Abstract
In primary sensory neocortical areas of the mammals, the distribution of sensory receptors is mapped with topographic precision and amplification in proportion to the peripheral receptor density. The visual, somatosensory and auditory cortical maps are established during a critical period in development. Throughout this window in time, the developing cortical maps are vulnerable to deleterious effects of sense organ damage or sensory deprivation. The rodent barrel cortex offers an invaluable model system to investigate mechanisms underlying the formation of topographic maps and their plasticity during development. Five rows of mystacial vibrissa (whisker) follicles on the snout and an array of sinus hairs are represented by layer IV neural modules (“barrels”) and thalamocortical axon terminals in the primary somatosensory cortex. Perinatal damage to the whiskers or the sensory nerve innervating them irreversibly alters the structural organization of the barrels. Earlier studies emphasized the role of sensory periphery in dictating whisker-specific brain maps and patterns. Recent advances in molecular genetics and analyses of genetically altered mice allow new insights into neural pattern formation in the neocortex and the mechanisms underlying critical period plasticity. Here we review the development and patterning of the barrel cortex and the critical period plasticity.
Keywords: cerebral cortex, trigeminal system, synaptic plasticity, whiskers, pattern formation, sensory deprivation, thalamus, activity-dependent mechanisms
The barrel cortex
In the primary somatosensory cortex of mice, layer IV neurons form cylindrical aggregates replicating the patterned array of whiskers on the contralateral snout. Rafael Lorente de Nó (1922) noted these cellular aggregates in layer IV of Golgi-stained mouse cortex and called them ‘glomérulos’ without localizing them to the somatosensory cortex (Fairén, 2007). Almost fifty years later Thomas A. Woolsey (1967) studied the mouse sensory cortex and associated Lorente de Nó’s glomeruli with whiskers. A few years later extensive studies performed by Thomas A. Woolsey and Hendrik Van der Loos (Woolsey and Van der Loos, 1970; Van der Loos and Woolsey 1973) revealed a one-to-one association of individual whiskers on the snout with cortical cellular aggregates, which they termed “barrels.” Around the same time, Herbert P. Killackey (1973), using anterograde axonal degeneration method, showed that in the rat, the barrels are filled with axon terminals arising from the ventroposteromedial (VPM) nucleus of the thalamus. Later studies in both species confirmed and detailed that layer IV stellate cells form the “barrel walls” and discrete patches or “bouquets” of VPM axon terminals fill them up (Killackey & Leshin, 1975; Erzurumlu & Jhaveri, 1990; Senft & Woolsey, 1991; Agmon et al., 1993; Rebsam et al., 2002).
The dendrites of barrel cells are preferentially oriented towards the barrel centers and embrace the thalamocortical terminals within the barrels (Woolsey et al., 1975, Steffen & Van der Loos, 1980; Jeanmonod et al., 1981; Datwani et al., 2002a). Pioneering electrophysiological recordings revealed that each barrel receives and processes information predominantly from a single whisker (Welker, 1971, 1976). Ensuing physiological studies consistently reported that neurons located within individual barrel columns are mostly responsive to deflection of the associated whiskers on the contralateral snout (Simons, 1978; Chapin & Lin, 1984; Simons, 1985; Armstrong-James & Fox 1987; Simons & Carvell, 1989; Armstrong-James et al., 1992; also reviewed in Petersen, 2007; Fox, 2008).
The distribution of barrels is best seen in tangential sections through the layer IV. The spatial arrangement of these neural modules across layer IV replicates that of the whiskers and sinus hairs on the snout. A similar but less distinct barrel-type patterning is present in the cortical areas representing the lower jaw, forepaw and hindpaw pads (Belford & Killackey, 1978; Dawson & Killackey, 1987; Killackey et al., 1995). When the mouse or rat cortex is flattened (Strominger & Woolsey, 1987; Dawson & Killackey, 1987), the entire face and body map and the barrel fields appear as a “musculus” or “ratunculus.” Today, the flattened cortex preparation is widely used to assess any alterations in patterning of the barrel cortex.
Development of subcortical whisker-specific neural patterns and the barrels in the cortex
The pioneering whisker follicle lesion studies of Van der Loos & Woolsey (1973) showed that each barrel corresponds to a single whisker on the contralateral face. The presence of whisker patterns in the subcortical structures and their malleability following whisker lesions were discovered later on (Van der Loos, 1976; Belford & Killackey 1979; 1981; Durham & Woolsey, 1984). The whisker-specific neural patterns in subcortical structures have been named “barreloids” in the VPM-VPL complex of the thalamus (Van der Loos, 1976) and “barrelettes” in the brainstem (Ma & Woolsey, 1984).
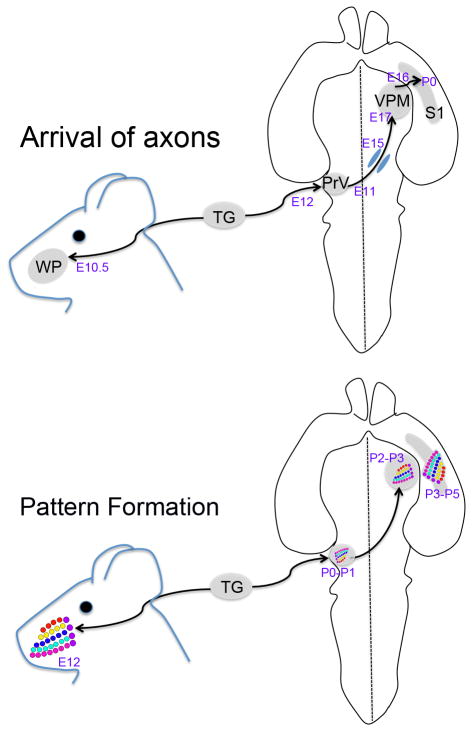
The topographic organization of the barrels in the neocortex is fashioned after the distribution of five rows of whiskers, each row with constant number of follicles. The infraorbital nerve (ION) of the maxillary division of the trigeminal nerve innervates all of the whiskers and sinus hairs on the whisker and furry buccal pads. Central axons of the trigeminal ganglion (TG) neurons contributing to the ION convey whisker-specific information to the trigeminal brainstem nuclei. Of these brainstem nuclei, the principal sensory nucleus of the trigeminal nerve (PrV) plays the main role in transferring whisker-specific patterning to the contralateral thalamus, the VPM nucleus (Killackey & Fleming, 1985). At each level of the central whisker pathways the afferent terminals are the first elements to form whisker-specific patterning. Barrelette cells in the brainstem, barreloid cells in the thalamus, and barrel cells in the cortex all develop a patterned organization of their dendrites after the clustering of presynaptic afferents. Furthermore, the pattern formation follows a sequential order from the periphery to the brainstem to the thalamus and finally the neocortex during the first few days after birth (reviewed in Sehara & Kawasaki, 2011; Figure 1, Table 1).
Figure 1.
Schematic diagram illustrating the timing of arrival of afferents along the whisker-barrel pathway and emergence of axonal and cellular patterns.
Table 1.
Chronology of events during development of the mouse whisker-barrel pathway
| E9 | Neurogenesis in the trigeminal ganglion from E8.5 to E13 (Davies & Lumsden, 1984; Wilkinson et al., 1996; Erzurumlu et al., 2010) |
| E10 |
E10 (30-somite-stage) A few pioneer axons of the ophthalmic branch of the trigeminal nerve reach past the eyecup, maxillary fibers begin target directed elongation; they are about 100 um away from the maxillary process (the site of the whisker pad) (Stainier & Gilbert, 1990). E10.5 (37-somite-stage) The pioneering maxillary (infraorbital nerve) axons reach the maxillary epithelium (presumptive whisker pad) and begin branching (Stainier & Gilbert, 1990). Central TG axons bifurcate upon entering the pontine flexure and each branch elongates to form the rostral (ascending) and caudal (descending) trigeminal tract. (Inferred from E12 rat data, Erzurumlu & Jhaveri, 1992b; see also Ding et al., 2003 for the mouse). The PrV cells emerge from the ventricular zone of the ventral aspect of the hindbrain around E10.5 and they express transcription factor Drg11 (Ding et al., 2003). |
| E11 | PrV cell axons cross the midline as soon as they are born (E10, E11) (Kivrak & Erzurumlu, 2012) and their midline crossing is guided by cooperative action of netrin-1 and slit proteins (Mirza et al., 2012). E11.5 Drg11-expressing PrV cells migrate ventrolaterally toward the region adjacent to the TG (Ding et al., 2003). E10.5-14.5 Neurogenesis in the dorsal thalamus (Wang et al., 2011) |
| E12 | Whisker follicles begin developing along five rows of skin ridges of a plate of thickened ectoderm (whisker pad) at E12. Individual follicles develop in a caudal to rostral sequence. A small nerve plexus is formed under the skin ridge and a dermal condensation occurs just above it; the epithelium over the dermal condensation thickens and grows down into the dermal condensation forming the follicle. (Yamakado & Yohro, 1979; Van Exan & Hardy, 1980. A gross topographic order in trigeminal projections to the whisker pad and the brainstem is present from the onset (Ding et al., 2003; Da Silva et al., 2011) |
| E13 | TCAs from the VPM arrive at the diencephalon-telencephalon boundary and by E13.5 pass through a permissive “corridor” en route to the neocortex (Lopez-Bendito & Molnar, 2003; Price et al., 2006; Lopez- Bendito et al., 2006). |
| E14 |
E14 Central TG axons emit radially oriented collaterals into the brainstem trigeminal nuclei (Ding et al., 2003). E14-E15 Layer IV neurons are born in the somatosensory cortex (Angevine & Sidman, 1961; Rebsam et al., 2002) |
| E15 | PrV is fully formed and central TG axons begin their arborization phase in the brainstem trigeminal nuclei (Ding et al., 2003; Erzurumlu et al., 2010) E15 PrV axons arrive in the midbrain (Erzurumlu & Kivrak, 2012) Pioneering TCAs reach the prospective somatosensory cortex (Molnar et al., 2003) |
| E16 | VPM axons extend along the internal capsule. |
| E17 | E17 PrV axons reach the VPM (Ding et al., 2003; Kivrak & Erzurumlu, 2012) |
| E18 |
E 18.5 central TG axons begin forming whisker-specific clustered terminal fields (Ding et al., 2003) E18-P0 PrV axons form bands (rows) in the VPM following an initially diffuse and exuberant invasion of the VPM (Kivrak & Erzurumlu, 2012) |
| E19-P0 | P0-P1Barrelette patterns emerge in the PrV (Ma, 1984; Li et al., 1994). TCAs arrive in the cortical plate (Senft & Woolsey, 1991; Agmon et al., 1993; Rebsam et al., 2002) |
| P1 | PrV axons form bands in VPM (Kivrak & Erzurumlu, 2012) |
| P2 | P2 patches within bands appear in VPM (Kivrak & Erzurumlu, 2012) TCA terminals form barrel rows in S1 cortex (Rebsam et al., 2002) |
| P3 | P3 Barreloids are visible in the VPM Durham & Woolsey, 1984; Kivrak & Erzurumlu, 2012) Whisker-specific TCA terminal patches appear within barrel rows (Rebsam et al., 2002; Lee et al., 2005a) P3-P4 end of critical period for structural plasticity following whisker follicle or ION damage (Durham & Woolsey, 1984; Datwani et al., 2002b; Rebsam et al., 2005) Closure for critical period plasticity for bilateral whisker trimming (Lee et al., 2009). |
| P4 | End of critical period for structural plasticity |
| P5 | P5-P7 Barrels as cellular aggregates become distinct (Rice et al., 1985 |
| P6 | Polarized dendritic orientation of barrel cells become apparent (Espinosa et al., 2009). |
| P7 | |
| P8 | |
| P9 | |
| P10 | P10-P14 Critical period plasticity for layer IV to II/III synapses (Single whisker experience-induced synaptic strength; Lendvai et al., 2000; Stern et al., 2001; Maravall et al., 2004a, b; Shokhet et al., 2005). |
| P11 | |
| P12 | |
| P13 | P13-P16 Critical period for horizontal connections between layer II/III neurons (Single whisker experience-induced synaptic strength; Wen & Barth, 2011) |
| P14 | Eyes open, active whisking starts (Landers & Ziegler, 2006) End of critical period for layer IV to II/III synapses. |
| P15 | |
| P16 | End of critical period for horizontal connections between layer II/III neurons. |
| P17 |
Whisker-specific patterning in the brainstem emerges by embryonic day (E)19-20 in the rat (Chiaia et al., 1992a; Waite et al., 2000), and at birth (P0) in mice (Ma, 1993; Figure 1, Table 1). From the earliest time of target invasion, terminal ION arbors have synaptically active boutons and the postsynaptic responses in the PrV show a predominant NMDA component (Waite et al., 2000). Polarized, asymmetrical dendritic orientation and whisker-specific patterning characterize the barrelette neurons. These small diameter cells display a transient K+ (IA) current and receive monosynaptic excitatory and disynaptic inhibitory inputs upon stimulation of the trigeminal tract (Lo et al., 1999).
The barrelette cells are also the trigeminothalamic projection cells (Erzurumlu et al., 1980). Their axons cross the midline, collect along the medial lemniscus, forming the trigeminal lemniscus. In the mouse, PrV neurons are derived from the rhombomeres 2 and 3 (Oury et al., 2006). Newly differentiated PrV neurons send axons across the midline at E11, as they are migrating to the presumptive PrV (Figure 1). These axons make a sharp rostral turn as soon as they traverse the midline and elongate rostrally towards the contralateral thalamus (Kivrak & Erzurumlu, 2012). The trigeminal lemniscus reaches the VPM by E17. These afferents initially develop expansive arbors within the boundaries of the VPM, which gradually coalesce into whisker row-specific curvilinear columns followed by single whisker-specific patches during the first few days after birth (Kivrak & Erzurumlu, 2012). Cellular patterns (barreloids) follow afferent patterns and are apparent by P3 (Figure 1). Axon guidance molecules netrin1 and slit proteins play a major role in guiding the initial formation of the trigeminal lemniscus (Mirza et al., 2012), but molecular guidance cues attracting and confining lemniscal axons to the VPM are not yet fully understood. The molecular mechanisms of pattern formation in the brainstem have been reviewed elsewhere (Erzurumlu et al., 2006; 2010; see also Pouchelon et al., this issue). In contrast to the development of terminal arbor patterning in VPM from initially diffuse to patchy, in both the upstream PrV and the downstream barrel cortex, whisker-related afferents develop topographically ordered terminal fields from the onset (Catalano & Killackey, 1991; Agmon et al., 1993; 1995; Waite et al., 2000; Lee et al., 2005a, b; but see Senft & Woolsey 1991 and Rebsam et al., 2002 for cortex).
Two decades ago, simultaneous labeling of thalamocortical axons, serotonergic raphe-cortical projections, postsynaptic cells, extracellular elements revealed that the whisker-specific patterning first occurs in the terminal arbors of the thalamocortical axons and other elements are patterned afterwards (Erzurumlu & Jhaveri, 1990; 1992b, Jhaveri et al., 1991; Blue et al., 1991). The whisker-specific patterning of thalamocortical terminals emerges during the 3 days after birth, first by formation of rows and then breaking up into individual whisker follicle representations (Erzurumlu & Jhaveri, 1990; Rhoades et al., 1990; Senft & Woolsey, 1991; Agmon et al., 1993; Rebsam et al., 2002; Lee et al., 2005b).
The early patterning of thalamocortical axons is dependent on the sensory periphery. This has been amply demonstrated by lesion studies performed in perinatal rodents (Van der Loos & Woolsey, 1970; Belford & Killackey, 1981; Durham & Woolsey, 1984; Bates & Killackey, 1985; Dawson & Killackey, 1987; Jensen & Killackey, 1987; Killackey & Dawson, 1989). Furthermore, in mice, selectively inbred for abnormal, additional whisker follicles (supernumerary whiskers) at different loci in the whisker pad, corresponding extra barrels formed in the somatosensory cortex (Van der Loos et al., 1984; 1986; Welker & Van der Loos, 1986). These and other lines of evidence, e.g., rerouting of visual pathways to the auditory cortex or visual pathways to the somatosensory cortex (Métin & Frost, 1989; Roe et al., 1990; Sur et al., 1990; Pallas, 2001) were interpreted as areal specification of the neocortex by incoming thalamic afferents (O’Leary, 1989; O’Leary et al., 1994). A recent study added another example of peripheral guidance of central patterns. Ephrins are expressed in a gradient along the developing whisker pad and in EphA4 knockout mice several ventroposterior whisker follicles are missing; consequently corresponding barrels in the caudal portions of rows D and E are also missing, as are the relevant subcortical patterns (North et al., 2010).
Areal specification of the barrel cortex
In 1994, an enhancer trap transgenic mouse line was produced in which beta-galactosidase expression was confined to layer-IV neurons of the primary somatosensory cortex (Cohen-Tannoudji et al., 1994). Heterotopic transplantation experiments further confirmed that this transgene (H-2Z1) is intrinsic to the neocortex and specific to the somatosensory region (Cohen-Tannoudji et al., 1994; Gitton et al., 1999a). These observations raised the possibility that the formation of the barrel cortex is guided by intrinsic cortical cues. The pattern of H-2Z1 expression in cortical explants in reeler and monoamine oxidase A (MAOA) deficient mice and in mice with neonatal whisker or thalamic lesions revealed that H-2Z1 expression develops autonomously an does not require sensory inputs from the periphery; but it is also under the influence of thalamic signals once these axons arrive in the cortical plate, as expression is strongly reduced by early thalamic lesions (Gitton et al., 1999b). Since then, genetic loss-of-function/gain-of-function and molecular profiling studies of the developing neocortex have revealed a complex interaction of numerous morphogens and transcription factors in localization of cortical maps, their orientation and size.
Signaling molecules such as fibroblast growth factor 8 (FGF8), bone morphogenetic proteins (BMPs), vertebrate orthologs of Drosophila wingless (WNTs), sonic hedgehog (SHH) are expressed from cortical patterning centers in a gradient and they define the topological coordinates of the cortical mantle and positioning of future territories of sensory and motor thalamocortical projections (Shimogori & Groove, 2006; Sur & Rubenstein, 2005; O’Leary & Sahara, 2008). For example, FGF8 is normally expressed in the anterior commissural plate and ectopic expression in the posterior cortex by in utero electroporation leads to mirror image, partial duplication of the whisker barrel maps (Fukuchi-Shimogori & Grove, 2001). Morphogens secreted by organizing centers of the cortical mantle (i.e., commissural plate, cortical hem) define areal fate by regulating differential expression of a variety of transcription factors in cortical progenitors.
Parcellation of SI body map and incoming thalamocortical afferents
While the areal specification of cortical maps is largely determined by intrinsic genetic programming of the neocortex, neural activity plays a role in allocation of cortical tissue to different components of the somatosensory body map. In mice with genetically reduced NMDA receptor function, the PrV in the brainstem shrinks and fails to develop barrelette patterns even though a full complement of whiskers is present on the snout (Lee & Erzurumlu, 2005). The downstream effects are absence of barreloids in the VPM and barrels in the face region of the neocortex and shrinkage of the overall face representation area in comparison to forepaw and hindpaw regions with digit-specific neural patterns and barrels (Lee & Erzurumlu, 2005).
Studies in mice with targeted gene deletions have identified several transcription factors that control thalamocortical development. These include Emx2, Tbr1, Gbx2, Mash1, Ebf1, Foxg1 and Pax6. In null mutations of Gbx2, Mash1, FoxG1 or Pax6 thalamic axons fail to innervate the neocortex (Lopez-Bendito & Molnar, 2003; Pratt & Price 2006; Price et al., 2006; also other articles in this issue). Transcription factors Lhx2, SCIP, and Emx1 and cadherins Cad6, Cad8, and Cad11 are all expressed in areal patterns independent of thalamocortical innervation, as seen in Mash-1 mutant mice which fail to develop a TCA projection (Nakagawa et al., 1999). A variety of axon guidance cues and growth-associated proteins also play roles during target-directed growth and invasion of thalamocortical axons. For example, Ephrins and their Eph receptors have been implicated in precision of patterning within topographically organized cortical face map (reviewed in Vanderhaeghen & Polleux, 2004; Uziel et al., 2006). GAP-43, a growth-associated phosphoprotein, is transiently expressed by developing thalamocortical axons and the thalamocortical topography is severely disrupted in GAP43 knockout mice (Erzurumlu et al., 1990; Maier et al., 1999). The numbers of experimentally identified intrinsic cortical and thalamic molecular cues are mounting. However, there is no evidence of sensory periphery-related topographic organization and patterning of the neocortex independent of thalamic innervation. Many studies point out to presence of distinct mechanisms underlying topographic ordering and periphery-related patterning along every station of the somatosensory pathway.
During their target-directed growth phase, thalamocortical axons interact with subplate (SP) neurons, which are some of the earliest generated cortical neurons and reside just above the white matter (reviewed in Kanold & Luhman, 2010). SP neurons form a scaffold for the developing reciprocal thalamocortical projections. Neurites arising from SP neurons are confined to barrel hollows during P4-6 and their patterned organization also appears to depend on an intact sensory periphery (Pinon et al., 2009).
Role of neural activity in patterning of the barrel cortex
Once the cortical territories are assigned to incoming thalamocortical axons and they are guided by molecular cues to their targets, neural activity plays a major role in patterning of the cortical sensory maps. Communication between thalamocortical axons and their postsynaptic partners occurs via glutamatergic neural transmission and it is regulated by monoamines (such as serotonin) and GABAergic inhibitory influences from local circuit neurons (Erzurumlu & Kind, 2001). As noted above, various forms of manipulations of the sensory periphery during a critical window in development induce plastic changes in the size and patterning of pre and postsynaptic elements in the barrel cortex.
In early studies, pharmacological blockade of action potentials or N-Methyl-D-Aspartate (NMDA) receptors, either in the neocortex or in the ION, did not affect cortical patterning (Chiaia et al., 1992b; Henderson et al., 1992; Schlaggar et al., 1993). However, later pharmacological blockade experiments noted significant alterations in single whisker responsiveness of barrel neurons and functional organization of cortical columns (Fox et al., 1996) and in the morphological integrity of barrels (Mitrovic et al., 1996). In spite of the inconsistent results from pharmacological perturbation studies, our current understanding of the role of activity in barrel formation comes from targeted gene manipulations in mice.
Genetic loss of function studies in mice revealed that NMDARs, metabotropic glutamate receptors, and altered levels of cortical serotonin (5-HT) yield defective barrel cortex phenotypes (reviewed in Erzurumlu & Kind, 2001; Erzurumlu & Iwasato, 2006; Inan & Crair, 2007; Wu et al., 2011).
Selective loss of the NR1 gene in cortical excitatory neurons genetically blocks NMDAR activity in the neocortex (Iwasato et al., 2000). In these mutants (CxNR1 knockout mice), the whiskers on the snout and all subcortical whisker-related patterns are similar to those seen in wild type mice but barrels as cellular aggregations are missing, the dendritic field bias of layer IV spiny stellate cells (barrel cells) is lost, and the thalamocortical axons form exuberant terminal arbors within which there is some clustering, i.e., rudimentary whisker-specific patterning (Iwasato et al., 2000; Datwani et al., 2002a; Lee et al., 2005a). Similar barrel cortex defects were also reported in genetically altered mice with defects in other components of the glutamatergic pathway such as metabotropic glutamate receptor 5 (mGluR5) and PLCβ (phospholipase C-β) (Hannan et al., 1998; 2001; Ballester-Rosado et al., 2010).
Cortical 5-HT augmentation (but not depletion) also affects barrel development (see Van Kleef et al., this issue). Initial pharmacological blockade experiments used p-chloroamphetamine (PCA), a selective 5-HT neurotoxin in postnatal rats and uncovered a delay in barrel formation (Blue et al., 1991), but a more dramatic effect was achieved in mice with 7–9-fold increase in 5-HT levels following disruption of the monoamine oxidase A (Maoa) gene. In Maoa knockout mice the thalamocortical axon terminal segmentation was completely blocked and barrels did not form, even though the whiskers on the snout and related patterning in the brainstem and thalamus were normal (Cases et al., 1996). Clorgyline (an MAOA inhibitor) administration during the postnatal period in wild type mice also mimicked the barrel cortex phenotype seen in the Maoa knockout mice (Cases et al., 1996; Vitalis et al., 1998). During a transient period in development the thalamocortical axons express 5-HT1B receptors (Bennett-Clarke et al., 1993) and the VPM neurons express the serotonin and the vesicular monoamine transporters (SERT/5-HTT/S16a4 and VMAT2) (Lebrand et al., 1996, 1998). 5-HTT knockout mice show partial patterning in the barrel cortex and thalamocortical axon patterning is severely disrupted in Maoa and 5-HTT double knockout mice. Genetic removal of 5-HT1B receptors in Maoa, 5-HTT knockout mice or in Maoa/5-HTT double knockout mice rescue the defects and both the thalamocortical axons and layer IV cortical cells form barrels (Salichon et al., 2001). The precise role of 5-HT in normal barrel development is still not clear (see Van Kleef et al., this issue for discussion). Electrophysiological recordings from thalamocortical slices indicated that 5-HT has a strong presynaptic inhibitory effect at the thalamocortical synapse (Rhoades et al., 1994; Laurent et al., 2002). During thalamocortical development, 5-HT1B-mediated signaling plays a multifaceted role, including autoregulation of glutamate release (Rhoades et al., 1994; Laurent et al., 2002), axonal growth (Lotto et al., 1999) and response to guidance molecules such as netrin 1 (Bonnin et al., 2007). However, pharmacological or genetic 5-HT depletion does not affect barrel formation other than a couple of days delay, which may be related to growth retardation (Van Kleef et al., this issue) and does not affect the duration of the critical period plasticity which follows neonatal whisker follicle damage (Blue et al., 1991; Osterheld-Haas and Hornung, 1996; Turlejski et al., 1997).
A spontaneous mouse mutant with a “barrelless” (brl) phenotype was identified in Lausanne (Welker et al., 1996). The mutation was later identified as a transposon insertion in the Adenylyl cyclase type I gene (AC1) (Abdel-Majid et al., 1998). In brl (or AC1 knockout) mice, thalamocortical axon arbors are broader and cellular barrels do not form (Welker et al., 1996; Gheorghita et al., 2006). The peripherally evoked synaptic responses of presumptive barrel cortex neurons are also abnormal with a prominent disruption of AMPA receptor surface expression and long-term potentiation (LTP) and long-term depression (LTD) in layer IV neurons (Welker et al., 1996; Lu et al., 2003). AC1 is a member of Adenylyl cyclases, it catalyzes the formation of cAMP and it is stimulated by guanine nucleotide-binding protein (Gs) coupled receptors and by Ca2 influx through voltage-sensitive Ca2 channels (reviewed in Hall & Cooper, 2011)
Brl mouse as a global AC1 knockout displays subcortical pattern defects. Cortex-specific AC1 deletion led to a much less severe barrel cortex phenotype (Iwasato et al., 2008). While minor alterations were noted in barrel cortex organization and postsynaptic function, gross morphological features and critical period plasticity were not altered suggesting that loss of presynaptic rather than postsynaptic AC1 function might be the major cause of defects seen in the brl mice.
Structural and functional defects were also observed in the barrel cortex of mice lacking one of the regulatory subunits (PKARIIβ) of PKA, a signaling molecule downstream from AC1 (Inan et al., 2006; Watson et al., 2006). These mice have postsynaptic defects in cellular organization and lack of LTP in layer IV; however, the thalamocortical axon patterning in these animals is present. Since PKARIIβ is postsynaptic the observed pattern deficits confirm the conclusion that the barrels as layer IV cellular aggregates can be perturbed independently of afferent patterning (Inan et al., 2006; Watson et al., 2006).
Involvement of presynaptic mechanisms in barrel formation was inferred from studies on monoamine oxidase A (Maoa; Rebsam et al., 2002), AC1 (Gheorghita et al., 2006) and RIM1α (Lu et al., 2006) knockout mice. A more recent study compared the effects of thalamic or cortical ablation of RIM1 and RIM2 proteins on barrel formation (Narboux-Nême et al., 2012). RIM proteins are located at the presynaptic active zone and control synaptic vesicle exocytosis (Deng et al., 2011; Kaeser et al., 2011). Thalamus-specific deletion of both RIM1 and RIM2 reduced neurotransmission efficacy by 67 % but did not affect whisker-specific patterning of thalamocortical axon terminals, albeit reduction in patch sizes (Narboux-Nême et al., 2012). On the other hand, in contrast to cortex-specific deletion, thalamus-specific deletion of RIMs yielded a barrelless phenotype with alterations in the dendritic orientation of layer IV neurons (Narboux-Nême et al., 2012). This underscores the importance of presynaptic thalamocortical mechanisms rather than cortico-cortical synapses in dendritic orientation of neurons in layer IV. Thus, at the thalamocortical synapse, both pre- and postsynaptic mechanisms are involved in barrel formation. Structural and functional defects in barrels often result from defective patterning of thalamocortical axons (see Wu et al., 2011 for a recent review). To date, no mouse mutants have been identified with a failure in thalamocortical axon terminal patterning but with a “normal” complement of barrels.
Critical Period Plasticity in the developing “whisker-barrel” pathway
Early sensory experience shapes the morphological and functional organization of developing brain circuits. Competition between synaptic inputs, balance between excitation and inhibition, interactions with the extracellular matrix environment all play roles in wiring of the brain during sensory system development (Hensch, 2004, 2005). Since the classical visual deprivation studies of Hubel & Wiesel (1964), it has been well established that the occlusion of one eye early in life leads to irreversible loss of visual acuity through the deprived eye (Daw 1995; Prusky & Douglas, 2003; Wiesel, 1982). Unbalanced visual experience and activity-dependent competition between eye-specific inputs lead to a rapid shift of neuronal responses in the primary visual cortex in favor of the open eye and this period of cortical malleability is known as the critical period for ocular dominance (Antonini et al., 1999; Hensch, 2005; Wiesel & Hubel, 1963). The concept of “critical period” indicates a time period during which the presence of specific external or internal condition is necessary for normal development and that the absence of such condition leads to irreversible alterations in the organism (Erzurumlu & Killackey, 1982). The critical period concept for the whisker-barrel pathway was inspired by ocular dominance plasticity studies of Hubel and Wiesel (Belford & Killackey, 1980; Durham & Woolsey, 1984; Van der Loos & Woolsey, 1973; Erzurumlu, 2010). However, there are significant differences between the development and the organization of these two sensory systems as well as in the sensory deprivation paradigms used. Occlusion of an eye by lid suture blocks natural visual stimulation without any damage to the retina and the visual pathways. Whisker follicle lesions or ION transection physically damages the sensory apparatus. Thus, “plasticity” as manifest in aberrant organization of the trigeminal pathway and the barrel field is a reflection of the vulnerability of the developing system to physical damage during its wiring rather than the critical nature of the sensory experience. Initially, the term “sensitive period” was used to describe structural alterations along the trigeminal pathway following neonatal whisker damage (Belford & Killackey, 1980). However, the term “critical period plasticity” has been widely adopted in reference to structural or functional or behavioral changes that take place following neonatal sensory peripheral damage or simple whisker trimming. Because of this, and due to differential effects of a variety of peripheral sensory manipulations on different cortical layers at morphological or functional levels, multiple critical periods have been delineated (Fox, 2002). In fact, such a wide concept of plasticity encompasses all forms of structural and functional malleability in the somatosensory cortex throughout life. In this review, we focused on developmental plasticity of the barrel cortex, seen during the first few postnatal weeks of life.
Rodent pups are born with a full set of fine whisker hairs that are curved back and are immobile. Active “whisking” behavior starts at the end of the second postnatal week, just before eye opening (Landers & Ziegler, 2006). Trigeminal ganglion cells are responsive to whisker stimulation before the onset of whisking (Shoykhet et al., 2003) and neonatal rats respond to whisker stimulation, and display tactile learning in a classical conditioning avoidance paradigm (Landers & Sullivan, 1999; Sullivan et al., 2003). Facial tactile experience of newborn rodent pups is mostly limited to physical contacts with their littermates and their mother. Whisker trimming between postnatal days 3–5 disrupts nipple attachment and huddling behaviors (Sullivan et al., 2003). Clearly morphological, physiological and behavioral maturation of the whisker-barrel system depends on sensory evoked activity during the postnatal life. However, as will be discussed below most of the “sensory deprivation” experiments in the whisker to barrel pathway consisted in lesioning the whisker follicles or the nerves. Whisker cautery or ION lesions clearly block peripherally evoked afferent activity along the pathway in addition to transsynaptic cell death and possible communication via yet to be identified “trophic” factors. Whisker plucking on the other hand, leads to lessened sensory activity but does not completely block it as the neonatal rodent pup gets its sensory receptors on the snout stimulated during huddling and suckling. Active whisking does not start until two weeks of age (Landers & Ziegler, 2006; Welker, 1964), the role of passive stimulation of developing whiskers and their follicles in shaping up the brain circuitries during a short period in postnatal development is still poorly understood.
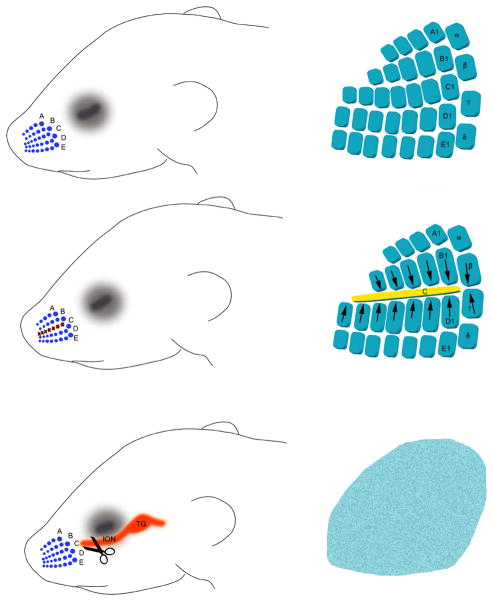
The spatial arrangement of whisker and sinus hair follicles on the snout provides a template for the formation of axonal and cellular patterns in the barrel cortex during a critical period in development. Five curvilinear arrays (rows A–E) of whisker follicles emerge before neurogenesis of the trigeminal ganglion or any other central trigeminal targets (Davidson & Hardy, 1952; Yamakado & Yohro, 1979; Van Exan & Hardy, 1980; Stainier & Gilbert, 1990). Whisker follicle cautery or lesions of the ION before P4 irreversibly alter the organization of central neural patterns. The classical example is from the original Van der Loos and Woolsey 1973 study where they electrocauterized the follicles of the middle row whiskers (row C) on the day of birth and observed that cortical barrels corresponding to row C whiskers shrunk (Figure 2). Fusion of the lesioned row C barrels and expansion of the neighboring rows D and E barrels have been demonstrated at the thalamocortical axon terminal, layer IV cortical cell and dendritic orientation levels for mice and rats (Woolsey & Wann, 1976; Belford & Killackey, 1980; Jeanmonod et al., 1981; Figure 2). If the whisker pad is completely denervated of its sensory input by ION transection, no barrels are seen in the cortex and the thalamocortical axons are distributed in an aberrant fashion (Belford & Killackey, 1980; Bates & Killackey, 1985; Jensen & Killackey, 1987). A similar defect in the dorsal column lemniscal pathway is also noted following embryonic paw amputations (Dawson & Killackey, 1987; Killackey et al., 1995). In contrast to physical damage to whisker follicles or the ION, simple whisker trimming or plucking in neonates do not alter the development of whisker-specific central patterns at the gross morphological level but has other consequences such as reducing dendritic complexity and spines of barrel neurons or the behavioral repertoire of the animal later on (Lee et al., 2010).
Figure 2.
Schematic diagram illustrating the classical structural plasticity in the barrel cortex following row C whisker lesions or infraorbital nerve transection. These effects are only seen when peripheral lesions are performed up to postnatal day 3. The patterns and deficits are routinely assessed by histochemical stains such as succinic dehydrogenase or cytochrome oxidase histochemistry or with immunohistochemistry for TCA markers such as 5-HTT or vesicular glutamate transporter 2 or by Nissl or Golgi stains for neuronal and dendritic organization.
Studies in rats and mice revealed that physical perturbation of the whisker follicles or the ION during a window of time in perinatal development leads to irreversible structural alterations in the patterning of the whisker-related neuronal circuits (Figure 2); this period abruptly ends by P4 (Van der Loos & Woolsey, 1973; Belford & Killackey, 1980; Durham & Woolsey, 1984). A recent study challenged the notion that sensory experience-dependent structural plasticity is confined to the first few days after birth. Thalamocortical axons arising from the VPM and/or posteromedial (POm) nucleus were labeled with virus-mediated fluorescent proteins and their distribution was examined after trimming all the whiskers except one or two for a period of P0-P96 (Wimmer et al., 2010). Whisker trimming induced about 30% decrease in the density of VPM axons in deprived cortical columns and these effects were reversible after regrowth of the trimmed whiskers, independent of the age at deprivation. No change was detected in POm projections. The differential effects of whisker follicle cautery and whisker trimming have been long noted and perhaps the notion of critical period should be evaluated differently depending on whether sensory deprivation is achieved through whisker trimming only or it also includes damage to the primary sensory neurons involved in mediating the sensory experience.
In the newborn rat, damage to the ION results in synaptic remodeling in the PrV. Trigeminal afferent patterning is lost and barrelette cell dendritic trees assume nonspecific symmetric distribution (Lo & Erzurumlu, 2002). Neonatal ION damage also leads to a significant apoptosis in the TG, the PrV and the VPM (Miller et al., 1991; Miller & Kuhn 1997; Sugimoto et al., 1998, 1999; Baldi et al., 2000). Despite significant cell loss, membrane properties of surviving PrV neurons, neurotransmitter release probability of trigeminal afferents and postsynaptic NMDA receptor subunit composition do not change (Lo & Erzurumlu 2001, 2011; Lo & Zhao 2011). However, postsynaptic responses of denervated PrV neurons are mostly silent synapses without functional AMPA receptors (Lo & Erzurumlu 2007). The molecular and functional changes at the first relay station of the PrV set the stage for pattern defects downstream in the VPM and the barrel cortex.
Another important caveat in whisker lesion-induced plasticity of the barrel cortex is that the whisker-specific patterning in the VPM and the barrel cortex is still under construction during the critical period. In other words, the so called “plastic” changes are not reorganization of already patterned presynaptic afferent terminals and oriented dendritic trees of postsynaptic cells but aberrant development of these elements from the onset in response to physical damage to the sensory periphery.
In search of axonally transported chemical signals
How does the disruption of the sensory periphery alter afferent clustering and dendritic organization of barrel cells across synapses? Molecular underpinnings of barrel cortex plasticity and its confinement to a critical period in development have been long sought. In this context two lines of thought have directed the search for molecular mechanisms of barrel cortex plasticity: Axonal transport of chemical signals and peripherally evoked activity (or lack of it) along the trigeminal pathway leading to the barrel cortex. Inhibition of trigeminal axonal transport with colchicine- or vinblastine impregnated implants in neonatal rats resulted in the absence of whisker-related cytochrome oxidase patterns in the PrV, VPM and the barrel cortex (Chiaia et al., 1996) but Na+ channel blockade with TTX or NMDA receptors with APV did not (Chiaia et al., 1992b; 1994; Henderson et al., 1992). These results suggested that chemical signals conveyed from the whisker follicles to the brainstem and transsynaptically onto the barrel cortex are necessary for the development of whisker-specific central neural patterns. An overlooked finding of the axonal transport blockade study is the fact that the pharmacological agents used also caused 90% reduction in TG cells (Chiaia et al., 1996).
Nerve growth factor (NGF) family of neurotrophins promotes survival and differentiation of primary sensory neurons and they are abundant in the developing whisker pad. Thus, they have been the prime candidates for patterning of the trigeminal pathway. In the mouse barrel cortex, neurons express brain-derived neurotrophic factor (BDNF) and trkB mRNA (Lush et al., 2005) and the expression levels decrease following row C cautery in newborns (Singh et al., 1997). A transient increase in BDNF mRNA levels has been detected in the barrel cortex following whisker stimulation (Rocamora et al., 1996). In BDNF knockout mice, thalamocortical synaptic communication is impaired (Itami et al., 2000). Barrel patterns form but thalamocortical patterning is delayed in both the BDNF and TrkB knockout mice (Lush et al., 2005). In another related study, gelfoam impregnated with NGF, BDNF or neurotrophin-3 (NT-3) was placed under the whisker pad following whisker row nerve transection (Calia et al., 2001). This study found that BDNF and NT-3 rescued development of the cortical barrels corresponding to the denervated whiskers and prevented the lesion-induced expansion of neighboring barrels of the intact whiskers (Calia et al., 2001). Application of BDNF and NGF to the damaged ION also rescues many VPM neurons from transneuronal death (Baldi et al., 2000). It is highly likely that peripheral infusion of neurotrophins acts first in the TG and prevents cell death in this experiment. This might then lead to maintenance of whisker patterns by the central trigeminal afferents and all along the pathway leading to the barrel cortex. Cortical levels of NGF, BDNF or NT-3 do not appear to play a major role in initial pattern formation and duration of the critical period plasticity. This conclusion is further supported by the presence of whisker-specific patterns in trkB knockout mice (Vitalis et al., 2002).
All of the presently available evidence suggests that whisker-specific neuronal patterns are first established in the PrV, and then transmitted faithfully to the VPM and the barrel cortex. Barrelette patterns fail to form in mice with loss of function mutations in NMDA receptor subunits NR1 and NR2D (Li et al., 1994; Kutsuwada et al., 1996) and in transcription factors Drg11 (Ding et al., 2003) and Lmx1-b (Xiang et al., 2010). So far no link has been identified between NMDA receptors and transcription factors Drg11 and Lmx1b. Several years ago it was noted that transforming growth factor β (TGF-β) family members activin and follistatin play a role in whisker development and patterning in the brainstem. Mice lacking the gene encoding for activin β do not have whiskers and follistatin-deficient mice develop thin and curled whiskers; both strains of mice die within 24 h after birth (Jhaveri et al., 1998). Activin βA knockout mice lack barrelettes and the barrelettes are not well developed in follistatin-deficient mice (Jhaveri et al., 1998). A recent study revealed that genetic disruption of TGF-β signaling in TG neurons (by conditional deletion of Smad4) results in the absence of or aberrant barrelette patterning in the brainstem trigeminal complex (da Silva et al., 2011). Smad4 is a nuclear transducer of TGF- β signaling, and TGF- β signaling has been linked to synaptic transmission (Sun et al., 2010) and NMDA receptor-mediated LTP in hippocampus (Müller et al., 2006). Thus, both chemical signaling from the sensory periphery and neural activity mediated through the ION could act cooperatively in setting up the initial scaffolding of barrel patterns two synapses below the neocortex, in the brainstem.
Neural activity and NMDA receptors in plasticity
At all levels of the trigeminal pathway NMDA mediated synaptic activity is recorded as soon as the presynaptic afferents arrive. (Leamey & Ho, 1998; Waite et al., 2000; Khazipov et al. 2004). In the newborn rat barrel cortex, thalamocortical inputs generate spindle bursts in the cortical plate and these are associated with large amplitude NMDA receptor-dependent delta waves (Minlebaev et al., 2009). Thus a physiological substrate is present for Hebbian type synapse consolidation via coactivation of cortical neurons by thalamic cells.
Spatially confined spindle burst activity is a prominent physiological feature of the barrel cortex in newborn rodents (Khazipov et al., 2004; Minlebaev et al., 2007; Yang et al., 2009). These spindle-bursts are driven by glutamatergic synapses involving mainly AMPA/kainate receptors, as well as NMDA receptors and gap junctions and they are compartmentalized by surround GABAergic inhibition (Minlebaev et al., 2007). Peripherally driven activity and postsynaptic NMDAR activation are required for normal development of receptive fields in barrel neurons (Fox et al., 1996; Foeller & Feldman, 2004). LTP at the thalamocortical synapses of the barrel cortex is NMDAR dependent and confined to the first postnatal week, but not restricted to the critical period ending at P3 (Crair & Malenka, 1995). At the thalamocortical synapse LTP results in a switch from slow kainate receptor-mediated synaptic transmission to fast AMPA receptor (AMPAR)-mediated transmission during this period (Kidd & Isaac, 1999). Thalamocortical synapses also express NMDA receptor-dependent LTD during development (Feldman et al., 1988). Thus both forms of synaptic plasticity must be in operation during formation and consolidation of barrel patterns initiated by the whisker-specific thalamocortical afferent terminals.
A recent study reported that early gamma oscillations (EGOs) synchronize neurons in a single thalamic barreloid and corresponding cortical barrel during barrel formation in neonatal rats (Minlabaev et al., 2011). It is suggested that sensory inputs from a single whisker lead to activation of gamma oscillator in the corresponding thalamic barreloid, which then imposes topographic feed-forward synchronization in the cortical barrel corresponding to that particular whisker (Minlabaev et al., 2011). In the context of critical period plasticity, EGOs can consolidate coactive inputs via repetitive synchronization of thalamic and cortical neurons, thus creating an activity-dependent competitive environment during pattern formation.
Is there a “molecular switch” which controls the timing of the critical period plasticity in the barrel cortex? This question still awaits an answer. Due to their coincidence detection properties and developmental regulation of their subunit composition NMDA receptors have attracted attention. NMDARs are heteromeric tetramers and each receptor contains the essential NR1 subunit, which binds glycine or D-serine and one or more of the NR2 subunits A–D, which bind glutamate (Wenthold et al., 2003). In the visual cortex, NR2B subunit expression shifts to NR2A subunit expression at the end of the critical period for ocular dominance plasticity (Carmignoto & Vicini, 1992). Dark rearing prolongs developmental NR2B expression and light exposure shifts in favor of NMDA receptors with NR2A subunit (Philpot et al., 2001; Quinlan et al., 1999a,b). There is also a developmental shift from NR2B to NR2A subunits in the thalamocortical segment of the whisker-barrel pathway (Liu et al., 2004), but no such change was found in the PrV at the brainstem level (Lo & Zhao, 2011). Whisker lesion-induced critical period plasticity has been investigated in NR2A knockout mice and even though the NMDA current kinetics remained immature, no change was observed in the closure of the critical period (Lu et al., 2001).
In cortex- specific NR1 knockout (CxNR1KO) mice barrels do not develop and the thalamocortical axon terminals show rudimentary patterning only in the region of large mystacial whiskers (Iwasato et al., 2000). In these mice too the duration of the row C whisker induced plasticity, as reflected by the patterning of thalamocortical axon terminals, did not go beyond P4 (Datwani et al., 2002b). Thus, postsynaptic NMDAR function in cortical excitatory neurons does not appear to be essential for thalamocortical axon plasticity. It remains to be seen whether this plasticity would be affected in thalamus-specific NR1 knockout mice, although unlikely.
Other key players of hippocampal synaptic plasticity, such as LTP and LTD, have been considered in studies of the barrel cortex critical period plasticity. For example, calcium/calmodulin-dependent kinase II (CaMKII) is an enzyme necessary for LTP and mice mutant for this enzyme lack NMDA receptor-dependent LTP in the barrel cortex (Glazewski et al., 1996; Glazewski et al., 2000; Hardingham et al., 2003). Postsynaptic activation of protein kinase C (PKC) increases synaptic strength and is also involved in LTP in the barrel cortex (Scott et al., 2007). However, none of these molecules have been shown to be essential in governing the timing of the lesion-induced critical period in the barrel cortex.
Glia, perineuronal nets and parvalbumin cells
A role for glial participation in barrel cortex plasticity has also been explored. Oligodendrocytes (OLs) appear in the mouse somatosensory cortex at the end of the critical period (Toda et al., 2008). In this context, critical period plasticity has been investigated in Olig1-deficient and jimpy mouse lines (Toda et al., 2008). In total absence of OLs (Olig1-deficient mice or in jimpy mice), the duration of the critical period is not delayed. Thus, potential involvement of OLs in barrel cortex plasticity is ruled out.
How about astrocytes? In the developing barrel cortex, expression of astrocyte-specific markers, such as the glutamate transporters GLAST and GLT-1 and the glutamine synthetase is confined to the barrel hollows (Voutsinos-Porche et al., 2003; Takasaki et al., 2008). Gap junction proteins, connexins associated with astrocytes (Cx43 and Cx30) also show an elevated expression pattern within the barrel hollows (Houades et al., 2008). Astrocyte-neuron interactions within the barrels are thought to play a major role in whisker-activated information processing in the barrel cortex and various forms of plasticity (reviewed in Giaume et al., 2009). Increased levels of glutamate transporter 1 (GLT1) in astrocytes have been noted during the critical period in the developing barrel cortex (Takasaki et al., 2008). Investigation of row C whisker lesion-induced plasticity in GLT1 knockout mice did not reveal any change in the duration of the critical period but a significantly lesser degree of plasticity in fusion of the row C barrels and expansion of the neighboring barrels was noted. Diminished lesion-induced plasticity was also observed in astrocyte-specific glutamate-aspartate transporter (GLAST) knockout mice (Takasaki et al., 2008). Thus astrocytes might play a role in levels of critical period plasticity by regulating glutamate transporters at the thalamocortical glutamatergic synapses.
An astrocyte-secreted protein thrombospondin promotes synaptogenesis; the neuronal thrombospondin receptor involved in synapse formation has been identified as alpha2delta-1, also the receptor for the anti-epileptic drug gabapentin (Eroglu et al., 2009). Gabapentin antagonizes thrombospondin binding to alpha2delta-1 and inhibits synapse formation. Examination of the whisker row C lesion induced plasticity in the barrel cortex of mice given gabapentin injections between P0-7 or in TSP1/2 double knockout mice revealed exaggerated plasticity and expansion of intact barrels but did not affect the timing of this response confined to the critical period (Eroglu et al., 2009).
Perineuronal nets formed by chondroitin sulfate proteoglycan aggregates, parvalbumin-positive GABAergic neurons and embryonic homeoprotein, Otx2, reportedly regulate the timing of the critical period for ocular dominance plasticity in the visual cortex (Pizzorusso et al., 2002; Sugiyama et al., 2008). A similar role for proteoglycan aggrecan and parvalbumin-positive neurons has been implicated for the barrel cortex plasticity following whisker trimming (McRae et al., 2007). The density of parvalbumin-positive cells and perineuronal nets was investigated in adolescent mouse barrel cortex and following removal of all but one whisker (univibrissa rearing or single whisker experience) on the snout or removal of every second whisker (chessboard deprivation) (Nowicka et al., 2009). Univibrissa rearing, but not chessboard deprivation, increased the density of perineuronal nets and parvalbumin-positive cells in the deprived barrels (Nowicka et al., 2009). These data suggest the involvement of parvalbumin-positive GABAergic neurons and perineuronal proteoglycan nets in plasticity of the barrel cortex in adolescent and adult mice as discussed in the next section.
Is the timing of the lesion-induced critical period plasticity set in subcortical structures?
In one of the pioneering studies (Durham and Woolsey, 1984) the timing of the critical period closure was reported to follow a succession from the brainstem to the barrel cortex between P0-P5. The only evidence strongly pointing to the subcortical regulation of critical period plasticity comes from studies involving excess serotonin in the barrel cortex. Pharmacological manipulations by administering MAOA inhibitor clorgyline in neonatal rats delay barrel formation with no apparent effects on the termination of the critical period by P4 (Boylan et al., 2001). As described earlier, in Maoa knockout mice thalamocortical axons do not segregate and the barrels do not form (Cases et al., 1996). When these mice are treated daily with parachlorophenylalanine (PCPA, an inhibitor of tryptophan hydroxylase that significantly lowers 5-HT levels), barrels form much later during development. When neonatal row C whisker lesions were performed in Maoa knockout mice with delayed barrel formation the end of the critical period plasticity (P3) did not shift to a later age, suggesting that the timing of the critical period is not set in the cortex (Rebsam et al., 2005). Thus, critical period plasticity responses of thalamocortical axons and their postsynaptic partners in the barrel cortex may be a simple transfer of plasticity effects coming from the subcortical trigeminal centers.
Multiplicity of barrel cortex plasticity
Whisker follicle or ION lesions are not the only experimental manipulations used in studying barrel cortex plasticity. Whisker pairing (clipping of all whiskers but two), univibrissa rearing (single whisker experience or trimming all whiskers except one), sparing of one row of whiskers and clipping all the others, or chessboard whisker deprivation have been used as forms of noninvasive sensory deprivation. These studies are more comparable to monocular lid suture or dark rearing experiments performed in the visual system research.
Peripheral manipulations without follicle or nerve damage do not yield robust structural defects in the organization of whisker patterns in the brain and the effects are not strictly confined to perturbations done during the first few days after birth (Simons & Land, 1987; Fox, 1992). In fact, sensory experience-dependent plasticity has been noted throughout the life of a rodent (Armstrong-James et al., 1994; Fox, 1992, 1994, 2002; Isaac et al., 1997; Lebedev et al., 2000; Maier et al., 2003; Rema et al., 2003). For example, whisker trimming in juvenile rats leads to long-lasting receptive field enlargement in the barrel cortex (Simons & Land, 1987). Poor rough surface discrimination is another consequence of bilateral whisker trimming from birth to 45 days of age (Carvell & Simons, 1996). Bilateral whisker trimming during the lesion-induced critical period leads to profound behavioral deficits later in life (Lee et al., 2010). P0-P3 whisker-trimmed adolescent rats display poor performance in a gap crossing test, increased locomotion in an open-field test and increased investigative and contact behaviors in a social interaction test (Lee et al., 2010). These results indicate that sensory experience is critical during the formative period of the barrels (P0-3) for behavioral development as well.
Layers II/III (supragranular) neurons receive inputs from the barrel cells and they too are susceptible to alterations of sensory inputs during development (Fox, 1992, 2002; Diamond et al., 1994; Glazewski & Fox, 1996). The timing of layers II/III critical period is not as well defined as the one for peripheral lesion-induced structural plasticity of the layer IV; it varies depending on the study but it is usually confined to the second and third postnatal weeks.
Alteration of sensory input by all but one whisker experience during development leads to plasticity effects in the strength of layer IV to layers II/III and intra layers II/III horizontal connections but with different timing; layer IV to II/III synapses show an earlier and shorter critical period between P10-14 (Lendvai et al., 2000; Stern et al., 2001; Maravall et al., 2004a, b; Shoykhet et al., 2005). A longer and later critical period window has been determined for the horizontal connections between layer II/III neurons (Wen & Barth, 2011).
Among the forms of plasticity seen in layer II/III is alterations in axonal projection fields of horizontal connections. Removing all but one whisker after the first postnatal week has significant plasticity effects in layers II/III indicating increased lateral transmission from the barrel corresponding to the experienced single whisker and failure of vertical transmission from deprived barrels (Fox, 1992). Pairing of whisker rows (i.e., trimming of all whiskers except those in select rows) during postnatal development (P7-15) also lead to increased horizontal projections from one spared row column to neighboring spared row columns and decreased horizontal projections from spared row columns to neighboring deprived row columns in supragranular layers (Broser et al., 2008). Thus, during the critical period for layers II/III horizontal corticocortical connections are susceptible to peripherally driven sensory experience.
Molecular mechanisms of layer II/III plasticity have received little attention. One study reported that type1 cannabinoid (CB1) receptors are involved in mediating whisker representation and critical period plasticity in layer II/III of the rat barrel cortex (Li et al., 2009). Pharmacological blockade of CB1 receptors during the early critical period for layer II/III (P12-16) disrupted functional whisker map development, led to impaired whisker tuning and prevented experience-dependent plasticity Blockade after P25 did not affect whisker tuning or expression of experience-dependent plasticity.
Defects in synaptic plasticity that follow learning rules have been linked to cognitive impairments. One such condition is the fragile X syndrome caused by mutations in the Fmr1 gene. Fmr1 knockout mice have been used to model this cognitive impairment syndrome. In the absence of fragile X mental retardation protein (FMRP) development of cortical glutamatergic synaptic transmission is impaired with persistence of silent synapses and the plasticity window is delayed in the barrel cortex (Harlow et al., 2010). More strikingly, plasticity in layers II/III was disrupted: strength of layer IV inputs was reduced and did not show experience-dependent plasticity (Bureau et al., 2008).
Concluding Remarks
The mouse barrel cortex has become an excellent model system to study cortical plasticity. Developmental studies have begun unraveling a complex genetic programming that directs areal specification of the neocortex. Numerous axon guidance molecules have been identified in wiring of the thalamus and neocortex. The roles of sensory activity and experience in shaping somatosensory cortical map and patterning of neural elements within the map have been underscored. Intracellular signaling pathways downstream from glutamatergic synaptic transmission are emerging. Various forms of developmental plasticity and layer-specific critical periods have been identified. Barrel cortex is also emerging as a model to investigate synaptic malfunction in developmental cognitive disorders. We do not yet know the molecular mechanisms underlying the critical period for structural plasticity of the barrel cortex and how the duration of this period is determined, even though the available evidence suggests that the timing of the critical period might be set in subcortical structures. Future studies aimed at identifying differential gene expression before, during and at the closure of the critical period at various levels of the trigeminal pathway will undoubtedly shed light on these questions.
Acknowledgments
Support: NIH/NINDS RO1 NS039050, NS037070 and Ecoles des Neurosciences de Paris (RSE);
INSERM, the Fondation Jerome Lejeune, the NERF (Région île de France); the European Commission (FP7-health-2007-A-201714) (PG)
Abbreviations
- AC1
Adenylyl cyclase type I
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor or quisqualate receptor
- BDNF
brain-derived neurotrophic factor
- CB1
type 1 cannabinoid receptors
- CaMKII
calcium/calmodulin-dependent kinase II
- E
embryonic day
- EGOs
early gamma oscillations
- FGF8
fibroblast growth factor 8
- FMRP
fragile X mental retardation protein
- GAP-43
growth-associated phosphoprotein
- GLAST
astrocyte-specific glutamate-aspartate transporter
- GLT1
glutamate transporter 1
- ION
Infraorbital branch of the trigeminal nerve
- LTP
long-term potentiation
- LTD
long-term depression
- mGluR5
metabotropic glutamate receptor 5
- MAOA
monoamine oxidase A
- NGF
Nerve growth factor
- NT-3
neurotrophin-3
- NMDA
N-methyl-D-Aspartate
- Ols
Oligodendrocytes
- P
postnatal day
- PCA
p-chloroamphetamine
- PCPA
parachlorophenylalanine
- PLC β
phospholipase C-β
- PKC
protein kinase C
- POm
posteromedial nucleus
- PrV
Principal sensory nucleus of the trigeminal nerve
- RIM
RAB interacting molecule, a protein controlling synaptic vesicle release
- SHH
sonic hedgehog
- SP
Subplate
- 5-HT
serotonin
- TCA
Thalamocortical axon
- TG
Trigeminal ganglion
- TGF-β
transforming growth factor β
- TSP
Thrombospondin
- TTX
Tetrodotoxin, a Na+ channel blocker
- VPM
Ventroposteromedial nucleus of the thalamus
- VPL
Ventroposterolateral nucleus of the thalamus
- WNT
Drosophila wingless
- WP
Whisker pad
Footnotes
The authors declare that they have no competing interests.
References
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- Agmon A, Yang LT, O’Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A, Yang LT, Jones EG, O’Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Antonini A, Fagiolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol. 1987;263:265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within the rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Diamond ME, Ebner FF. An innocuous bias in whisker use in adult rats modifies receptive fields of barrel cortex neurons. J Neurosci. 1994;14:6978–6991. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi A, Calia E, Ciampini A, Riccio M, Vetuschi A, Persico AM, Keller F. Deafferentation-induced apoptosis of neurons in thalamic somatosensory nuclei of the newborn rat: critical period and rescue from cell death by peripherally applied neurotrophins. Eur J Neurosci. 2000;12:2281–2290. doi: 10.1046/j.1460-9568.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- Ballester-Rosado CJ, Albright MJ, Wu CS, Liao CC, Zhu J, Xu J, Lee LJ, Lu HC. mGluR5 in cortical excitatory neurons exerts both cell-autonomous and -nonautonomous influences on cortical somatosensory circuit formation. J Neurosci. 2010;30:16896–16909. doi: 10.1523/JNEUROSCI.2462-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates CA, Killackey HP. The organization of the neonatal rat’s brainstem trigeminal complex and its role in the formation of central trigeminal patterns. J Comp Neurol. 1985;240:265–287. doi: 10.1002/cne.902400305. [DOI] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. Anatomical correlates of the forelimb in the ventrobasal complex and the cuneate nucleus of the neonatal rat. Brain Res. 1978;158:450–455. doi: 10.1016/0006-8993(78)90688-1. [DOI] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. The sensitive period in the development of the trigeminal system of the neonatal rat. J Comp Neurol. 1980;193:335–350. doi: 10.1002/cne.901930203. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Pro Natl Acad Sci USA. 1993;90:153–157. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue ME, Erzurumlu RS, Jhaveri S. A comparison of pattern formation by thalamocortical and serotonergic afferents in the rat barrel field cortex. Cereb Cortex. 1991;1:380–389. doi: 10.1093/cercor/1.5.380. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- Boylan CB, Kesterson KL, Bennett-Clarke CA, Chiaia NL, Rhoades RW. Neither peripheral nerve input nor cortical NMDA receptor activity are necessary for recovery of a disrupted barrel pattern in rat somatosensory cortex. Dev Brain Res. 2001;129:95–106. doi: 10.1016/s0165-3806(01)00163-8. [DOI] [PubMed] [Google Scholar]
- Broser P, Grinevich V, Osten P, Sakmann B, Wallace DJ. Critical period plasticity of axonal arbors of layer 2/3 pyramidal neurons in rat somatosensory cortex: layer-specific reduction of proteins into deprived cortical columns. Cereb Cortex. 2008;18:1588–1603. doi: 10.1093/cercor/bhm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calia E, Persico AM, Baldi F, Keller F. BDNF and NT-3 applied in the whisker pad reverse cortical changes after peripheral deafferentation in neonatal rats. Eur J Neurosci. 1998;10:3194–31200. doi: 10.1046/j.1460-9568.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Abnormal tactile experience in early life disrupts active touch. J Neurosci. 1996;16:2750–2757. doi: 10.1523/JNEUROSCI.16-08-02750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Robertson RT, Killackey HP. Rapid alteration of thalamocortical axon morphology follows peripheral damage in the neonatal rat. Proc Natl Acad Sci USA. 1995;92:2549–2552. doi: 10.1073/pnas.92.7.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano SM, Robertson RT, Killackey HP. Individual axon morphology and thalamocortical topography in developing rat somatosensory cortex. J Comp Neurol. 1996;367:36–53. doi: 10.1002/(SICI)1096-9861(19960325)367:1<36::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetetized and awake rats. J Comp Neurol. 1984;229:199–213. doi: 10.1002/cne.902290206. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Bennett-Clarke CA, Eck M, White FA, Crissman RS, Rhoades RW. Evidence for prenatal competition among the central arbors of trigeminal primary afferent neurons. J Neurosci. 1992a;12:62–76. doi: 10.1523/JNEUROSCI.12-01-00062.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaia NL, Fish SE, Bauer WR, Bennett-Clarke CA, Rhoades RW. Postnatal blockade of cortical activity by tetrodotoxin does not disrupt the formation of vibrissa-related patterns in the rat’s somatosensory cortex. Dev Brain Res. 1992b;66:244–250. doi: 10.1016/0165-3806(92)90086-c. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Fish SE, Bauer WR, Figley BA, Eck M, Bennett-Clarke CA, Rhoades RW. Effects of postnatal blockade of cortical activity with tetrodotoxin upon the development and plasticity of vibrissa-related patterns in the somatosensory cortex of hamsters. Somatosens Mot Res. 1994;11:219–28. doi: 10.3109/08990229409051390. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Bennett-Clarke CA, Crissman RS, Zheng L, Chen M, Rhoades RW. Effect of neonatal axoplasmic transport attenuation in the infraorbital nerve on vibrissae-related patterns in the rat’s brainstem, thalamus and cortex. Eur J Neurosci. 1996;8:1601–1612. doi: 10.1111/j.1460-9568.1996.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Cohen-Tannoudji M, Babinet C, Wassef M. Early determination of a mouse somatosensory cortex marker. Nature. 1994;368:460–463. doi: 10.1038/368460a0. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Da Silva S, Hasegawa H, Scott A, Zhou X, Wagner AK, Han BX, Wang F. Proper formation of whisker barrelettes requires periphery-derived Smad4-dependent TGF-β signaling. Proc Natl Acad Sci USA. 2011;108:3395–3400. doi: 10.1073/pnas.1014411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cell Neurosci. 2002a;21:477–492. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. Lesion-induced thalamocortical axonal plasticity in the S1 cortex is independent of NMDA receptor function in excitatory cortical neurons. J Neurosci. 2002b;22:9171–9175. doi: 10.1523/JNEUROSCI.22-21-09171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P, Hardy MH. The development of mouse vibrissae in vivo and in vitro. J Anatomy. 1952;86:342–356. [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Lumsden AGS. Relation of target encounter and neuronal death to nerve growth factor responsiveness in the developing mouse trigeminal ganglion. J Comp Neurol. 1984;223:124–137. doi: 10.1002/cne.902230110. [DOI] [PubMed] [Google Scholar]
- Daw N. Visual Development. Plenum Publishing Corp; New York: 1995. [Google Scholar]
- Dawson DR, Killackey HP. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. J Comp Neurol. 1987;256:246–256. doi: 10.1002/cne.902560205. [DOI] [PubMed] [Google Scholar]
- Deng L, Kaeser PS, Xu W, Südhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MF, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Yin J, Xu HM, Jacquin MF, Chen ZF. Formation of whisker-related principal sensory nucleus-based lemniscal pathway requires a paired homeodomain transcription factor, Drg11. J Neurosci. 2003;23:7246–7254. doi: 10.1523/JNEUROSCI.23-19-07246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Effects of neonatal whisker lesions on mouse central trigeminal pathways. J Comp Neurol. 1984;223:424–447. doi: 10.1002/cne.902230308. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–92. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS. Mechanisms of plasticity in the development of cortical somatosensory maps. In: Blumberg MS, Freeman JH, Robinson SR, editors. Developmental and Comparative Neuroscience: Epigenetics, Evolution, & Behavior. Oxford University Press; 2010. [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Emergence of connectivity in the embryonic rat parietal cortex. Cereb Cortex. 1992a;2:336–352. doi: 10.1093/cercor/2.4.336. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992b;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Iwasato T. Patterning of the somatosensory maps with NMDA receptors. In: Erzurumlu R, Guido W, Molnár Z, editors. Development and plasticity in sensory thalamus and cortex. New York: Springer; 2006. [Google Scholar]
- Erzurumlu RS, Killackey HP. Critical and sensitive periods in neurobiology. Curr Top Dev Biol. 1982;17:207–240. doi: 10.1016/s0070-2153(08)60522-0. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Bates CA, Killackey HP. Differential organization of thalamic projection cells in the brainstem trigeminal complex of the rat. Brain Res. 1980;198:427–433. doi: 10.1016/0006-8993(80)90756-8. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S, Benowitz LI. Transient patterns of GAP-43 expression during the formation of barrels in the rat somatosensory cortex. J Comp Neurol. 1990;292:443–456. doi: 10.1002/cne.902920310. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Chen ZF, Jacquin MF. Molecular determinants of the face map development in the trigeminal brainstem. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:121–134. doi: 10.1002/ar.a.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Murakami Y, Rijli FM. Mapping the face in the somatosensory brainastem. Nat Rev Neurosci. 2010;11:252–263. doi: 10.1038/nrn2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62:205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairén A. Cajal and Lorente de Nó on cortical interneurons: Coincidences and progress. Brain Res Rev. 2007;55:430–444. doi: 10.1016/j.brainresrev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, Isaac JT. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron. 1998;21:347–357. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- Foeller E, Feldman DE. Synaptic basis for developmental plasticity in somatosensory cortex. Curr Opin Neurobiol. 2004;14:89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. The cortical component of experience-dependent synaptic plasticity in the rat barrel cortex. J Neurosci. 1994;14:7665–7679. doi: 10.1523/JNEUROSCI.14-12-07665.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Fox K. Barrel Cortex. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Fox K, Schlaggar BL, Glazewski S, O’Leary DD. Glutamate receptor blockade at cortical synapses disrupts development of thalamocortical and columnar organization in somatosensory cortex. Proc Natl Acad Sci USA. 1996;93:5584–5589. doi: 10.1073/pnas.93.11.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Gheorghita F, Kraftsik R, Dubois R, Welker E. Structural basis for map formation in the thalamocortical pathway of the barrelless mouse. J Neurosci. 2006;26:10057–10067. doi: 10.1523/JNEUROSCI.1263-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Maravall M, Welker E, Bonvento G. The barrel cortex as a model to study dynamic neuroglial interaction. Neuroscientist. 2009;15:351–366. doi: 10.1177/1073858409336092. [DOI] [PubMed] [Google Scholar]
- Gitton Y, Cohen-Tannoudji M, Wassef M. Specification of somatosensory identity in cortical explants. J Neurosci. 1999a;19:4889–4898. doi: 10.1523/JNEUROSCI.19-12-04889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitton Y, Cohen-Tannoudji M, Wassef M. Role of thalamic axons in the expression of H-2Z1, a mouse somatosensory cortex specific marker. Cereb Cortex. 1999b;9:611–620. doi: 10.1093/cercor/9.6.611. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Chen CM, Silva A, Fox K. Requirement for alpha-CaMKII in experience-dependent plasticity of the barrel cortex. Science. 1996;272:421–423. doi: 10.1126/science.272.5260.421. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Giese KP, Silva A, Fox K. The role of alpha-CaMKII autophosphorylation in neocortical experience-dependent plasticity. Nat Neurosci. 2000;3:911–918. doi: 10.1038/78820. [DOI] [PubMed] [Google Scholar]
- Halls ML, Cooper DMF. Regulation by Ca2+-Signaling Pathways of Adenylyl Cyclases. Cold Spring Harb Perspect Biol. 2011;3(1):a004143. doi: 10.1101/cshperspect.a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan AJ, Kind PC, Blakemore C. Phospholipase C-beta1 expression correlates with neuronal differentiation and synaptic plasticity in rat somatosensory cortex. Neuropharmacology. 1998;37:593–605. doi: 10.1016/s0028-3908(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Blakemore C, Katsnelson A, Vitalis T, Huber KM, Bear M, Roder J, Kim D, Shin HS, Kind PC. PLC-beta1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat Neurosc,i. 2001;4:282–288. doi: 10.1038/85132. [DOI] [PubMed] [Google Scholar]
- Hardingham N, Glazewski S, Pakhotin P, Mizuno K, Chapman PF, Giese KP, Fox K. Neocortical long-term potentiation and experience-dependent synaptic plasticity require alpha-calcium/calmodulin-dependent protein kinase II autophosphorylation. J Neurosci. 2003;23:4428–4436. doi: 10.1523/JNEUROSCI.23-11-04428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TA, Woolsey TA, Jacquin MF. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Dev Brain Res. 1992;66:146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- Houades V, Koulakoff A, Ezan P, Seif I, Giaume C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J Neurosci. 2008;28:5207–5217. doi: 10.1523/JNEUROSCI.5100-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Effects of monocular deprivation in kittens. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964;248:492–497. doi: 10.1007/BF00348878. [DOI] [PubMed] [Google Scholar]
- Inan M, Crair MC. Development of cortical maps: perspectives from the barrel cortex. Neuroscientist. 2007;13:49–61. doi: 10.1177/1073858406296257. [DOI] [PubMed] [Google Scholar]
- Inan M, Lu HC, Albright MJ, She WC, Crair MC. Barrel map development relies on protein kinase A regulatory subunit II beta-mediated cAMP signaling. J Neurosci. 2006;26:4338–4349. doi: 10.1523/JNEUROSCI.3745-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Itami C, Mizuno K, Kohno T, Nakamura S. Brain-derived neurotrophic factor requirement for activity-dependent maturation of glutamatergic synapse in developing mouse somatosensory cortex. Brain Res. 2000;857:141–150. doi: 10.1016/s0006-8993(99)02352-5. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Inan M, Kanki H, Erzurumlu RS, Itohara S, Crair MC. Cortical adenylyl cyclase 1 is required for thalamocortical synapse maturation and aspects of layer IV barrel development. J Neurosci. 2008;28:5931–5943. doi: 10.1523/JNEUROSCI.0815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmonod D, Rice FL, Van der Loos H. Mouse somatosensory cortex: alterations in the barrelfield following receptor injury at different early postnatal ages. Neuroscience. 1981;6:1503–1535. doi: 10.1016/0306-4522(81)90222-0. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Killackey HP. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. II The altered morphology of thalamocortical afferents following neonatal infraorbital nerve cut. J Neurosci. 1987;7:3544–3553. doi: 10.1523/JNEUROSCI.07-11-03544.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri S, Erzurumlu RS, Crossin K. Barrel construction in rodent neocortex: role of thalamic afferents versus extracellular matrix molecules. Proc Natl Acad Sci USA. 1991;88:4489–4493. doi: 10.1073/pnas.88.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri S, Erzurumlu RS, Chiaia N, Kumar TR, Matzuk MM. Defective whisker follicles and altered brainstem patterns in activin and follistatin knockout mice. Mol Cell Neurosci. 1998;12:206–219. doi: 10.1006/mcne.1998.0710. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- Killackey HP. Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res. 1973;51:326–331. doi: 10.1016/0006-8993(73)90383-1. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Dawson DR. Expansion of the central hindpaw representation following fetal forelimb removal in the rat. Eur J Neurosci. 1989;1:210–221. doi: 10.1111/j.1460-9568.1989.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Fleming K. The role of the principal sensory nucleus in central trigeminal pattern formation. Brain Res. 1985;354:141–145. doi: 10.1016/0165-3806(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Leshin S. The organization of specific thalamocortical projections to the posteromedial barrel subfield of the rat somatic sensory cortex. Brain Res. 1975;86:469–472. doi: 10.1016/0006-8993(75)90897-5. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Kivrak BG, Erzurumlu RS. Development of the principal nucleus trigeminal lemniscal projections in the mouse. 2012 doi: 10.1002/cne.23183. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. Vibrissae-evoked behavior and conditioning before functional ontogeny of the somatosensory vibrissae cortex. J Neurosci. 1999;19:5131–5137. doi: 10.1523/JNEUROSCI.19-12-05131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers M, Zeigler PH. Development of rodent whisking: trigeminal input and central pattern generation. Somatosens Mot Res. 2006;23:1–10. doi: 10.1080/08990220600700768. [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev MA, Mirabella G, Erchova I, Diamond ME. Experience-dependent plasticity of rat barrel cortex: redistribution of activity across barrel-columns. Cereb Cortex. 2000;10:23–31. doi: 10.1093/cercor/10.1.23. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Aldebrecht C, Doye A, Alvarez C, El-Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Leamey CA, Ho SM. Afferent arrival and onset of functional activity in the trigeminothalamic pathway of the rat. Dev Brain Res. 1998;105:195–207. doi: 10.1016/s0165-3806(97)00170-3. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Erzurumlu RS. Altered parcellation of neocortical somatosensory maps in N-methyl-D-aspartate receptor-deficient mice. J Comp Neurol. 2005;485:57–63. doi: 10.1002/cne.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol. 2005a;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LJ, Lo FS, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci. 2005b;25:2304–2311. doi: 10.1523/JNEUROSCI.4902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LJ, Chen WJ, Chuang YW, Wang YC. Neonatal whisker trimming causes long-lasting changes in structure and function of the somatosensory system. Exp Neurol. 2009;219:524–532. doi: 10.1016/j.expneurol.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Li L, Bender KJ, Drew PJ, Jadhav SP, Sylwestrak E, Feldman DE. Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex. Neuron. 2009;64:537–549. doi: 10.1016/j.neuron.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whiskerrelated neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. Neonatal deafferentation does not alter membrane properties of trigeminal nuc;eus principalis neurons. J Neurophysiol. 2001;85:1088–1096. doi: 10.1152/jn.2001.85.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. L-type calcium channel-mediated plateau potentials in barrelette cells during structural plasticity. J Neurophysiol. 2002;88:794–801. doi: 10.1152/jn.2002.88.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. Conversion of functional synapses into silent synapses in the trigeminal brainstem after neonatal peripheral nerve transection. J Neurosci. 2007;27:4929–4934. doi: 10.1523/JNEUROSCI.5342-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Zhao S. N-methyl-D-aspartate receptor subunit composition in the rat trigeminal principal nucleus remains constant during postnatal development and following neonatal denervation. Neuroscience. 2011;178:240–249. doi: 10.1016/j.neuroscience.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Guido W, Erzurumlu RS. Electrophysiological properties and synaptic responses of cells in the trigeminal principal sensory nucleus of postnatal rats. J Neurophysiol. 1999;82:2765–2775. doi: 10.1152/jn.1999.82.5.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Molnár Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorento de Nó R. La corteza cerebral del ratón. Trab Lab Investig Biol (Madrid) 1922;20:41–78. [Google Scholar]
- Lotto B, Upton L, Price DJ, Gaspar P. Serotonin receptor activation enhances neurite outgrowth of thalamic neurons in rodents. Neurosci Let. 1999;269:87–90. doi: 10.1016/s0304-3940(99)00422-x. [DOI] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Lu HC, She WC, Plas DT, Neumann PE, Janz R, Crair MC. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical ‘barrel’ map development. Nat Neurosci. 2003;6:939–947. doi: 10.1038/nn1106. [DOI] [PubMed] [Google Scholar]
- Lu HC, Butts DA, Kaeser PS, She WC, Janz R, Crair MC. Role of efficient neurotransmitter release in barrel map development. J Neurosci. 2006;26:2692–2703. doi: 10.1523/JNEUROSCI.3956-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush ME, Ma L, Parada LF. TrkB signaling regulates the developmental maturation of the somatosensory cortex. Int J Dev Neurosci. 2005;23:523–536. doi: 10.1016/j.ijdevneu.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Ma PM. Barrelettes: architectonic vibrissal representations in the brainstem trigeminal complex of the mouse: II, normal postnatal development. J Comp Neurol. 1993;327:376–397. doi: 10.1002/cne.903270306. [DOI] [PubMed] [Google Scholar]
- Ma PM, Woolsey TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Res. 1984;306:374–379. doi: 10.1016/0006-8993(84)90390-1. [DOI] [PubMed] [Google Scholar]
- Maier DL, Mani S, Donovan SL, Soppet D, Tessarollo L, McCasland JS, Meiri KF. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci USA. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier DL, Grieb GM, Stelzner DJ, McCasland JS. Large-scale plasticity in barrel cortex following repeated whisker trimming in young adult hamsters. Exp Neurol. 2003;184:737–45. doi: 10.1016/S0014-4886(03)00335-2. [DOI] [PubMed] [Google Scholar]
- Maravall M, Stern EA, Svoboda K. Development of intrinsic properties and excitability of layer 2/3 pyramidal neurons during a critical period for sensory maps in rat barrel cortex. J Neurophysiol. 2004;92:144–156. doi: 10.1152/jn.00598.2003. [DOI] [PubMed] [Google Scholar]
- Maravall M, Koh IY, Lindquist WB, Svoboda K. Experience-dependent changes in basal dendritic branching of layer 2/3 pyramidal neurons during a critical period for developmental plasticity in rat barrel cortex. Cereb Cortex. 2004;14:655–64. doi: 10.1093/cercor/bhh026. [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métin C, Frost DO. Visual responses of neurons in somatosensory cortex of hamsters with experimentally induced retinal projections to somatosensory thalamus. Proc Natl Acad Sci USA. 1989;86:357–361. doi: 10.1073/pnas.86.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Kuhn P. Neonatal transection of the infraorbital nerve increases the expression of protein related to neuronal death in the principal sensory nucleus of the trigeminal nerve. Brain Res. 1997;796:233–244. doi: 10.1016/s0006-8993(97)00713-0. [DOI] [PubMed] [Google Scholar]
- Miller MW, al-Ghoul WM, Murtaugh M. Expression of ALZ-50 immunoreactivity in the developing principal sensory nucleus of the trigeminal nerve: effect of transecting the infraorbital nerve. Brain Res. 1991;560:132–138. doi: 10.1016/0006-8993(91)91223-n. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J Neurophysiol. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. NMDA receptors pattern early activity in the developing barrel cortex in vivo. Cereb Cortex. 2009;19:688–696. doi: 10.1093/cercor/bhn115. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R. Early γ oscillations synchronize developing thalamus and cortex. Science. 2011;334:226–229. doi: 10.1126/science.1210574. [DOI] [PubMed] [Google Scholar]
- Mirza R, Kivrak B, Erzurumlu RS. Cooperative slit and netrin signaling in contralateralization of the mouse trigeminothalamic pathway. 2012 doi: 10.1002/cne.23188. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic N, Mohajeri H, Schachner M. Effects of NMDA receptor blockade in the developing rat somatosensory cortex on the expression of the glia-derived extracellular matrix glycoprotein tenascin-C. Eur J Neurosci. 1996;8:1793–1802. doi: 10.1111/j.1460-9568.1996.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Higashi S, Lopez-Bendito G. Choreography of early thalamocortical development. Cereb Cortex. 2003;13:661–669. doi: 10.1093/cercor/13.6.661. [DOI] [PubMed] [Google Scholar]
- Müller MR, Zheng F, Werner S, Alzheimer C. Transgenic mice expressing dominant-negative activin receptor IB in forebrain neurons reveal novel functions of activin at glutamatergic synapses. J Biol Chem. 2006;281:29076–29084. doi: 10.1074/jbc.M604959200. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Johnson JE, O’Leary DD. Graded and areal expression patterns of regulatory genes and cadherins in embryonic neocortex independent of thalamocortical input. J Neurosci. 1999;19:10877–10885. doi: 10.1523/JNEUROSCI.19-24-10877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narboux-Nême N, Evrard A, Ferezou I, Erzurumlu RS, Kaeser PS, Lainé J, Rossier J, Ropert N, Südhof TC, Gaspar P. Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex. 2012 doi: 10.1523/JNEUROSCI.0343-12.2012. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North HA, Karim A, Jacquin MF, Donoghue MJ. EphA4 is necessary for spatially selective peripheral somatosensory topography. Dev Dyn. 2010;239:630–638. doi: 10.1002/dvdy.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka D, Soulsby S, Skangiel-Kramska J, Glazewski S. Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur J Neurosci. 2009;30:2053–2063. doi: 10.1111/j.1460-9568.2009.06996.x. [DOI] [PubMed] [Google Scholar]
- Ohsaki K, Nakamura S. Instructive role of a peripheral pattern for the central patterning of the trigeminal projection at the brainstem and thalamus revealed by an artificially altered whisker pattern. Neuroscience. 2006;141:1899–1908. doi: 10.1016/j.neuroscience.2006.04.082. [DOI] [PubMed] [Google Scholar]
- Ohsaki K, Osumi N, Nakamura S. Altered whisker patterns induced by ectopic expression of Shh are topographically represented by barrels. Dev Brain Res. 2002;137:159–170. doi: 10.1016/s0165-3806(02)00462-5. [DOI] [PubMed] [Google Scholar]
- O’Leary DD. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989;12:400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Sahara K. Genetic regulation of arealization of the neocortex. Curr Opin Neurobio,l. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Schlaggar BL, Tuttle R. Specification of neocortical areas and thalamocortical connections. Annu Rev Neurosci. 1994;17:419–439. doi: 10.1146/annurev.ne.17.030194.002223. [DOI] [PubMed] [Google Scholar]
- Osterheld-Haas MC, Hornung JP. Laminar development of the mouse barrel cortex: effects of neurotoxins against monoamines. Exp Brain Res. 1996;110:183–195. doi: 10.1007/BF00228550. [DOI] [PubMed] [Google Scholar]
- Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313:1408–1413. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]
- Pallas SL. Intrinsic and extrinsic factors shaping cortical identity. Trends Neurosci. 2001;24:417–423. doi: 10.1016/s0166-2236(00)01853-1. [DOI] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Piñon MC, Jethwa A, Jacobs E, Campagnoni A, Molnár Z. Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. Physiol. 2009;587(Pt 9):1903–1915. doi: 10.1113/jphysiol.2008.167767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pratt T, Price DJ. Dual roles of transcription factors in forebrain morphogenesis and development of axonal pathways. In: Erzurumlu R, Guido W, Molnár Z, editors. Development and plasticity in sensory thalamus and cortex. New York: Springer; 2006. pp. 19–xx. [Google Scholar]
- Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnar Z. The development of cortical connections. Eur J Neurosci. 2006;23:910–920. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci. 2003;17:167–173. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experiencedependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice. J Neurosc,i. 2002;22:8541–8552. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Dissociating barrel development and lesion-induced plasticity in the mouse somatosensory cortex. J Neurosci. 2005;25:706–710. doi: 10.1523/JNEUROSCI.4191-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rema V, Armstrong-James M, Ebner FF. Experience-dependent plasticity is impaired in adult rat barrel cortex after whiskers are unused in early postnatal life. J Neurosci. 2003;23:358–366. doi: 10.1523/JNEUROSCI.23-01-00358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Bennett-Clarke CA, Chiaia NL, White FA, Macdonald GJ, Haring JH, Jacquin MF. Development and lesion induced reorganization of the cortical representation of the rat’s body surface as revealed by immunocytochemistry for serotonin. J Comp Neurol. 1990;293:190–207. doi: 10.1002/cne.902930204. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Bennett-Clarke CA, Shi MY, Mooney RD. Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J Neurophysiol. 1994;72:2438–2450. doi: 10.1152/jn.1994.72.5.2438. [DOI] [PubMed] [Google Scholar]
- Rice FL, Gomez C, Barstow C, Burnet A, Sands P. A comparative analysis of the development of the primary somatosensory cortex: interspecies similarities during barrel and laminar development. J Comp Neurol. 1985;236:477–495. doi: 10.1002/cne.902360405. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Welker E, Pascual M, Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AW, Pallas SL, Hahm JO, Sur M. A map of visual space induced in primary auditory cortex. Science. 1990;250:818–820. doi: 10.1126/science.2237432. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Fox K, O’Leary DD. Postsynaptic control of plasticity in developing somatosensory cortex. Nature. 1993;364:623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- Scott HL, Braud S, Bannister NJ, Isaac JTR. Synaptic strength at the thalamocortical input to layer IV neonatal barrel cortex is regulated by protein kinase C. Neuropharmacology. 2007;52:185–192. doi: 10.1016/j.neuropharm.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Sehara K, Kawasaki H. Neuronal circuits with whisker-related patterns. Mol Neurobiol. 2011;43:155–162. doi: 10.1007/s12035-011-8170-8. [DOI] [PubMed] [Google Scholar]
- Senft SL, Woolsey TA. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991;1:308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Grove EA. Subcortical and neocortical guidance of area-specific thalamic innervation. In: RErzurumlu R, Guido W, ZMolnár Z, editors. Development and plasticity in sensory thalamus and cortex. New York: Springer; 2006. pp. 42–53. [Google Scholar]
- Shoykhet M, Land PW, Simons DJ. Whisker trimming begun at birth or on postnatal day 12 affects excitatory and inhibitory receptive fields of layer IV barrel neurons. J Neurophysiol. 2005;94:3987–3995. doi: 10.1152/jn.00569.2005. [DOI] [PubMed] [Google Scholar]
- Shoykhet M, Shetty P, Minnery BS, Simons DJ. Protracted development of responses to whisker deflection in rat trigeminal ganglion neurons. J Neurophysiol. 2003;90:1432–1437. doi: 10.1152/jn.00419.2003. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Temporal and spatial integration in the rat S1 vibrissa cortex. J Neurophysiol. 1985;54:615–635. doi: 10.1152/jn.1985.54.3.615. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformations in the rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987;326:694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Singh TD, Mizuno K, Kohno T, Nakamura S. BDNF and trkB mRNA expression in neurons of the neonatal mouse barrel field cortex: normal development and plasticity after cauterizing facial vibrissae. Neurochem Res. 1997;22:791–797. doi: 10.1023/a:1022075508176. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc Natl Acad Sci USA. 1990;87:923–927. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen H, Van der Loos H. Early lesions of mouse vibrissal follicles: their influence on dendrite orientation in the cortical barrelfield. Exp Brai. 1980;40:419–431. doi: 10.1007/BF00236150. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Strominger RN, Woolsey TA. Templates for locating the whisker area in fresh flattened mouse and rat cortex. J Neurosci Meth. 1987;22:113–118. doi: 10.1016/0165-0270(87)90004-5. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Xiao C, Takeyama A, He YF, Takano-Yamamoto T, Ichikawa H. Apoptotic cascade of neurons in the subcortical sensory relay nuclei following the neonatal infraorbital nerve transection. Brain Res. 1999;824:284–290. doi: 10.1016/s0006-8993(99)01237-8. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers MS, Flemming J, Vaught C, Young TA, Jonathan Polan H. Characterizing the functional significance of the neonatal rat vibrissae prior to the onset of whisking. Somatosens Mot Res. 2003;20:157–162. doi: 10.1080/0899022031000105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Gewirtz JC, Bofenkamp L, Wickham RJ, Ge H, O’Connor MB. Canonical TGF-β Signaling Is Required for the Balance of Excitatory/Inhibitory Transmission within the Hippocampus and Prepulse Inhibition of Acoustic Startle. J Neurosci. 2010;30:6025–6035. doi: 10.1523/JNEUROSCI.0789-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Rubenstein JLR. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Sur M, Pallas SL, Roe AW. Cross-modal plasticity in cortical development: differentiation and specification of sensory neocortex. Trends Neurosci. 1990;13:227–233. doi: 10.1016/0166-2236(90)90165-7. [DOI] [PubMed] [Google Scholar]
- Takasaki C, Okada R, Mitani A, Fukaya M, Yamasaki M, Fujihara Y, Shirakawa T, Tanaka K, Watanabe M. Glutamate transporters regulate lesion-induced plasticity in the developing somatosensory cortex. J Neurosci. 2008;28:4995–5006. doi: 10.1523/JNEUROSCI.0861-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Hayakawa I, Matsubayashi Y, Tanaka K, Ikenaka K, Lu QR, Kawasaki H. Termination of lesion-induced plasticity in the mouse barrel cortex in the absence of oligodendrocytes. Mol Cell Neurosci. 2008;39:40–49. doi: 10.1016/j.mcn.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Turlejski K, Djavadian RL, Kossut M. Neonatal serotonin depletion modifies development but not plasticity in rat barrel cortex. Neuroreport. 1997;8:1823–1828. doi: 10.1097/00001756-199705260-00007. [DOI] [PubMed] [Google Scholar]
- Uziel D, Garcez P, Lent R, Peuckert C, Niehage R, Weth F, Bolz J. Connecting thalamus and cortex: the role of ephrins. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:135–142. doi: 10.1002/ar.a.20286. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Polleux F. Developmental mechanisms patterning thalamocortical projections: intrinsic, extrinsic and in between. Trends Neurosci. 2004;27:384–391. doi: 10.1016/j.tins.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Van der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 1976;2:1–6. doi: 10.1016/0304-3940(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Dorfl H, Welker E. Variation in pattern of mystacial vibrissae in mice. A quantitative study of ICR stock and several inbred strains. J Hered. 1984;75:326–336. [PubMed] [Google Scholar]
- Van der Loos H, Welker E, Dorfl J, Rumo G. Selective breeding for variations in patterns of mystacial vibrissae of mice. Bilaterally symmetrical strains derived from ICR stock. J Hered. 1986;77:66–82. doi: 10.1093/oxfordjournals.jhered.a110201. [DOI] [PubMed] [Google Scholar]
- Van Exan RJ, Hardy MH. A spatial relationship between innervation and the early differentiation of vibrissa follicles in the embryonic mouse. J Anat. 1980;131:643–656. [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Callebert J, Launay JM, Price DJ, Seif I, Gaspar P. Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: determination of a sensitive developmental period. J Comp Neurol. 1998;393:169–184. doi: 10.1002/(sici)1096-9861(19980406)393:2<169::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Gillies K, Hanoun N, Hamon M, Seif I, Gaspar P, Kind P, Price DJ. Interactions between TrkB signaling and serotonin excess in the developing murine somatosensory cortex: a role in tangential and radial organization of thalamocortical axons. J Neurosci. 2002;22:4987–5000. doi: 10.1523/JNEUROSCI.22-12-04987.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, et al. Glial glutamatetransporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Waite PM, Ho SM, Henderson TA. Afferent ingrowth and onset of activity in the rat trigeminal nucleus. Eur J Neurosci. 2000;12:2781–2792. doi: 10.1046/j.1460-9568.2000.00161.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Bluske KK, Dickel LK, Nakagawa Y. Basal progenitor cells in the embryonic mouse thalamus--their molecular characterization and the role of neurogenins and Pax6. Neural Dev. 2011;6:35. doi: 10.1186/1749-8104-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RF, Abdel-Majid RM, Barnett MW, Willis BS, Katsnelson A, Gillingwater TH, McKnight GS, Kind PC, Neumann PE. Involvement of protein kinase A in patterning of the mouse somatosensory cortex. J Neurosci. 2006;26:5393–5401. doi: 10.1523/JNEUROSCI.0750-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971;26:259–275. [PubMed] [Google Scholar]
- Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neuro. 1976;166:173–189. doi: 10.1002/cne.901660205. [DOI] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J Neurosci. 1986;6:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E, Armstrong-James M, Bronchti G, Ourednik W, Gheorghita-Baechler F, Dubois R, Guernsey DL, Van der Loos H, Neumann PE. Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science. 1996;271:1864–1867. doi: 10.1126/science.271.5257.1864. [DOI] [PubMed] [Google Scholar]
- Wen JA, Barth AL. Input-specific critical periods for experience-dependent plasticity in layer 2/3 pyramidal neurons. J Neurosci. 2011;31:4456–4465. doi: 10.1523/JNEUROSCI.6042-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wilkinson GA, Fariñas I, Backus C, Yoshida CK, Reichardt LF. Neurotrophin-3 is a survival factor in vivo for early mouse trigeminal neurons. J Neurosci. 1996;16:7661–7669. doi: 10.1523/JNEUROSCI.16-23-07661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Broser PJ, Kuner T, Bruno RM. Experience-induced plasticity of thalamocortical axons in both juveniles and adults. J Comp Neurol. 2010;518:4629–4648. doi: 10.1002/cne.22483. [DOI] [PubMed] [Google Scholar]
- Woolsey TA. Somatosensory, auditory and visual cortical areas of the mouse. Johns Hopkins Med J. 1967;121:91–112. [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Wann JR. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. J Comp Neurol. 1976;170:53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Dierker ML, Wann DF. Mouse SmI cortex: qualitative and quantitative classification of golgi-impregnated barrel neurons. Proc Natl Acad Sci USA. 1975;72:2165–2169. doi: 10.1073/pnas.72.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Ballester Rosado CJ, Lu HC. What can we get from ‘barrels’: the rodent barrel cortex as a model for studying the establishment of neural circuits. Eur J Neurosci. 2011;34:1663–676. doi: 10.1111/j.1460-9568.2011.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Zhang KH, Yin J, Arends JJ, Erzurumlu RS, Jacquin MF, Chen ZF. The transcription factor, Lmx1b, is necessary for the development of the principal trigeminal nucleus-based lemniscal pathway. Mol Cell Neurosci. 2010;44:394–403. doi: 10.1016/j.mcn.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakado M, Yohro T. Subdivision of mouse vibrissae on an embryological basis with descriptions of variations in the number and arrangement of sinus hairs and cortical barrels in BALB/c (nu/+; nude, nu/nu) and hairless (hr/hr) strains. Amer J Anat. 1979;155:153–174. doi: 10.1002/aja.1001550202. [DOI] [PubMed] [Google Scholar]
- Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]