Abstract
Silver nanoparticles (Ag NPs) are becoming increasingly prevalent in consumer products as antibacterial agents. The increased use of Ag NP-enhanced products may lead to an increase in toxic levels of environmental silver, but regulatory control over the use or disposal of such products is lagging due to insufficient assessment on the toxicology of Ag NPs and their rate of release into the environment. In this article we discuss recent research on the transport, activity and fate of Ag NPs at the cellular and organismic level, in conjunction with traditional and recently established methods of nanoparticle characterization. We include several proposed mechanisms of cytotoxicity based on such studies, as well as new opportunities for investigating the uptake and fate of Ag NPs in living systems.
Keywords: cell uptake, characterization methods, imaging, physiological sensing, silver nanoparticles, toxicology
Silver nanoparticles (Ag NPs) have become increasingly popular as antibiotic agents in textiles and wound dressings, medical devices and appliances such as refrigerators and washing machines. They are traditionally defined as particles with overall dimensions below 100 nm, but the term ‘nanosilver’ is also becoming widely adopted, especially in the context of commercial products that contain nanomaterials with a large fraction of silver. The number of Ag NP-containing products has grown from less than 30 in 2006 to over 300 at the beginning of 2011 [301] and they are most often employed as bacteriostatic coatings for preventing infection or as deodorants. It is estimated that approximately 280 tons of Ag NPs were produced for use in commercial or industrial products and that number is expected to quadruple by 2015 [1]. However, an adequate assessment of the long-term effects of Ag NP exposure on human physiology and their release into the environment is lagging behind the rapid increase in the commercialization of Ag NP products. Most of the scientific literature on the toxicology of Ag NPs has only been published in the past decade [2–4]. Many of these studies have revealed Ag NPs to have noticeable toxicity against several cell lines as well as a number of aquatic organisms, but the mechanistic basis of these toxic effects is now an area of active research. In particular, the bioavailability of silver ions (Ag+) from Ag NPs, considered by many as a major factor in Ag-mediated toxicity, remains poorly understood [5]. For example, certain algal species are more sensitive to Ag NPs than to free Ag+, but the addition of cysteine (known to form complexes with Ag+) reduces the toxic effects of both Ag sources [6]. Such studies underscore the need to understand the transport, uptake and degradation of Ag NPs under physiological conditions, to accurately assess the relative benefits and risks of using Ag NPs in commercial products.
Traditional methods in toxicology research and assessments have focused mostly on chemical agents and were not originally designed to encompass nanoparticles, so determining the toxicological effects of Ag NPs raises several challenges. For instance, Ag NPs and Ag-oxides have strong optical extinctions at visible wavelengths and can interfere with colorimetric assays such as the MTT assay, which is used to measure cell viability based on mitochondrial activity [7]. Another issue is the large variation in physicochemical characteristics, depending on the source and type of Ag NPs; variations in particle size, shape and surface chemistry can have significant impacts on toxicity. It is necessary to characterize Ag NPs both prior to use and also during the course of a study, because significant temporal changes will often occur during an experimental trial [8,9]. In fact, particle agglomeration is commonly observed in studies involving Ag NPs, particularly when diluted in media with high ionic strength (> 10 mM). This agglomeration can affect their bioavailability by reducing their rate of degradation or cell uptake, as larger aggregates are less efficiently internalized [8]. These variables impose significant challenges for designing in vivo toxicological studies, which must be supported by numerous control studies in order to identify the most important experimental parameters.
On the contrary, the strong optical properties of Ag NPs can be useful in the context of biosensing and biological imaging and offer excellent opportunities to study NP uptake and biodistribution, in vivo as well as in vitro [10,11]. Ag NPs support localized surface plasmons that give rise to resonant light scattering and other optical properties [12], and can support a variety of bioanalytical sensing and imaging modalities. Recent applications of Ag NPs include the detection of biomarkers in Alzheimer’s disease [13], the targeted imaging of cancer cells [14] and the identification of pathogens by surface-enhanced Raman scattering [15]. The plasmon-enhanced optical activities of Ag NPs enable them to be tracked in real time without the need for additional labels, as well as a handle for evaluating their eventual degradation.
The goal of this article is to examine potential fate or exposure pathways of Ag NPs, to discuss recent findings related to the mechanisms of Ag NP-mediated toxicity and to present current and emerging methods for assessing the transport, uptake and fate of Ag NPs in biological systems. In relation to the latter, we include methods that have not yet been applied toward toxicological studies, but have strong potential to monitor individual Ag NPs in real time, particularly when comparing their effects to their ionic counterparts. This article is not intended to be fully comprehensive in any one area, but rather to highlight the most recent findings and novel approaches for measuring Ag NP uptake and toxicity. We conclude with a section covering the current gaps in knowledge and future research needs.
Environmental fate & exposure pathways of Ag NPs
Silver ions have long been known for their antimicrobial properties. Early Romans used Ag+ to disinfect potable water and Ag+ was the most common antimicrobial agent until the largescale development of synthetic antibiotics in the mid 20th century. Nevertheless, Ag+ remains a common biocide in household products, biomedical instruments, drinking water filters and appliances [16–18]. A comprehensive review by Silver summarizes the many uses and misuses of silver-containing products and the downstream biological impact of Ag+ on prokaryotic and eukaryotic cells [19].
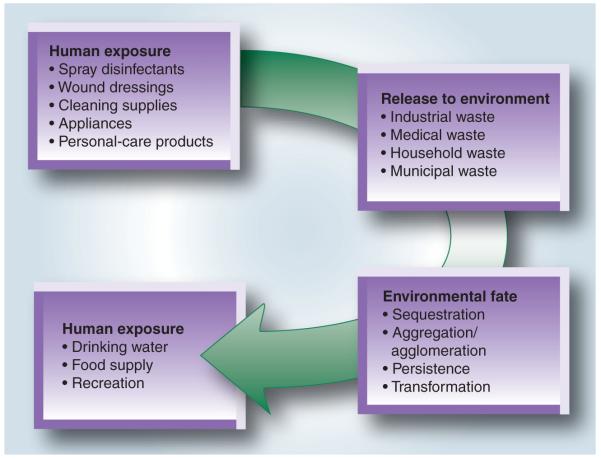
Silver nanoparticles have notable biocidal activity, due in part to the sustained release of Ag+, but further enabled by its surface and photocatalytic properties that can facilitate oxidative damage in nearby cells [20–24]. Ag NPs are significantly more toxic than Ag+ to prokaryotic cells and have been shown to be effective bactericides at nanomolar concentrations, compared with micromolar levels for Ag+ [25–27]. For these reasons, Ag NPs have been incorporated into numerous textile products and surface coatings as a bacteriostat. Unfortunately, these commercial activities have resulted in the unintended but worrisome consequence of Ag NPs moving into the ecosystem [23,28,29], raising recent concerns over the bioaccumulation of Ag NPs and the increased risk of human exposure (Figure 1) [30].
Figure 1.
Potential human and environmental exposure routes for silver nanoparticles.
There are no detailed studies of the release of Ag NPs from medical or household sources. In large part, this is due to the lack of tools capable of measuring the various types of Ag NPs, which includes metallic nanoparticles [31], Ag-zeolites [32], Ag–dendrimer complexes [33] and many other forms reviewed by Marambio-Jones and Hoek [34].
Blaser and coworkers predicted that Ag+ and Ag NPs comprise up to 15% of the biocidal compounds released from the plastics and textile industries entering waterways [35]. If Ag NPs enter municipal wastewater treatment plants, it has been estimated that approximately 7% leave the facility bound in sludge [35]. However, the subsequent fate of the Ag NP is largely unknown and depends on the sludge management approach used by individual municipalities (e.g., landfilling, incineration and land application).
As discussed by Marambio-Jones and Hoek, the increased use of Ag NPs in medical devices, clothing, household water filters, contraceptives, antibacterial sprays, cosmetics, detergents, cooking utensils, cell phones, computers and children’s toys is likely to result in an increase in the concentration of Ag NP discharge to our ecosystems [34]. Some studies have noted the potential for bioaccumulation of Ag NPs in various organisms, such as biofilms [36] and Mytilus edulis [37], a marine mussel. One study found that Daphnia magna, an aquatic invertebrate, accumulated nanoparticles from aqueous as well as foodborne exposure [38]; however, to date no large-scale bioaccumulation studies have been conducted.
Recent studies have investigated the effects of acute Ag NP exposure to humans from commercial products, such as disinfectant sprays [201,202], wound dressings [39], kitchen utensils [40] and cleaning supplies [41]. However, there is only anecdotal information concerning the fate of Ag NPs released by direct industrial discharge, wastewater treatment effluent, and medical or consumer waste, and no systematic studies on their environmental and toxicological impact. While there is some evidence to suggest that Ag+ released from Ag NPs is rendered less toxic by complexation with organic ligands and sulfur [42], there remain large gaps in knowledge on the chemistry and biochemistry of Ag NP biodegradation, which is necessary to address the toxicological impact of Ag NPs and Ag+ introduced by anthropogenic activities [43].
Assessments of Ag NP release into the environment
Current models indicate that environmental Ag+ and Ag NPs comprise nearly 15% of the biocidal compounds released as a point source from the plastics and textile industries [35]. Ag NPs have also been identified in the effluent waste from mining, photographic and electronic industrial processes [28,29]. Although there are currently no published data concerning the fate of Ag NPs in hospital waste, the potential for accumulation of Ag NPs from this industry is large considering the increased use of Ag NPs in wound dressings [39], surgical equipment [44], catheters [45,46] and even paint on the walls of hospital rooms [47]. It is estimated that the amount of Ag NPs released into aquatic ecosystems is currently on the order of 10–100 ng/l, a level that is likely to be exceeded within the next decade [48].
Silver nanoparticles are commonly discharged into the environment as a point-source pollutant and thus may be collected in municipal wastewater treatment plants (WWTPs) [26]. However, approximately 7% of Ag NPs entering WWTPs will be accumulated in sludges that are later deployed as agricultural fertilizers [35]. A majority of the Ag NPs in sludge remain in the upper soil layers and can potentially enter surface waters via runoff or groundwater tables [49]. The fate and transport of Ag NPs is also complicated by the fact that while some materials can be complexed as silver sulfide [42], removed by precipitation [50] or agglomeration [21,51], other forms may pass through WWTPs unaltered [35] and/or released as Ag+ [28].
It is important to note that in addition to the potentially adverse effects of increased biological exposure to Ag NPs, there is a risk that an increased release of Ag NPs into the environment may stimulate a rise in bacterial strains with heightened resistance to silver [19]. At present, most studies have focused on planktonic microbes [24,29,49] and very little information is available to outline the effect of Ag NPs on aquatic or terrestrial microbial populations [17]. One study did find a high sensitivity of a crucial soil microbe, Bradyrhizobium canariense, to Ag NPs [52]. However, there is a large body of literature describing the penetration of chemical toxins into bacterial biofilms [24,53,54], with suggestions that such environmental pressures may encourage the development of ‘persister cells’ with high levels of antimicrobial resistance [55]. In fact, the increased resistance to antimicrobials [53] and community-based defense mechanisms [56] are hallmarks of microbial biofilms, a topic that has been studied for decades [57]. The relationship between Ag NPs and increased Ag+ resistance has not been studied, perhaps hindered in part by limitations in technologies to enable such investigations.
Mechanisms of Ag NP toxicity
Silver nanoparticles are frequently touted as being highly effective as antimicrobial agents while being nontoxic to mammals. However, numerous in vitro studies have demonstrated the toxic effects of Ag NPs on rat liver (BRL3A) and neuronal cells [58,59], human lung epithelial cells [60] and murine stem cells [61]. Ag NPs have also exhibited toxicity in aquatic organisms, including vertebrates [11,62–64]. There is also strong evidence that microorganisms and plants are capable of concentrating nanoparticulate materials, which increases the potential for Ag NPs to accumulate in the food chain [65]. It should be noted, however, that many of the studies to date have used concentrations of Ag NPs that are much higher (>1 ppm) than what could be considered environmentally relevant. So far, most of the information relevant to the mechanisms of Ag NP toxicity has been derived from in vitro studies. Only a handful of mechanistic studies have been conducted in vivo, reflecting a significant gap in knowledge on a topic of increasing concern to the environment as well as human health.
The mechanisms of Ag+ toxicity are well understood, with many studies published over the past 50 years [302]. There is a general consensus that mitochondria are a primary target of Ag+ and are vulnerable to the ‘permeability transition pathway’, characterized by the formation of proteinaceous pores in mitochondrial membranes. In rat liver mitochondria, this increased permeability results in mitochondrial swelling, aberrant metabolism and eventually cellular apoptosis [66]. The lowest observed adverse effect level for Ag+ in mammalian cells has been reported to be in the range of 222 to 362 mg Ag/kg-day [67,68].
By contrast, there is less agreement on the factors that enable Ag NPs to deliver toxic effects to cells and organisms. In addition to size-dependent physical properties that can affect the release rate of Ag+, Ag NPs also exhibit size-dependent mechanisms of cell uptake that greatly influences their bioavailability. This mode of action has been referred to as the ‘Trojan Horse’ mechanism [23,24]: Ag NPs that permeate cell membranes can produce higher levels of intracellular Ag+, causing cytotoxic and genotoxic effects by the disruption of cell transport and local depletion of glutathione and other anti-oxidants [58,69–71]. Ag NPs smaller than 5 nm can passively penetrate cell walls and membranes, while larger NPs are generally internalized by endosomal mechanisms [22,23]. Therefore, considerable attention should be directed toward the transport and fate of Ag NPs, in order to better understand its toxicological effect on cells and organisms.
In vitro studies
The cytotoxic effects of Ag NPs have mostly been characterized in terms of oxidative stress, DNA damage and modulation of cytokine production. The cell uptake of Ag NPs can stimulate the production of radical oxygen species (ROS), resulting in oxidative stress and genotoxic effects. ROS are produced owing to a disruption in the flux of ions and electrons across the mitochondrial membrane; if produced in sufficiently high amounts, ROS can induce cell death by either apoptosis or necrosis [58,66,72–74]. Studies have shown Ag NP toxicity to be both size and shape dependent; for example, one study with alveolar macrophages indicated that Ag NPs with a mean size of 15 nm induced the greatest loss in mitochondrial activity [73]. However, contradictory data exists on the influence of Ag NP size and mitochondrial toxicity [58], suggesting that such effects may be case or species dependent, so a wider range of studies are needed before any generalities can be assumed.
With regard to genotoxicity, Ag NPs can damage DNA (in this case from human lung fibroblasts, IMR-90 and human glioblastoma cells, U251) indirectly by increasing ROS production or by decreasing ATP production (related again to mitochondrial damage), which impairs energy-dependent DNA repair mechanisms [74]. Direct DNA damage by Ag+ (released by Ag NPs) or by Ag NPs themselves have also been reported [75–78]. The latter case has been measured in mouse embryonic and fibroblast cells, indirectly by the increased expression in DNA repair proteins (Rad51 and H2AX) and an upregulation of p53, a cell-cycle checkpoint protein [79].
Silver nanoparticles have been reported to have both stimulatory and suppressive effects on the production of cytokines associated with the inflammatory response and again are likely to be dependent on case, dose and cell type. For instance, alveolar macrophages exposed to Ag NPs responded with an increase in the production of proinflammatory response mediators (TNF-α, MIP-2 and IL-1β) [73] and human epidermal cells exposed to Ag NPs produced an increase in IL-1β, IL-6, IL-8 and TNF-α [7]. By contrast, human mesenchymal stem cells exposed to Ag NPs exhibited both declines (IL-6 and IL-8) and increases (IL-8) in proinflammatory factors [80]; the latter was only observed when cells were exposed to less than 5 μg/ml Ag NP.
Recently, a fourth chemometric of Ag NP-induced toxicity has been reported. Rat coronary endothelial cells exposed for 24 h to high doses of Ag NPs (100 μg/ml) responded with an increased production of nitric oxide, which also increased cell proliferation [81]. At lower doses (<10 μg/ml), only a decrease in mitochondrial function was observed. Nitric oxide is known to have an important role in the cardiovascular system, suggesting another direction for the biological effects of Ag NPs. n In vivo studies Compared with in vitro studies, significantly less information is available on the potential mechanisms of toxicity of Ag NPs from in vivo studies. As reviewed below, exposure of laboratory rodents to Ag NPs has resulted in a myriad of toxicological responses, including effects on circulatory, respiratory, central nervous and hepatic systems. Effects on dermal tissues have also been reported after topical administration of Ag NPs.
In vivo studies
Compared with in vitro studies, significantly less information is available on the potential mechanisms of toxicity of Ag NPs from in vivo studies. As reviewed below, exposure of laboratory rodents to Ag NPs has resulted in a myriad of toxicological responses, including effects on circulatory, respiratory, central nervous and hepatic systems. Effects on dermal tissues have also been reported after topical administration of Ag NPs.
Ingestion or inhalation of Ag NPs results in their transport to the circulatory system [22,82]. Only one whole animal study is available on the effects of Ag NPs on hematological parameters. Mice injected with Ag NPs responded with a decrease in platelet aggregation [83]. The mechanisms of such a response remain unknown. With regard to adverse respiratory effects of Ag NPs, only one complete study has been reported [84]. In this study, rats were exposed to 18–19 nm Ag NPs at a concentration of 0.7–2.9 × 106 particles/cm3 for 90 days. Lung function, measured as tidal volume, minute volume and peak inspiration flow, was impaired in the highest concentration tested. Inflammatory responses (total protein, alveolar wall thickening and macrophage infiltration) were also increased in some animals [84]. A second, independent study indicated a rapid clearance of silver from rat lungs after an acute (6 h) inhalation exposure to Ag NPs, but an autopsy revealed the translocation of Ag NPs to the brain a week after initial exposure [85]. A pharmacokinetic study of Ag NPs injected into the bloodstream also confirmed their movement through the blood–brain barrier with subsequent accumulation in the brain, accompanied by neuronal d egeneration and necrosis [86].
Recent evidence indicates that liver and bile ducts are targets of toxicity for Ag NPs: Ag+ has been found to accumulate in liver following exposure to Ag NPs [82,84,87,88]. Histopathological analyses of liver and bile ducts of mice after Ag NP exposure also revealed vacuolization and hepatic focal necrosis, hyperplasia of bile ducts, increased infiltration of inflammatory cells and dilation of central veins. Increases in the expression of genes involved in apoptotic and inflammatory pathways have also been detected in mice livers exposed to Ag NPs [75].
Toxic effects were also observed during zebrafish development. Mortality, heart rate and hatching rate were all impacted by Ag NPs. Each of these end points was affected in a dose-dependent manner (5–100 μg/l Ag NPs). Changes in morphology, edema and an overall slowing of development were also detected. In this study, uniform distribution of the nanoparticles within the zebrafish embryos was also observed [89].
A single study is available on the morphological alterations of skin cells following exposure to Ag NPs [7]. In this study, pigs were topically dosed with Ag NPs (20–50 nm, 0.34–34 μg/ml) for 14 days. The highest doses caused edema, epidermal hyperplasia and focal inflammation.
In summary, most of the information available on the mechanisms of toxicity and associated effects of Ag NPs comes from in vitro studies, with only limited information from in vivo studies. Three main mechanisms of toxicity of Ag NPS have been proposed: oxidative stress, DNA damage and cytokine induction. Results from in vivo studies have shown that exposure of Ag NPs can result in effects in different major organs. It is important to mention that the studies summarized here used different formulations of Ag NPs (most were generated in the laboratory and some were purchased commercially). Very few studies have evaluated the mechanisms and associated toxicity effects of Ag NPs ‘leached’ from current commercial products.
Physicochemical assessment techniques
The physicochemical properties of Ag NPs are relevant to their toxicology; namely size, shape and surface chemistry. For example, a number of studies have correlated the size and shape of Ag NPs with their bactericidal properties [17,23,90,91]. A variety of methods are available to quantify these properties, several of which are summarized in Table 1; other approaches have been described in recent articles and reviews [92,93]. It has been noted, however, that while the physicochemical characterization of Ag NPs is necessary for a comprehensive ana lysis of its biological uptake and interactions with cells, such measurements are not sufficient for predicting nanotoxicological effects [94]. Indeed, this issue should be viewed in the opposite direction: toxicological studies are critical for correlating the physicochemical properties of nanoparticles and their interactions with living systems.
Table 1.
Methods for characterization of silver nanoparticles.
| Method of characterization | Attribute measured |
Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Transmission electron microscopy |
Size, shape |
Measures absolute size and shape |
Preparation subject to artifacts | [18,80,84–86] |
| Dynamic light scattering | Size | Measures effect size | Polydispersity and irregular shapes skew results |
[80,84,87–91] |
| Atomic force microscopy | Size, shape |
Measures effect size and shape |
Limited by cantilever tip size and particle surface chemistry |
[88,92–95] |
| Zeta potential | Surface charge |
Indicates stability and surface chemistry |
Affected by capping agents and shape |
[25,86,87,89,96,97] |
| Brunauer Emmett Teller | Specific surface area | Measures total area available for interaction |
No size distribution | [84,85,98–100] |
Transmission electron microscopy
Transmission electron microscopy (TEM) is the most common method of characterizing NP size and shape [91,95]. TEM images of NPs are typically acquired in brightfield mode, based on the contrast generated by electron scattering from heavy atoms, then subjected to image ana lysis software to obtain statistical distributions in particle size and ellipticities. Individual NPs can also be assessed for uniformity in shape or lack of thereof [17,96]. However, TEM ana lysis is limited by sample size (typically <200 particles) and also by variability in sample preparation methods, which are typically performed by casting and drying droplets of NPs in solution onto polymer-coated grids. This practice can skew the true size distribution of particles in suspension, as well as their state of agglomeration [97]. For these reasons, size ana lysis by TEM is best conducted in conjunction with other methods that c haracterize particles in their equilibrium states.
Dynamic light scattering
Dynamic light scattering (DLS) is a method of particle size ana lysis based on light scattering and the Brownian motion of particles in solution [91,95,98]. Whereas TEM defines particle size by differences in electron scattering, DLS measures the hydrodynamic radius of particles based on their rates of translational diffusion. DLS measurements will often produce larger values than those obtained by TEM, because they include the influence of the organic surface coating in the size estimates and the particle size distribution includes aggregates as well as individually dispersed particles. For these reasons, DLS is considered by many to be a more accurate estimate of the effective size of particles in solution [99,100]. However, DLS also has some drawbacks: the values are dependent on particle concentration, due to its sensitivity to aggregation effects, and it cannot provide an accurate assessment of particle shape without considerable parameterization. Furthermore, nonspherical particles are subject to additional motional behaviors that are easily misinterpreted. For example, DLS measurements of monodispersed gold nanorods can produce two peaks, corresponding either to rotational diffusion [101] or to an anisotropic orientation relative to the light source [102].
Atomic force microscopy
Scanning probe microscopies, such as atomic force microscopy, can provide complementary size and surface ana lysis of NPs bound to substrates, in liquid states as well as in air. Nanometer-sized probes are mounted on cantilevers and rastered across the sample, recording changes in forces as the probe tip interacts with the surface [103]. The lateral resolution of atomic force microscopy is lower than that of TEM due to limitations in tip size and shape; on the other hand, it is highly sensitive in the z-direction and is especially useful for depth profiling with nanometer resolution [104]. The cantilevers can also be functionalized to increase its sensitivity to surface properties and has been used to map electrostatic and chemical interactions [105,106]. Topological analyses based on such interactions do not necessarily reflect the true dimensions of nanostructured features, but rather an effective size based on physicochemical interactions [99].
Zeta potential
Another important factor in the transport and fate of Ag NPs is their electrokinetic or zeta potential, measured in millivolts. This is typically defined by the electrostatic double layer surrounding each NP, which in turn is influenced by surfactant coatings and the ionic strength of the supporting medium [107]. NPs can form stable suspensions in aqueous solutions when their zeta potentials (typically negative) are above 30 mV. This is usually the case for particles dispersed in low ionic strength solutions, but their zeta potentials are reduced at higher ionic strength because the cations are more closely associated with the particle surface, which reduces the electrostatic double layer [98,108] and can lead to agglomeration [26]. It should be noted that the zeta potential is not the sole factor in NP stabilization; particles can also be sterically stabilized by organic surface coatings, independent of surface charge [100].
Brunauer Emmet Teller
Specific surface area is yet another size-dependent factor in NP toxicity [96,109,110]. The specific surface area of NPs is much greater than that of their bulk counterparts and is an especially important issue in the case of aerosolized particles, which can enter the body through inhalation. The BET method is the most common method of quantifying exposed surface and has been used to measure the specific surface area of Ag NPs [95,109]. This method is based on the absorption of gas molecules onto the surface of the target analyte at a specified pressure. The specific surface area is then obtained as the ratio of the total surface area to the weight of NPs (in m2/g) [111].
Real-time physiological sensing
The unique properties of Ag NPs have been found to interfere with some of the more traditional toxicological assays. To accommodate this interference researchers in the field of nanotoxicology have had to incorporate new techniques or modify existing techniques. Some of these techniques were recently reviewed [2]; however, this article will focus on the use of microsensors for real-time physiological sensing and the incorporation of advanced imaging techniques into toxicological studies.
A logical first step for understanding the mechanisms of Ag NP toxicity is to compare the adverse effects with that of its ionic counterpart. The differential responses to Ag+ and Ag NPs can provide insights into the relationship between cell/tissue physiology and any size-dependent phenomena attributable to Ag NPs. Such information can be expected to be useful for guiding future regulations, as there are no rules currently in place to control the commercialization of Ag NP enhanced products [112]. However, efforts to obtain this knowledge is limited by available technologies for monitoring physiological changes during Ag NP exposure. While semiquantitative physiological assessment methods are widely employed and still very useful, one major drawback is that they are destructive (i.e., organisms need to be sacrificed). This imposes significant limits on the amount and quality of information that can be obtained from an individual specimen during experimental trials.
Over the last few decades the use of electrochemical microsensors and nanosensors has become more prominent for biological research applications [113]. Used in both intracellular and extracellular applications, these sensors have allowed us to measure analytes related to metabolism, stress and cell communication/signaling in ways which were not previously conceivable. Traditional use of nano/microsensors has involved penetration into cells/tissues or extracellular measurements along the surface of cells/ tissues. Intracellular micro- and nano-sensors have been shown to cause membrane damage and cytotoxicity, respectively [114]. While recent developments in intracellular nanosensors have allowed them to be used in minimally invasive formats [115], most extracellular micro/nanosensors are still used invasively due to low signal-to-noise ratio and a lack of multidimensional spatial resolution.
One extracellular technique that has alleviated these problems is the self-referencing microsensor technique. This sensor modality significantly improves signal-to-noise ratio and provides direct measurement of dynamic analyte flux, increasing spatial resolution with minimal sacrifice of temporal resolution [116]. While the use of microsensors in self-referencing modality has been around for decades [117], its potential for real-time physiological sensing has yet to be fully realized. The operation of sensors in self-referencing mode involves the oscillation of a single microsensor between two points, separated by a constant distance via computer-controlled stepper motors. Flux information can be obtained in real time by measuring Ag concentrations at each point, based on Fick’s first law of diffusion. The combination of a dc-coupled amplification scheme and oscillatory movement of a single electrode produces significant noise filtration and an increase in signal-to-noise ratio [116].
While there are currently no microsensors for the direct detection of Ag NPs, other sensors can be employed for monitoring physiological responses to Ag NPs. For instance, Ag+ can affect the uptake of H+ and various ions (e.g., Na+ and K+), simple sugars (e.g., glucose) and metabolic analytes necessary for cellular growth and development [118]. Self-referencing micro sensors can be used to monitor the real-time flux of glucose [119,120], glutamate [121], indoleacetic acid [122] and hydrogen peroxide. The latter case has been used to detect an increase in H2O2 efflux after exposing an excised, murine spinal cord to citrate-stabilized Ag NPs at 1 ppm (Figure 2). This release of H2O2 was most likely the result of oxidative stress induced by Ag NPs, in agreement with previous experiments [123,124]. While others have noted net increases in H2O2 efflux due to Ag NP exposure, the self-referencing microsensor technique provides a higher resolution of temporal and spatial data not obtainable with previous techniques. This high degree of resolution enables the use of metrics such as time to response, duration of response, peak efflux and total efflux, m easured as integrated flux (Table 2) [122].
Figure 2. Real-time hydrogen peroxide efflux from a murine spinal cord segment exposed to 1 mg/l silver nanoparticles.

The gap in the graph is a result of having to reposition the sample and probe after the addition of Ag NPs. A spike in efflux is visible approximately 7 min after the addition.
Ag NP: Silver nanoparticles.
Reproduced with permission from [Stensberg et al., Unpublished Data].
Table 2.
Summary of H2O2 efflux from exposure of murine spinal cord to 1 mg/l citrate stabilized silver nanoparticles.
| Treatment | H2O2 iFlux (fmol cm-2) |
Peak response (fmol cm-2s-1) |
Time to response (min) |
Duration of response (min) |
|---|---|---|---|---|
| Control (DMEM) | 7907 | N/A | N/A | N/A |
| 1 mg/l Ag NPs | 32381 | 364.2 | 5.2 | 5.7 |
iFlux values for the nanoparticle dose were much larger than the control value (control was integrated over the same time span as the duration of response) [122].
Ag NP: Silver nanoparticles; iFlux: Integrated flux.
Reproduced with permission from [Stensberg et al., Unpublished Data].
Amperometric sensors have also been used with enzymes that produce electroactive species generated from redox reactions. For example, glucose oxidase converts glucose into gluconic acid and H2O2, which can be measured by amperometry [125]. Enzyme-based biosensors can also be used in the self-referencing modality near cell/tissue surfaces and measure the effect of Ag NPs on physiological transport of various analytes.
Another type of microsensor that can be used in a self-referencing modality is the ion-selective electrode (ISE), which can detect a vast array of biologically relevant cations [125]. With regard to Ag NP and Ag+ toxicity, self-referencing ISEs have been used to evaluate toxicity based on the aberrant flux of Na+ and H+ [118]. For example, H+ efflux was measured from Daphnia magna embryos dosed with silver nitrate (Figure 3a) and Ag NPs (Figure 3b). Increases in time to response, peak response and total integrated flux were observed for Ag NP exposure when compared with embryos exposed to Ag+ (Table 3).
Figure 3. Proton efflux from Daphnia magna embryos when dosed with 130 and 650 ng/l (A) AgNO3 and (B) silver nanoparticles.
Note the differences in scale on the Y axis between the two graphs.
Ag NP: Silver nanoparticles.
Reproduced with permission from [Stensberg et al., Unpublished Data].
Table 3.
Summary of proton flux from exposure of Daphnia magna embryos to AgNO3 and silver nanoparticles.
| Treatment | H+ iFlux (pmol cm-2) |
Peak response (pmol cm-2 s-1) |
Time to response (min) |
Duration of response (min) |
|---|---|---|---|---|
| Control (hard water) | 1015 | N/A | N/A | N/A |
| 130 ng/l AgNO3 | 1313 | 2.4 | <1 | 6.4 |
| 650 ng/l AgNO3 | 5831 | 11.8 | <1 | 17.3 |
| 130 ng/l Ag NPs | 1552 | 698.4 | 3.4 | 2.1 |
| 650 ng/l Ag NPs | 25,363 | 2452.6 | 3.3 | 27.4 |
iFlux values for the high nanoparticle dose was much larger than the comparable dose of AgNO3 [122].
Ag NP: Silver nanoparticles; iFlux: Integrated flux.
Reproduced with permission from [Stensberg MC et al., Manuscript in Preparation].
A micro-ISE for Ag+ has been recently developed and demonstrated in self-referencing mode, with detection limits below 100 nm [126]. The sensor can monitor the rate of Ag+ uptake without interference from Ag NPs (Figure 4) and is thus particularly useful for separating the physiological effects of Ag+ from those of Ag NPs. In particular, any physiological response that does not correlate directly with Ag+ influx can be attributed specifically to Ag NP toxicity. The ability to noninvasively segregate the effects of Ag NPs and Ag+ on physiological transport will be crucial for establishing a mode of action for Ag NPs.
Figure 4. Real-time ionic silver flux measured at the surface of a Pseudomonas aeruginosa biofilm exposed to 9 μM (1.5 ppm) silver nitrate.

Reproduced with permission from [126].
Finally, optical microsensors have been used in a self-referencing modality [127,128]. These have some advantages over electrochemical sensors, as they are relatively unaffected by electrical or mechanical noise. The self-referencing optical microsensors have been used to measure real-time O2 flux as a metric for physiological stress in fathead minnows, exposed to several environmental contaminants [129]. However, preliminary data on Daphnia magna embryos exposed to Ag NPs indicate little or no effect on O2 consumption (Figure 5 & Table 4).
Figure 5. Proton efflux from Daphnia magna embryos when dosed with 130 and 650 ng/l (A) AgNO3 and (B) silver nanoparticles.
No notable differences were observed between the two treatments.
NP: Nanoparticle.
Reproduced with permission from [Stensberg et al., Unpublished Data].
Table 4.
Summary of oxygen flux from exposure of Daphnia magna embryos to AgNO3 and Ag NPs.
| Treatment | O2 iFlux (pmol cm-2) | Peak response (pmol cm-2 sec-1) |
|---|---|---|
| Control (hard water) | 244 | N/A |
| 130 ng/l AgNO3 | 265 | 16.1 |
| 650 ng/l AgNO3 | 288 | 17.3 |
| 130 ng/l Ag NPs | 265 | 22.1 |
| 650 ng/l Ag NPs | 260 | 16.2 |
Integrated influx values (iFlux) were calculated over the same timespan at points before and after each dose. Time to response and duration of response values were not discernable [122].
Ag NP: Silver nanoparticles; iFlux: Integrated flux.
Reproduced with permission from [Stensberg MC et al., Manuscript in Preparation].
Biological imaging with Ag NPs
Silver nanoparticles exhibit a strong optical activity due to plasmon resonance, an electrodynamic phenomenon based on the excitation of conduction electrons at specific frequencies of light [12]. These plasmon resonances enhance the detection and tracking of Ag NPs by a number of optical imaging methods, from simple light scattering to multiphoton luminescence, surface-enhanced Raman scattering (SERS) and in vivo biomedical imaging modalities. With major exception to the darkfield imaging studies, most of these cases can be considered exploratory in their use of Ag NPs, but also demonstrate their potential utility in the design of in vitro and in vivo studies that address downstream toxicological effects. Ultimately, the accumulation of toxicological data will determine the scope and limitations in developing Ag NPs as imaging agents for clinical use. On the other hand, these novel imaging tools present opportunities for tracing the various pathways and fates of Ag NPs in biological systems.
Ag NPs in optical darkfield microscopy
Colloidal Ag NPs are widely recognized as optical labels for biosensing and imaging applications based on light scattering [12]. Ag NPs below 50 nm typically support strong extinctions between 400 and 500 nm (blue–green region of the visible spectrum), although individual NPs as small as 2.6 nm can be detected under optimal conditions [130]. The plasmon resonances can shift toward longer wavelengths if the particles are larger than 50 nm or are anisotropic in shape [131]. This wavelength-dependent scattering enables Ag NPs to be distinguished according to their physical characteristics allowing independent tracking of NP uptake rate as a function of size or shape. Xu and coworkers have investigated the uptake of Ag-coated gold NPs by several different organisms using darkfield optical imaging [132]. In one such study involving Pseudomonas aeruginosa, an opportunistic bacterial pathogen, the active uptake and efflux of single NPs as large as 80 nm in diameter were monitored with transport times ranging from minutes to hours, depending on the particle size [10]. Differences in NP efflux activities could also be discerned between various strains of Pseudomonas and was attributed to the existence of yet-unidentified membrane pumps. Remarkably, the bacteria were proficient at excreting NPs of all sizes and their viabilities were unaffected by the low (picomolar) level of Ag-coated NPs used in this study.
Darkfield imaging has also been used to investigate the dynamics of Ag NP uptake by zebrafish embryos [11,132]. Early-stage embryos (8–64 cells) were exposed to unfunctionalized Ag NPs of various sizes (mean diameter 11.6 nm) and at subnanomolar concentrations (1010 to 4 × 1011 NPs/ml). Single-particle tracking confirmed that most Ag NPs were sufficiently dispersed to diffuse freely into the embryo by passage through the chorion pore canals, followed by their penetration into the inner mass (Figure 6). Single-particle tracking also revealed changes in the diffusion coefficients of Ag NPs over time, attributable to local gradients in viscosity as the particles continued to permeate through the embryonic tissue. However, the passive transport of Ag NPs into the chorionic space could be affected by the clogging of pores caused by particle aggregation, as determined by the localized accumulation of NPs with a redshift in scattering. With respect to organismic toxicity, Ag NPs did not appear to have a detrimental effect on the development of embryos exposed to particle conbe found in all parts of the developed zebrafish embryo at 120 h postfertilization. However, embryos exposed to higher concentrations of Ag NPs experienced a high death rate, accompanied by multiple developmental malformations whose frequency of occurrence increased with Ag NP levels (Figure 7). It is worth noting that a complementary study using similarly sized Au NPs indicated far fewer adverse effects on embryonic development, meaning that the developmental abnormalities induced by Ag NPs are not simply due to particle size [133].
Figure 6. Single-particle tracking (A–F) of a silver nanoparticles (in dashed circle) toward the chorionic space of a zebrafish embryo, using optical darkfield microscopy.
Rectangular outline in (A) includes chorionic pore channels; scale bar = 15 μm.
Reproduced with permission from [11].
Figure 7. Selected images of zebrafish larvae with various developmental deformities.
Reproduced with permission from [11].
Darkfield optical imaging is equally useful for obtaining insights into the cytotoxic effects of Ag NPs in mammalian cells [134]. Unfunctionalized Ag NPs were found to inhibit the growth of L929 cells (derived from a murine fibrosarcoma) at a concentration of 0.46 nM (22 μg/ml). Darkfield microscopy indicated that the amount of Ag NPs increased in both the cytoplasm and nuclei over time, with the relative concentration several fold higher in the former versus the latter. Most cells exhibited abnormal morphologies after a 72 h exposure with either oversized nuclei or multiple nuclei, all of which contained higher quantities of DNA than cells with normal nuclei. This suggests that while Ag NPs may be directly responsible for inhibiting cytokinesis, they do not interfere with DNA replication.
Ag NPs as fluorescent & nonlinear optical labels
Recent advances in synthesis and optical ana lysis have enabled researchers to determine that Ag NPs can also be luminescent and provide excellent contrast under various types of fluorescence imaging. Small (<2 nm) Ag nanoclusters (NCs) have an intrinsic capacity to produce photoluminescence, similar to semicondutor quantum dot NPs, whereas colloidal Ag NPs can produce luminescence under two-photon excitation conditions at plasmon resonance. Ag NPs can also contribute to fluorescence imaging by providing a local electromagnetic field to increase the rate of emission of nearby fluorophores, a mechanism known as surface-enhanced fluorescence. These imaging modalities are readily applicable toward toxicological studies, although such studies have yet to be reported.
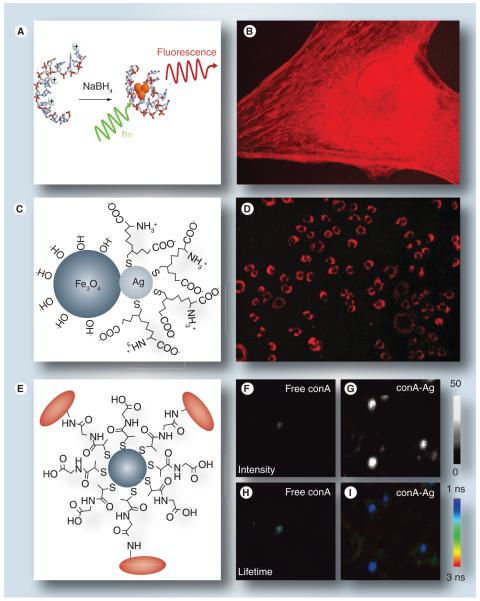
The luminescent properties of Ag NCs were first reported by Dickson and coworkers [135]. Ag NCs can be synthesized by several different methods, including biosynthesis in live cells, and for in situ fluorescence imaging studies [136], and are easily functionalized with biomolecular ligands. For example, Ag NCs have been synthesized in the presence of avidin-conjugated DNA, then used as fluorescent labels of biotinylated NIH 3T3 cells [137] (Figure 8a & 8b). The Ag NCs are well suited for in vitro fluorescence imaging and are superior to conventional dye molecules in both emission intensity and photostability. Variations in chemistry enable Ag NCs to be used as fluorescent labels for various cellular components, such as actin, microtubule filaments and specific surface proteins [138]. The emission wavelength of Ag NCs is highly size dependent and can even be tuned to near-infrared (NIR) wavelengths. For example, Ag NCs synthesized in the presence of oligocytosine DNA can be used as NIR-active biomarkers to monitor their transfection of live cells [139].
Figure 8. Silver nanoclusters and nanoparticles as fluorescent contrast agents.
fluorescence; (C & D) two-photon excited luminescence; (E–I) surface-enhanced fluorescence imaging. (A) Production of DNA-encapsulated silver nanoclusters (Ag NCs); (B) fluorescence imaging of live NIH 3T3 cells with anti-actin silver nanoclusters (Ag NCs); (C & D) Ag–Fe3O4 nanoparticle (NP) heterodimer as a contrast agent for two-photon excited luminescence imaging in macrophage cells; (E) plasmon-coupled Ag NP–dye probe; (F & G) demonstration of 20–30-fold enhancement in fluorescence intensity, in the presence of Ag NPs; (H & I) Ag NP-enhanced fluorescence lifetime imaging.
(A & B) Reproduced with permission from [137].
(C & D) Reproduced with permission from [145].
(F & G) Reproduced with permission from [147].
(H & I) Reproduced with permission from [148].
Colloidal Ag NPs are also capable of photoluminesence, although their quantum yields are much smaller than that of Ag NCs. Nevertheless, they can still be used as fluorescent labels if the excitation intensity is sufficiently high. For instance, 36-nm Ag NPs encapsulated in polymer shells have been used as drug delivery vehicles and tracked by fluorescence imaging inside of B16F10 cells [140]. Metallic nanoshells with 50-nm silica cores and 10-nm Ag shells have also been reported as fluorescent labels for detecting CXCR4 chemokine receptors on the surfaces of T lymphocytes [141].
Metal nanoparticles can also produce a two-photon excited luminescence (TPL) by ultrafast pulsed laser excitation. This has been particularly well studied in NIR-resonant NPs, such as Au nanorods [142], but the TPL activity of Ag NPs has also been reported with applications toward biological imaging [143,144]. For example, TPL has been used to monitor the nonspecific uptake of Ag–Fe3O4 NPs into macrophages (Figure 8C & 8D) [145]. Ag NPs as small as 10 nm could produce strong TPL signals with femto-second NIR laser excitation, whereas the magnetic component allowed cells to be manipulated by external magnetic field gradients.
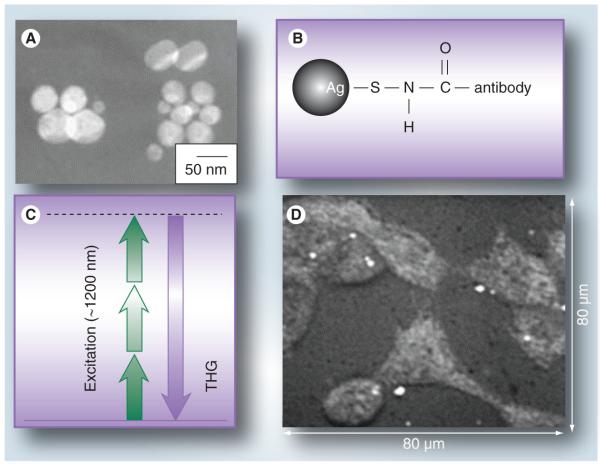
Third-harmonic generation (THG) is another nonlinear optical technique for imaging Ag NPs, one that is more efficient than TPL because it does not require excited states for energy conversion. Ag NPs are ideal contrast agents for THG owing to their large third-order susceptibility and the overlap of their plasmon resonance with the tripling of the NIR frequencies used to excite THG signals [146]. THG imaging has been applied toward in vitro cancer cell detection, using antibody-labeled Ag NPs incubated with mouse bladder carcinoma cells (Figure 9) [14]. While TPL and THG are excellent imaging tools for in vitro studies involving Ag NPs, their short working distances are a drawback for whole-animal imaging, so their application toward nanotoxicology is best served at the cellular level.
Figure 9. Silver nanoparticles used as contrast agents for third-harmonic generation imaging.
(A) TEM images of silver nanoparticles (Ag NPs). (B) Ag NPs conjugated with anti-Her2 antibodies. (C) Transition state for THG. (D) THG image of mouse bladder carcinoma cells (MBT2) marked with antibody-labeled Ag NPs. THG: Third-harmonic generation.
Reproduced with permission from [14].
Surface-enhanced fluorescence & Raman imaging
Although colloidal Ag NPs are less efficient than Ag NCs as fluorescent markers, they can indirectly support fluorescence imaging by enhancing the emission rates of nearby dye molecules by a process termed surface-enhanced fluorescence. Ag NP–dye conjugates have been demonstrated as fluorescent probes for cellular imaging by conjugating fluorescently labeled lectins onto 20-nm Ag NPs, which were then incubated with HEK 293A cells (Figure 8e) [147,148]. Cells labeled with the Ag NP-coupled probes produced fluorescence signals 20–30-times brighter than those labeled with organic dyes alone (Figure 8F & 8g). Further studies demonstrated that the lifetime of the coupled Ag NP–dye is significantly modified compared with uncoupled dye molecules, which extends the application of Ag NP–dye conjugates to fluorescence lifetime imaging (Figure 8H & 8i).
The plasmon resonances of Ag NPs can also be applied toward imaging modalities based on SERS. Close-packed or aggregated metal NPs form electromagnetic ‘hot spots’ that can enhance Raman signals by several orders of magnitude, to the extent that their emissions are comparable to fluorescence. Bacteria adsorbed onto Ag nanostructures can be detected by their characteristic Raman vibrational spectra using SERS microscopy, with limits of detection as low as ten bacteria/ml [149,150]. SERS-active Ag NP ‘tags’ can also be prepared using appropriate surface chemistries, often through biophysical inter action with proteins or other biomolecular species. For instance, Ag NPs conjugated to ligands bearing cyano groups (C≡N) have been used to image membrane receptor clustering on Hela cells through protein-mediated NP aggregation [151]. Alkyne- and carborane-functionalized Ag NPs can also produce large SERS signals and provide characteristic Raman signatures for antibody-targeted cell imaging [152,153]. SERS is even useful for detecting low-molecular-weight species and has been used to monitor adrenergic signaling to cardiac myocyte cells, which control the contraction and subsequent beating of heart muscles [154]. This ability to track molecular signatures may prove useful for cellular toxicology studies involving Ag NPs, by correlating their presence with nearby s tress-induced metabolites or biomarkers.
Ag NPs as contrast agents in biomedical imaging
Near-infrared-resonant Ag nanostructures, such as Ag nanoshells, have been investigated as optical contrast agents for photoacoustic imaging and other clinically relevant imaging modalities. Emelianov and coworkers proved the concept by injecting submicron Ag nanoshells with silica cores into porcine pancreas, with detection by multimodal ultrasound and photoacoustic imaging [155]. The Ag nanoshells not only increased photoacoustic contrast, but also enabled greater imaging depth into the tissue (Figure 10a & 10b).
Figure 10. Silver nanoparticles as contrast agents in biomedical imaging.
(A) Scanning electron microscope image of Ag nanoshells. (B) Ultrasound (left), photoacoustic (middle) and merged image of Ag nanoshells in porcine pancreas (right). (C) Transmission electron microscope image of 125I-labeled 12-nm silver nanoparticles. (D) CT-SPECT images of 125I-labeled Ag NPs in rats at different time points after intravenous administration.
(A) Reproduced with permission from [155].
(B) Reproduced with permission from [157].
Colloidal Ag NPs have also been examined as contrast agents for computed tomography, based on their large x-ray absorption coefficients. Dendrimer-stabilized Ag NPs (16 nm) were injected subcutaneously under mouse skin tissue and determined to attenuate x-ray transmission at levels comparable to iodine-based x-ray contrast agents used in clinical settings [156]. Ag NPs (12 nm) have also been functionalized with the radiotracer 125I, followed by systemic administration in Balb/c mice for pharmacokinetic studies (Figure 10C & 10D) [157]. The in vivo biodistribution of these Ag NPs were evaluated by single-photon emission computerized tomography imaging, which indicated the spleen and liver as the primary organs of NP uptake 24 h after injection (41.5 and 24.5% ID/g, respectively). This study provides useful information about the near-term in vivo accumulation of Ag NPs, complementary to toxicity studies discussed earlier.
Conclusion
It is expected that the number of applications for Ag NPs will continue to grow, but there is still much that needs to be understood with respect to their fate and accumulation in the environment and their potential long-term effects on humans and other organisms. Recent studies have shown that the release of Ag NPs into the environment is increasing, yet there are large gaps in our understanding of how these particles are transported through ecosystems and migrate into the food chain and their consequences on human health.
At the cellular level, a variety of mechanisms of Ag NP toxicity have been reported, including ROS generation, DNA damage and cytokine induction during in vitro studies. The few in vivo studies that have been conducted so far indicate the potential for adverse effects at the organismic level, with vulnerabilities in the circulatory, respiratory, central nervous, hepatic and dermal systems. Many more studies are needed to determine the biodistribution and subsequent toxicity of Ag NPs using in vivo systems. These future studies should include modeling of the toxicological impact of Ag NPs leached from textiles, a major source of anthropogenic silver. The physicochemical properties of Ag NPs are also important factors and should be monitored during the course of a toxicological study to assess the effects of any physical changes on NP uptake and bioavailability.
While Ag NPs present some challenges for traditional toxicological assays, they also have unique qualities that enable entirely new approaches to examine their toxicological impact on cells and organisms. This includes the use of self-referencing microsensors for real-time physiological sensing and novel imaging modalities that take advantage of the strong plasmon resonances produced by Ag NPs, permitting their tracking in a label-free manner. These recently developed tools can easily be incorporated into experimental designs that will enhance the quality of risk assessment of Ag NPs.
Future perspective
The commercialization of Ag-enhanced products with antibacterial properties is increasing at an accelerated pace. The number of toxicological studies involving Ag NPs continues to grow as well, but most of these are performed in vitro on cell cultures, with lower-order lifeforms or with embryonic organisms. Toxicological assessments of Ag NPs on higher-order organisms lag far behind and may be limited by the availability of appropriate tools for in vivo characterization and assessment. The movement of Ag NPs from consumer-related activities through the environment also remains poorly understood and is in need of more comprehensive studies.
One important goal for future nanotoxicology research is to establish better models to assess the long-term effects of Ag NPs in mammalian systems, thereby enabling the design of in vivo studies with definable end points. The recently developed imaging modalities described above are ideally suited for monitoring the in vivo transport and fate of Ag NPs and may be able to contribute toward new insights into t oxicological mechanisms.
In closing, we hope that future discussions on the health and risk benefits of Ag NPs will be driven by sound scientific evidence, produced from carefully designed studies using the appropriate tools. Such studies are vital to ensure that the eventual regulation of Ag-enhanced products will be determined by facts rather than by alarm or ignorance.
Executive summary.
Environmental exposure
▪ Silver nanoparticle (Ag NP)-enhanced commercial products have become a major source of environmental silver.
▪ Almost no data exists on the environmental concentrations of Ag NPs.
Mechanisms of toxicity
▪ Reported mechanisms of Ag NP toxicity include DNA toxicity, cytokine induction and oxidative stress.
▪ Some in vivo studies have reported Ag NP toxicity in respiratory, circulatory, central nervous, hepatic and dermal systems.
Nanoparticle characterization methods
▪ Nanoparticle characterization contributes toward our understanding of nanotoxicology, but does not define it.
▪ Standard NP characterization methods can be applied, including transmission electron microscopy, dynamic light scattering and zeta-potential measurements.
Physiological sensing
▪ More attention should be shifted to real-time quantitative ana lysis (e.g., self-referencing microsensors), which offer high spatial and temporal resolution.
▪ Electrochemical or optical self-referencing sensors can be used to monitor analyte fluxes that may be affected by Ag+ or Ag NP exposure.
Nanoparticle-based imaging
▪ Ag NPs exhibit strong plasmon resonances at visible wavelengths and can support a wide variety of optical imaging modalities.
▪ Biomedical imaging modalities are highly useful for characterizing the in vivo biodistribution of Ag NPs, with high impact on toxicological studies.
Acknowledgments
The authors gratefully acknowledge financial support from the NIH (RC1-CA147096) and The National Science Foundation (CBET-0854036).
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.The Silver Institute . The future demand of silver: industrial demand. The Silver Institute; Washington DC, USA: 2011. pp. 27–32. [Google Scholar]
- 2.Dhawan A, Sharma V. Toxicity assessment of nanomaterials: methods and challenges. Anal. Bioanal. Chem. 2010;398:589–605. doi: 10.1007/s00216-010-3996-x. [DOI] [PubMed] [Google Scholar]
- 3.Duran N, Marcato PD, de Conti R, Alves OL, Costa FTM, Brocchi M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Brazil. Chem. Soc. 2010;21:949–959. [Google Scholar]
- 4.Kahru A, Dubourguier H. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269:105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Lubick N. Nanosilver toxicity: ions, nanoparticles – or both? Environ. Sci. Technol. 2008;42:8617. doi: 10.1021/es8026314. [DOI] [PubMed] [Google Scholar]
- 6.Navarro E, Piccapietra F, Wagner B, et al. Toxicity of silver nanoparticles to Chlaydomonas reinhardtii. Environ. Sci. Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- 7.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ. Health. Persp. 2010;118:407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skebo JE, Grabinski CM, Schrand AM, Schlager JJ, Hussain SM. Assessment of metal nanoparticle agglomeration, uptake, and interaction using a high-illuminating system. Int. J. Toxicol. 2007;26:135–141. doi: 10.1080/10915810701226248. [DOI] [PubMed] [Google Scholar]
- 9.Stebounova LV, Guio E, Grassian VH. Silver nanoparticles in simulated biological media: a study of aggregation, sedimentation, and dissolution. J. Nanopart. Res. 2011;13:233–244. [Google Scholar]
- 10.Xu XHN, Chen J, Jeffers RB, Kyriacou S. Direct measurement of sizes and dynamics of single living membrane transporters using nano-optics. Nano Lett. 2002;2:175–182. [Google Scholar]
- 11.Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XHN. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–143. doi: 10.1021/nn700048y. ▪ Darkfield microscopy was used to monitor the transport of single silver nanoparticles (Ag NPs) into zebrafish embryos. Developmental abnormalities were found to be highly dependent on Ag NP dose, with a critical concentration of 0.19 nM.
- 12.Yguerabide J, Yguerabide EE. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. Anal. Biochem. 1998;262:157–176. doi: 10.1006/abio.1998.2760. [DOI] [PubMed] [Google Scholar]
- 13.Haes AJ, Hall WP, Chang L, Klein WL, Van Duyne RP. A localized surface plasmon resonance biosensor: first steps toward an assay for Alzheimer’s disease. Nano. Lett. 2004;4:1029–1034. [Google Scholar]
- 14.Tai SP, Wu Y, Shieh DB, et al. Molecular imaging of cancer cells using plasmonresonant-enhanced third-harmonicgeneration in silver nanoparticles. Adv. Mater. 2007;19:4520–4523. ▪ Ag NPs can produce strong third-harmonic generation contrast using a pulsed near-infrared laser, enabling the detection of single particles on cell membranes.
- 15.Wang Y, Lee K, Irudayaraj J. Silver nanosphere SERS probes for sensitive identification of pathogens. J. Phys. Chem. C. 2010;114:16122–16128. [Google Scholar]
- 16.Savage N, Diallo MS. Nanomaterials and water purification: opportunities and challenges. J. Nanopart. Res. 2005;7:331–342. [Google Scholar]
- 17.Fabrega J, Fawcett S, Renshaw J, Lead J. Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ. Sci. Technol. 2009;43:7285–7290. doi: 10.1021/es803259g. [DOI] [PubMed] [Google Scholar]
- 18.Zodrow K, Brunet L, Mahendra S, et al. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009;43:715–723. doi: 10.1016/j.watres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Silver S. Bacterial silver resistance: molecular biology. FEMS Microbiol. Rev. 2003;27:341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- 20.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 21.Limbach LK, Wick P, Manser P, Grass RN, Bruinink A, Stark WJ. Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007;41:4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Pitts B, Stewart PS, Camper A, Yoon J. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrob. Agents Chemother. 2008;52:1446–1453. doi: 10.1128/AAC.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi O, Hu Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008;42:4586–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- 24.Choi O, Deng KK, Kim NJ, Ross L, Surampalli RY, Hu ZQ. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008;42:3066–3074. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Lok C, Ho C, Chen R, et al. Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2006;12:527–534. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 26.Cumberland S, Lead J. Particle size distributions of silver nanoparticle at environmentally relevant conditions. J. Chromatogr. A. 2009;1216:9099–9105. doi: 10.1016/j.chroma.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Choi OK, Hu ZQ. Nitrification inhibition by silver nanoparticles. Wat. Sci. Technol. 2009;59:1699–1702. doi: 10.2166/wst.2009.205. [DOI] [PubMed] [Google Scholar]
- 28.Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- 29.Geranio L, Heurberger M, Nowack B. The behavior of silver nanotextiles during washing. Environ. Sci. Technol. 2009;43:8113–8118. doi: 10.1021/es9018332. [DOI] [PubMed] [Google Scholar]
- 30.Hansen SF, Michelson ES, Kamper A, Borling P, Stuer-Lauridsen F, Baun A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology. 2008;17:438–447. doi: 10.1007/s10646-008-0210-4. [DOI] [PubMed] [Google Scholar]
- 31.Kvitek L, Panacek A, Soukupova J, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles. J. Phys. Chem. C. 2008;112:5825–5834. [Google Scholar]
- 32.Cowan M, Abshire K, Houk S, Evans S. Antimicrobial efficacy of a silver-zeolite matrix coating on stainless steel. J. Ind. Microbiol. Biotechnol. 2003;30:102–106. doi: 10.1007/s10295-002-0022-0. [DOI] [PubMed] [Google Scholar]
- 33.Balogh L, Swanson D, Tomalia D, Hagnauer G, McManus A. Dendrimer–silver complexes and nanocomposites as antimicrobial agents. Nano Lett. 2001;1:18–21. [Google Scholar]
- 34.Marambio-Jones C, Hoek EMV. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010;12:1531–1551. [Google Scholar]
- 35.Blaser SA, Scheringer M, MacLeod M, Hungerbühler K. Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008;390:396–409. doi: 10.1016/j.scitotenv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Fabrega J, Renshaw J, Lead JR. Interactions of silver nanoparticles with Psuedomonas putida biofilms. Environ. Sci. Technol. 2009;43:9004–9009. doi: 10.1021/es901706j. [DOI] [PubMed] [Google Scholar]
- 37.Zuykov M, Pelletier E, Demers S. Colloidal complexed silver and silver nanoparticles in extrapallial fluid of Mytilus edulis. Mar. Environ. Res. 2011;17:17–21. doi: 10.1016/j.marenvres.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Zhao CM, Wang WX. Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ. Toxicol. Chem. 2011;30:885–892. doi: 10.1002/etc.451. [DOI] [PubMed] [Google Scholar]
- 39.Dworniczek E, Nawrot U, Seniuk A, Wlodarczyk K, Bialynicki-Birula R. The in vitro effect of a silver-containing dressing on biofilm development. Adv. Clin. Exp. Med. 2009;18:277–281. [Google Scholar]
- 40.US Environmental Protection Agency . Inventory of nanotechnology-based consumer products. The Project on Emerging Nanotechnologies; Mar, 2009. [Google Scholar]
- 41.Mueller NC, Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008;42:4447–4453. doi: 10.1021/es7029637. [DOI] [PubMed] [Google Scholar]
- 42.Kim B, Park CS, Murayama M, Hochella MF. Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products. Environ. Sci. Technol. 2010;44:7509–7514. doi: 10.1021/es101565j. [DOI] [PubMed] [Google Scholar]
- 43.Nowack B. Nanosilver revisited downstream. Science. 2010;330:1054–1055. doi: 10.1126/science.1198074. [DOI] [PubMed] [Google Scholar]
- 44.Vasilev K, Cook J, Griesser HJ. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Dev. 2009;6:553–567. doi: 10.1586/erd.09.36. [DOI] [PubMed] [Google Scholar]
- 45.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem. Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 46.Hachem R, Reitzel R, Borne A, et al. Novel antiseptic urinary catheters for prevention of urinary tract infections: correlation of in vivo and in vitro test results. Antimicrob. Agents Chemother. 2009;53:5145–5149. doi: 10.1128/AAC.00718-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naddafi K, Jabbari H, Chehrehei M. Effect of nanosilver painting on control of hospital air-transmitted microorganisms. Iran J. Environ. Health. 2010;7:223–228. [Google Scholar]
- 48.Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- 49.Hu Z, Chandran K, Grasso D, Smets B. Impact of metal sorption and internalization on nitrification inhibition. Environ. Sci. Technol. 2003;37:728–734. doi: 10.1021/es025977d. [DOI] [PubMed] [Google Scholar]
- 50.Tiede K, Boxall ABA, Wang XM, et al. Application of hydrodynamic chromatography-ICP-MS to investigate the fate of silver nanoparticles in activated sludge. J. Anal. Atom. Spectrom. 2010;25:1149–1154. [Google Scholar]
- 51.Zhang L, Yu JC, Yip HY, et al. Ambient light reduction strategy to synthesize silver nanoparticles and silver-coated TiO2 with enhanced photocatalytic and bactericidal activities. Langmuir. 2003;19:10372–10380. [Google Scholar]
- 52.Kumar N, Shah V, Walker VK. Perturbation of an artic soil microbial community by metal nanoparticles. J. Hazard. Mater. 2011;190:816–822. doi: 10.1016/j.jhazmat.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Stewart PS, Rayner J, Roe F, Rees WM. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl. Microbiol. 2001;91:525–532. doi: 10.1046/j.1365-2672.2001.01413.x. [DOI] [PubMed] [Google Scholar]
- 54.Toner B, Manceau A, Marcus MA, Millet DB, Sposito G. Zinc sorption by a bacterial biofilm. Environ. Sci. Technol. 2005;39:8288–8294. doi: 10.1021/es050528+. [DOI] [PubMed] [Google Scholar]
- 55.Lewis K. Persister cells. Annu. Rev. Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 56.Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Bryers JD. Medical biofilms. Biotechnol Bioeng. 2009;100:1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In vitro. 2005;19:975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 59.Hussain SM, Javorina MK, Schrand AM, Duhart HM, Ali SF, Schlager JJ. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006;92:456–463. doi: 10.1093/toxsci/kfl020. [DOI] [PubMed] [Google Scholar]
- 60.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 61.Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005;88:412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niyogi S, Wood CM. The biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ. Sci. Technol. 2004;38:6177–6192. doi: 10.1021/es0496524. [DOI] [PubMed] [Google Scholar]
- 63.Laban G, Nies L, Turco R, Bickham J, Sepúlveda M. The effects of silver nanoparticles on fathead minnow (Pimephales promelas) embryos. Ecotoxicology. 2010;19:185–195. doi: 10.1007/s10646-009-0404-4. [DOI] [PubMed] [Google Scholar]
- 64.Gaiser BK, Fernandes TF, Jepson M, Lead JR, Tyler CR, Stone V. Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ. Health UK. 2009;8:1–4. doi: 10.1186/1476-069X-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Persp. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Almofti MR, Ichikawa T, Yamashita K, Terada H, Shinohara Y. Silver ion induces a cyclosporine A-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J. Biochem. 2003;134:43–49. doi: 10.1093/jb/mvg111. [DOI] [PubMed] [Google Scholar]
- 67.Walker F. Experimental argyria: a model for basement membrane studies. Brit. J. Exp. Pathol. 1971;52:589–593. [PMC free article] [PubMed] [Google Scholar]
- 68.Matuk Y, Ghosh M, McCulloch C. Distribution of silver in the eyes and plasma proteins of the albino rat. Can. J. Ophthalmol. 1981;16:145–150. [PubMed] [Google Scholar]
- 69.Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 70.Tran C, Donaldson K, Stones V, et al. A scoping study to identify hazard data needs for addressing the risks presented by nanoparticles and nanotubes. Institute of Occupational Medicine; Edinburgh, UK: 2005. Research Report. [Google Scholar]
- 71.Suzuki T, Guo Y, Inoue S, Zhao X, Ohkochi M, Ando Y. Multiwalled carbon nanotubes mass-produced by dc arc discharge in He-H2 gas mixture. J. Nanopart. Res. 2007;8:279–285. [Google Scholar]
- 72.Wang MG, Katayama H, Ohgaki S. Inactivation of Legionella pneumophila and Pseudomonas aeruginosa: evaluation of the bactericidal ability of silver cations. Water Res. 2007;41:4097–4104. doi: 10.1016/j.watres.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 73.Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J. Phys. Chem. B. 2008;112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 74.Asharani PV, Mun G Low Kah, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. ▪ Toxicity of starch-coated Ag NPs was studied in vitro using two human cell lines. Ag NPs induced mitochondrial damage, oxidative stress (radical oxygen species), and DNA damage in a dose-dependent manner. The authors propose the following mechanism of toxicity for Ag NPs: disruption of the mitochondrial respiratory chain, leading to production of radical oxygen species and interruption of ATP synthesis, which in turn cause DNA damage.
- 75.Cha K, Hong H, Choi Y, et al. Comparison of acute responses of mice livers to short-term exposure to nano-sized or micro-sized silver particles. Biotechnol. Lett. 2008;30:1893–1899. doi: 10.1007/s10529-008-9786-2. [DOI] [PubMed] [Google Scholar]
- 76.Chi Z, Liu R, Zhao L, et al. A new strategy to probe the genotoxicity of silver nanoparticles combined with cetylpyridine bromide. Spectrochim Acta A. 2009;72:577–581. doi: 10.1016/j.saa.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 77.Kumari M, Mukherjee A, Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009;407:5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 78.Yang W, Shen C, Ji Q, et al. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology. 2009;20:1–7. doi: 10.1088/0957-4484/20/8/085102. [DOI] [PubMed] [Google Scholar]
- 79.Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM. DNA damage response to different surface chemistry of silver nanoparticles. Toxicol. Appl. Pharm. 2008;233:404–410. doi: 10.1016/j.taap.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Greulich C, Kittler S, Epple M, Muhr G, Köller M. Studies on the biocompatibility and the interaction of silver nanoparticles with human mesenchymal stem cells (hMSCs) Langenbeck Arch. Surg. 2009;394:495–502. doi: 10.1007/s00423-009-0472-1. [DOI] [PubMed] [Google Scholar]
- 81.Rosas-Hernández H, Jiménez-Badillo S, Martĺnez-Cuevas PP, et al. Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicol. Lett. 2009;191:305–313. doi: 10.1016/j.toxlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Sung JH, Ji JH, Park JD, et al. Subchronic inhalation toxicity of silver nanoparticles. Toxicol. Sci. 2009;108:452–461. doi: 10.1093/toxsci/kfn246. ▪ In vivo study that evaluated the subchronic toxicity of Ag NPs (18–19 nm) in rats exposed (via inhalation) to three doses (1.4–3.0 × 106 particles/cm3) for 6 h/day for a total of 13 weeks. Bile-duct hyperplasia increased with dose. In addition, several lesions in the lungs were observed. This study is one of the first to show hepatic damage due to Ag NP exposure via inhalation.
- 83.Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. 2009;3:1357–1364. doi: 10.1021/nn900277t. [DOI] [PubMed] [Google Scholar]
- 84.Sung JH, Ji JH, Yoon JU, et al. Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal. Toxicol. 2008;20:567–574. doi: 10.1080/08958370701874671. [DOI] [PubMed] [Google Scholar]
- 85.Takenaka S, Karg E, Roth C, et al. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Persp. 2001;4:547–551. doi: 10.1289/ehp.01109s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang J, Xiong L, Wang S, et al. Influence of silver nanoparticles on neurons and blood–brain barrier via subcutaneous injection in rats. Appl. Surf. Sci. 2008;255:502–504. [Google Scholar]
- 87.Ji JH, Jung JH, Kim SS, et al. Twenty-eightday inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2007;19:857–871. doi: 10.1080/08958370701432108. [DOI] [PubMed] [Google Scholar]
- 88.Kim YS, Kim JS, Cho HS, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- 89.Asharani PV, Wu YL, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19:1–8. doi: 10.1088/0957-4484/19/25/255102. [DOI] [PubMed] [Google Scholar]
- 90.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Yacaman MJ. The bacterial effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 91.Martinez-Castanon GA, Nino-Martinez N, Martinez-Gutierrez F, et al. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008;10:1343–1348. [Google Scholar]
- 92.Liu J, Sonshine DA, Shervani S, Hurt RH. controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4(11):6903–6913. doi: 10.1021/nn102272n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sadik OA, Zhou AL, Kikandi S, Du N, Wang Q, Varner K. Sensors as tools for quantitation, nanotoxicity and nanomonitoring assessment of engineered nanomaterials. J. Environ. Monit. 2009;11(10):1782–1800. doi: 10.1039/b912860c. [DOI] [PubMed] [Google Scholar]
- 94.Warheit DB. Debunking some misconceptions about nanotoxicology. Nano Lett. 2010;10(12):4777–4782. doi: 10.1021/nl103432w. [DOI] [PubMed] [Google Scholar]
- 95.Wani IA, Khatoon S, Ganguly A, Ahmed J, Ganuli AK, Ahmad T. Silver nanoparticles: large scale solvothermal synthesis and optical properties. Mater. Res. Bull. 2010;45:1033–1038. [Google Scholar]
- 96.Henglein A, Giersig M. Formation of colloidal silver nanoparticles: capping action of citrate. J. Phys. Chem. B. 1999;103:9533–9539. [Google Scholar]
- 97.Domingos R, Baalousha M, Ju-Nam U, et al. Characterizing manufactured nanoparticles in the environment: multimethod determination of particle sizes. Environ. Sci. Technol. 2009;43:7277–7284. doi: 10.1021/es900249m. ▪ Used a broad array of techniques to study a few different types of nanoparticles.
- 98.Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B. 2003;107:668–677. [Google Scholar]
- 99.Jensen TR, Duval ML, Kelly KL, Lazarides AA, Schatz GC, Van Duyne RP. Nanosphere lithography: effect of the external dielectric medium on the surface plasmon resonance spectrum of a periodic array of silver nanoparticles. J. Phys. Chem. B. 1999;103:9846–9853. [Google Scholar]
- 100.Kvitek L, Prucek R, Panacek A, Novotny R, Hrbac J, Zboril R. The influence of complexing agents on particle size in the process of SERS active silver colloid synthesis. J. Mater. Chem. 2005;15:1099–1105. [Google Scholar]
- 101.Leonov AP, Zheng J, Clogston JD, Stern ST, Patri AK, Wei A. Detoxification of gold nanorods by treatment with polystyrenesulfonate. ACS Nano. 2008;2(12):2481–2488. doi: 10.1021/nn800466c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez-Fernandez J, Perez-Juste J, Liz-Marzan LM, Lang PR. Dynamic light scattering of short Au rods with low aspect ratios. J. Phys. Chem. C. 2007;111:5020–5025. [Google Scholar]
- 103.Riskin M, Basnar B, Chegel VI, et al. Switchable surface properties through the electrochemical or biocatalytic generation of Ag+ nanoclusters on monolayer-functionalized electrodes. J. Am. Chem. Soc. 2006;128:1253–1260. doi: 10.1021/ja0561183. [DOI] [PubMed] [Google Scholar]
- 104.Jiang X, Zeng Q, Yu A. Thiol-frozen shape evolution of triangular silver nanoplates. Langmuir. 2007;23(4):2218–2223. doi: 10.1021/la062797z. [DOI] [PubMed] [Google Scholar]
- 105.Gracia-Pinilla MA, Perez-Tijerina E, Garcia JA, et al. On the structure and properties of silver nanoparticles. J. Phys. Chem. C. 2008;112:13492–13498. [Google Scholar]
- 106.Langry KC, Ratto TV, Rudd RE, McElfresh MW. The AFM measured force required to rupture the dithiolate linkage of thioctic acid to gold is less than the rupture force of a simple gold-alkyl thiolate bond. Langmuir. 2005;21(26):12064–12067. doi: 10.1021/la0513555. [DOI] [PubMed] [Google Scholar]
- 107.Sarkar A, Kapoor S, Mukherjee T. Preparation, characterization, and surface modification of silver nanoparticles in formamide. J. Phys. Chem. B. 2005;109:7698–7704. doi: 10.1021/jp044201r. [DOI] [PubMed] [Google Scholar]
- 108.Mukherjee B, Weaver JW. Aggregation and charge behavior of metallic and nonmetallic nanoparticles in the presence of competing similarly-charged inorganic ions. Environ. Sci. Technol. 2010;44:3332–3338. doi: 10.1021/es903456e. [DOI] [PubMed] [Google Scholar]
- 109.Park S, Kim B. A study of NO removal of activated carbon fibers with deposited silver nanoparticles. J. Colloid Interf. Sci. 2005;282:124–127. doi: 10.1016/j.jcis.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 110.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interf. Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 111.Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938;60:309–319. [Google Scholar]
- 112.Faunce T, Watal A. Nanosilver and global public health: international regulatory issues. Nanomedicine. 2010;5:617–632. doi: 10.2217/nnm.10.33. [DOI] [PubMed] [Google Scholar]
- 113.Oldenziel WH, van der Zeyden M, Dijkstra G, et al. Monitoring extracellular glutamate in hippocampal slices with a microsensor. J. Neurosci. Meth. 2007;160:37–44. doi: 10.1016/j.jneumeth.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 114.Overly CC, Lee KD, Berthiaume E, Hollenbeck PJ. Quantitative measurement of intraorganelle pH in the endosomal-lysosomal pathway in neurons by using ratiometric imaging with pyranine. Proc. Natl Acad. Sci. USA. 1995;92:3156–3160. doi: 10.1073/pnas.92.8.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buck SM, Xu H, Brasuel M, Philbert MA, Kopelman R. Nanoscale probes encapsulated by biologically localized embedding (PEBBLEs) for ion sensing and imaging in live cells. Talanta. 2004;63:41–59. doi: 10.1016/j.talanta.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 116.Porterfield DM. Measuring metabolism and biophysical flux in the tissue, cellular and sub-cellular domains: recent developments in self-referencing amperometry for physiological sensing. Biosens. Bioelectron. 2007;22:1186–1196. doi: 10.1016/j.bios.2006.06.006. ▪ Reviewed the various types of self-referencing microsensors and potential applications.
- 117.Khütrieber WM, Jaffe LF. Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J. Cell Biol. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bianchini A, Grosell M, Gregory S, Wood C. Acute silver toxicity in aquatic animals is a function of sodium uptake rate. Environ. Sci. Technol. 2002;36:1763–1766. doi: 10.1021/es011028t. [DOI] [PubMed] [Google Scholar]
- 119.McLamore ES, Shi J, Jaroch D, et al. A self-referencing enzyme-based microbiosensor for real time measurement of physiological glucose transport. Biosens. Bioelectron. 2011;26:2237–2245. doi: 10.1016/j.bios.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi J, McLamore ES, Jaroch D, Claussen JC, Rickus JL, Porterfield DM. Oscillatory glucose flux in INS 1 pancreatic β cells: a self-referencing microbiosensor study. Anal. Biochem. 2011;411:185–193. doi: 10.1016/j.ab.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McLamore ES, Mohanty S, Shi J, Rickus JL, Porterfield DM. Real time neuronal glutamate flux during potassium stimulation. J. Neurosci. Meth. 2010;189:14–22. doi: 10.1016/j.jneumeth.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 122.McLamore ES, Diggs A, Marzal P Calvo, et al. Non-invasive quantification of endogenous root auxin transport using an integrated flux microsensor technique. Plant J. 2010;63:1004–1016. doi: 10.1111/j.1365-313X.2010.04300.x. [DOI] [PubMed] [Google Scholar]
- 123.Ivask A, Bondarenk O, Jepihhina N, Kahru A. Profiling of the reactive oxygen species-related ecotoxicity of CuO, ZnO, TiO2, silver and fullerene nanoparticles using a set of recombinant luminescent Escherichia coli strains: differentiating the impact of particles and solubilized metals. Anal. Bioanal. Chem. 2010;398:701–716. doi: 10.1007/s00216-010-3962-7. [DOI] [PubMed] [Google Scholar]
- 124.Liu W, Wu Y, Wang C, et al. Impact of silver nanoparticles on human cells: effect on particle size. Nanotoxicology. 2010;4:319–330. doi: 10.3109/17435390.2010.483745. [DOI] [PubMed] [Google Scholar]
- 125.McLamore E, Porterfield DM, Banks MK. Noninvasive self-referencing electrochemical sensors for quantifying real-time biofilm analyte flux. Biotechnol. Bioeng. 2009;102:791–799. doi: 10.1002/bit.22128. [DOI] [PubMed] [Google Scholar]
- 126.McLamore ES, Stensberg MC, Sepúlveda MS, Zhang W, Banks MK, Porterfield DM. A self-referencing microelectrode for real time measurements of silver flux. Sensor. Actuat. B Chem. 2011;153:445–452. [Google Scholar]
- 127.McLamore ES, Zhang W, Porterfield DM, Banks MK. Real time, non-invasive biofilm physiology during chemical toxin exposure. Environ. Sci. Technol. 2010;44:7050–7057. doi: 10.1021/es1012356. [DOI] [PubMed] [Google Scholar]
- 128.McLamore ES, Jaroch D, Chatni MR, Porterfield DM. Self-referencing optrodes for measuring real time oxygen flux in plant roots and photosynthetic microbial mats. Planta. 2010;232:1087–1099. doi: 10.1007/s00425-010-1234-6. [DOI] [PubMed] [Google Scholar]
- 129.Sanchez B, Ochoa-Acuna H, Porterfield D, Sepúlveda M. Oxygen flux as an indicator of physiological stress in fathead minnow (Pimephales promelas) embryos: a real-time bio-monitoring system of water quality. Environ. Sci. Technol. 2008;42:7010–7017. doi: 10.1021/es702879t. [DOI] [PubMed] [Google Scholar]
- 130.Huang T, Nallathamby PD, Xu XHN. Photostable single-molecule nanoparticle optical biosensors for real-time sensing of single cytokine molecules and their binding reactions. J. Am. Chem. Soc. 2008;130:17095–17105. doi: 10.1021/ja8068853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mock JJ, Barbic M, Smith DR, Schultz DA, Schultz S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002;116:6755–6759. [Google Scholar]
- 132.Nallathamby PD, Lee KJ, Xu X-HN. Design of stable and uniform single nanoparticle photonics for in vivo dynamics imaging of nano environments of zebrafish embryonic fluids. ACS Nano. 2008;2:1371–1380. doi: 10.1021/nn800048x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Browning LM, Lee KJ, Huang T, Nallathamy PD, Lowman JE, Xu XHN. Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale. 2009;1:138–152. doi: 10.1039/b9nr00053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nallathamby PD, Xu X-HN. Study of cytotoxic and therapeutic effects of stable and purified silver nanoparticles on tumor cells. Nanoscale. 2010;2:942–952. doi: 10.1039/c0nr00080a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Petty JT, Zheng J, Hud NV, Dickson RM. DNA-templated Ag nanocluster formation. J. Am. Chem. Soc. 2004;126:5207–5212. doi: 10.1021/ja031931o. [DOI] [PubMed] [Google Scholar]
- 136.Yu J, Patel SA, Dickson RM. In vitro and intracellular production of peptide-encapsulated fluorescent silver nanoclusters. Angew. Chem. Int. Ed. 2007;46:2028–2030. doi: 10.1002/anie.200123456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu J, Choi S, Richards CI, Antoku Y, Dickson RM. Live cell surface labeling with fluorescent Ag nanocluster conjugates. Photochem. Photobiol. 2008;84:1435–1439. doi: 10.1111/j.1751-1097.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu J, Choi S, Dickson RM. Shuttle-based fluorogenic silver-cluster biolabels. Angew. Chem. Int. Ed. 2009;48:318–320. doi: 10.1002/anie.200804137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Antoku Y, Hotta J-I, Mizuno H, Dickson RM, Hofkens J, Vosch T. Transfection of living HeLa cells with fluorescent poly-cytosine encapsulated Ag nanoclusters. Photochem. Photobiol. Sci. 2010;9:716–721. doi: 10.1039/c0pp00015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu W, Zhou T, Berliner A, Banerjee P, Zhou SQ. Smart core-shell hybrid nanogels with Ag nanoparticle core for cancer cell imaging and gel shell for pH-regulated drug delivery. Chem. Mater. 2010;22:1966–1976. [Google Scholar]
- 141.Zhang J, Fu Y, Li G, Zhao RY, Lakowicz JR. Detection of CXCR4 receptors on cell surface using a fluorescent metal nanoshell. J. Biomed. Opt. 2011;16:016011. doi: 10.1117/1.3528623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tong L, Wei Q, Wei A, Cheng JX. Gold nanorods as contrast agents for biological imaging: surface conjugation, two-photon luminescence, and photothermal effects. Photochem. Photobiol. 2009;85:21–32. doi: 10.1111/j.1751-1097.2008.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gunn JM, Ewald M, Dantus M. Polarization and phase control of remote surface-plasmon-mediated two-photon-induced emission and waveguiding. Nano Lett. 2006;6:2804–2809. doi: 10.1021/nl0619150. [DOI] [PubMed] [Google Scholar]
- 144.Patel SA, Richards CI, Hsiang JC, Dickson RM. Water-soluble Ag nanoclusters exhibit strong two-photon-induced fluorescence. J. Am. Chem. Soc. 2008;130:11602–11603. doi: 10.1021/ja804710r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang J, Gu H, Shao H, Devlin E, Papaefthymiou GC, Ying JY. Bifunctional Fe3O4–Ag heterodimer nanoparticles for two-photon fluorescence imaging and magnetic manipulation. Adv. Mater. 2008;20:4403–4407. [Google Scholar]
- 146.Liu TM, Tai SP, Yu CH, et al. Measuring plasmon-resonance enhanced third-harmonic chi(3) of Ag nanoparticles. Appl. Phys. Lett. 2006;89:043122. [Google Scholar]
- 147.Zhang J, Fu Y, Lakowicz JR. Single cell fluorescence imaging using metal plasmon-coupled probe. Bioconjugate Chem. 2007;18:800–805. doi: 10.1021/bc0603384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang J, Fu Y, Liang D, Nowaczyk K, Zhao RY, Lakowicz JR. Single-cell fluorescence imaging using metal plasmon-coupled probe 2, single-molecule counting on lifetime image. Nano Lett. 2008;8:1179–1186. doi: 10.1021/nl080093z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y, Lee K, Irudayaraj J. Silver nanosphere SERS probes for sensitive identification of pathogens. J. Phys. Chem. C. 2010;114:16122–16128. [Google Scholar]
- 150.Preciado-Flores S, Wheeler DA, Tran TM, et al. SERS spectroscopy and SERS imaging of Shewanella oneidensis using silver nanoparticles and nanowires. Chem. Comm. 2011;47:4129–4131. doi: 10.1039/c0cc05517d. [DOI] [PubMed] [Google Scholar]
- 151.Hu Q, Tay LL, Noestheden M, Pezacki JP. Mammalian cell surface imaging with nitrile-functionalized nanoprobes: biophysical characterization of aggregation and polarization anisotropy in SERS imaging. J. Am. Chem. Soc. 2006;129:14–15. doi: 10.1021/ja0670005. ▪ Ag NPs functionalized with a Raman-active cyano (-CN) group were targeted to the surface of HeLa cells. Receptor-induced NP clustering could be detected by SERS imaging.
- 152.Kennedy DC, Duguay DR, Tay LL, Richeson DS, Pezacki JP. SERS detection and boron delivery to cancer cells using carborane labelled nanoparticles. Chem. Comm. 2009;44:6750–6752. doi: 10.1039/b916561d. [DOI] [PubMed] [Google Scholar]
- 153.Kennedy DC, McKay CS, Tay LL, Rouleau Y, Pezacki JP. Carbon-bonded silver nanoparticles: alkyne-functionalized ligands for SERS imaging of mammalian cells. Chem. Comm. 2011;47:3156–3158. doi: 10.1039/c0cc05331g. [DOI] [PubMed] [Google Scholar]
- 154.Kennedy DC, Tay LL, Lyn RK, et al. Nanoscale aggregation of cellular β2-Adrenergic receptors measured by plasmonic interactions of functionalized nanoparticles. ACS Nano. 2009;3:2329–2339. doi: 10.1021/nn900488u. [DOI] [PubMed] [Google Scholar]
- 155.Homan K, Shah J, Gomez S, et al. Combined ultrasound and photoacoustic imaging of pancreatic cancer using nanocage contrast agents. Proc. SPIE. 2009;7177:71771M. [Google Scholar]
- 156.Liu H, Wang H, Guo R, et al. Size-controlled synthesis of dendrimer-stabilized silver nanoparticles for x-ray computed tomography imaging applications. Polymer Chem. 2010;1:1677–1683. [Google Scholar]
- 157.Chrastina A, Schnitzer JE. Iodine-125 radiolabeling of silver nanoparticles for in vivo SPECT imaging. Int. J. Nanomedicine. 2010;5:653–659. doi: 10.2147/IJN.S11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patents
- 201.Holladay RJ, Christensen H, Moeller WD. US09946834 2004
- 202.Sawafta R, Haik Y, Hitchcock W, Kuturu V, Ciubotaru I, Lee YS. US11671675 2008
Websites
- 301.Woodrow Wilson International Center for Scholars (WWICS) Project on emerging nanotechnologies. 2011 www.nanotechproject.org/inventories/consumer/ana lysis_draft/
- 302.ATSDR . Agency for Toxic Substances and Disease Registry. Atlanta, GA: 1990. Toxicological profile for silver. 7440–22–4 www.atsdr.cdc.gov/toxprofiles/tp146.html. [PubMed] [Google Scholar]