Abstract
The topographic organization of the thalamocortical axons (TCAs) in the barrel field (BF) in the rodent primary somatosensory cortex (S1) results from a succession of temporally and spatially precise developmental events. Prenatally, growth and guidance mechanisms enable TCAs to navigate through the forebrain and reach the cortex. Postnatally, TCAs grow into the cortex and the refinement of their terminal arborization pattern in layer IV creates barrel-like structures. The combined results of studies performed over the past 20 years clearly show that serotonin (5-hydroxytryptamine; 5-HT) signaling modulates these pre- and early postnatal developmental processes. In this context, 5-HT signaling can purposely be described as ‘modulating’ rather than ‘controlling’ because developmental alterations of 5-HT synthesis, uptake or degradation either have a dramatic, moderate or no effect at all on TCA pathway and BF formation. In this review we summarize and compare the outcomes of diverse pharmacological and genetic manipulations of 5-HT signaling on TCA pathway and BF formation, in an attempt to understand these discrepancies.
Keywords: mouse, serotonin, somatosensory cortex, thalamus, axon guidance, barrel field, genetic models, 5-HT1B, 5-HT1D, 5-HT receptors, SERT, MAOA, VMAT2, development
Serotonin and thalamocortical pathway formation
The simple and clear topographic organization of the rodent barrel field (BF), in the primary somatosensory cortex (S1), makes it a convenient model to study cortical map development (See Erzurumlu & Gaspar in this issue). Briefly, peripheral receptors of the whiskers are isomorphically related to the barrels in layer IV of the S1, relaying downstream sensory relay stations in the trigeminal complex of the brainstem (barrelettes) and the ventrobasal complex (VB) of the thalamus (barreloids). In S1, the larger whiskers are represented in the posteromedial barrel subfield (PMBSF) and the smaller ones in the anterior snout (AS)(Woolsey & Van der Loos, 1970; Dawson & Killackey, 1987). The barrels themselves consist of a ring of granular neurons surrounding an area of lower cell density (Wu et al., 2011). Glutamatergic TCAs from the VB instruct the formation of the BF (Erzurumlu & Jhaveri, 1990; Erzurumlu & Gaspar, this issue). In mice, TCAs first reach the primitive S1 by embryonic day 18.5 (E18.5; (Molnar et al., 2003)) after growing through the intermediate zone in the cortex. At birth, many TCAs send collateral branches to the infragranular layers, but only a few traverse the cortical plate and reach the marginal zone. By postnatal day 1 (P1), the number of axons in the cortical plate has increased, and separations between the three principal domains of S1 (sensory afferents from the forepaw and hindpaw pads, from the lower jaw, and PBMSF/AS) start to become visible (Rebsam et al., 2002). The patterning of S1 becomes progressively more marked during subsequent days, with a complete barrel pattern visible by P5. Consistent with the instructive role of ingrowing TCAs in BF formation, there is no observable pre- or early postnatal “pre-patterning” in the cerebral cortex (Erzurumlu & Jhaveri, 1990; Senft & Woolsey, 1991; Agmon et al., 1993).
The idea that serotonin (5-HT) may play a role in BF formation stems from earlier observations that there is abundant 5-HT immunostaining in the early postnatal rodent S1, where it shows a segmented pattern in cortical layer IV that overlaps with the BF (Fujimiya et al., 1986; D’Amato et al., 1987; Rhoades et al., 1990; Bennett-Clarke et al., 1991; Blue et al., 1991; Bennett-Clarke et al., 1994a; Killackey et al., 1995; Dori et al., 1996). 5-HT is a neurotransmitter produced by neurons located in the raphe nuclei. In the adult, 5-HT neurons display a widespread innervation pattern, reaching many brain structures (Lidov & Molliver, 1982). Many studies have shown that 5-HT signaling affects several developmental events, including cell division, neuronal migration, cell differentiation, axon growth and guidance and synaptogenesis (Gaspar et al., 2003). Two decades ago, the observation that 5-HT axons and TCA patterns overlap in the early postnatal S1 suggested that 5-HT signaling might contribute to BF formation (Fujimiya et al., 1986; D’Amato et al., 1987). In mice, 5-HT immunohistochemistry revealed a dense accumulation of 5-HT fibers in the cortical subplate at P1, which became bi-laminar by P3 (Fujimiya et al., 1986). Subsequently, dense accumulations of 5-HT immunoreactive fibers shaped as “barrel-like” structures were observed just above cortical layer V at P5. By P7, these structures became smaller and located in layer IV. Eventually, the barrel-like pattern of 5-HT immunoreactive fibers disappeared and fibers were distributed over the entire S1, progressively fading from P10 to adulthood. A similar temporal and spatial serotonergic innervation of the S1 cortex was observed in rat pups using autoradiographic imaging of 5-HT uptake sites and 5-HT immunochemistry (D’Amato et al., 1987; Rhoades et al., 1990; Blue et al., 1991).
Thus, although serotonergic innervation in the adult brain is rather widespread, there appeared to be a transient developmental timeframe during which 5-HT axons are topographically organized, precisely matching TCA patterning in S1. This transient relationship between 5-HT immunoreactive axons and TCAs in S1 was later investigated (Lebrand et al., 1996). These elegant studies demonstrated that the dense transient innervation of 5-HT immunoreactive fibers did not originate in the raphe nuclei, but in the VB. The 5-HT immunoreactive pattern could be accounted for by the thalamic neurons that transiently express the plasma membrane 5-HT transporter (slc6a4; SERT) and thus TCAs can uptake and temporarily contain 5-HT. Importantly, Lebrand et al. (1996) demonstrated that thalamic neurons do not express tryptophan hydroxylase (Tph) and thus cannot produce 5-HT, but rather ‘borrow’ it through uptake from their extracellular environment. The transient expression of SERT lasts from E15 to P10 (Hansson et al., 1998; Lebrand et al., 1998). During this time, VB neurons also express the vesicular monoamine transporter 2 (VMAT2), suggesting that they are able to store the 5-HT into vesicles, protecting it from degradation by monoamine oxydase A (MAOA) and possibly for later release. Consistent with the possibility that signaling by this ‘borrowed’ 5-HT could play instructive roles in BF formation, the expression of several 5-HT receptors (e.g. 5-HT1B and 5-HT2A receptors (Leslie et al., 1992; Bennett-Clarke et al., 1993; Mansour-Robaey et al., 1998)) also show a barrel-like pattern in S1 during this time (D’Amato et al., 1987; Cases et al., 1996; Mansour-Robaey et al., 1998).
Importantly, the transient postnatal nature of 5-HT immunostaining, SERT and specific 5-HT receptors expression in the BF and thalamic neurons suggested a purely developmental function, distinct from a later functional role in adulthood. This hypothesis was supported by studies showing that mouse lines carrying targeted mutations that affect 5-HT signaling pathways display abnormal cortical BF formation (Cases et al., 1995; Cases et al., 1996; Cases et al., 1998; Vitalis et al., 1998; Persico et al., 2001; Alvarez et al., 2002; Rebsam et al., 2002; 2005; Altamura et al., 2007; Murphy & Lesch, 2008). For instance, blocking 5-HT degradation by knocking out MAOA (MAOA-KO) or 5-HT uptake by knocking out SERT (SERT-KO) in mice dramatically increases extracellular 5-HT during early postnatal brain development, and also profoundly alters TCA segregation in the cortex and the patterning of their terminals into barrel-like structures. Although these observations suggested that a set level of 5-HT signaling during development was required for normal BF formation (Trowbridge et al., 2011), the Pet1-KO mutant mouse line in which extracellular brain 5-HT is abnormally low (Hendricks et al., 2003) does not show obvious BF formation defects (see below and Fig. 1). In the following sections, we summarize the BF-related phenotypes observed in various mutant mouse lines with elevated or decreased 5-HT signaling during development.
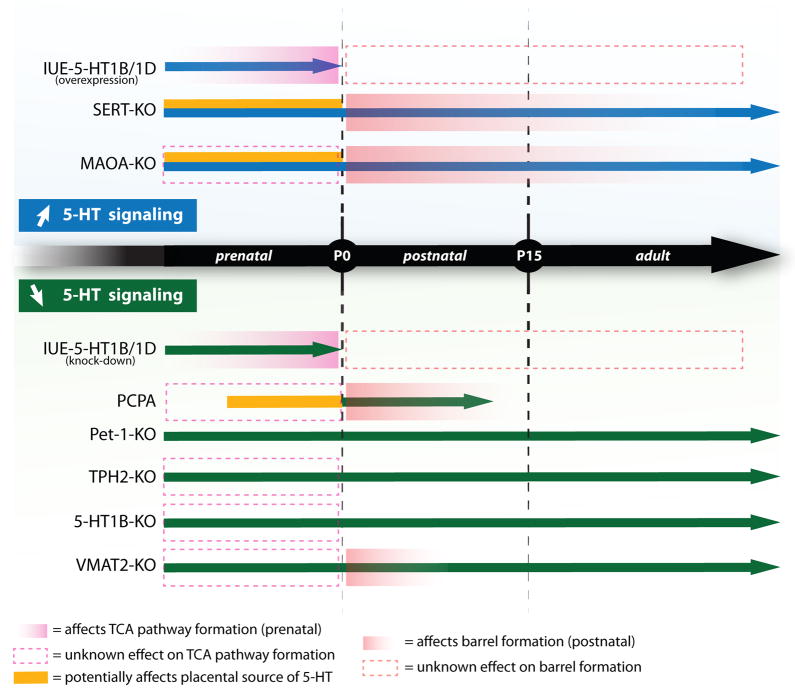
Figure 1.
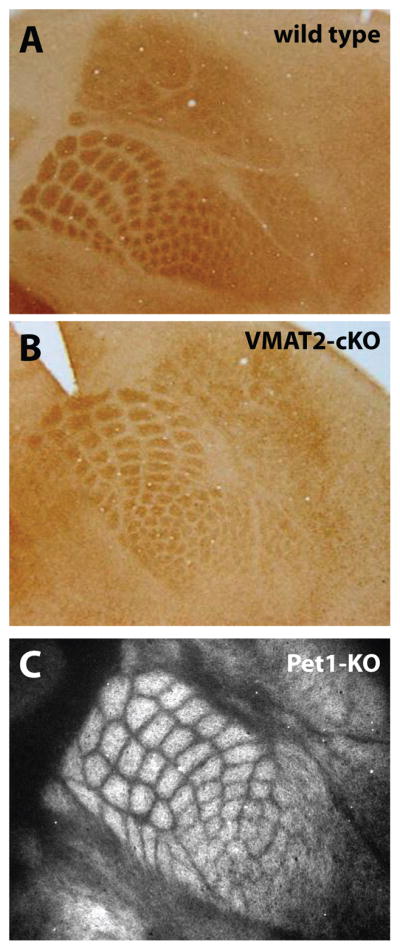
Decreased extracellular brain serotonin (5-HT) does not affect cortical barrel field (BF) formation. Despite the profound reduction of brain 5-HT in VMAT2-cKO mice (see text), the BF appears grossly normal at P5 (B), similar to wild type (A). (C), similarly in the Pet1-KO mice where brain 5-HT concentration is reduced by ~70%, BF pattern appears normal at P7. BF formation was visualized in flattened cortex preparations stained with a 5-HTT (SERT) antibody.
Different models of developmental 5-HT signaling dysfunction
The multiple rodent models used over time to investigate 5-HT signaling role in BF formation are listed in Table 1 (in chronological order). Altered 5-HT signaling was obtained either by blocking endogenous synthesis, uptake, storage or degradation of the amine, or by changing the expression level of specific receptors or transcription factors. As discussed later in the text, some important pitfalls to consider when comparing these different models are the differences in timing and pattern of expression of the targeted genes or the nature, route and time of exposure of the pharmacological agent used. Thus, developmental alterations in 5-HT signaling were generated at different stages of pre- and postnatal development, and in different regions of the brain and periphery. Although there is spatial and temporal overlap in 5-HT signaling alterations across these models, a careful consideration of the differences may explain the discrepancies reported regarding the role of 5-HT in BF formation. The timeline of these different alterations and their effects on prenatal TCA pathway and postnatal BF formation is illustrated in Fig. 2.
Table 1.
Summary of genetic/pharmacological manipulations affecting 5-HT signaling and their effect on TCA pathway and BF formation.
| Model name | Manipulation/Gene affected | Duration | Effect on extracellular brain 5-HT concentration | Effects on TCAs (prenatal) | Effects on BF (postnatal) | References |
|---|---|---|---|---|---|---|
| PCPA | Pharmacological blocker of TPH1/2; inhibits 5-HT synthesis non-specifically | P0–P10 | Decrease | n.d. | - Reduction of BF areas in cortical layer IV (S1) - Delayed barrel pattern maturation |
(Persico et al., 2000) |
| MAOA-KO | MAOA gene not functional; decreased 5-HT degradation | Lifelong | Increase | n.d. | - PMBSF and AS fused - BF surface area in S1 enlarged by 20%. |
(Cases et al., 1995; Cases et al., 1996; Rebsam et al., 2002) |
| SERT-KO | SERT (slc6a4) gene knockout; reduced 5-HT uptake | Lifelong | Increase | n.d. | - Only few PMBSF barrels visible - Thinning of cortical layer IV - Altered cortical development |
(Persico et al., 2001; Altamura et al., 2007; Murphy & Lesch, 2008) |
| VMAT2-KO | VMAT2 (slc18a2) gene knockout; reduced intracellular 5-HT uptake. | Lifelong (die at P5) | Decrease | n.d. | - Delayed emergence of barrel patterning - Development of upper cortical layers severely delayed |
(Persico et al., 2001; Alvarez et al., 2002) |
| TPH2-KO | TPH-2 gene knockout; no central 5-HT synthesis | Lifelong | Decrease (CNS only) | n.d. | Normal | (Gutknecht et al., 2008) |
| Pet1-KO | Pet1 gene knockout; 80% of 5-HT neurons fail to differentiate | Lifelong | Decrease (CNS/PNS) | Normal | Normal at P7 (see Fig. 1) | Gaspar, unpublished observations |
| 5-HT1B-KO | 5-HT1B (htr1b) gene knockout; reduced 5-HT signaling in subsets of neurons (incl. thal/raphe). | Lifelong | n.d. (decreased 5-HT signaling) | n.d. | normal | (Salichon et al., 2001) |
| 5-HT1B/D- in utero electro-poration | In utero electroporation of 5-HT1B/1D receptors siRNAs; decreased both receptor expression | E12.5–E18.5 | n.d. (decreased 5-HT signaling in thalamic neurons only) | Ventrolateral shift of TCAs in IC and cortex | n.d. | (Bonnin et al., 2007) |
| 5-HT1B/D- in utero electro-poration | In utero electroporation of 5-HT1B/1D receptors expression plasmids; increased both receptor expression | E12.5–E18.5 | n.d. (increased 5-HT signaling in thalamic neurons only) | Dorsomedial shift of TCAs in IC and cortex | n.d. | (Bonnin et al., 2007) |
Figure 2.
Timeline of the genetic and pharmacological alterations of 5-HT signaling and their effects on thalamocortical axons (TCA) pathway and cortical barrel field (BF) formation. Manipulations leading to increased 5-HT signaling during development are illustrated in the top panel. Overexpression of 5-HT1B/1D receptors (IUE: in utero electroporation) alters TCA pathway prenatally; the effect on postnatal BF formation is unknown (dotted box). In the SERT-KO and MAOA-KO mice, extracellular brain 5-HT concentration is increased and BF formation is altered postnatally. The blue arrows represent the duration of the effect on 5-HT signaling. The bottom panel illustrates manipulations leading to a decrease of 5-HT signaling prenatally (IUE) and/or pre- and postnatally (knockout mice and p-chlorophenylalanine (PCPA) treatment). Knocking down 5-HT1B/1D receptors expression by in utero electroporation alters TCA pathway prenatally, with unknown effect on postnatal BF formation. In most of the genetic mouse models, prenatal effects on TCA pathway formation were not analyzed in detail. The potential prenatal effects on the placental source of 5-HT, inferred from the targeted genes being expressed in the placenta, are indicated (yellow line).
Pharmacological 5-HT depletion
One of the most popular ways to deplete serotonin is the use of the false neurotransmitter, p-Chlorophenylalanine (PCPA), which acts as an irreversible inhibitor of Tph. PCPA is usually administered systemically, it crosses the blood brain barrier, and also to some extent the placental barrier, and therefore can inhibit brain 5-HT synthesis at fetal and postnatal stages of development (Lauder et al., 1985). It acts on both Tph1 and Tph2 isoforms and therefore depletes the 5-HT stores in both the brain, peripheral tissues and blood. PCPA is not entirely specific since it can also inhibit tyrosine hydroxylase, however, at lower doses it was found not to significantly affect brain norepinephrine or dopamine concentrations (Koe & Weissman, 1966; Persico et al., 2000; Jha et al., 2006). The other major serotonin depleting agent that has been used is 5–7-dihydroxytryptamine (5,7-DHT)(Baumgarten & Lachenmayer, 1972). 5–7-DHT is transported preferentially into serotonergic cells via the serotonin transporter (Choi et al., 2004), and at higher doses also into catecholaminergic neurons. The specificity for 5-HT neurons is therefore not absolute but can be enhanced by co-administration of selective monoaminergic reuptake blockers, such as desipramine (Osterheld-Haas et al., 1994). However, during development 5,7-DHT may be taken up into neurons that transiently express the transporter, such as the thalamic neurons. Finally, some studies have relied on amphetamine-like agents that act as 5-HT releasers, such as fenfluramine, or p-chloroamphetamine (PCA) with a secondary effect of 5-HT stores depletion (Costa et al., 1971; Trulson & Jacobs, 1976).
Consistent with 5-HT signaling playing a role in BF formation, overall postnatal 5-HT depletion obtained through these different agents caused a reduction of BF areas in cortical layer IV, which was interpreted as a delay in barrel pattern maturation (Blue et al., 1991; Bennett-Clarke et al., 1994b; Osterheld-Haas et al., 1994). However a more recent study questioned the causal role of 5-HT depletion in this effect. In this study, Persico and collaborators compared the effects of 3 different serotonin-depleting conditions (PCA, PCPA injections and hypo-proteic diet) on cortical barrel development. These experimental conditions cause a variable level of 5-HT depletion, and of general cortical growth. This enabled them to test whether changes of barrel size were correlated to changes in 5-HT levels or to general body and brain growth. This thorough analysis revealed that barrel growth is less sensitive to 5-HT depletion per se than to the concomitant drug-induced growth impairment (Persico et al., 2000). Thus, a conclusion of this newer study was that postnatal brain growth is a major determinant of barrel field development, rather than postnatal reduction in 5-HT brain levels. Other models of pharmacological 5-HT depletion have also resulted in some degree of alteration of BF formation; for example, large brain 5-HT reductions around birth obtained either by 5,7-DHT injections (neurotoxic to 5-HT neurons) or by injections of the 5-HT releaser fenfluramine (Bennett-Clarke et al., 1994b; Bennett-Clarke et al., 1995), produced a 20–30% reduction in the size of TCA patches in S1 (measured after diI tracing of TCA), although there was no growth impairment of the pups. The change in patch size was maintained into adulthood, even after brain 5-HT concentration returned to normal. Like most pharmacological approaches, a significant pitfall is that the drugs may have off-target effects. For instance, PCPA blocks 5-HT synthesis non-specifically in the brain and periphery; although a direct role of peripheral 5-HT production in early postnatal brain development remains to be investigated. Off-target effects are an important caveat when drugs are administered prenatally; intraperitoneal injections of PCPA in pregnant dams was shown to have more widespread effects on brain and cortical development (Lauder et al., 1985; Vitalis et al., 2007), but it also blocks peripheral maternal 5-HT synthesis and placental 5-HT synthesis (Bonnin & Levitt, 2011). Blocking these early exogenous sources of 5-HT could non-specifically affect fetal development (Cote et al., 2003; Cote et al., 2007), and thus render the interpretation of the phenotype difficult. Thus, pharmacological approaches that disrupt brain 5-HT levels during development and also affect BF formation suggest, but do not necessarily prove, causality. However, genetic mouse models described in the next sections provide more direct evidences of 5-HT involvement in BF formation.
Increased 5-HT/MAOA-KO
The MAOA enzyme degrades 5-HT, norepinephrine (NE), epinephrine and dopamine (DA). The MAOA-KO (generated by accidental insertion of the interferon β minigene in the open reading frame of the MAOA gene; (Cases et al., 1995)) was the first mouse line in which the effect of elevated 5-HT levels on BF formation was investigated (Cases et al., 1995). Inactivation of MAOA induces a nine-fold increase in brain 5-HT levels at P1 and a six-fold increase at P12 (Cases et al., 1995) and extends the period during which 5-HT immunoreactivity is observed in thalamic nuclei, from E15 to P15 (when it is normally observed from P1 to P7 in wild type mice)(Cases et al., 1998). Elevated 5-HT levels in MAOA-KO mice prevent barrel formation in cortical layer IV, leading to a continuous band of TCAs and layer IV neurons although no changes in barrelettes and barreloids were noted in these mice (Cases et al., 1996). The PMBSF and AS representations appeared to be fused, and the surface area occupied by S1 was enlarged by 20%. These alterations were permanent and persisted into adulthood. Consistent with the effect being mediated by elevated brain 5-HT levels, reducing 5-HT levels with PCPA rescued barrel development and administration of the irreversible MAOA inhibitor, clorgyline, to wild type mice was sufficient to partially phenocopy the cortical barrel defects (Cases et al., 1996). In a subsequent study, a pharmacological approach was used to determine the timeline of the elevated 5-HT effect on BF formation. Blocking MAOA activity during pregnancy and early postnatal period with clorgyline produced a three- to eight-fold increase in brain 5-HT concentration, and increased 5-HT immunoreactivity in S1 (Vitalis et al., 1998). The most severe alterations of BF formation (i.e. absence of pattern, except for some fused barrels in the PMBSF) were observed in pups treated with clorgyline from E15 to P7, or from P0 to P7. Interestingly, a cumulative effect of pre- and postnatal MAOA inhibition was observed, whereas no obvious changes of barrel pattern were visible in pups treated only prenatally (E15 to birth), or only later postnatally (P4 to P7). It was then concluded that, besides prenatal influences, the first five postnatal days constitute a critical developmental period for 5-HT effect on BF formation. It was then hypothesized that increased 5-HT levels may disrupt the ingrowth of TCAs into the cortical layer IV. However, SERT immunostaining in MAOA-KO mice revealed that TCA development and laminar distribution within the cortex appear grossly normal until P1 (Rebsam et al., 2002). However, after P1, differences in the tangential distribution of TCAs resulted in a diffuse barrel pattern. This suggested that increases in brain 5-HT levels affect mainly the patterning of TCA terminals postnatally. Furthermore, single axon labeling of TCA at P7 indicated that this lack of segregation resulted from alterations in individual terminal arborization, such as a reduced number of terminal endpoints in layer IV and an increased tangential extent of TCA arbors (Rebsam et al., 2002). The proposed interpretation was that a lack of retraction of misplaced axon branches as well as reduced collateral branch formation in MAOA-KO mice lead to the altered BF patterning. The narrow postnatal timeframe for this mechanism was supported by more recent studies showing that lowering brain 5-HT levels by PCPA injection between P4 and P11 in the MAOA-KO mice can partially rescue the phenotype (Rebsam et al., 2005). This study also revealed that the plasticity of BF patterning (i.e. the capacity of TCAs to form new collaterals and to retract misplaced branches) is limited to the first two postnatal weeks.
Thus, the MAOA-KO mice provided invaluable clues regarding 5-HT influence on BF development. The precise mechanisms by which chronic, elevated extra- and intracellular 5-HT concentration affects this process is not entirely elucidated. However, there is clear evidence suggesting that the over-activation of the presynaptic hetero-receptor 5-HT1B (htr1b), which is transiently expressed by thalamic neurons (Bennett-Clarke et al., 1993; Young-Davies et al., 2000; Salichon et al., 2001; Bonnin et al., 2007), contributes to this effect. The clearest evidence is genetic: 5-HT1B receptor deletion partially rescued the MAOA-KO phenotype (see below and (Salichon et al., 2001); (Rebsam et al., 2002)). The chronic increased ligand availability in MAOA-KO mice triggers a partial down-regulation of 5-HT1B receptor expression in TCAs (Cases et al., 1996) and a significant reduction of 5-HT1A receptor binding (Lanoir et al., 2006), which does not allow a complete rescue of the phenotype. However, similarly to pharmacological treatments, potential pitfalls that may be due to peripheral effects of the MAOA-KO have to be considered since elevation of 5-HT concentration is not brain specific. For instance during pregnancy, MAOA is strongly expressed in syncytiotrophoblasts of the placenta (cells of fetal origin; (Nagai et al., 2010)). Although its role there is still unknown, it may be important to control vasoconstriction in and around the placenta by removing any free maternal blood 5-HT (Balkovetz et al., 1989). The absence of 5-HT degradation in the placenta could therefore indirectly trigger a cascade of hypoxic response pathways in the fetus, with potential impact on fetal brain development not directly related to local 5-HT signaling. Thus, complementary approaches using time- and region-specific deletion of the MAOA gene are needed. Interestingly, as discussed above, similar barrel alterations could be observed following pharmacological inhibition of MAOA after birth, suggesting that a specific role of MAOA in barrel patterning may be more important post- than prenatally; this is also supported by studies in which a forebrain- and postnatal-specific expression of MAOA gene rescued barrel patterning in MAOA-KO mice (Chen et al., 2007).
Increased extracellular 5-HT/SERT-KO
SERT (slc6a4) is a Na+-dependent transporter that resides at the plasma membrane and removes 5-HT from the extracellular space, thereby terminating its action on postsynaptic targets (Blakely et al., 1991). The SERT-KO mouse (in which exon 2 of the SERT gene was replaced with a PGK-neo gene cassette by homologous recombination (Bengel et al., 1998)) is another useful model to investigate the effects of elevated extracellular 5-HT level on brain development. In the adult brain, the absence of 5-HT reuptake, mainly in serotonergic neurons, causes a 14-fold increase in extracellular 5-HT levels (Fabre et al., 2000). The effects on early postnatal brain development were reported in several studies. BF development was altered in SERT-KO pups, although to a lesser degree than in MAOA-KO mice (Persico et al., 2001). 5-HT immunohistochemistry revealed an absence of transient barrel pattern in the P7 SERT-KO pups. Cytochrome oxydase staining showed that only few of the largest, most caudal barrels were visible in the PMBSF. Interestingly, the absence of SERT induced a thinning of cortical layer IV (Altamura et al., 2007) and generally appeared to alter cortical development itself (e.g. increased cell density in the cortex; (Murphy & Lesch, 2008)). As in the MAOA-KO mice, the barrel pattern could be rescued by lowering 5-HT brain levels by early postnatal PCPA injections (Persico et al., 2001). All these observations suggested that, similar to the MAOA-KO phenotype, increased extracellular 5-HT levels in the SERT-KO disrupt BF development. Interestingly, the BF disruption appeared overall less severe in the SERT-KO than in the MAOA-KO, suggesting that, as mentioned above, prenatal (e.g. placental) effects could contribute to the phenotype in the latter. Although SERT is also expressed in the placenta, its function there is still unknown: others and our recent results (Bonnin et al., 2011) suggest it could function, in conjunction with MAOA, to control 5-HT level in the maternal and/or fetal blood within the placenta in order to prevent vasoconstriction (Bottalico et al., 2004). The very strong expression of the norepinephrine transporters (NET) in the placenta (our unpublished observations) could provide some level of compensatory uptake in the absence of SERT, like it does in the adult brain (Mossner et al., 2006). Thus, the placental contribution to the SERT-KO phenotype may be less pronounced than in the MAOA-KO, where 5-HT degradation is severely impaired. As mentioned above for the MAOA-KO, there are potential pitfalls in SERT-KO phenotype interpretation. The generalized deletion of SERT may produce elevated extracellular 5-HT levels in the entire brain; however, the transient down-regulation of SERT expression in the thalamus and TCAs may be sufficient to cause alterations in BF development. This possibility is being addressed in current studies by examining BF development in SERT conditional knockdown, where gene deletion is restricted to thalamic neurons.
5-HT depletion/VMAT2-KO
In axon terminals, the neural specific vesicular monoamine transporter 2 (VMAT2) packages 5-HT (and also NE, DA) into vesicles after its cytoplasmic synthesis. This packaging is important for vesicular release of 5-HT and serotonergic neurotransmission at large, and interestingly could also prevent cytotoxicity by controlling cytoplasmic levels of free monoamines (Mooslehner et al., 2001). In early postnatal VMAT2-KO mice (which die by P5), 5-HT remains mainly cytoplasmic and is rapidly degraded by MAOA, which is expressed in the raphe during development (Vitalis et al., 2003). Thus, brains of VMAT2-KO mice show a near complete 5-HT depletion, particularly in serotonergic axons and terminals (Fon et al., 1997; Alvarez et al., 2002). Since VMAT2-mediated transport is not 5-HT specific, other monoamine (such as DA and NE) levels are also extremely low in VMAT2-KO brains. In these pups, axonal segregation of TCAs into periphery-related patterns in S1 is delayed by 1 day but appears normal by P5. Importantly, the development of upper cortical layers (II-IV) was reduced and proper barrels did not form in layer IV (Alvarez et al., 2002). Similarly, no significant alteration in thalamocortical patterning development was found in another VMAT2-KO mouse strain (Persico et al., 2001). A 5-fold increase in brain 5-HT levels obtained by generating VMAT2-MAOA-DKO (double knockout) mice did not rescue the VMAT2-KO phenotype but instead phenocopied the MAOA-KO, with a complete absence of BF (Alvarez et al., 2002). Overall, these mutants showed that a genetically mediated depletion of 5-HT, DA, and NE delays the development of TCAs but does not alter BF patterning, which is consistent with the effect of pharmacologically depleting 5-HT (see above). There are two major pitfalls in interpreting the contribution of 5-HT depletion to the VMAT2-KO phenotype: one is the severe growth retardation and the second is the concomitant depletion of DA and NE. The early postnatal mortality, made it impossible to investigate the long-term effects of this mutation.
Other genetic 5-HT depletion models
Several new genetic models of 5-HT depletion have been generated and recently reviewed (Trowbridge et al., 2011). The effect on BF formation has yet to be investigated in most of them; for instance in Tph1- and Tph2-KO mice, 5-HT synthesis is almost completely abolished in peripheral and central tissues, respectively (Cote et al., 2003; Walther & Bader, 2003; Gutknecht et al., 2008; Savelieva et al., 2008; Alenina et al., 2009), yet BF formation was not examined in detail. Neuronal-specific 5-HT depletion has also been achieved by deletion of the transcription factors necessary for 5-HT neuron differentiation. For instance, Pet1 (Fev in humans) is required to establish the final identity of 5-HT neurons in the raphe nuclei (Hendricks et al., 1999; Maurer et al., 2004; Lillesaar et al., 2009). In the Pet1-KO mice, most neuronal precursors in the raphe nuclei fail to differentiate into serotonergic neurons, leading to a nearly complete 5-HT depletion in late prenatal and early postnatal brains (Hendricks et al., 2003; Bonnin et al., 2011; Kiyasova et al., 2011). No major abnormalities in overall brain development have been observed in this model. In particular, BF formation appears normal in the Pet1-KO at P7 as illustrated in Fig. 1 (Gaspar, unpublished observations). It should be mentioned that in these mice, even though most 5-HT neurons are missing, the placenta appears to provide sufficient amounts of 5-HT to maintain normal brain concentrations at early prenatal stages of development (Bonnin et al., 2011). Thus, 5-HT signaling may not be disrupted until later stages of development, minimizing the potential impact on early TCA pathway formation (see section below and Bonnin et al. (2007)). Lastly, another mouse model of brain-specific 5-HT depletion has been recently generated. Since VMAT2-KO mice are severely hypomorphic and die during the first postnatal week, a conditional raphe neuron-specific (VMAT2;;Sert-crecKO) mouse line has been generated (Narboux-Neme et al., 2011). In these mice also, the BF appears normal (see Fig. 1, Narboux-Neme and Gaspar, unpublished observation). Overall, the absence of major BF defect in these mouse lines is consistent with the pharmacological and genetic models of 5-HT depletion described in the previous sections. It should be noted, however, that the timing of BF formation has not been studied in detail in any of those lines.
5-HT1B-KO
So far the models suggest that an excess, but not absence, of extracellular 5-HT significantly affects BF formation. This suggests that over-activation of 5-HT receptors either on TCAs or in their target region (S1) may be responsible for the effect. 5-HT1B receptors are Gi-coupled, and transiently expressed on TCAs during pre- and early postnatal development (Bennett-Clarke et al., 1993; Bonnin et al., 2006). A knockout mouse line (5-HT1B-KO) was generated to test the hypothesis that 5-HT1B over-activation could mediate the effects of increased extracellular 5-HT levels on BF formation. Consistent with this possibility, the absence of this receptor (in the MAOA/5-HT1B-Double KO mouse) largely rescued the MAOA-KO phenotype (Salichon et al., 2001; Rebsam et al., 2002). Similarly, a normal barrel pattern was observed in SERT/5-HT1B-DKO and MAOA/SERT/5-HT1B-Triple KO mice (Salichon et al., 2001). Although the segregation of cortical cells in layer IV of S1 was still incomplete in these lines, a complete rescue could be achieved by further PCPA treatment between P0 and P8. Thus, even in the absence of 5-HT1B receptor, elevated 5-HT levels still disturb cortical cell organization, suggesting the contribution of another 5-HT receptor. 5-HT1D and 5-HT1B receptors belong to the same subfamily (Pullar et al., 2004), are expressed in similar transient patterns in these regions, and thus may be good candidates (Bonnin et al., 2006; Bonnin et al., 2007). Thus, 5-HT1D receptors could provide redundant signaling and maintain normal BF development in the single 5HT1B-KO mice. This possibility is illustrated in Fig. 3. These genetic models support the idea that over-activation of 5-HT receptors causes the alterations in TCA and BF development. The mechanism by which such over-activation affects TCA patterning is not fully understood. A first possibility is that over-activation of 5-HT1B receptors inhibits the glutamatergic thalamocortical transmission postnatally, thereby disrupting BF refinement locally in S1 (Rhoades et al., 1994; Laurent et al., 2002). Another possibility would be a direct overstimulation of the 5-HT1B receptors trophic effects on growing TCAs (Lieske et al., 1999; Lotto et al., 1999; Persico et al., 2006), leading them to ‘overshoot’ their targets in S1.
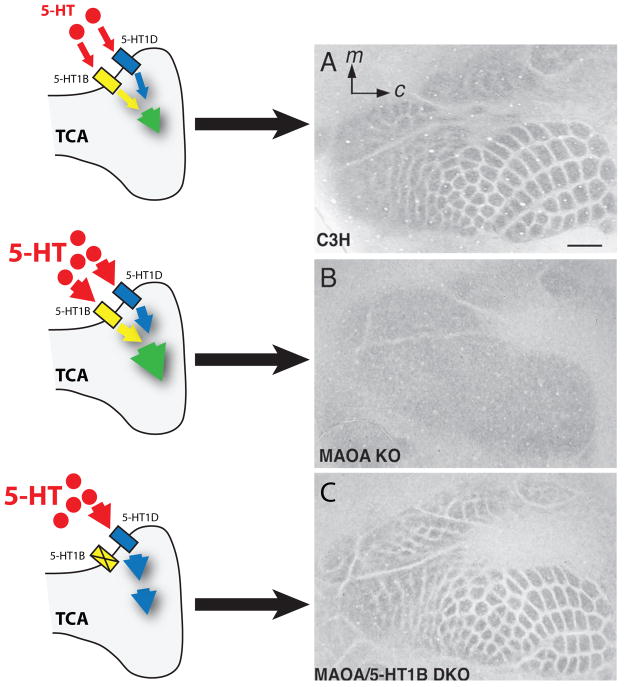
Figure 3.
Potential mechanism by which increased extracellular serotonin (5-HT) in the MAOA-KO mice affects cortical barrel field (BF) formation. (A), in wild type (C3H; C3H/HeJ background) mice 5-HT signals simultaneously through both 5-HT1B and 5-HT1D receptors, which are transiently expressed by thalamic neurons and present on axon terminals (see text). (B), increased extracellular 5-HT concentration in the MAOA-KO mice leads to over-activation of 5-HT1B and 5-HT1D receptors, and to an absence of clear BF formation as observed at P7 in flattened cortex preparations stained with a 5-HT antibody. (C), in the absence of 5-HT1B receptors (MAOA/5-HT1B –Double KO), the MAOA-KO phenotype is rescued, suggesting that residual 5-HT signaling is sufficient for normal BF formation, despite the increased extracellular 5-HT concentration. Since BF patterning is not altered in 5-HT1B-KO mice (see text), the model proposes that 5-HT1D receptors may provide sufficient signaling for normal BF formation in the absence of 5-HT1B receptors, with or without elevated extracellular 5-HT concentration. BF formation (right panels) was visualized in flattened cortex preparations stained with CO histochemistry.
There is in vivo evidence that 5-HT1B and 5-HT1D signaling, in concert, play important role for the prenatal TCA pathway formation. The similarity of 5-HT1B and 5-HT1D receptor transcripts expression patterns in the thalamus with that of the netrin-1 receptors Unc5c and DCC (Braisted et al., 2000; Garel & Rubenstein, 2004) suggested that 5-HT signaling could be important for TCA pathway formation, prenatally (E12.5 to E16.5). This hypothesis was tested in vitro, and explant studies showed that a 5-HT1B/1D-mediated decrease in intracellular cAMP level in thalamic neurons switches their axons response to netrin-1 from attraction to repulsion (Bonnin et al., 2007). The importance of 5-HT signaling in thalamic axons response to guidance cues was confirmed in vivo. In utero electroporation was used to knockdown or overexpress 5-HT1B/1D receptors simultaneously and specifically in the thalamus; this targeted genetic manipulation allowed to decrease or increase both receptors expression simultaneously starting at E12.5, without affecting transcript expression in other brain regions. Two opposing effects of decreasing or increasing 5-HT signaling in thalamic neurons in vivo were observed. Knocking down 5-HT1B/1D receptors expression with siRNAs led to a ventrally shifted, or expanded path of TCAs in the internal capsule before entering the cortex. Conversely, overexpressing both receptors led to an abnormally restricted dorso–medial path of TCAs in the internal capsule (Bonnin et al., 2007). Based on 5-HT effects in vitro and on netrin-1 expression in this region, it was proposed that changing 5-HT signaling in TCAs affects their positioning within netrin-1 gradient in the internal capsule (Bonnin et al., 2007). Thus, 5-HT signaling through 5-HT1B/1D receptors in vivo can modulate the patterning of TCA pathway in the fetal brain. The long-term effect of these manipulations on postnatal BF formation was not investigated, although the observed changes in TCA pathway in the internal capsule and cortex suggest they could lead to ventral or dorsal shifts in BF positioning in S1. To date, the exact mechanism by which over-activation of 5-HT1B (and possibly other) receptors affects TCAs and BF patterning is not fully understood. Yet, the prenatal manipulations of 5-HT1B/1D receptors expression and in vitro results suggest that intracellular cAMP could be an important mediator of 5-HT modulation of TCAs pathway and, possibly, BF formation (Gheorghita et al., 2006). Interestingly, despite their similarities with 5-HT1B receptors in terms of expression pattern, timing of expression in thalamic neurons and downstream signalling pathway, the involvement of 5-HT1D receptors in postnatal BF formation remains to be investigated.
Should manipulations leading to opposite effects on 5-HT signaling have opposite consequences?
Here, we summarized and compared different manipulations that led to changes in brain 5-HT concentration during development. These manipulations have been achieved pharmacologically, genetically by using multiple KO mouse models, and by acute in vivo alterations of 5-HT receptors expression. The results generally converge to show that developmental 5-HT signaling is important for TCA growth and guidance (prenatally) and for BF refinement (postnatally), although 5-HT may play temporally distinct roles in these processes.
The fact that an excess, but not absence, of extracellular 5-HT affects TCA pathway and BF formation suggests that 5-HT signaling may have a modulatory, rather than instructive, role. The existence of a threshold in the amount of extracellular 5-HT above which BF formation is compromised, and below which other compensatory signals are triggered that maintain normal BF formation (e.g. signaling through 5-HT1D in the absence of 5-HT1B) may explain the apparent discrepancy between increased and decreased 5-HT signaling effects. In addition, the temporal and regional specificity in the expression of genes deleted in the various mouse lines described above, could contribute to the observed phenotypic differences. For instance, the increase in extracellular 5-HT levels resulting from MAOA deletion may start earlier and be more generalized than in the SERT-KO. Adding to this complexity, there is now evidence of multiple and changing sources of 5-HT, particularly during prenatal development (Bonnin & Levitt, 2011; Trowbridge et al., 2011), and also of dynamic (temporal/spatial) expression patterns of multiple 5-HT receptors (Gaspar et al., 2003; Bonnin et al., 2006). Thus, altering the expression of a single gene, early in prenatal development, may trigger compensatory responses in the expression of related genes in the 5-HT signaling pathway, limiting the impact on brain development. Furthermore, there is recent evidence that excessive levels of intracellular 5-HT can trigger receptor-independent events, such as covalent coupling of 5-HT by transglutaminases to small GTPases (termed ‘serotonylation’; (Paulmann et al., 2009)). This mechanism was shown to modulate insulin secretion from pancreatic β-cells, suggesting that intracellular 5-HT can trigger direct cellular responses besides the known receptor-mediated signaling. The intriguing possibility that serotonylation of small GTPases in thalamic neurons could contribute to the effect of massive 5-HT increase, and phenotypes, observed in some of the genetic models remains an open question.Overall, the constitutive KO models described in this review may not be entirely specific to 5-HT (e.g. VMAT2-KO), can affect both brain and periphery 5-HT levels (e.g. MAOA-KO), and alter the 5-HT level at different developmental stages and thus provide improved, but incomplete demonstration of the specific involvement of 5-HT in TCA pathway and BF formation. The current development of conditional or inducible KO models (Gross et al., 2002; Liu et al., 2010) will provide more specific spatio-temporal control over 5-HT signaling disruption and provide a better assessment of 5-HT role in brain development.
Acknowledgments
E.S.B.v.K. is supported by a grant from the Honours Programme Beyond the Frontiers (Radboud Honours Academy; Radboud University, Nijmegen, the Netherlands). P.G. is funded by ANR (MNP-neur-032), European Commission, INSERM, and University Paris 06. A.B. is supported by the NICHD (grant 5R21HD065287) and NARSAD.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- Agmon A, Yang LT, O’Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura C, Dell’Acqua ML, Moessner R, Murphy DL, Lesch KP, Persico AM. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb Cortex. 2007;17:1394–1401. doi: 10.1093/cercor/bhl051. [DOI] [PubMed] [Google Scholar]
- Alvarez C, Vitalis T, Fon EA, Hanoun N, Hamon M, Seif I, Edwards R, Gaspar P, Cases O. Effects of genetic depletion of monoamines on somatosensory cortical development. Neuroscience. 2002;115:753–764. doi: 10.1016/s0306-4522(02)00484-0. [DOI] [PubMed] [Google Scholar]
- Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. The Journal of biological chemistry. 1989;264:2195–2198. [PubMed] [Google Scholar]
- Baumgarten HG, Lachenmayer L. 5,7-dihydroxytryptamine: improvement in chemical lesioning of indoleamine neurons in the mammalian brain. Z Zellforsch Mikrosk Anat. 1972;135:399–414. doi: 10.1007/BF00307184. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Chiaia NL, Crissman RS, Rhoades RW. The source of the transient serotoninergic input to the developing visual and somatosensory cortices in rat. Neuroscience. 1991;43:163–183. doi: 10.1016/0306-4522(91)90425-n. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Hankin MH, Leslie MJ, Chiaia NL, Rhoades RW. Patterning of the neocortical projections from the raphe nuclei in perinatal rats: investigation of potential organizational mechanisms. The Journal of comparative neurology. 1994a;348:277–290. doi: 10.1002/cne.903480209. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Lane RD, Rhoades RW. Fenfluramine depletes serotonin from the developing cortex and alters thalamocortical organization. Brain research. 1995;702:255–260. doi: 10.1016/0006-8993(95)00867-5. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:153–157. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW. Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat’s somatosensory cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994b;14:7594–7607. doi: 10.1523/JNEUROSCI.14-12-07594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Blue ME, Erzurumlu RS, Jhaveri S. A comparison of pattern formation by thalamocortical and serotonergic afferents in the rat barrel field cortex. Cereb Cortex. 1991;1:380–389. doi: 10.1093/cercor/1.5.380. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–794. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nature neuroscience. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslen B, Marsal K, Hansson SR. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25:518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O’Leary DD. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Chen K, Cases O, Rebrin I, Wu W, Gallaher TK, Seif I, Shih JC. Forebrain-specific expression of monoamine oxidase A reduces neurotransmitter levels, restores the brain structure, and rescues aggressive behavior in monoamine oxidase A-deficient mice. The Journal of biological chemistry. 2007;282:115–123. doi: 10.1074/jbc.M609830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Jonak E, Fernstrom JD. Serotonin reuptake inhibitors do not prevent 5,7-dihydroxytryptamine-induced depletion of serotonin in rat brain. Brain research. 2004;1007:19–28. doi: 10.1016/j.brainres.2003.12.044. [DOI] [PubMed] [Google Scholar]
- Costa E, Groppetti A, Revuelta A. Action of fenfluramine on monoamine stores of rat tissues. Br J Pharmacol. 1971;41:57–64. doi: 10.1111/j.1476-5381.1971.tb09935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, Dandolo L, Hamon M, Mallet J, Vodjdani G. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato RJ, Blue ME, Largent BL, Lynch DR, Ledbetter DJ, Molliver ME, Snyder SH. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DR, Killackey HP. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. The Journal of comparative neurology. 1987;256:246–256. doi: 10.1002/cne.902560205. [DOI] [PubMed] [Google Scholar]
- Dori I, Dinopoulos A, Blue ME, Parnavelas JG. Regional differences in the ontogeny of the serotonergic projection to the cerebral cortex. Exp Neurol. 1996;138:1–14. doi: 10.1006/exnr.1996.0041. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain research. Developmental brain research. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. The European journal of neuroscience. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Kimura H, Maeda T. Postnatal development of serotonin nerve fibers in the somatosensory cortex of mice studied by immunohistochemistry. The Journal of comparative neurology. 1986;246:191–201. doi: 10.1002/cne.902460205. [DOI] [PubMed] [Google Scholar]
- Garel S, Rubenstein JL. Intermediate targets in formation of topographic projections: inputs from the thalamocortical system. Trends Neurosci. 2004;27:533–539. doi: 10.1016/j.tins.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature reviews Neuroscience. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gheorghita F, Kraftsik R, Dubois R, Welker E. Structural basis for map formation in the thalamocortical pathway of the barrelless mouse. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:10057–10067. doi: 10.1523/JNEUROSCI.1263-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Waider J, Kraft S, Kriegebaum C, Holtmann B, Reif A, Schmitt A, Lesch KP. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008;115:1127–1132. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998;83:1185–1201. doi: 10.1016/s0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Jha S, Rajendran R, Davda J, Vaidya VA. Selective serotonin depletion does not regulate hippocampal neurogenesis in the adult rat brain: differential effects of p-chlorophenylalanine and 5,7-dihydroxytryptamine. Brain research. 2006;1075:48–59. doi: 10.1016/j.brainres.2005.12.110. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe BK, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- Lanoir J, Hilaire G, Seif I. Reduced density of functional 5-HT1A receptors in the brain, medulla and spinal cord of monoamine oxidase-A knockout mouse neonates. The Journal of comparative neurology. 2006;495:607–623. doi: 10.1002/cne.20916. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Towle AC, Patrick K, Henderson P, Krebs H. Decreased serotonin content of embryonic raphe neurons following maternal administration of p-chlorophenylalanine: a quantitative immunocytochemical study. Brain research. 1985;352:107–114. doi: 10.1016/0165-3806(85)90092-6. [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. The Journal of comparative neurology. 1998;401:506–524. [PubMed] [Google Scholar]
- Leslie MJ, Bennett-Clarke CA, Rhoades RW. Serotonin 1B receptors form a transient vibrissa-related pattern in the primary somatosensory cortex of the developing rat. Brain research Developmental brain research. 1992;69:143–148. doi: 10.1016/0165-3806(92)90132-g. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain research bulletin. 1982;8:389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- Lieske V, Bennett-Clarke CA, Rhoades RW. Effects of serotonin on neurite outgrowth from thalamic neurons in vitro. Neuroscience. 1999;90:967–974. doi: 10.1016/s0306-4522(98)00501-6. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Stigloher C, Tannhauser B, Wullimann MF, Bally-Cuif L. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1 expression. The Journal of comparative neurology. 2009;512:158–182. doi: 10.1002/cne.21887. [DOI] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nature neuroscience. 2010;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotto B, Upton L, Price DJ, Gaspar P. Serotonin receptor activation enhances neurite outgrowth of thalamic neurones in rodents. Neurosci Lett. 1999;269:87–90. doi: 10.1016/s0304-3940(99)00422-x. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Mechawar N, Radja F, Beaulieu C, Descarries L. Quantified distribution of serotonin transporter and receptors during the postnatal development of the rat barrel field cortex. Brain research Developmental brain research. 1998;107:159–163. doi: 10.1016/s0165-3806(98)00016-9. [DOI] [PubMed] [Google Scholar]
- Maurer P, Rorive S, de Kerchove d’Exaerde A, Schiffmann SN, Salmon I, de Launoit Y. The Ets transcription factor Fev is specifically expressed in the human central serotonergic neurons. Neurosci Lett. 2004;357:215–218. doi: 10.1016/j.neulet.2003.12.086. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Higashi S, Lopez-Bendito G. Choreography of early thalamocortical development. Cereb Cortex. 2003;13:661–669. doi: 10.1093/cercor/13.6.661. [DOI] [PubMed] [Google Scholar]
- Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS, Emson PC. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol Cell Biol. 2001;21:5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner R, Simantov R, Marx A, Lesch KP, Seif I. Aberrant accumulation of serotonin in dopaminergic neurons. Neurosci Lett. 2006;401:49–54. doi: 10.1016/j.neulet.2006.02.081. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nature reviews Neuroscience. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Nagai A, Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. Cellular expression of the monocarboxylate transporter (MCT) family in the placenta of mice. Placenta. 2010;31:126–133. doi: 10.1016/j.placenta.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Narboux-Neme N, Sagne C, Doly S, Diaz SL, Martin CB, Angenard G, Martres MP, Giros B, Hamon M, Lanfumey L, Gaspar P, Mongeau R. Severe serotonin depletion after conditional deletion of the vesicular monoamine transporter 2 gene in serotonin neurons: neural and behavioral consequences. Neuropsychopharmacology. 2011;36:2538–2550. doi: 10.1038/npp.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterheld-Haas MC, Van der Loos H, Hornung JP. Monoaminergic afferents to cortex modulate structural plasticity in the barrelfield of the mouse. Brain research Developmental brain research. 1994;77:189–202. doi: 10.1016/0165-3806(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Altamura C, Calia E, Puglisi-Allegra S, Ventura R, Lucchese F, Keller F. Serotonin depletion and barrel cortex development: impact of growth impairment vs. serotonin effects on thalamocortical endings. Cereb Cortex. 2000;10:181–191. doi: 10.1093/cercor/10.2.181. [DOI] [PubMed] [Google Scholar]
- Persico AM, Di Pino G, Levitt P. Multiple receptors mediate the trophic effects of serotonin on ventroposterior thalamic neurons in vitro. Brain research. 2006;1095:17–25. doi: 10.1016/j.brainres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar IA, Boot JR, Broadmore RJ, Eyre TA, Cooper J, Sanger GJ, Wedley S, Mitchell SN. The role of the 5-HT1D receptor as a presynaptic autoreceptor in the guinea pig. Eur J Pharmacol. 2004;493:85–93. doi: 10.1016/j.ejphar.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:8541–8552. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Dissociating barrel development and lesion-induced plasticity in the mouse somatosensory cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:706–710. doi: 10.1523/JNEUROSCI.4191-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Bennett-Clarke CA, Chiaia NL, White FA, Macdonald GJ, Haring JH, Jacquin MF. Development and lesion induced reorganization of the cortical representation of the rat’s body surface as revealed by immunocytochemistry for serotonin. The Journal of comparative neurology. 1990;293:190–207. doi: 10.1002/cne.902930204. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Bennett-Clarke CA, Shi MY, Mooney RD. Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J Neurophysiol. 1994;72:2438–2450. doi: 10.1152/jn.1994.72.5.2438. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft SL, Woolsey TA. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991;1:308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- Trowbridge S, Narboux-Neme N, Gaspar P. Genetic models of serotonin (5-HT) depletion: what do they tell us about the developmental role of 5-HT? Anat Rec (Hoboken) 2011;294:1615–1623. doi: 10.1002/ar.21248. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Behavioral evidence for the rapid release of CNS serotonin by PCA and fenfluramine. Eur J Pharmacol. 1976;36:149–154. doi: 10.1016/0014-2999(76)90266-1. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Alvarez C, Chen K, Shih JC, Gaspar P, Cases O. Developmental expression pattern of monoamine oxidases in sensory organs and neural crest derivatives. The Journal of comparative neurology. 2003;464:392–403. doi: 10.1002/cne.10804. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Callebert J, Launay JM, Price DJ, Seif I, Gaspar P. Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: determination of a sensitive developmental period. The Journal of comparative neurology. 1998;393:169–184. doi: 10.1002/(sici)1096-9861(19980406)393:2<169::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. The European journal of neuroscience. 2007;26:331–344. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain research. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Wu CS, Ballester Rosado CJ, Lu HC. What can we get from ‘barrels’: the rodent barrel cortex as a model for studying the establishment of neural circuits. The European journal of neuroscience. 2011;34:1663–1676. doi: 10.1111/j.1460-9568.2011.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Davies CL, Bennett-Clarke CA, Lane RD, Rhoades RW. Selective facilitation of the serotonin(1B) receptor causes disorganization of thalamic afferents and barrels in somatosensory cortex of rat. The Journal of comparative neurology. 2000;425:130–138. doi: 10.1002/1096-9861(20000911)425:1<130::aid-cne11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]