Abstract
The purpose of the present study was to investigate moderate intensity progressive resistance exercise (PRE) in growing adolescent rats and its effect on muscle hypertrophy (defined as an increase in fiber cross-sectional area). We hypothesized that in adolescent animals moderate intensity PRE would increase: 1) fiber cross-sectional area (CSA); 2) myosin heavy chain (MyHC) content; and 3) expression and phosphorylation of cell signaling molecules involved in translational regulation, compared to age-matched sedentary controls (SED). In the PRE group, three-week old male rats were trained to climb a vertical ladder as a mode of PRE training such that by 10 weeks, all animals in the PRE group had progressed to carry an additional 80% of body weight per climb. In agreement with our hypotheses, we observed that 10 weeks of moderate PRE in adolescent animals was sufficient to increase CSA of muscle fibers and increase MyHC content. Average muscle fiber CSA increased by greater than 10% and total MyHC content increased by 35% (p<0.05) in the PRE group compared to SED animals. Concurrently, we investigated sustained changes in the expression and phosphorylation of key signaling molecules that are previously identified regulators of hypertrophy in adult animal models. Contrary to our hypotheses, expression and phosphorylation of the translational regulators mTOR and Akt were not increased in the PRE group. In addition, we observed that the ratio of phosphorylated-to-unphosphorylated ribosomal protein S6 (rpS6) was reduced over six-fold in PRE animals (p<0.05) and total rpS6 protein levels were unchanged between PRE and sedentary animals (p>0.05). We conclude that moderate intensity PRE is sufficient to induce muscle hypertrophy in adolescent animals while the signaling mechanisms associated with muscle hypertrophy may differ between growing adolescents and adults.
Keywords: hypertrophy, growth, mTOR, ribosomal protein S6, resistance exercise
Introduction
Progressive resistance training (PRE) is recommended for adolescents to improve health, fitness and strength (6). Unlike adults, adolescents make strength gains as a normal course of growth and maturation. Muscle growth is rapid during adolescence as muscle fibers increase almost double in diameter (24). In addition, with adequate PRE, strength gains in adolescents may occur above normal development (6). Strength gains during adolescence correlates with an increase in muscle fiber size, but it is assumed that PRE related gains in strength (∼30% gains in 8 week programs) are greatly due to neurological adaptation rather than an increase in muscle cross sectional area (CSA) or hypertrophy (26). However, studies of muscle hypertrophy in human adolescents have been imprecise and therefore, in adolescents, it is unclear if PRE elicits hypertrophy of muscle above and beyond normal muscle growth. It is possible that strength gains from moderate intensity PRE prescribed for adolescents may not be distinguishable from normal growth, especially in terms of muscle hypertrophy. Interestingly in human adolescents, moderate loading may actually be more advantageous than heavy loading in terms of strength gains and safety (7).
PRE interventions in adolescent rodents have provided more precise investigations of muscle adaptation. Data from rodents have shown that hypertrophy of muscle does occur with PRE in adolescents animals with heavy loading regimens. Using a climbing apparatus and tail weights, Yarasheski et al. (31) reported ∼15% increase in fiber CSA across muscles following an 8-week PRE program in 3-week old rats that achieved loads of up to 250% above animal body weight. It is noteworthy that these findings were similar in different fiber types, with a ∼13% increase in CSA for rectus femoris fibers classified as type IIb (expressing MyHC2B) and ∼16% increase in type IIa fibers (expressing MyHC2A) in the PRE animals compared to controls. Duncan et al. (5) implemented a progressive PRE protocol for 26 weeks using 3-week old rats, and these rats progressed to carrying loads equivalent to 140% of their final body weight. CSA across muscle fibers was reported to increase ranging from 21-59% gains between various muscle groups investigated. It is important to recognize that these rodent studies utilized heavy loading which may not be representative of more moderate loads recommended for human adolescents. The effect of more moderate loading on muscle hypertrophy in adolescent rodents has not been explored.
The mechanism by which RT stimulates hypertrophy in adolescents is presumed to occur via endogenous growth factors as testosterone levels are already substantially increasing during this time (26). In adults, several growth-factor dependent signaling proteins have been observed to become phosphorylated within 24 hours of resistance exercise including mTOR, Akt, p70S6 kinase, and ribosomal protein S6 (rpS6) and the phosphorylation of these molecules correlates with increases in muscle protein synthesis and hypertrophy (3, 17, 29). Following eight weeks of human strength training, mTOR mRNA levels elevate suggesting that mTOR protein levels may become chronically elevated as well (16). The significantly greater growth rates occurring in adolescents may elicit different signaling mechanisms in response to PRE compared to adults. Because muscles are actively growing during adolescence, it is possible that all (or some) of these signaling pathways are already maximally activated. Exercise-induced muscle hypertrophy in adolescents could involve increased expression and/or more sustained activation of these pathways compared to adults. The interaction between training regimens and adolescent growth has not been explored with respect to this. Relatively little has been reported on PRE in adolescents with regard to mechanisms of muscle adaptation. Specifically, it is presently unknown whether adolescent exercise results in sustained elevations in the phosphorylation levels of signaling molecules such as mTOR, Akt, p70S6 Kinase, or rp S6 and/or increased expression of these proteins.
The purpose of the present study was to investigate whether moderate PRE stimulates muscle hypertophy in adolescent animals and to concurrently investigate the effects of PRE on mTOR, Akt, and rpS6 protein expression and phosphorylation. The rat flexor hallucis longus (FHL) muscle was examined because it was previously shown to hypertrophy following PRE in adults (12). Consistent with expected muscle hypertrophy, we hypothesized that a 10-week PRE regimen starting at three-weeks postnatally would increase: 1) fiber CSA and 2) myosin heavy chain (MyHC) content. In addition, we hypothesized that the expression and/or phosphorylation levels of mTOR, Akt, and rpS6 will increase following 10-weeks of PRE compared to sedentary controls of similar age.
Methods
Experimental Approach to the Problem
We hypothesized that moderate intensity PRE in adolescent animals (up to 80% body weight) would stimulate muscle hypertrophy (i.e., an increase in fiber cross sectional area - CSA) in a growth factor-dependent manner. In order to precisely investigate muscle CSA and to obtain biochemical information regarding myosin heavy chain (MyHC) expression and growth factor signaling, we used young adolescent rodents. Although several animal models (e.g, hindlimb unloading or synergist ablation) produce robust muscle hypertrophy in remaining muscles, these models preclude analyses of normal adolescent growth. PRE is a training regimen in which exercise intensity is optimized by gradually increasing muscle load over time and induces muscle hypertrophy within six to eight weeks (6-12 repetition maximums with multiple sets, 2-3 days per week) (1), making PRE a useful model for studies of the interaction between resistance training and adolescent muscle growth. In this study, we utilized a ladder climbing apparatus and gradually added mass to the tails of rats over time in order to simulate a PRE program comparable to that in humans as utilized by other investigators (5, 12, 31). Control animals were sedentary (SED) animals that were caged in the exact same manner as the PRE group and were not provided that PRE training. Comparisons were made between groups to establish changes in muscle CSA, MyHC expression, and changes in growth factor-dependent signaling molecules.
Subjects
All experimental procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee. Mayo Clinic conducts research in a manner consistent with the guidelines of the NIH (#A3291-01), USDA (#41-R-0006), and AAALAC (#000717). Sixteen, 3-week old (∼56 g), male Sprague-Dawley rats (Harlan Laboratories, Madison, WI) were randomly assigned to either a PRE trained group (PRE) or a sedentary group (SED). Both groups of rats were allowed free access to food and water. Animals were housed in a 12:12 light-dark cycle environment with stable room temperature (22°C). The PRE rats were removed from the housing room three times per week to be trained and were trained at the same time each day to minimize variability. The SED animals were also removed from the housing room, but remained in their cages for the duration of the training. Both groups of animals were weighed weekly.
Animal training protocol
Three-week old, male Sprague Dawley rats (55.3 ± 1.1 g) were trained to climb a vertical ladder as a mode for PRE. Trained animals began climbing with a body-weight only load and an external load was progressively increased each week such that at the end of 10 weeks, all animals in the PRE group had progressed to carry an additional 80% of body weight per climb (Figure 1 B). This resistance workload is equivalent to ∼3 J at week one and ∼24 J per exercise session at week ten. Both SED and PRE were free-fed and increased in body weight during 10-week study period, consistent with expected postnatal growth. The PRE training protocol utilized a vertical ladder (40 cm tall and 30 cm wide) comprising 54 wooden rungs (5 mm in diameter) with a platform at the top. Rats in the PRE group had a 50-ml conical tube taped to the base of their tail (containing a variable mass of steel shot) and were placed on the bottom rung of the ladder allowing them to climb to the top platform (Figure 1 A) three times per week. The training protocol consisted of 3 sets of ten repetitions with 2 minutes of rest between sets. Rats that did not successfully climb to the platform in less than 10 s were placed on the bottom rung, and if a rat refused to climb for 3 consecutive attempts, the exercise set was terminated. The resistance workload was increased ∼10% each week (by increasing the mass of steel shot) only if the rat was able to complete all 30 repetitions.
Figure 1.
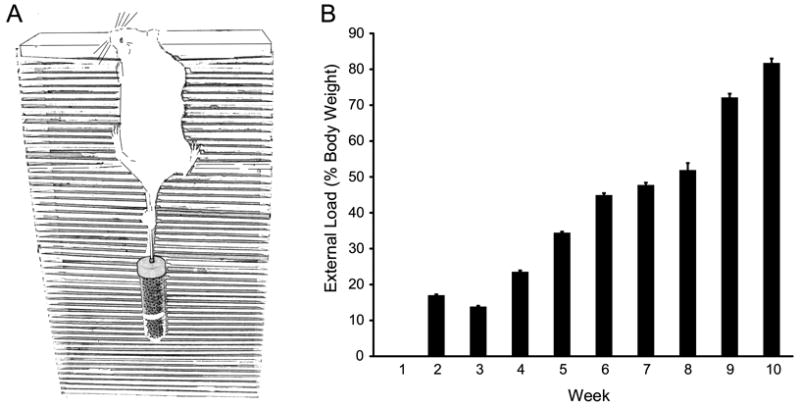
Animals in the progressive resistance exercise (PRE) group were trained to voluntarily climb to a platform located at the top of a 40 cm ladder consisting of 54 rungs (5 mm diameter) as depicted in A. Once animals reached the platform, they were manually placed on the lower rungs. Animals were allowed to climb up to 10 repetitions or to exhaustion. Mass (copper shot) was added to a conical tube attached to the base of the rat's tail using athletic tape, and increased progressively over the 10-week training protocol. B. Relative load progressed from body-weight only (week 1) to 82 ± 1% above body weight (week 10).
Procedures
Tissue preparation
The FHL muscle was removed from the animals 24 hours after the final training session(17). Animals were anesthetized with xylazine (10 mg/kg) and ketamine (90 mg/kg) and the entire FHL muscle was dissected from the flexor digitorum longus and posterior tibialis muscles of the posterolateral leg on each side. One FHL muscle was quickly frozen for protein analyses while the other was processed for histochemical analyses after freezing at optimal length in melting isopentane.
Muscle fiber type proportions and cross-sectional area (CSA)
Serial cross sections from the midportion of each muscle were cut at 10-μm thickness with a cryostat (Reichert-Jung, model 2800E). Sections were reacted with mouse primary antibodies against MyHC isoforms as previously reported (19, 30, 33). Antibodies against MyHCSlow (IgG, Novocastra), MyHC2A (IgG, hybridoma - Blau A4.74), MyHC2B (IgM, hybridoma - Schiaffino BFF3) as well as BF-35 antibody (IgG, hybridoma; which reacts with all MyHC isoforms but MyHC2X) were diluted 1:200 in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (5 mg/ml). Muscle sections were incubated in primary antibody for ∼2 h at room temperature, washed in PBS and reacted with Cy3-conjugated secondary antibodies (goat anti-mouse IgG or goat anti-mouse IgM) for 1 h. Sections incubated with only the secondary antibodies served as controls for non-specific reactivity of all secondary antibodies (10). The slides were imaged with an Olympus Fluoview confocal microscope mounted on a BX50WI microscope (Olympus America, Melville, NY) using a green HeNe laser (543 nm). Representative sections were imaged with an Olympus DApo 40×/1.3 NA oil immersion objective in arrays of 800 × 600 pixels (400 × 300 μm). Fiber type proportions and CSA measurements were performed in at least 8 sections per muscle. All fibers in the image were analyzed such that ∼100 fibers were fiber type-identified per muscle. Fiber type proportions were determined directly using the total number of fibers of each type in all sections obtained from a muscle.
MyHC content
The procedure for myosin protein determination from skeletal muscle was described previously (8-10). Basically, MyHC isoforms were separated by SDS-PAGE gel electrophoresis. Known concentrations of purified rabbit myosin (Sigma, St. Louis, MO), verified with the BioRad-DC kit according to the manufacturer's protocol, were run in parallel to permit quantification of MyHC content in FHL samples. Expression of different MyHC isoforms was quantified by densitometry. MyHC content was then normalized by tissue mass.
Protein expression and phosphorylation of translational regulators
We examined signal transduction proteins that are positive regulators of protein translation (2, 13, 22, 25) including mTOR, Akt, and S6 ribosomal protein (rpS6). We hypothesized that each of these signaling proteins would have higher expression and/or greater phosphorylation levels (of critical amino acid motifs) in PRE compared to SED animals. We also examined AMPK, a reported antagonist of mTOR signaling (15). We hypothesized that expression and phosphorylation of AMPK would not be different between groups. Two hundred mg of FHL was lysed on ice using a glass mortar and pestle and diluted in one ml cell lysis buffer (in mM: Tris-HCl [pH 7.5] 20, NaCl 150, Na2EDTA 1, EGTA 1, Na4P2O7 2.5, β-glycerophosphate 1, Na3VO4 1, and 1% Triton) containing 1 μg/ml leupeptin and phosphatase inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Total protein concentration was determined using the Bio-Rad Dc Assay and 200 μg of protein was loaded per well on either 7% or 15% Bio-Rad Criterion SDS-PAGE gels. Proteins were resolved by gel electrophoresis and transferred to PVDF membranes as described by the manufacturer (BioRad Criterion Gel System). Membranes were blocked in 5% milk in 1× Tris-buffered saline (pH = 7.4, TBS; Biorad) for 30 minutes and incubated at 4 °C with primary antibody overnight. Primary antibodies were used at a dilution of 1:1000 in 5% milk in 1× TBS (all from Cell Technology Inc.) and included both phosphorylated and total Akt (#9271/9272), 5′ AMP-activated protein kinase (#2603/2535), mammalian target of rapamycin (#2971/2974), and S6 ribosomal proteins (#4858/2217). Membranes were then washed three times with TBS for 5 min and probed with anti-rabbit horse radish peroxidase (HRP)-conjugated secondary antibody in TBS for 1 h. Membranes were wash three times with TBS for 5 min and protein was detected using Super Signal Dura enhanced chemiluminescence as described by the manufacturer (Pierce, Thermo Fisher Scientific). Membranes were probed and stripped serially for phosphorylated forms and total for each of the signaling proteins as described above. Restore Western Blot Stripping Buffer (Pierce, Thermo Fisher Scientific) was used according to manufacturer instructions to remove previously applied antibodies. Membranes were also probed for β-tubulin (Santa Cruz-58886) using methods described above to provide a loading control. Native signaling proteins were normalized to β-tubulin for comparison.
Cortisol levels
One-ml blood samples were collected from the femoral vein at the time of FHL dissection. Samples were placed in a 1.5-ml microfuge tube and blood was allowed to coagulate at room temperature for 15 minutes. Serum was separated from the clot by centrifugation at 10,000 g for 5 minutes. Serum was placed in a fresh tube and stored at -80 degree C until analysis. Cortisol was quantified using the Parameter Cortisol Assay Kit (R&D Systems, Inc., Minneapolis, MN) following the procedures recommended by the manufacturer.
Statistical Analyses
Changes in body weight over time were analyzed using repeated-measures MANOVA with grouping variables being the experimental assignment and time. Average fiber CSA was determined for each animal according to fiber type. At least 100 fibers per section (8 sections) were analyzed for each animal. Two-way ANOVA was used for comparisons of fiber CSA and distribution, with grouping variables being the experimental assignment and MyHC isoform expression. Data were analyzed for normality. No outlier exclusion was necessary. One-way ANOVA was used for comparisons of FHL muscle mass, serum cortisol levels, and protein levels. These measurements were done in triplicate; with coefficient of variance <10%. Post-hoc analyses were conducted using Tukey-Kramer honestly significant difference (HSD) test as appropriate. JMP software (version 8.0, SAS Inc.) was used for all comparisons. All data are reported as mean ± SE, unless otherwise specified. A p<0.05 was considered significant.
Results
Training effect on body weight
Following a ten-week PRE regimen, there was a significant group and time interaction for changes in animal body weight (p=0.02). After 4 weeks, a significant difference in body weight was evident across groups, and by 10 weeks, age-matched SED animals weighed 363 ± 7 g compared to 325 ± 5 g for the PRE group (∼12 % difference; p<0.05). Serum cortisol levels were not significantly different between groups (93 ± 8 vs. 81 ± 11 ng/ml in the PRE and SED groups, respectively), suggesting that hormonal influences or stress levels did not differ across groups.
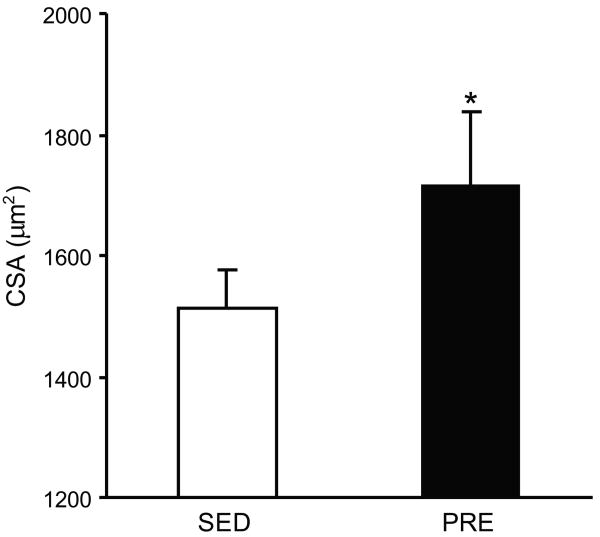
Effect of resistance training on muscle hypertrophy (CSA)
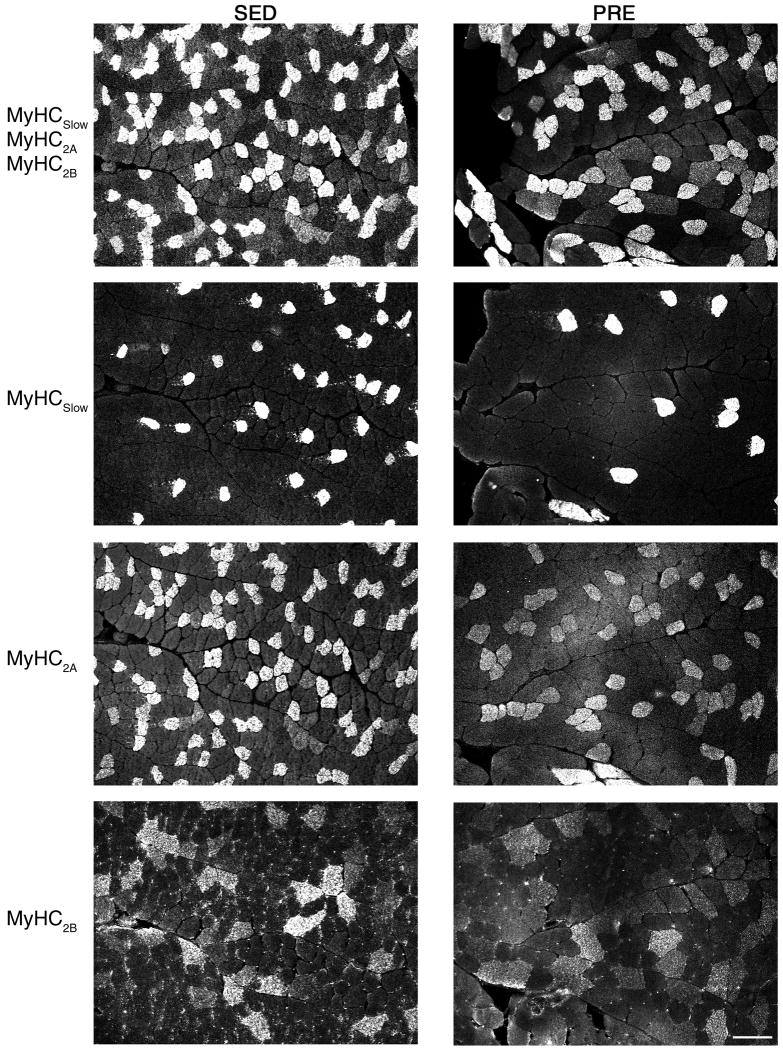
The FHL muscle comprises fibers expressing all four MyHC isoforms (Figure 2), with fibers expressing MyHC2X and MyHC2A present in greater proportion than those expressing MyHC2B or MyHCSlow (Table 1). Overall, fibers in the FHL muscle were of larger CSA in PRE animals (1714 ± 125 μm2) compared to SED animals (1512 ± 66 μm2;, p=0.01).(Figure 3). There were differences in fiber CSA according to fiber type (two-way ANOVA; p<0.001) and no group vs. fiber type interaction (p=0.69). Overall, the CSA of FHL fibers expressing MyHCSlow, MyHC2A, MyHC2B and MyHC2X was 10-20% greater in PRE vs. SED animals (Table 1), although only overall differences were statistically significant (Figure 3). Importantly, there were no significant differences in the proportion of fibers expressing different MyHC isoforms following 10-weeks of PRE when compared to SED controls indicative of normal postnatal growth in both groups. Overall, ∼80% of FHL fibers expressed MyHC2X or MyHC2A independent of group assignment.
Figure 2.
Representative cross-sectional area (CSA) of FHL from SED (left panels) or PRE animals (right panels). Fibers were type-idenfied by immunoflourescent labeling of myosin heavy chain (MyHC) isoforms. Primary antibodies were used to detect MyHCSlow, MyHC2A, and MyHC2B fibers. Note that an antibody is not available for the MyHC2X isoform. Therefore, MyHC2X (top row) were identified as the non-fluorescent fibers following MyHCSlow, MyHC2A, and MyHC2B triple labeling.
Table 1. FHL muscle fiber cross-sectional area (CSA) and fiber type composition according to myosin heavy chain (MyHC) isoform in the sedentary control (SED) and progressive resistance exercise (PRE) training groups.
| CSA (μm2) | Fiber type (%) | |||
|---|---|---|---|---|
| MyHC | SED | PRE | SED | PRE |
| Slow | 1165 ± 75 | 1396 ± 117 | 9.2 ± 2.1 | 11.7 ± 1.4 |
| 2A | 1030 ± 46 | 1135 ± 96 | 38.1 ± 1.6 | 35.6 ± 2.7 |
| 2X | 1737 ± 110 | 2042 ± 204 | 43.6 ± 1.3 | 45.1 ± 3.0 |
| 2B | 2688 ± 167 | 3134 ± 221 | 9.2 ± 1.0 | 7.6 ± 1.2 |
Fiber CSA and type proportion were obtained for each animal. Values are mean ± SE across animals in each experimental group.
Figure 3.
Cross sectional area of FHL fibers from SED (open bar) or PRE animals (filled bar). Average CSA by MyHC isoform are listed in Table 1. * indicates statistically significant difference (p<0.05).
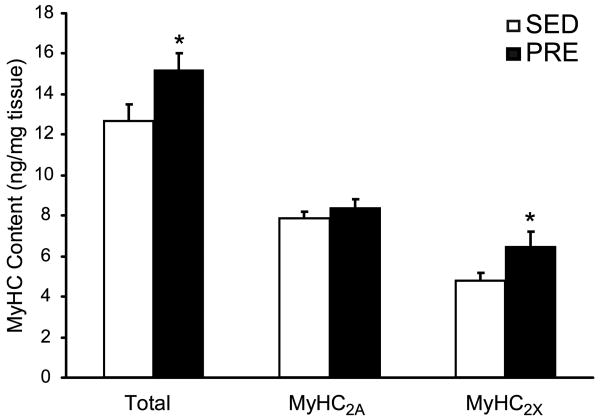
Effect of resistance training on MyHC content
Following PRE, there were significant group differences in MyHC content (two-way ANOVA, p=0.003; with no group vs. fiber type interaction, p=0.26). Total MyHC content increased by 35% (p<0.05) in the PRE compared to SED animals (Figure 4). Expression of MyHC2X increased significantly (∼20%; p<0.05), and although MyHC2A expression increased by 7%, this difference was not statistically significant (Figure 4). Consistent with analyses of fiber type distribution in the FHL muscle (Table 1), MyHC isoforms MyHC2X and MyHC2A comprised ∼90% of total MyHC. Both MyHCSlow and MyHC2B isoforms (less than 10% each) were close to the limit of detection of the protein analysis method, precluding comparisons of these isoforms between groups.
Figure 4.
Myosin heavy chain (MyHC) isoforms expression in the FHL from SED (open bars) and PRE (closed bars) animals. MyHC isoforms were separated by gel electrophoresis and quantified by silver staining using myosin standards (see Methods for details). Total MyHC increased in the PRE group compared to the SED control group primarily as the result the increased expression of the MyHC2X isoform. * indicates statistically significant difference (p<0.05).
Effect of resistance training on translational regulatory proteins
Following 10 weeks of training, mTOR, Akt, and AMPK protein levels were expressed equivalently across PRE and SED groups (Figure 5). Phosphorylation of specific serine (ser) or threonine (thr) residues of mTOR (ser-2481), Akt (ser-473), and AMPK (thr-172) were also not different between groups. Accordingly, when protein quantities were expressed as ratios of phosphorylated to total protein levels, relative phosphorylation levels of mTOR, Akt and AMPK did not differ between groups (Figure 5). However, the ratio of phosphorylated-to-unphosphorylated rpS6 protein was reduced over six-fold in PRE animals (p<0.05). Total rpS6 protein levels were unchanged between groups (p>0.05).
Figure 5.
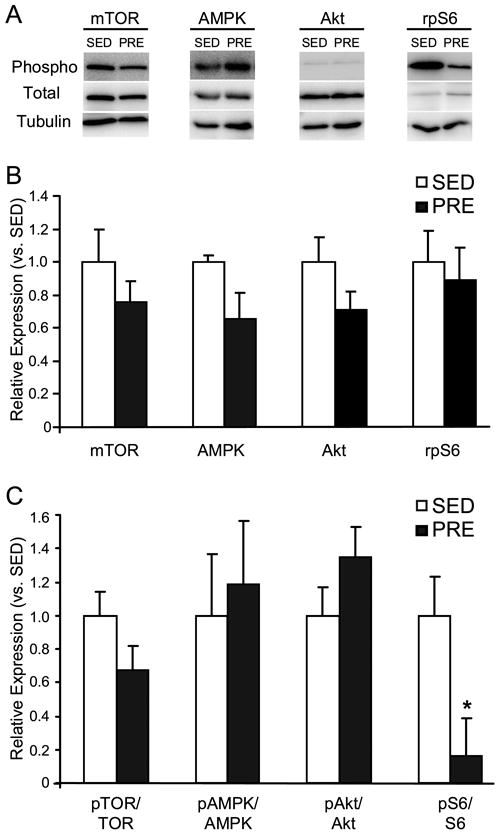
A. Representative immunoblots of phosphorylated (phospho) and total mTOR, Akt, AMPK and rpS6 proteins in the FHL from SED (left panels) or PRE animals (right panels). Tubulin is shown as a loading control and PRE expression is shown relative to control. B. Expression of total mTOR, Akt, AMPK, and rpS6 proteins in the FHL of PRE (white bars) and SED animals (black bars). C. Relative expression of phosphorylated to total mTOR, Akt, AMPK, and rpS6 proteins in the FHL of PRE (white bars) and SED animals (black bars). * indicates statistically significant difference between training groups (p<0.05).
Discussion
In agreement with our hypotheses, both FHL fiber CSA and MyHC content were increased following a moderate intensity PRE training program. In the present study, we observed a 10-20% increase in FHL fiber CSA when adolescent animals were trained to carry ∼80% above body weight over 10 weeks. These results are generally consistent with previous studies using similar models of PRE in rats, but several important methodological differences must be considered. For instance, Hornberger and Farrar (12) investigated FHL adaptations following 8 weeks of progressive ladder climbing, beginning PRE training with mature, adult rats (∼370 g). They indirectly calculated a 17% increase in CSA based on muscle weight and estimated fiber length, similar to our direct measurements of CSA in individual fibers types. By beginning PRE with mature adult animals (∼370 g), Hornberger and Farrar (12) were able to increase loading faster and achieved up to 332% of body weight after 8 weeks of PRE. The similar gains in CSA with heavier loading may relate to differences in animal age and weight as well as the response to training. Using a climbing apparatus and tail weights similar to that in our experiments, Yarasheski et al. (31) reported ∼15% increase in fiber CSA across muscles following an 8-week PRE program in 3-week old rats that achieved loads of up to 250% above body weight. It is noteworthy that these findings were similar in different fiber types, with a ∼13% increase in CSA for rectus femoris fibers classified as type IIb (expressing MyHC2B) and ∼16% increase in type IIa fibers (expressing MyHC2A) in the PRE animals compared to controls. Duncan et al. (5) implemented a progressive PRE protocol for 26 weeks using 3-week old rats, and these rats progressed to carrying loads equivalent to 140% of their final body weight. They reported a similar increase in fiber CSA across muscle fibers of different succinate dehydrogenase enzyme (SDH) activity. Low SDH activity fibers of the trained group increased 21% in the extensor digitorum longus, 37% in the plantaris and 37% in the rectus femoris muscle. Fibers with high and intermediate SDH activity in the soleus muscle of trained animals displayed a 23% and 59% increase in CSA, respectively.
Although several studies have investigated physiological adaptations to muscle hypertrophy in response to high intensity PRE (5, 12, 31), these studies did not examine the intracellular pathways implicated in muscle hypertrophy (e.g., translational regulators such as rpS6). Following 10 weeks of PRE, we investigated sustained changes in the expression and phosphorylation of key signaling molecules that are identified regulators of hypertrophy in adult animal models. The Akt-mTOR signaling molecules and their downstream effects have been implicated as critical regulators of protein synthesis and hypertrophy in varying models of muscle hypertrophy including synergist ablation, growth factor treatment, and mechanical loading in adults (2, 13, 22, 25). However, few if any animal models have explored signaling during hypertrophy in adolescent animals using PRE. It is unclear if hypertrophy in adolescents induced by PRE also depends on Akt-mTOR signaling cascades.
In adolescent animals, we hypothesized that sustained elevations in mTOR, Akt, and rpS6 protein expression may occur as an adaptive response since PRE-induced hypertrophy would occur simultaneously with sustained, rapid growth rates. This is a reasonable given that mTOR mRNA levels increase with chronic PRE (16) and that by increasing the expression of constitutively active Akt protein, muscle hypertrophy is significantly increased (2). However, we observed that critical regulators of protein synthesis and translational events—Akt and mTOR—were not elevated in terms of protein expression or phosphorylation levels in the adolescent rat FHL muscle following PRE. These results are consistent with prior investigations of chronic PRE and long term adaptation observed in adult animals (14, 32).
Both mTOR and Akt are upstream activators of translational regulators such as p70 S6-kinase, an enzyme that phosphorylates rpS6 (4). The rpS6 protein is a component of the early translation initiation complex utilized in the synthesis of new proteins and is a suspected downstream candidate for mTOR-induced hypertrophy (27). In contrast to our hypothesis, we observed a reduction in rpS6 phosphorylation levels and no change in rpS6 expression levels. Thus current results indicate that chronic elevations in the expression or the phosphorylation of translational regulators, such as rpS6, are not evident for FHL hypertrophy during PRE in rapidly growing adolescents. We speculate that in adolescent animals, mTOR, Akt, and rpS6 response to PRE may be muted in the presence of a chronic state of rapid muscle growth. Postnatal growth of skeletal muscle fibers is substantial in young adolescent animals with fractional protein synthesis rate approximately two-three times greater in three-week old animals compared to 44- and 105-week old animals, respectively (18). This may be due to naturally rising levels of testosterone and insulin-like growth factor-1 (26).
In chronically trained adults, rpS6 signals may also become muted over time. Karagounis et al have observed in adults that the acute rpS6 phosphorylation response decreases with repeated bouts of resistance exercise (14). Importantly, in this study, animals were exercised three times over a five day period with a day of rest between exercise bouts, and rpS6 phosphorylation was elevated 3 hours after the first and second bout of resistance exercise, but not following the third bout of exercise. Thus, rpS6 signaling likely desensitizes rapidly following repeated bouts of exercise and may be regulated independently of mTOR and Akt. Thus it appears that rpS6 is activated immediately following acute bouts of PRE in naïve adult animals but that the persistence of translational signals is limited with chronic training. (14). Therefore, these results suggest that muscle cell signaling pathways may change over time in response to chronic PRE. We speculate that in adolescent animals, rpS6 activity may be muted in the presence of a chronic state of rapid muscle growth. Thus, rpS6 signaling likely desensitizes rapidly following repeated bouts of exercise and may be regulated independently of mTOR and Akt. Genetic studies add complexity to the issue as fibroblasts derived from mutant mice lacking rpS6 exhibit enhanced rates of protein synthesis (28), The role of rpS6 in the regulation of sustained global protein synthesis is thus unclear and it is suggested rpS6 is a fine tuner of global protein synthesis with both positive and negative regulation of protein synthesis and growth (27). Interestingly, mTOR and Akt phosphorylation is muted at basal levels with repeated bouts of activity as well(14)
AMP kinase (AMPK) is an important regulator of energy metabolism and has been shown to inhibit mTOR phosphorylation (15, 20, 21). AMPK signaling may increase with physical activity (11) which could potentially inhibit mTOR, therefore we also investigated AMPK phosphorylation levels. We observed that both AMPK protein and phosphorylation levels were unchanged between PRE and SED groups. The lack of a detectable change in AMPK signaling following PRE is consistent with its primary role as a metabolic sensor (23) likely involved in regulation of the response to aerobic exercise, not PRE. Alternatively, high metabolic rates in growing adolescent animals may obscure any additional stimuli imposed by PRE.
Practical Applications
This study highlights that adolescents are capable of PRE-induced muscle hypertrophy with moderate loading. These results are consistent with strength gains observed with high-repetition, moderate intensity PRE in children(7). This suggests that lower intensity PRE protocols are sufficient to increase muscle hypertrophy above normal growth rates in adolescents, which has generally been dismissed in the past by indirect studies (26). Since high repetition, moderate intensity loading is considered safer for children due to lower loading stress on joints, tendon, and muscle, the coach and personal trainer may prefer moderate intensity PRE for preventing injury in adolescent populations. This study prompts the need to re-address whether PRE-induced muscle hypertrophy is an underlying mechanism of strength gain in adolescent children.
Acknowledgments
We thank Ms. Yun-Hua Fang for her technical assistance with immunohistochemistry and Thomas Keller and Rebecca L. Macken for technical assistance with MyHC content analyses.
GRANTS: Supported by NIH grant R01 AR51173 and the Mayo Clinic.
References
- 1.American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 2.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 3.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–46. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–9. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 5.Duncan ND, Williams DA, Lynch GS. Adaptations in rat skeletal muscle following long-term resistance exercise training. Eur J Appl Physiol Occup Physiol. 1998;77:372–8. doi: 10.1007/s004210050347. [DOI] [PubMed] [Google Scholar]
- 6.Faigenbaum AD, Kraemer WJ, Blimkie CJ, Jeffreys I, Micheli LJ, Nitka M, Rowland TW. Youth resistance training: updated position statement paper from the national strength and conditioning association. J Strength Cond Res. 2009;23:S60–79. doi: 10.1519/JSC.0b013e31819df407. [DOI] [PubMed] [Google Scholar]
- 7.Faigenbaum AD, Westcott WL, Loud RL, Long C. The effects of different resistance training protocols on muscular strength and endurance development in children. Pediatrics. 1999;104:e5. doi: 10.1542/peds.104.1.e5. [DOI] [PubMed] [Google Scholar]
- 8.Geiger PC, Bailey JP, Zhan WZ, Mantilla CB, Sieck GC. Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol. 2003;95:611–619. doi: 10.1152/japplphysiol.00862.2002. [DOI] [PubMed] [Google Scholar]
- 9.Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J Appl Physiol. 2001;90:380–388. doi: 10.1152/jappl.2001.90.1.380. [DOI] [PubMed] [Google Scholar]
- 10.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- 11.Glynn EL, Lujan HL, Kramer VJ, Drummond MJ, DiCarlo SE, Rasmussen BB. A chronic increase in physical activity inhibits fed-state mTOR/S6K1 signaling and reduces IRS-1 serine phosphorylation in rat skeletal muscle. Appl Physiol Nutr Metab. 2008;33:93–101. doi: 10.1139/h07-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornberger TA, Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29:16–31. doi: 10.1139/h04-002. [DOI] [PubMed] [Google Scholar]
- 13.Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380:795–804. doi: 10.1042/BJ20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karagounis LG, Yaspelkis BB, 3rd, Reeder DW, Lancaster GI, Hawley JA, Coffey VG. Contraction-induced changes in TNFalpha and Akt-mediated signalling are associated with increased myofibrillar protein in rat skeletal muscle. Eur J Appl Physiol. 2010 doi: 10.1007/s00421-010-1427-5. [DOI] [PubMed] [Google Scholar]
- 15.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38:1958–64. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 16.Lamas L, Aoki MS, Ugrinowitsch C, Campos GE, Regazzini M, Moriscot AS, Tricoli V. Expression of genes related to muscle plasticity after strength and power training regimens. Scand J Med Sci Sports. 20:216–25. doi: 10.1111/j.1600-0838.2009.00905.x. [DOI] [PubMed] [Google Scholar]
- 17.Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2006;576:923–33. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis SE, Kelly FJ, Goldspink DF. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem J. 1984;217:517–26. doi: 10.1042/bj2170517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki M, Esser KA. Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J Appl Physiol. 2009;106:1367–73. doi: 10.1152/japplphysiol.91355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M, Viollet B. Important role for AMPKalpha1 in limiting skeletal muscle cell hypertrophy. Faseb J. 2009;23:2264–73. doi: 10.1096/fj.08-119057. [DOI] [PubMed] [Google Scholar]
- 22.Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol. 2005;289:C1457–65. doi: 10.1152/ajpcell.00165.2005. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–41. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- 24.Oertel G. Morphometric analysis of normal skeletal muscles in infancy, childhood and adolescence. An autopsy study. J Neurol Sci. 1988;88:303–13. doi: 10.1016/0022-510x(88)90227-4. [DOI] [PubMed] [Google Scholar]
- 25.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 26.Rowland TW. Children's exercise physiology. 2nd. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 27.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–8. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 24:2857–72. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- 30.Sieck GC, Zhan WZ, Prakash YS, Daood MJ, Watchko JF. SDH and actomyosin ATPase activities of different fiber types in rat diaphragm muscle. J Appl Physiol. 1995;79:1629–1639. doi: 10.1152/jappl.1995.79.5.1629. [DOI] [PubMed] [Google Scholar]
- 31.Yarasheski KE, Lemon PW, Gilloteaux J. Effect of heavy-resistance exercise training on muscle fiber composition in young rats. J Appl Physiol. 1990;69:434–7. doi: 10.1152/jappl.1990.69.2.434. [DOI] [PubMed] [Google Scholar]
- 32.Zanchi NE, de Siqueira Filho MA, Lira FS, Rosa JC, Yamashita AS, de Oliveira Carvalho CR, Seelaender M, Lancha AH., Jr Chronic resistance training decreases MuRF-1 and Atrogin-1 gene expression but does not modify Akt, GSK-3beta and p70S6K levels in rats. Eur J Appl Physiol. 2009;106:415–23. doi: 10.1007/s00421-009-1033-6. [DOI] [PubMed] [Google Scholar]
- 33.Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 1997;82:1145–53. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]