 (Anderson and Price, 2002). Other studies have shown, however, that over a longer time course, axons did terminate correctly in the presence of TTX, although arborisation was more elaborate with a greater number of branches (Wilkemeyer and Angelides, 1996). These results are consistent with the notion that synaptic activity might regulate the expression of the various surface molecules that provide permissive or stop signals necessary to adopt proper thalamocortical circuitry (Takemoto et al., 2002; Yamamoto et al., 2000b).
Genetic alterations of proteins involved in synaptic transmission provide an alternative means to dissect the role of neural activity patterns during brain development (Rizo and Sudhof, 2002). In particular, induced mutations of the gene encoding SNAP-25 (Synaptosome associated protein of 25 kDa) have been useful in distinguishing between action potential-dependent and –independent neurotransmitter release (Washbourne et al., 2002), as well as changes in synaptic plasticity during postnatal maturation of the brain in mice (Bark et al., 2004).
Because Snap25 null mutant mice do not survive birth (Washbourne et al., 2002), our previous studies were limited to fetal brain and could only establish that the initial topographical placement of thalamocortical and corticofugal fibres proceeds correctly in the absence of SNAP-25, and hence action potential evoked synaptic activity (Molnár et al., 2002). This investigation, therefore could not address the further development of thalamocortical circuitry that occurs during early neonatal periods (P2—P4), which includes migration of layer 4 neurons to their final laminar positions (Agmon et al., 1993; Rebsam et al., 2002), followed by the growth of TCAs to these layer 4 cells where they stop to elaborate complex axonal arbours. To extend our examination of the role of synaptic activity to these later steps of thalamic fibre stopping and initial layer detection we have exploited the use of an ex vivo heterochronic explant system (Yamamoto et al., 1989; Molnár and Blakemore, 1991, 1999; Bolz et al., 1992; Yamamoto et al., 1992). The specific termination and branching of TCAs invading layer 4 are preserved, although the area specificity of these connections appears to be lost in the ex vivo system (Molnár and Blakemore, 1999; Yamada et al., 1999, 2000; Yamamoto et al., 2000a,b; Yamamoto, 2002; Poskanzer et al., 2003). Using this explant co-culture system, we demonstrate here that the ex vivo development of thalamocortical connections, extending from SNAP-25 deficient thalamus into wild type cortex, proceeds normally in the absence of Ca2+-triggered, action potential-dependent neuroexocytosis, suggesting that the innervation of thalamic projections and their initial recognition of appropriate cortical landmarks does not require regulated synaptic transmission.
Methods
Animals
All animal experiments were approved by a local ethical review committee and conducted in accordance with personal and project licenses under the U.K. Animals (Scientific Procedures) Act (1986).
PCR genotyping of SNAP-25 deficient and control fetuses
Snap25−/− mutant fetuses, removed by caesarean section at E16–18, were initially distinguished from control heterozygote and homozygote wild type littermates by their lack of spontaneous movement and response to a pinch to a limb. To confirm the genotype of “non-moving” fetuses and to determine the genotype of the control fetuses, PCR genotyping was performed on genomic DNA prepared from ear or post-mortem tail biopsies. For technical details please see Washbourne et al., (2002).
Preparation, maintenance and analysis of thalamus/cortex heterochronic co-cultures
Co-cultures were prepared as described as generally described in previously published protocols (Yamamoto et al., 1989, 1992; Molnár and Blakemore, 1991, 1999; Rennie et al., 1994). As depicted in Fig.1, the heterochronic co-cultures consisted of thalamic explants taken from fetuses (E16–E17) resulting from time mated pregnancies of Snap25+/− × Snap25+/− mice (backcrossed for 7 generations to C57BL/6; Washbourne et al., 2002) and cortical explants were obtained from P3–P4 wild type C57BL/6 mice.
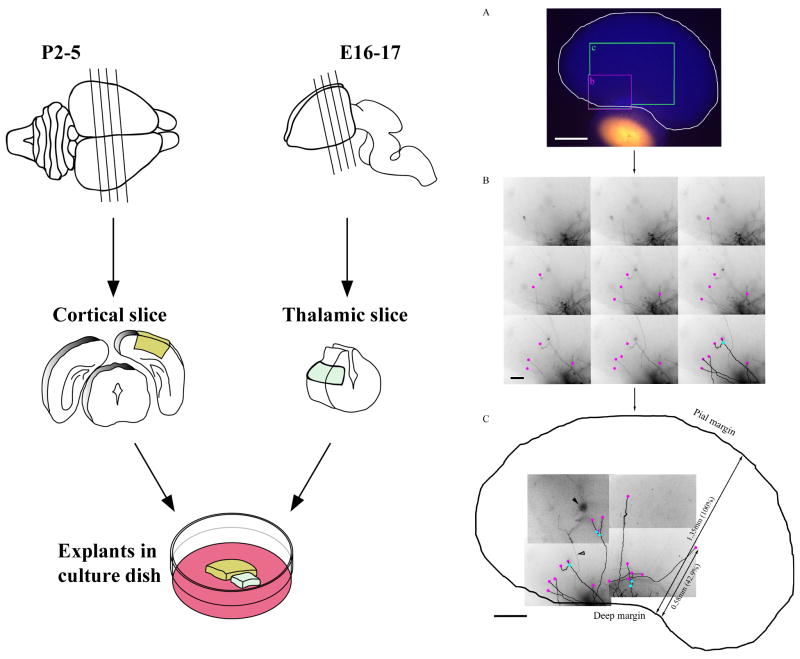
Figure 1. Preparation of the cultures (left) and an example for the measurement of thalamic fibres (right).
Left panels: Schematic diagram illustrating the preparation of heterochronic thalamocortical co-cultures. P2–5 wild-type C57 Bl/6 cortical slices (300 to 500 μm) were prepared by manual dissection using a microscalpel. Cortical pieces from the putative somatosensory cortices were dissected and transferred to prepared tissue culture inserts. E16–17 brains (from Snap25 wild-type, heterozygote, or knockout mice) were dissected so as to remove the dorsolateral aspect of the dorsal thalamus (putatively containing the LGN and VB). These explants were positioned adjacent (that is ≤ 2 mm) to the white matter side of the cortical slice in a culture dish and grown at 5% CO2, 100% humidity, and 37°C for 7 days.
Right panels: Example of a co-culture in which the thalamocortical axon architecture was measured. Co-cultures were grown and labelled as illustrated in left panels. A. Low power digital micrographs of both bis-benzimide (appear blue) and DiI (appear orange) labelling of the co-cultures. An outline figure showing the whole co-culture, thalamic explant, and cortical slice was generated from the bis-benzimide labelling pattern using Adobe Photoshop. B. Axons were visualised through the cortical slice thickness, using a 20× objective lens and N3 filter cubes and assessed in terms of clarity and obstruction by cortical cell neurites. Adobe Photoshop was used to assist in observing the axons converting the colour images to greyscale, negative inverting (i.e. black becomes white and vice versa), and adjusting each frame as a whole for gamma balance. By aligning the images, axons could be followed through the series and their termination and bifurcation points were marked (purple and cyan, respectively). C. In order to follow axons throughout the entire co-culture a montage of z-series were acquired. This was a vital component of the method as it allowed the differentiation between thalamocortical and corticofugal axons, note the corticofugal axon (open arrowhead) emanating from the cortical cell (filled arrowhead). Each termination and bifurcation point was measured with respect to the pial and deep margin of the cortical extract. To account for different cortical thickness this was expressed as a percentage rather than the absolute value. Scale bars: A – 500 μm, B – 100 μm, and C – 250 μm
Fetal dorsal thalamic explants
Time mated pregnant mice were killed by cervical dislocation and the embryos removed by caesarean section. To identify homozygous Snap25 null mutant (Snap25−/−) fetuses, the mice were tested for reaction to tactile stimuli and those unable to move in reaction to a mild forceps pinch were assumed to be Snap25 null. All the genotypes were subsequently confirmed by PCR analysis using genomic DNA obtained post mortem from tails (see Washbourne et al., 2001). The number of mice with genotypes Snap25+/+, Snap25+/−, Snap25−/− used for this study were: E16 (8,10,16; E17 (12, 14, 24); E18 (2,8,6); P2–4 (35,0,0) for genotypes respectively. The embryos were placed in ice cold oxygenated artificial cerebro-spinal fluid (ACSF) and all subsequent manipulations were performed in this solution. The brains were dissected from the heads and dorsal thalamic explants were obtained by manually dissecting the brain.
Cortices were pushed anterior-laterally to reveal the dorsal thalamus and the pia was removed to expose the dorsal thalamus. Thalamic regions containing the VB and LGN (but aiming not to include putative P0, see Molnár and Blakemore, 1999) were dissected using a micro-surgical scalpel into pieces typically 800 μm3 that were further cut into at least two pieces. All tissue from prepared by either manual dissection or microtome sectioning were collected in ice cold, oxygenated ACSF.
Neonatal cortical explants
Postnatal mice, P2–P4, were anaesthetised on ice and decapitated. The brains were dissected in ice cold ACSF and cortical slice explants were prepared from somatosensory cortex approximately 400 μm apart (see Molnár and Blakemore, 1999 for technical details).
Preparation of the culture plates, positioning the explants, and incubation
6 well tissue culture plates were prepared using either 30 mm Millicell Organotypic PTFE 0.4 μm pore size inserts (Millipore, Billerica, MA USA) or 24mm Transwell-COL Collagen-Coated 0.4 μm pore PTFE Membrane Insert (Corning, Acton, MA USA). The inserts were pre-incubated for at least 1 hour at 37°C (5% CO2) with tissue culture medium: DMEM/F-12 Ham (Sigma, Poole, UK) supplemented with 2.4 mg/ml D-glucose, 50 mg/ml penicillin, 50 mg/ml streptomycin, 5% fetal calf serum, 1% N2 supplement, Gibco/Invitrogen, Paisley, UK). Sufficient media was added to the well to maintain a thin film over the surface of the insert membrane during pre-equilibration. Four cortical and four thalamic explants were placed on each membrane. We did not observe any noticeable differences in cultures using E16,17 or 18 thalamic explants (similarly as described in Molnár and Blakemore, 1999). The co-cultures were grown for between 5 and 14 days with the medium being changed every 48–72 h. After this period the cultures were fixed with 4% PFA at room temperature for at least 2 h. Prior to carbocyanine dye tracing they were stored at 4°C in 4% PFA or PBSA.
Labelling and visualisation of the co-cultures
Following the fixation of the co-cultures in 4% PFA, fibres emanating from the thalamic into the cortical explant were visualised by DiI labelling (Molnár et al., 2006). Small crystals of DiI (1,1-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate, Invitrogen) were inserted into the thalamic explant and the co-culture was incubated for 7 days at 37°C in 2% PFA or PBSA to allow tracer diffusion. The cultures were then counterstained with bis-benzimide (2.5 μg/ml in PB for 10 min), mounted on gelatine subbed slides, coverslipped under PB and sealed to prevent evaporation. The cultures were visualised with a Leica DM upright fluorescent microscope. Where TCAs were visible images were acquired using a 20X objective with a Leica DC500 digital camera with IM50 software in a z-series to allow the path of axons to be followed throughout the thickness of the culture. We used this image acquisition rather than confocal microscopy because of the considerable thickness of the cultures, that would have required large number of focal planes for the analysis).
Quantitative assessment of axonal architecture and statistical analysis
Using Adobe Photoshop, and referring to 5× and 10× power images, 20× objective lens z-series stacks from individual co-cultures were arranged to form composite pseudo three-dimensional micrographs, which were superimposed onto an outline of the entire co-culture (Fig. 1). In order to compare the complexity of axon arborisation, the fibres were first categorised into four groups: “unbranched”, “simple” (3 or fewer branches), “complex” (4 or more branches), and “marginal” that corresponded to TCAs which progressed around the margin of the cortical slice explant regardless of the number of branch points; examples are shown in Fig. 2.
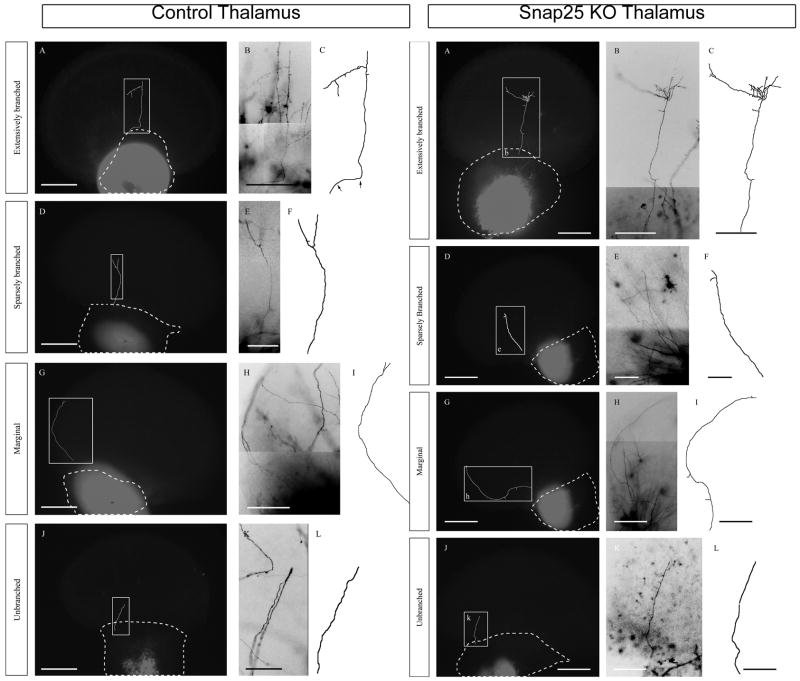
Figure 2. Examples of axon outgrowth patterns from control (Snap25+/+ and Snap25+/−) thalamic explant (left columns) and Snap25−/− thalamic explant (right columns) co- cultures.
P2 wild-type cortical slices were co-cultured with either control E16 (Snap25+/− or Snap25+/+) left panels or E16 Snap25−/− dorsal thalamic explants (left panels) for 7 days in vitro. Following fixation, DiI crystals were placed in the thalamic regions of the cultures to label axons connecting the thalamus and cortex; biz-benzimide counterstaining was also utilised to label the cytoarchitecture of the co-cultures. Dotted lines depict the boundaries of thalamic explants. Axons observed projecting from the thalamic explant into the cortical slice were drawn from high power images, and mapped onto outline images of the co-culture. The examples in both left and right panels illustrated represent the typical range of axon morphology seen, which were arbitrarily categorised based on the complexity of their axonal arbours and direction of growth into the four groups shown (extensively branched, sparsely branched, marginal, unbranched). The examples illustrated represent the typical range of axon morphology. The first column in both Control and Snap25 KO panels shows a lower power image of the co-cultures (DiI labelling appear white in black and white images) superimposed are boxes representing the enlarged regions shown in the second column (colour inverted and greyscaled micrographs) and the reconstructed axon in column three. Panels A–C: “extensively branched” axons projected directly into the mid-layer of the cortex, and elaborated many axon branches. Arrows in this panel of control show the region of the axon that progressed tangential to the pial surface prior to turning to grow radially into the cortical slice. D–F: “sparsely branched” axons projected directly into the mid-layer of the cortex but only elaborated one of two branches. G–I: “marginal” axons projected into cortex but extended along the edge of the slice, occasional bifurcations were observed. J–K “unbranched” axons projected directly into the cortical slice and terminated in the deep through middle layers however they did not form branches. The proportions of these thalamic fibre growth and branch patterns were similar when cultured with control or Snap25 KO thalami. Scale bars: A, D, G and J – 500 μm, B, C, H, and I – 250 μm, E, F, K, and L – 125 μm.
To score the position of axon bifurcation and termination, each point was marked on the outline view (Fig. 1) and measured with respect to the distance from edge of the thalamic block. The position was then expressed as a percentage of the total distance between the thalamic block and pial margin of the cortical explant. Concurrently to marking these features of axonal architecture, thalamocortical fibres were reconstructed in 2-dimensions using drawing features of Adobe Photoshop and Adobe Illustrator. Comparison of the different groups was performed using the Students t-test for statistical significance. This was done in three ways: averaged per culture, per axon, or, per branch or termination point. An example of this analysis is shown in Fig. 1 for the quantification of 10 axons identified in a cortical slice of a representative co-culture with a Snap25−/− thalamic explant (depicted in Fig. 1, panels A–C). To visualise the distribution of the axon branch or termination points through the cortical thickness, data were pooled between groups (i.e. genotype for Snap25−/− or control cultures) and then placed into 5 bins, each representing 20% of the cortical thickness. The distribution of total points was then represented graphically. To provide a further analysis of axon morphology, axons were traced and the number of branch points per fibre was counted. The mean numbers of branch points were calculated and the control and Snap25−/− groups were compared using the Mann-Whitney non-parametric test.
Results
Development and general characteristics of the thalamocortical co-cultures
Thalamocortical heterochronic co-cultures were set up to provide an ex vivo model of the thalamic axon termination and initial branching that occurs during the first week of postnatal development. In these cultures, explants from either Snap25 null mutant or control (heterozygous or wild type) fetuses were placed adjacent to cortical sections isolated from wild type P2–4 wild type neonates. In order to analyze in detail only those co-cultures that successfully reflected normal development over this period, we established several criteria. First, co-cultures had to appear healthy, free of contamination and therefore remained attached to the filter substrate throughout processing. Secondly, we eliminated all co-cultures that showed exuberant cortico-thalamic axon ingrowth evidenced by backlabelled cortical cells that obscured any TCAs, (supplementary figure S1, panels A and B). Based on these criteria, we selected and performed detailed quantitative analysis on 15 control and 13 knockout co-cultures.
At 7 DIV, the co-cultures were collected, fixed with paraformaldehyde, and labelled with thalamic explant DiI crystal placements, before counterstaining with bis-benzimide (see Methods). Our initial examination showed that both control and knockout thalamus co-cultures exhibited a large range of axonal morphology that extended from deeply penetrating fibres with extensive terminal arbours to short, unbranched axons. Based on these observations we developed four categories that were used to describe individual axonal processes. Representative examples of projections that were distinguished and classified in this manner from both control and Snap25−/− thalamus co-cultures are shown in Figure 1 left and right panels, respectively. Tracings of individual axons showed that thalamic projections typically entered into the cortical slice and then grew radially towards the pial margin (Fig. 2, panels C, F and L). In a few cases, however, fibres were observed to have grown around the lateral periphery of the cortical slice and were consequently termed marginal (Fig. 2I). The number and the relative proportion of reconstructed fibres assigned to each of these groups in both control and SNAP-25 deficient projections were very similar. The number of reconstructed axons were: extensive branching (11,11); sparse branching (21, 19); unbranched (75,50); marginal (6,2) for control and for Snap25−/− respectively. What was clear from this initial characterization is the largest proportion of fibers that were traced, both innervating from mutant and wild type, were unbranched projections, followed by those categorized by sparse branching, and that projections exhibiting extensive branching was only reflected by a minority of projections from either geneotype.
DiI labels neural fibres bidirectionally by virtue of lateral diffusion through the cell membrane, independent from the direction of normal cell trafficking (Godement et al., 1987; Molnár et al., 2006). This property often led to backfilling of cortical neurons and labelling of axons that projected into the thalamic region of the co-culture. The number of neurons stained in this manner varied greatly between co-cultures. In some co-cultures, the labelled cells were so numerous that they obscured TCAs and prevented their analysis, thereby eliminating that co-culture from this study. Interestingly, in these cases the distribution of the labelled neurons appeared broad, in both the ventricular to pial and medial to lateral direction across the cortical thickness (supplementary Fig. S1). However, in the upper apical region of the cortical explant, corresponding to layers 1–4, these neurons were relatively sparse. We examined several of these neurons in more detail and found that they were typically pyramidal, with varying dendritic morphology, but could be differentiated by lateral branching of tufts at the distal end of their apical dendrite. Nevertheless we saw no noticeable difference in the number or distribution of backfilled cortical neurons, or their projections, in cultures made with either Snap25−/−mutant or control thalamic explants (suppl. Fig. S1 panels B, D, F).
Quantitative analysis of co-cultures
In contrast to development in vivo, the characteristic lamination pattern of the P2–4 cortex was not readily seen in all of the cortical slices of co-cultures after 7 DIV similarly as reported previously (Molnár and Blakemore, 1999). Although careful observation did reveal evidence for residual cortical layering in most cultures, cellular density and cytoarchitecture from the ventricular to the pial margin appeared homogenous when assessed by fluorescent microscopy after bis-benzimide staining. Because this presented a difficulty in assigning cortical layers based on distinctive morphometric landmarks that remain in acute brain slices, we therefore devised a whole slice thickness method to compare axonal architecture phenotypes between Snap25 null and heterozygote or wild-type co-cultures. For this purpose, we estimated the depth of the projection from the position of the terminus, and the position of the branchpoints relative to the thickness of the cortical section (expressed as % distance).
In addition to calculating the mean positions of termination and branch points, the distribution of termination and branch points were evaluated by splitting the cortical sheet into 5 bins of equal proportional width (i.e. 0–20%, 20–40%, etc). The number of features in each bin was counted, and the distribution of each feature was expressed as a percentage of the total number counted within each bin.
Position of termination and bifurcation of control and SNAP-25 deficient thalamic projections
The anatomical features of the co-cultures were measured, as described above, blind to their genotype. They were then sorted for comparison into control (Snap25+/+ and Snap25+/−)and SNAP-25 deficient (Snap25−/−) thalamic explant co-cultures. Initially, the mean position and number of termination and branch points in each co-culture were calculated and then averaged to derive the population mean for control and SNAP-25 deficient co-cultures. There was no significant difference between the mean axon termination point of projections from control and Snap25−/− null mutant thalamic explants (control 34.1% ± 4.24, n=15; Snap25−/− 35.6% ± 2.8 n=13; p=0.773, unpaired Students t-test).
The mean length of projection into the cortex was also calculated based on the total individual termination points per genotype, which favours co-cultures with more distinguishable projections. Again, this analysis showed no difference between the projections from control and SNAP-25 deficient thalamus (control, 38.7% ± 1.34 n=201; Snap25−/− 41.2% ± 1.32 n=184; p=0.180)
The position of the axon bifurcation points were compared both on the basis of cultures analysis and total individual events as described above. Similarly, these measurements also showed no significant difference where control and SNAP-25 deficient thalamic projections branched in the overlaying cortical section. The position of branch points averaged from each co-culture was 41.1% ± 4.73 (n=10) for controls compared to 37.0% ± 4.43 (n=10) for Snap25−/− thalamus co-cultures (p=0.528). Virtually the same result was obtained when means were calculated on the basis of total branch points, where the mean position for control axon branch points was 42.9% ± 2.17 (n=74) and for Snap25−/−axons was 43.3% ± 1.68 (n=94) (p=0.90).
Distribution of termination and bifurcation points of thalamic projections
Because the above analyses of the mean termination and branch point positions did not show any significant differences between the two groups of co-cultures, we considered whether this was due to the large range and broad distribution of these features of axonal architecture throughout the cortical slice. We therefore analyzed the distribution of the axon termination and branch points, as described in the Methods, by dividing the cortex into 5 layers of equal width, starting from the edge juxtaposed to the thalamic explant, and counting within each layer the number of these features made by the DiI labelled innervating thalamic projections. As previously performed for the position of these features, we calculated the distribution of bifurcation and termination points both by averaging the number of these events observed in independent cultures and on the basis of the total scored as individual events.
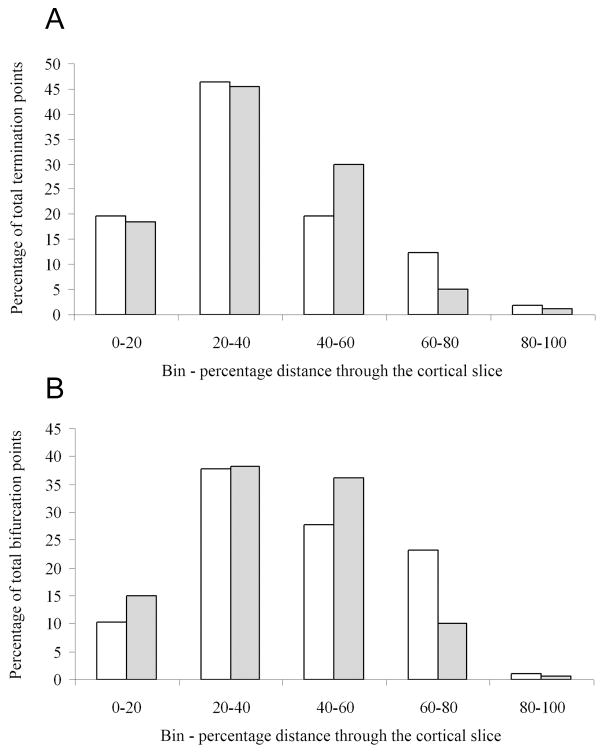
As shown in Fig. 3A, the termination points of thalamic projections, calculated on the basis of total observed terminals scored for control and SNAP-25 deficient co-cultures (white and grey bars respectively), was widely distributed across the cortical slice, with endpoints observed in all five bins. Nevertheless, the relative number of termination points for axonal projections of both control and null mutant co-cultures peaked in the 20–40% bin, with 46% and 45% of the endpoints, respectively. Importantly, approximately equal amounts axonal termination points were observed for control and SNAP-25 deficient projections throughout the more superficial regions, albeit almost exclusively in the 40%–80% bins, confirming that SNAP-25 deficient projections, as controls, were able to effectively innervate the outer layers of the cortical slices. When the same analysis was performed for the distribution of branch points (Fig. 3B), we observed a similar general trend as seen for termination points although the distribution appeared broader with a greater percentage of bifurcations located in the superficial layers (e.g. bins 40–60% and 60–80%). Interestingly, the relative proportion of SNAP-25 deficient projections either terminating or branching within the outer level of the cortical slice (demarcated as the 60–80% bin) appeared relatively decreased (with a concomitant increase in the 40–60% bin), with respect to controls. While this may suggest that the projections from the null mutant thalamus could have a tendency to innervate neurons populating somewhat deeper layers of the developing cortex, this observation clearly shows that axons lacking SNAP-25 and unable to develop action potential-dependent synaptic transmission, do not simply extend towards the pial surface.
Figure 3. A and B: Histograms show the distribution of termination (A) and branch points (B) through the cortical slice of Snap25 control (white bars, wild-type P2 cortex and wild-type or Snap25−/+E16 thalamic explant) and knockout (filled bars, wild-type P2 cortex and Snap25−/−E16 thalamic explant) co-cultures.
The distributions of termination and branch points of thalamic axons in the cortical slice were plotted for each of 5 equal bins representing 20% of the cortical thickness. The figures show the mean percentage of points per bin, which was calculated using the standardised data from each co-culture.
A: Termination points show a broad distribution and both control (white bars) and knockout (filled bars) fibres and they both peaked in the 20–40% bin. Control cultures N = 15, knockout cultures N = 13, control culture termination points N = 201, knockout culture termination points N = 184.
B: Distribution of branch points. White bars represent control axons, filled bars – knockout axons. The branch points mirrored the terminations described above, although in both control and knockout fibres the peak was broader essentially covering both the 20–40% and 40–60% bins. Control cultures N = 10, knockout cultures N = 10, control culture termination points N = 74, knockout culture termination points N = 90.
Analysis of axon branch number
Although the previous analyses of the mean positions and of the distribution of the termination and bifurcation points showed no significant differences between control and Snap25−/− thalamic co-cultures several reports have indicated that neural activity has an instructive role, not only the laminar termination and branch pattern, but also the frequency of arborisation (Wilkemeyer and Angelides, 1996; Lee et al., 2005b). In order to investigate this facet of TCA development in the co-cultures, we performed a more detailed morphometric analysis on a selection of axons traced onto outlines of co-cultures to calculate the number of branch points per axon. For comparative analysis, we evaluated both sets of axons that either contained or excluded unbranched axons, which represented the majority of axons in control and mutant co-cultures (Table 1). The distribution of number of branches scored per axon and the mean number of branch points per axon for control and SNAP-25 deficient co-cultures was similar (presented in Table 1). Non-parametric analysis of the mean number of branch points, and the distribution of the frequency of the these branch points, demonstrated no significant difference between control and Snap25 null mutant projections, whether or not unbranched axons were included (U=3537, p=0.42 for all axons; U=757, p=0.18 branched axons only, Mann-Whitney U test; See Table 1 for details).
Discussion
The ex vivo approach of using a heterochronic explant co-culture system has allowed us to extend our earlier investigation of the role of action potential-driven synaptic activity in forming thalamocortical circuitry into the initial postnatal period of brain development in the mouse (Molnár et al., 2002). The use of this paradigm was necessitated by the fact that Snap25 null mutant mice die at or before birth, presumably from respiratory failure (Washbourne et al., 2002), a finding common to other mouse mutants lines bearing disrupted genes encoding presynaptic proteins (Verhage et al., 2000; Varoqueaux et al., 2002; Washbourne et al., 2002; Varoqueaux et al., 2005; Schoch et al., 2001). This strategy could therefore be useful to evaluate the effects of these other mutations that disrupt neurotransmission at different steps of synaptic vesicle exocytosis, resulting, for example, in variable effects on spontaneous action potential–independent quantal release (Verhage et al., 2000; Varoqueaux et al., 2002; Washbourne et al., 2002; Varoqueaux et al., 2005). Moreover, the use of heterochronic co-culture systems would complement the development and use of regional-specific, temporally regulated conditional mutations.
Within our timeframe studied, we observed that the laminar position and distribution of fibre termination and branch points, and number of branches per axon made by SNAP-25 deficient thalamus into cortical slices were indistinguishable from control thalamic explants derived from heterozygous and wild type fetal mice. This result was somewhat unexpected given prior studies using TTX to block synaptic activity in the developing cat brain (Herrmann and Shatz, 1995), and in similar thalamocortical co-cultures (Wilkemeyer and Angelides, 1996; Anderson and Price, 2002). These studies have shown that TTX mediated blockade prevents the proper termination pattern of TCAs leading to their unrestrained growth laterally through layer 4 ultimately reaching the pial surface. Several observations suggest that the apparent termination of thalamic projections in the co-culture system was unlikely to result from limitations of the ex vivo culture system. First, the growth rate of axons within the developing cortex, 20–40 μm/h (Yamamoto et al., 1997; Skaliora et al., 2000), would provide ample time within seven days of culture to reach the pia. Secondly, we found that the conditions of culturing were sufficiently permissive to allow extension of several fibres to the most superficial regions of the cortex, including the pial surface. Although clearly this was seen for very few fibres, the number of these projections within the outer most 20% of the cortical slice appeared comparable between mutant and control groups (see Fig. 3A), indicating that lack of SNAP-25 did not increase the frequency of ectopic extension. Interestingly, although we found that the overall distribution of these termination points did not differ by genotype, fibres from control thalamus appeared to be more likely than SNAP-25 deficient fibres to terminate in the next superficial layer corresponding the 60–80% bin of the cortical section. In fact this would be in contrast to the expectation that SNAP-25 deficient projections, by virtue of not being capable of evoked synaptic transmission, would not be able to elicit and recognize the appropriate cortical landmarks and thereby extend without guidance to the outermost surface of the cortex.
Activity dependent regulation of growth permissive and restrictive molecules has been postulated several years ago, and many of these have been shown to be present in membrane preparations (Takemoto et al., 2002; Yamamoto et al., 2000b). TCAs have been shown, for example, to be able to terminate and branch with layer specificity in fixed cortical slices where the expression of these molecules is preserved (Yamamoto et al., 2000a; Yamamoto et al., 2000b). In this approach, and other further reduced systems growing thalamic explants on substrates bearing membrane extracts (Mann et al., 1998; Mann et al., 2002; Takemoto et al., 2002) thalamic axons are exposed to fixed growth signals, whereas our co-culture of heterochronic explants allows for a dynamic interchange of signalling between the outgrowing fibres and their presumptive targets (Uesaka et al., 2006; Yamada et al., 2010).
Snap25 null mice provides a refined model to address the role of neuroexocytosis in neural development. For example, although previous studies reported that knockdown of Snap25 by antisense oligonucleotides could prevent neurite outgrowth (Osen-Sand et al., 1993), detailed analysis of SNAP-25 deficient mice showed various neural systems formed projections normally (Molnár et al., 2002; Washbourne et al., 2002). Further, electrophysiological analyses of central and peripheral Snap25−/− synapses showed that while the action potential-evoked neurotransmission was essentially ablated, axons were still capable of conducting stimulated action potentials (Molnár et al., 2002) and of spontaneously releasing neurotransmitter, albeit to varying extents at different synapses (Washbourne et al., 2002; Tafoya et al., 2006; Degardo-Martinez et al., 2007; Bronk et al., 2008).
Our experiments raise the question of what cellular function(s), if any, is retained by SNAP-25 deficient thalamic projections that allows them to navigate a correct topographical trajectory to invade the cortex (Molnár et al., 2002) and subsequently recognize appropriate stop signals once they have successfully begun their radial extension to the developing layer 4 of the cortex (this paper). In the native system, synaptic interactions between TCAs and the cortex can be detected from E18 in rats (equivalent to E17 in mice), these develop over the course of the first postnatal week (Higashi et al., 2002; 2005; Molnár et al., 2003). Spontaneously generated signals in the retina system which occur in the late stages of embryogenesis in mice and rats (Mooney et al., 1993; Wong et al., 1993; Mooney et al., 1996) are known to alter the development of thalamocortical fibre deployment (Huberman et al., 2006) indicating that the thalamus already acts as a relay nucleus at an embryonic stage of development. In contrast, the somatosensory system is more mature at birth (Erzurumlu and Killackey, 1983; Schlaggar and O’Leary, 1994), and therefore direct stimulation from the periphery is likely to influence TCA development. Measurements of neural firing patterns in ex vivo cultures, however, are relatively sparse, although both thalamic and cortical neurons in co-cultures are capable of firing action potentials (Yamamoto et al, 2007; Yamada et al., 2010) and that NMDA mediated synaptic transmission develops between the thalamus and the cortical explants (Yamada et al., 1999). In the case of our chimeric explant co-cultures we can not exclude the role of evoked release of transmitter or guidance factors from the wild type cortical neurons encountered by thalamic projections. Nevertheless, because SNAP-25 deficient neurons are unable to release neurotransmitter in response to the arrival of action potentials at presynaptic terminals, this would suggest that axonal navigation through the cortex and recognition of layer 4 followed by initial arborisation is not dependent on synaptic signalling from these invading fibres. As our study only explored the stop and initial branch development in a cortical layer-detection culture essay (Molnár and Blakemore, 1999), longer culturing periods would be required to study further steps of branch elaboration and remodelling (Uesaka et al., 2007). Nevertheless, these results are compatible with other systems (e.g. retino-tectal projections) where it has been demonstrated that exocytosis-deficient munc18-1−/− axons are able to select their targets appropriately (Nicol et al., 2007).
Beyond action potential triggered synaptic transmission, other signalling events may be expressed by thalamic axons to communicate with cortical cells and solicit instructive guidance. For example, the demonstrated effect of TTX leading to aberrant axonal extension may reflect the need for transmission of neural impulses along growing fibres for appropriate TCA laminar targeting. This could act through electrical synapses; however, observations of early thalamocortical interactions, including the transient subplate interactions do show that the post-synaptic responses are likely to be chemically mediated (Friauf et al., 1990; Hanganu et al., 2001, 2002; Higashi et al., 2002; Molnár et al., 2003; Kanold, 2004). Alternatively, long-term effects of TTX blockade of action potentials could act by limiting depolarization-dependent Ca2+ influx at presynaptic terminals which has been shown to down regulate protein ubiquitination and turnover of synaptic proteins (Chen et al., 2003). This could lead to decreased levels of those proteins interpreting instructive signals in thalamic axons or those proteins expressed by cortical cells which issue these instructions. Evidence suggests that non-synaptic spontaneous activity changes both transcriptional programs (Spitzer, 2012) and second messenger signaling downstream of axon guidance molecules (Ming et al., 2002; Nicol et al., 2007; Nicol et al., 2011). The idea that spontaneous, action potential-independent synaptic transmission might be sufficient to elicit guidance signals from cortical neurons should be considered.
Supplementary Material
Acknowledgments
We are grateful to Dr Juan Small and Dr Guillermina López-Bendito for their help with the initial attempts of the co-culture experiments. ZM is grateful to Eleanor Grant for her help with Photoshop. The work was supported from the Wellcome Trust Graduate Studentship (DB), MRC Program Grant and McDonald-Pew Centre of Cognitive Neuroscience, Oxford (Z.M.), and the NIH (R01 MH48989 M.C.W.).
References
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Agmon A, Yang LT, O’Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Price DJ. Layer-specific thalamocortical innervation in organotypic cultures is prevented by substances that alter neural activity. Eur J Neurosci. 2002;16:345–349. doi: 10.1046/j.1460-9568.2002.02069.x. [DOI] [PubMed] [Google Scholar]
- Arber S. Subplate neurons: bridging the gap to function in the cortex. Trends Neurosci. 2004;27:111–113. doi: 10.1016/j.tins.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Bark IC, Wilson MC. Regulated vesicular fusion in neurons: snapping together the details. Proc Natl Acad Sci U S A. 1994;91:4621–4624. doi: 10.1073/pnas.91.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J, Novak N, Staiger V. Formation of specific afferent connections in organotypic slice cultures from rat visual cortex co-cultured with lateral geniculate nucleus. J Neurosci. 1992;12:3054–3070. doi: 10.1523/JNEUROSCI.12-08-03054.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J, Uziel D, Muhlfriedel S, Gullmar A, Peuckert C, Zarbalis K, Wurst W, Torii M, Levitt P. Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. J Neurobiol. 2004;59:82–94. doi: 10.1002/neu.10346. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Robertson RT, Killackey HP. Early ingrowth of thalamocortical afferents to the neocortex of the prenatal rat. Proc Natl Acad Sci U S A. 1991;88:2999–3003. doi: 10.1073/pnas.88.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER, An S, Barton N, Jahn R. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994;269:27427–27432. [PubMed] [Google Scholar]
- Chen H, Polo S, Di Fiore PP, De Camilli PV. Rapid Ca2+-dependent decrease of protein ubiquitination at synapses. Proc Natl Acad Sci U S A. 2003;100(25):14908–13. doi: 10.1073/pnas.2136625100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Killackey HP. Development of order in the rat trigeminal system. J Comp Neurol. 1983;213:365–380. doi: 10.1002/cne.902130402. [DOI] [PubMed] [Google Scholar]
- Fairen A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol. 1986;251:67–83. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- Fox K, Schlaggar BL, Glazewski S, O’Leary DD. Glutamate receptor blockade at cortical synapses disrupts development of thalamocortical and columnar organization in somatosensory cortex. Proc Natl Acad Sci U S A. 1996;93:5584–5589. doi: 10.1073/pnas.93.11.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, McConnell SK, Shatz CJ. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. Segregation of geniculocortical afferents during the critical period: a role for subplate neurons. J Neurosci. 1994;14:3862–3880. doi: 10.1523/JNEUROSCI.14-06-03862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. Spontaneous synaptic activity of subplate neurons in neonatal rat somatosensory cortex. Cereb Cortex. 2001;11:400–410. doi: 10.1093/cercor/11.5.400. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci. 2002;22:7165–7176. doi: 10.1523/JNEUROSCI.22-16-07165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43(5):687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Shatz CJ. Blockade of action potential activity alters initial arborization of thalamic axons within cortical layer 4. Proc Natl Acad Sci U S A. 1995;92:11244–11248. doi: 10.1073/pnas.92.24.11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Antonini A, Shatz CJ. Ultrastructural evidence for synaptic interactions between thalamocortical axons and subplate neurons. Eur J Neurosci. 1994;6:1729–1742. doi: 10.1111/j.1460-9568.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Higashi S, Molnár Z, Kurotani T, Toyama K. Prenatal development of neural excitation in rat thalamocortical projections studied by optical recording. Neuroscience. 2002;115:1231–1246. doi: 10.1016/s0306-4522(02)00418-9. [DOI] [PubMed] [Google Scholar]
- Higashi S, Hioki K, Kurotani T, Kasim N, Molnár Z. Functional thalamocortical synapse reorganization from subplate to layer IV during postnatal development in the reeler-like mutant rat (shaking rat Kawasaki) J Neurosci. 2005;25:1395–1406. doi: 10.1523/JNEUROSCI.4023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Speer CM, Chapman B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in v1. Neuron. 2006;52:247–254. doi: 10.1016/j.neuron.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO. Transient microcircuits formed by subplate neurons and their role in functional development of thalamocortical connections. Neuroreport. 2004;15:2149–2153. doi: 10.1097/00001756-200410050-00001. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Krug K, Smith AL, Thompson ID. The development of topography in the hamster geniculo-cortical projection. J Neurosci. 1998;18:5766–5776. doi: 10.1523/JNEUROSCI.18-15-05766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land PW, Kandler K. Somatotopic organization of rat thalamocortical slices. J Neurosci Methods. 2002;119:15–21. doi: 10.1016/s0165-0270(02)00150-4. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Lo FS, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci. 2005a;25:2304–2311. doi: 10.1523/JNEUROSCI.4902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol. 2005;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Molnár Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Lund RD, Mustari MJ. Development of the geniculocortical pathway in rats. J Comp Neurol. 1977;173:289–306. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Fenstermaker V, Watson BO, Yuste R. A visual thalamocortical slice. Nat Methods. 2006;3:129–134. doi: 10.1038/nmeth849. [DOI] [PubMed] [Google Scholar]
- Mann F, Zhukareva V, Pimenta A, Levitt P, Bolz J. Membrane-associated molecules guide limbic and nonlimbic thalamocortical projections. J Neurosci. 1998;18:9409–9419. doi: 10.1523/JNEUROSCI.18-22-09409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Peuckert C, Dehner F, Zhou R, Bolz J. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129:3945–3955. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29(2):441–52. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417(6887):411–8. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Blakemore C. Lack of regional specificity for connections formed between thalamus and cortex in co-culture. Nature. 1991;351:475–477. doi: 10.1038/351475a0. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Blakemore C. Development of signals influencing the growth and termination of thalamocortical axons in organotypic culture. Exp Neurol. 1999;156:363–393. doi: 10.1006/exnr.1999.7032. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Blakey D, Bystron I, Carney RSE. Tract-Tracing in Developing Systems and in Postmortem Human Material Using Carbocyanine Dyes. In: Zaborszky L, Wouterlood FG, Lanciego JL, editors. Neuroanatomical Tract-Tracing 3: Molecules, Neurons, and Systems. New York: Springer Science+Business Media, Inc; 2006. pp. 366–393. [Google Scholar]
- Molnár Z, Kurotani T, Higashi S, Yamamoto N, Toyama K. Development of functional thalamocortical synapses studied with current source-density analysis in whole forebrain slices in the rat. Brain Res Bull. 2003;60:355–371. doi: 10.1016/s0361-9230(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Molnár Z, López-Bendito G, Small J, Partridge LD, Blakemore C, Wilson MC. Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity. J Neurosci. 2002;22:10313–10323. doi: 10.1523/JNEUROSCI.22-23-10313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Madison DV, Shatz CJ. Enhancement of transmission at the developing retinogeniculate synapse. Neuron. 1993;10:815–825. doi: 10.1016/0896-6273(93)90198-z. [DOI] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Moore JW, Scott WR. Tetrodotoxin Blockage Of Sodium Conductance Increase In Lobster Giant Axons. J Gen Physiol. 1964;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Muzerelle A, Rio JP, Métin C, Gaspar P. Requirement of adenylate cyclase 1 for the ephrin-A5-dependent retraction of exuberant retinal axons. J Neurosci. 2006;26(3):862–72. doi: 10.1523/JNEUROSCI.3385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Hong KP, Spitzer NC. Spatial and temporal second messenger codes for growth cone turning. Proc Natl Acad Sci U S A. 2011;108(33):13776–81. doi: 10.1073/pnas.1100247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Voyatzis S, Muzerelle A, Narboux-Nême N, Südhof TC, Miles R, Gaspar P. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10(3):340–7. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, Grenningloh G, Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnár Z. The development of cortical connections. Eur J Neurosci. 2006;23:910–920. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice. J Neurosci. 2002;22:8541–8552. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie S, Lotto RB, Price DJ. Growth-promoting interactions between the murine neocortex and thalamus in organotypic co-cultures. Neuroscience. 1994;61:547–564. doi: 10.1016/0306-4522(94)90433-2. [DOI] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- Scheller RH. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, O’Leary DD. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994;346:80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol. 1978;281:267–83. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaliora I, Adams R, Blakemore C. Morphology and growth patterns of developing thalamocortical axons. J Neurosci. 2000;20:3650–3662. doi: 10.1523/JNEUROSCI.20-10-03650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci. 2012;13(2):94–106. doi: 10.1038/nrn3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Fukuda T, Sonoda R, Murakami F, Tanaka H, Yamamoto N. Ephrin-B3-EphA4 interactions regulate the growth of specific thalamocortical axon populations in vitro. Eur J Neurosci. 2002;16:1168–1172. doi: 10.1046/j.1460-9568.2002.02166.x. [DOI] [PubMed] [Google Scholar]
- Uesaka N, Hirai S, Maruyama T, Ruthazer ES, Yamamoto N. Activity dependence of cortical axon branch formation: a morphological and electrophysiological study using organotypic slice cultures. J Neurosci. 2005;25:1–9. doi: 10.1523/JNEUROSCI.3855-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Hayano Y, Yamada A, Yamamoto N. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J Neurosci. 2007;27(19):5215–23. doi: 10.1523/JNEUROSCI.4685-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Ruthazer ES, Yamamoto N. The role of neural activity in cortical axon branching. Neuroscientist. 2006;12(2):102–6. doi: 10.1177/1073858405281673. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Polleux F. Developmental mechanisms patterning thalamocortical projections: intrinsic, extrinsic and in between. Trends Neurosci. 2004;27:384–391. doi: 10.1016/j.tins.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Sons MS, Plomp JJ, Brose N. Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse. Mol Cell Biol. 2005;25:5973–5984. doi: 10.1128/MCB.25.14.5973-5984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Bortoletto N, Graham ME, Wilson MC, Burgoyne RD, Montecucco C. Botulinum neurotoxin E-insensitive mutants of SNAP-25 fail to bind VAMP but support exocytosis. J Neurochem. 1999;73:2424–2433. doi: 10.1046/j.1471-4159.1999.0732424.x. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Cansino V, Mathews JR, Graham M, Burgoyne RD, Wilson MC. Cysteine residues of SNAP-25 are required for SNARE disassembly and exocytosis, but not for membrane targeting. Biochem J. 2001;357:625–634. doi: 10.1042/0264-6021:3570625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, López-Bendito G, Molnár Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Wilkemeyer MF, Angelides KJ. Addition of tetrodotoxin alters the morphology of thalamocortical axons in organotypic co-cultures. J Neurosci Res. 1996;43:707–718. doi: 10.1002/(SICI)1097-4547(19960315)43:6<707::AID-JNR7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yamamoto N, Toyama K. Development of membrane properties of rat neocortical neurons studied in organotypic co-cultures. Neurosci Lett. 1999;275:65–68. doi: 10.1016/s0304-3940(99)00745-4. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yamamoto N, Toyama K. Development of NMDA and non-NMDA receptor-mediated excitatory synaptic transmission in geniculocortical and corticocortical connections in organotypic co-culture preparations. Eur J Neurosci. 2000;12:3854–3862. doi: 10.1046/j.1460-9568.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- Yamada A, Uesaka N, Hayano Y, Tabata T, Kano M, Yamamoto N. Role of pre- and postsynaptic activity in thalamocortical axon branching. Proc Natl Acad Sci U S A. 2010;107(16):7562–7. doi: 10.1073/pnas.0900613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Higashi S, Toyama K. Stop and branch behaviors of geniculocortical axons: a time-lapse study in organotypic co-cultures. J Neurosci. 1997;17:3653–3663. doi: 10.1523/JNEUROSCI.17-10-03653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Tamada A, Murakami F. Wiring of the brain by a range of guidance cues. Prog Neurobiol. 2002;68:393–407. doi: 10.1016/s0301-0082(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Yamada K, Kurotani T, Toyama K. Laminar specificity of extrinsic cortical connections studied in co-culture preparations. Neuron. 1992;9:217–228. doi: 10.1016/0896-6273(92)90161-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Matsuyama Y, Harada A, Inui K, Murakami F, Hanamura K. Characterization of factors regulating lamina-specific growth of thalamocortical axons. J Neurobiol. 2000a;42:56–68. doi: 10.1002/(sici)1097-4695(200001)42:1<56::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Inui K, Matsuyama Y, Harada A, Hanamura K, Murakami F, Ruthazer ES, Rutishauser U, Seki T. Inhibitory mechanism by polysialic acid for lamina-specific branch formation of thalamocortical axons. J Neurosci. 2000b;20:9145–9151. doi: 10.1523/JNEUROSCI.20-24-09145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Maruyama T, Uesaka N, Hayano Y, Takemoto M, Yamada A. Molecular mechanisms of thalamocortical axon targeting. Novartis Found Symp. 2007;288:199–208. doi: 10.1002/9780470994030.ch14. discussion 208–11, 276–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.