Abstract
Background
Thiazide-type diuretics are associated with an increased incidence of diabetes as compared to other anti-hypertension medications. In this study we determined long-term cardiovascular disease (CVD) consequences of incident diuretic-associated diabetes compared to the effects of incident diabetes associated with calcium channel and ACE inhibitor use.
Methods and Results
22,418 participants from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial with baseline diabetes, incident diabetes (7.5% with chlorthalidone, 5.6% with amlodipine, and 4.3% with lisinopril), or no diabetes at 2 years of in-trial follow-up were followed for a mean total of 6.9 years (2.9 years in-trial and 4 additional years post-trial through the use of national data bases). The primary outcome was CVD mortality (death due to coronary heart disease [CHD], stroke, heart failure, or other CVD). Among other outcomes were all-cause mortality, non-CVD mortality, and CHD (nonfatal myocardial infarction/fatal CHD). Participants on chlorthalidone with incident diabetes versus no diabetes had consistently lower, non-significant risk for CVD mortality (hazard ratio [HR] 1.04, 95% confidence interval (CI 0.74–1.47), all-cause mortality (HR 1.04, 95% CI 0.82–1.30), and non-CVD mortality (HR 1.05, 95% CI 0.77–1.42) than participants with incident diabetes on amlodipine or lisinopril (HR’s 1.22–1.53). Participants with incident diabetes had elevated CHD risk compared to those with no diabetes (HR 1.46, 95% CI 1.09–1.96) but those on chlorthalidone had significantly lower risk than those on lisinopril (HR 1.18 versus 2.57, p for interaction = 0.04).
Conclusions
Our findings suggest that thiazide-related incident diabetes has less adverse long-term CVD impact than incident diabetes that develops on other antihypertensive medications.
Keywords: diabetes mellitus, diuretics, cardiovascular diseases, mortality, ALLHAT
INTRODUCTION
In placebo-controlled and active-controlled clinical trials with an average follow-up of up to 5 years, diuretic therapy for the treatment of hypertension (HTN) has been associated with more favorable clinical cardiovascular outcomes than other HTN medications.1 Based on decades of rigorously conducted clinical trials, diuretic therapy has been endorsed as first-line therapy.2 Despite these benefits, the long-term use of diuretics (>5 years) is still questioned, because they are associated with potentially unfavorable metabolic consequences, especially increased risk of incident diabetes mellitus (DM).3 In the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) the risk of incident DM at four years follow-up was 15–30% higher with the thiazide-like diuretic chlorthalidone (11%) versus the calcium blocker amlodipine (9.3%), or the ACE inhibitor lisinopril (7.8%).4 Any deleterious impact of incident DM worsens with time, suggesting that there could be an attenuation of the medium-term salutary effects of diuretic therapy on cardiovascular disease (CVD) over longer periods of follow-up.5
Here we examine long-term CVD mortality and morbidity, total mortality, and end stage renal disease, in individuals who during ALLHAT were classified as having DM at baseline, incident DM (based on 2-year glucose levels), or no DM at baseline or 2 years. Participants were passively followed for 4 years after trial completion (mean total follow up 8.9 years; 6.9 years after the 2-year diabetes determination). We hypothesize that the effects of incident DM on CVD and renal outcomes is less with chlorthalidone than with amlodipine or lisinopril, as was found in the in-trial period.4
METHODS
Details of the ALLHAT design and results have been published.4 The in-trial period lasted from 1994 through 2002. All participants signed informed consent upon study entry. After trial completion, the post-trial follow-up of participants through 2006 was accomplished using national databases (see below). The IRB of The University of Texas Health Science Center approved the long-term follow-up study.
ALLHAT Participants and Laboratory Testing
Eligibility criteria for ALLHAT have been previously published.4 The dose of each step 1 blinded medication (chlorthalidone, amlodipine, and lisinopril) was titrated to achieve a target blood pressure <140/90 mmHg. If the blood pressure could not be controlled using the maximum dose of step 1 medication, open-label step 2 and step 3 medications (atenolol, reserpine, clonidine, and hydralazine) were added. Other drugs, including low doses of open-label step 1 drug classes, were permitted if clinically indicated or blood pressure was not controlled. Potassium supplementation was mandated when a local recheck confirmed potassium level <3.2 mEq/L and was encouraged for levels consistently <3.5 mEq/L. After initial titration visits, participants were seen routinely every 3 months during the first year of follow-up and every 4 months throughout the rest of the active phase of the trial.
Baseline central laboratory test results for glucose, lipid, creatinine, and potassium levels were obtained; a sample obtained after >8 hours without food was considered a fasting sample. At years 2, 4, and 6, glucose levels were evaluated again. Serum potassium and creatinine levels were measured at 1 month and at years 1, 2, 4, and 6.
Cohorts for Analysis
Baseline DM was defined as a physician diagnosis or a baseline fasting glucose (FG) ≥126 mg/dl or non-fasting glucose ≥200 mg/dl. Participants with no history of DM with either a FG <126 mg/dl or non-fasting glucose <100 mg/dl were classified as non-diabetic at baseline. Participants with non-fasting glucose 100–199 mg/dl could not be classified, and were excluded. Diagnostic evidence confirming diabetic history was not systematically sought. Participants who were non-diabetic at baseline were further classified as incident DM (follow-up FG ≥126 or follow-up non-fasting glucose ≥200 mg/dl) or no DM (follow-up FG<126 or follow-up non-fasting glucose <100 mg/dl) based on glucose testing at year 2. We chose the more conservative criterion of 100–199 mg/dl for non-fasting glucose levels (and not 126–199 mg/dl) so as to exclude diabetic participants with non-fasting glucose levels of 100–125 mg/dl whose glucose levels were obtained less than 8 hours after eating and did not reach the >126 mg/dl criterion. The associations of baseline DM and incident DM compared with no DM with the risks of primary and secondary outcomes were evaluated based on DM status at 2 years because: (1) this was the first FG available during follow up; (2) more complete glucose data were available at 2 years than at 4 years or 6 years, and (3) there was more follow-up time for events. Average follow-up time was 4.9 years in-trial and 8.9 years including the extended follow-up (6.9 years post-2-year incident DM determination).
A fourth arm in the ALLHAT study, randomized to doxazosin, was terminated early due to a nearly two-fold higher risk of incident heart failure and a low probability of reaching a statistically significant difference in the primary endpoint.4, 6 Due to differences in follow-up time, comparisons of the impact of incident DM on CVD outcomes between doxazosin and chlorthalidone are not reported.
Extended Follow-up End Point Definition and Determination
For the post-trial period, data are not available on treatments, blood pressure levels, outpatient morbidity or laboratory values.
Mortality endpoints
Mortality data were available for the entire cohort during both in-trial and post-trial periods, except for Canadian participants (due to lack of access to databases). During the trial, most causes of death were determined by the investigator. Additional in-trial and all post-trial all-cause and cause-specific mortality were ascertained from the National Death Index (NDI) using social security number, name, sex, and date of birth as matching criteria (see below).
CVD mortality (death due to coronary heart disease [CHD], stroke, heart failure (HF), or other CVD) was designated a priori as the primary endpoint of the extended follow-up. Total mortality and its components, including CHD death, were pre-specified and assessed as secondary outcomes.
A death identified through passive surveillance was defined as a possible match through NDI or Social Security that was verified at the coordinating center after receipt of a death certificate from the state. Death certificates were used only for verification of participant identity. Causes of death (ICD-10 coding) were obtained from NDI Plus and collapsed into the categories used in these analyses. NDIPlus (fact of death plus cause) were initally provided under the ICD-9 revision; for deaths occurring in 1999 forward this was changed to ICD-10 revision. To continue with the original conversion scheme of ICD-9 to ALLHAT category, the World Health Organization Two-way Translator for the 9th and 10th Revisions was used to convert ICD-10 codes to ICD-9 codes.7
Secondary endpoints
Morbidity data for hospitalized events were available for both in-trial and post-trial periods. During the in-trial period events were ascertained by the investigator and confirmed by the Coordinating Center Endpoints Department based on discharge summaries. Nonfatal events were also ascertained from Centers for Medicare and Medicaid Services (CMS [formerly HCFA]) and the United States Renal Data System (USRDS). During the post-trial period, nonfatal events (except renal events) were ascertained from CMS only for participants with valid Medicare or Social Security numbers who were not enrolled from Veterans Affairs (VA) sites (69% of all non-Canadian participants). Data on renal events were obtained from the USRDS; which includes VA participants. Due to the lack of access to databases, Canadian and VA participants were not included in any combined morbidity/mortality analyses (except ESRD analysis for VA individuals). Lack of access to a VA database involved administrative issues relating to informed consent for post-trial follow-upspecific to VA patients.
The following fatal/non-fatal outcomes were pre-specified as secondary endpoints: Total CVD (mortality or hospitalized non-fatal myocardial infarction [MI]), stroke [fatal or non-fatal hospitalized], or HF [fatal or non-fatal hospitalized]), CHD (mortality or hospitalized non-fatal MI), stroke (fatal or non-fatal hospitalized), and ESRD.
Analysis
Contingency tables and z-tests were used to compare baseline characteristics of participants assigned to amlodipine or lisinopril versus chlorthalidone within baseline DM group, and to compare glucose values and DM incidence during follow-up. Evaluations of the effect of baseline and incident DM compared with no DM on the risks for the primary and secondary outcomes subsequent to 2 years were performed using Cox regression, by treatment groups and for all treatment groups combined, adjusted for baseline age, gender, race, BMI, cigarette smoking, atherosclerotic CVD, LVH, baseline systolic blood pressure (SBP), baseline diastolic blood pressure (DBP), HDL-cholesterol, and LDL-cholesterol. Comparisons of the hazard ratios for baseline DM and incident DM compared with no DM for each treatment group were evaluated using treatment x diabetes status interaction terms in Cox regressions; comparisons were also done for incident DM compared with baseline DM. The Cox proportional hazards regression model assumption was examined by using loglog plots and testing a treatment x time (time-dependent) interaction term.
In addition, among those with no DM at baseline, the occurrence of outcomes before and after 2 years was examined by treatment group and by DM status at 2 years (no DM, incident DM, unknown status [death before year 2 follow-up or glucose level unavailable after baseline]) using contingency table analysis (chi-square or Fisher’s exact test) to examine if there were any treatment differences over the entire follow-up period. These diabetes status subgroups are subject to confounding, as they are based on a post-randomization measurement.
Given the many multivariate, subgroup, and interaction analyses performed, statistical significance at the .05 level should be interpreted with caution.
RESULTS
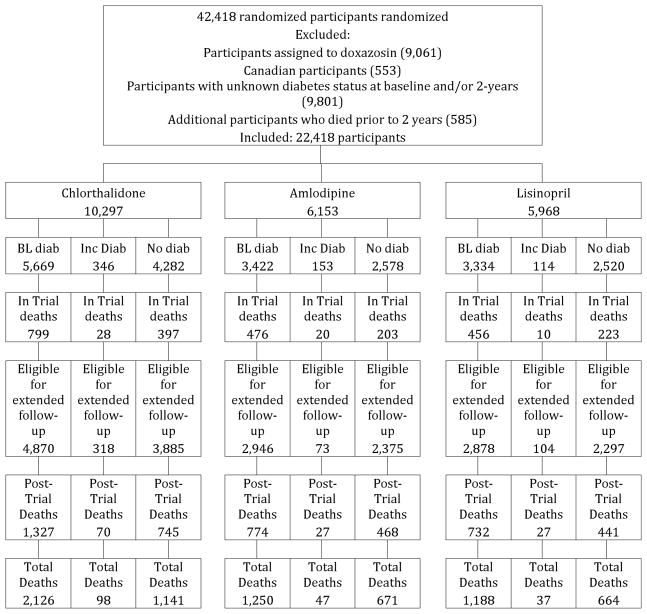
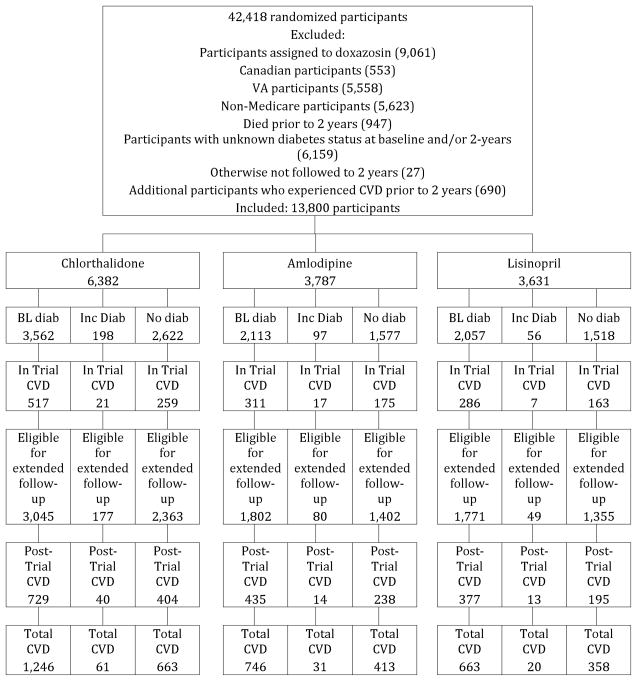
For analyses of mortality endpoints, 20,000 participants from 42,418 randomized participants were excluded who were assigned to doxazosin (9,061), were Canadian (553), , who died prior to 2 years (585), or whose baseline and/or 2-year DM status could not be determined (9,801). Of the 9,801 excluded due to unknown diabetes status, 7,105 were due to missing glucose values, and 2,696 were due to nonfasting glucose values between 100 and 199 ml/dL. The remaining 22,418 participants assigned to chlorthalidone, amlodipine, or lisinopril were classified according to baseline and 2-year DM status (Figure 1a). For analyses of fatal/nonfatal total CVD, an additional 8618 participants were excluded because we lacked the data or they experienced a CVD event prior to 2 years (Figure 1b). Analyses of other events included varying numbers of participants, depending on how many participants experienced those events prior to 2 years.
Figure 1.
Figure 1a. CONSORT Diagram for All-Cause Mortality & Renal Events – Amlodipine, Lisinopril, and Chlorthalidone
Figure 1b. CONSORT Diagram for CVD – Amlodipine, Lisinopril, and Chlorthalidone
Baseline Characteristics (Table 1)
Table 1.
Baseline Characteristics of ALLHAT Participants by Diabetes Status at Year 2 of Follow-up and by Treatment Group - Chlorthalidone, Amlodipine, and Lisinopril (All-Cause Mortality Cohort)
| Chlorthalidone | Amlodipine | Lisinopril | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diab BL | IDM | No Diab | Diab BL | IDM | No Diab | Diab BL | IDM | No Diab | Diab BL | IDM | No Diab | |
| N | 5669 | 346 | 4282 | 3422 | 153 | 2578 | 3334 | 114 | 2520 | 12,425 | 613 | 9380 |
| Age, y-mean(sd) | 66.4(7.3) | 66.1(7.4) | 66.7(7.5)* | 66.3(7.3) | 65.4(7.0) | 66.9(7.5)† | 66.5(7.4) | 66.2(7.5) | 66.7(7.5) | 66.4(7.3) | 65.9(7.3) | 66.8(7.5)† |
| Women-n(%) | 2844(50.2) | 115(33.2) | 1838(42.9)† | 1694(49.5) | 67(43.8) | 1121(43.5)† | 1628(48.8) | 34(29.8) | 1041(41.3)† | 6166(49.6) | 215(35.2) | 4000(42.6)† |
| Black-n(%) | 2211(39.0) | 106(30.6) | 1202(28.1)† | 1345(39.3) | 48(31.4) | 749(29.1)† | 1310(39.3) | 32(28.1) | 728(28.9)† | 4866(39.2) | 186(30.3) | 2679(28.6)† |
| Current smoker-n(%) | 756(13.3) | 91(26.3) | 1149(26.8)† | 457(13.4) | 40(26.1) | 659(25.6)† | 457(13.7) | 26(22.8) | 652(25.9)† | 1670(13.4) | 157(25.6) | 2460(26.2)† |
| CHD-n(%) | 1140(20.3) | 116(33.7) | 1267(29.8)† | 615(18.2) | 43(28.1) | 750(29.3)† | 646(19.5) | 35(30.7) | 725(29.1)† | 2401(19.5) | 194(31.8) | 2742(29.5)† |
| ASCVD§-n(%) | 2027(35.8) | 219(63.3) | 2617(61.1)† | 1160(33.9) | 99(64.7) | 1564(60.7) | 1202(36.1) | 74(64.9) | 1535(60.9)† | 4389(35.3) | 392(64.0) | 5716(60.9)† |
| LVH|| by ECG-n(%) | 500(10.5) | 35(11.3) | 538(13.7) | 305(10.7)† | 18(12.7) | 355(15.1) | 285(10.3)† | 16(15.5) | 360(15.7) | 1090(10.5)† | 69 (12.4) | 1253(14.6)† |
| Blood pressure, mmHG, mean(sd) | ||||||||||||
| SBP | 146.4(15.6) | 147.3(15.5) | 145.6(15.7)* | 146.4(15.6) | 148.5(15.3) | 145.6(16.0)* | 146.7(15.1) | 147.8(15.1) | 145.4(15.7)† | 146.5(15.4) | 147.7(15.4) | 145.5(15.8)† |
| DBP | 83.0(9.9) | 84.3(10.7) | 84.5(10.0)† | 82.7(10.1) | 85.2(9.4) | 84.4(10.1)† | 83.0(9.9) | 86.0(9.6) | 84.4(10.0)† | 82.9(10.0) | 84.7(10.2) | 84.5(10.0)† |
| Body mass index, kg/m2– mean(sd) | 31.1(6.4) | 31.1(6.3) | 28.6(5.6)† | 31.3(6.2) | 29.8(5.4) | 28.6(5.6)† | 31.2(6.2) | 29.8(5.2) | 28.6(5.8)† | 31.2(6.3) | 30.5(5.9) | 28.6(5.7)† |
| Cholesterol, mg/dL-mean(sd) | ||||||||||||
| Total | 215.6(45.8) | 215.4(39.3) | 216.8(41.2) | 216.8(45.3) | 217.6(37.5) | 215.3(42.2) | 215.2(44.4) | 216.0(42.4) | 215.2(39.7) | 215.8(45.2) | 216.0(39.4) | 216.0(41.1) |
| HDL | 44.8(13.2) | 43.0(13.6) | 48.3(15.8)† | 45.2(14.0) | 42.0(12.0) | 48.7(14.9)† | 44.6(13.0) | 42.4(11.6) | 47.7(15.2)† | 44.8(13.4) | 42.6(12.9) | 48.3(15.4)† |
| LDL | 134.2(38.3) | 136.0(34.6) | 137.5(35.9)† | 134.8(37.7) | 137.8(33.0) | 136.2(36.2)† | 134.0(36.6) | 136.6(36.0) | 137.4(35.6) | 134.3(37.7) | 136.6(34.4) | 137.1(35.9)† |
| Fasting glucose, mg/dL-mean(sd) | 169.1(70.0) | 105.4(12.7) | 91.8(10.2)† | 167.2(67.2) | 105.8(12.4) | 91.9(10.5)† | 168.3(65.3) | 105.5(13.3) | 92.6(10.8)† | 168.4(67.8) | 105.5(12.7) | 92.0(10.5)† |
Diab = diabetes, BL = baseline, IDM = incident diabetes
p<.05 comparing diabetes subgroups
p<.01 comparing diabetes subgroups
Atherosclerotic cardiovascular disease
Left ventricular hypertrophy: tall R wave on ECG with or without ST segment depression or T wave inversion
Of 22,418 randomized participants, 46% were women, 81% were ≥60 years (mean 66±7.5 years), 35% were Black and 19% were Hispanic, and 55% had a history of type 2 DM. Table 1 shows baseline characteristics for the mortality cohort by treatment groups within diabetes strata. Compared to participants with no DM at baseline or at 2 years, participants with DM at baseline were younger, had higher SBP and lower DBP, higher BMI, and lower HDL and LDL cholesterol; they were more likely to be female or Black, and less likely to be current smokers, have a history of CHD or atherosclerotic CVD, or have baseline LVH. Participants with incident DM at 2 years were younger and had higher SBP, higher BMI, and lower HDL-cholesterol compared with participants with no DM at baseline or at 2 years; they were less likely to be female. Similar findings were found in the cohorts used for total CVD morbidity analysis (Appendix Table 1).
Incident DM
Table 2 depicts incident diabetes in the mortality cohort. During follow-up, mean glucose levels increased from baseline in all treatment groups for those who did not have baseline DM. 7.5% of participants assigned to chlorthalidone met the fasting or non-fasting glucose criteria for incident DM at 2 years, compared with 5.6% assigned to amlodipine (p=.002) and 4.3% assigned to lisinopril (p<.001). Between years 2 and 6, incident DM developed at similar rates in those treated with amlodipine, lisinopril, and chlorthalidone (1.1%, and 1.0%, and +0.9%, respectively).
Table 2.
Incident Diabetes During the In-Trial Period – Chlorthalidone, Amlodipine, and Lisinopril – Mortality Cohort (Glucose values in mg/dl)
| Chlorthalidone | Amlodipine* | Lisinopril* | |
|---|---|---|---|
| Fasting + nonfasting glucose criteria† | |||
| 2 Years | |||
| N | 4,628 | 2,731 | 2,634 |
| Mean(sd) glucose (fasting+nonfasting), mg/dl | 98.7(28.3) | 95.9(23.5), p<.001 | 94.1(19.5), p<.001 |
| New diabetes, n(%)-fasting+nonfasting† | 346(7.5%) | 153(5.6%), p=.002 | 114(4.3%), p<.001 |
| 4 Years | |||
| N | 3,503 | 2,097 | 1,982 |
| Mean(sd) glucose (fasting+nonfasting), mg/dl | 103.6(29.8) | 101.4(31.5), p=.008 | 98.9(20.4), p<.001 |
| New diabetes, n(%)-fasting+nonfasting† | 268(7.7%) | 126(6.0%), p=.02 | 97(4.9%), p<.001 |
| 6 Years | |||
| N | 1,037 | 628 | 620 |
| Mean(sd) glucose (fasting+nonfasting), mg/dl | 106.4(28.2) | 103.5(26.5), p=.04 | 103.6(30.5), p=.06 |
| New diabetes, n(%)-fasting+nonfasting† | 87(8.4%) | 42(6.7%), p=.21 | 33(5.3%), p=.02 |
| Ever | |||
| N(fasting + nonfasting) | 4,628 | 2,731 | 2,634 |
| New diabetes, n(%)-fasting + nonfasting† | 588(12.7%) | 259(9.5%), p<.001 | 209(7.9%), p<.001 |
| Fasting glucose criteria only† | |||
| 2 Years | |||
| N | 3,386 fasting | 1,974 fasting | 1,904 fasting |
| Mean(sd) fasting glucose, mg/dl | 101.4(26.1) | 98.3(22.0), p<.001 | 96.8(19.7), p<.001 |
| New diabetes, n(%)-fasting only† | 314(9.3%) | 139(7.0%), p=.005 | 107(5.6%), p<.001 |
| Fasting change from baseline, mean(sd, N), mg/dl | +8.4(24.5, 2,993) | +5.4(20.3, 1,737), p<.001 | +3.5(18.9, 1,693), p<.001 |
| Fasting change ≥+10mg/dl - n(%) | 1,054(35.1%) | 506(29.1%), p<.001 | 434(25.6%), p<.001 |
| 4 Years | |||
| N | 2,337 fasting | 1,392 fasting | 1,289 fasting |
| Mean(sd) fasting glucose, mg/dl | 103.9(29.3) | 101.2(27.0), p=.005 | 99.0(18.4), p<.001 |
| New diabetes , n(%)-fasting only† | 251(10.7%) | 113(8.1%), p=.009 | 90(7.0%), p<.001 |
| Fasting change from baseline–mean(sd, N), mg/dl | +10.6(27.2, 2,096) | +8.5(25.6, 1,247), p=.03 | +6.3(17.6, 1,156), p<.001 |
| Fasting change ≥+10mg/dl – n(%) | 852(40.7) | 440(35.3), p=.002 | 381(33.0%), p<.001 |
| 6 Years | |||
| N | 601 fasting | 363 fasting | 346 fasting |
| Mean(sd) fasting glucose, mg/dl | 106.0(26.4) | 105.2(27.4), p=.67 | 103.3(23.4), p=.12 |
| New diabetes, n(%)-fasting only† | 79(13.1%) | 40(11.0%), p=.33 | 31(9.0%), p=.053 |
| Fasting change from baseline–mean(sd, N), mg/dl | +12.0(24.3, 551) | +12.1(27.4, 327), p=.93 | +9.2(20.3, 315), p=.08 |
| Fasting change ≥+10mg/dl, n(%) | 251(45.6%) | 130(39.8%), p=.09 | 119(37.8%), p=.03 |
| Ever | |||
| N(fasting) | 3,297 | 1,928 | 1,876 |
| New diabetes, n(%)–fasting only† | 490(14.9%) | 216(11.2%), p<.001 | 186(9.9%), p<.001 |
Comparisons are with the chlorthalidone group
Diabetes: fasting glucose 126+ or nonfasting glucose 200+ mg/dl
Outcome Results (Table 3; Appendix Figures 1 and 2)
Table 3.
Hazard Ratios and Outcomes by Antihypertensive Treatment Group and Diabetic Status (defined at 2 years) – Chlorthalidone, Amlodipine, Lisinopril – adjustments are over all treatment groups combined
| Total Number of Events/Participants | Unadjusted 8-Year Risk per 100 Population (SE) [Adjusted rates in brackets] |
Adjusted HR (95% CI)* Compared with No Diabetes at Baseline or at Year 2 p value | Adjusted HR (95% CI)* Comparing Inc Diab with Diab BL p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No Diab | Inc diab | Diab BL | No Diab | Inc diab | Diab BL | Inc diab | Diab BL | ||
| CVD mortality | |||||||||
| Total | 1,063/9,380 | 86/613 | 1,986/12,425 | 11.3(0.4) [8.7] |
13.8(1.5) [10.5] |
16.9(0.4) [15.5] |
1.21(0.94–1.55) P=.13 |
1.85(1.69–2.02) P<.001 |
0.66(0.52–0.85) 0=.001 |
| Chlorthalidone | 479/4,282 | 42/346 | 934/5,669 | 11.3(0.5) [8.9] |
11.6(1.8) [9.2] |
17.6(0.6) [15.8] |
1.04(0.74–1.47) P=.82 |
1.86(1.63–2.12) P<.001 |
0.57(0.40–0.80) P=.001 |
| Amlodipine | 305/2,578 | 26/153 | 551/3,422 | 11.6(0.7) [8.9] |
17.1(3.2) [13.4] |
16.6(0.7) [15.0] |
1.53(0.96–2.45) P=.07 |
1.74(1.47–2.07) P<.001 |
0.91(0.57–1.46) P=.70 |
| Lisinopril | 279/2,520 | 18/114 | 501/3,334 | 10.9(0.7) [8.3] |
16.5(3.7) [10.9] |
16.1(0.7) [15.2] |
1.33(0.76–2.34) P=.31 |
1.90(1.59–2.26) P<.001 |
0.69(0.39–1.20) P=.19 |
| Non-CVD mortality | |||||||||
| Total | 1,354/9,380 | 92/613 | 2,398/12,425 | 14.6(0.4) [11.8] |
15.1(1.6) [13.3] |
20.4(0.4) [19.9] |
1.14(0.90–1.43) P=27 |
1.77(1.64–1.92) P<.001 |
0.66(0.52–0.83) P<.001 |
| Chlorthalidone | 634/4,282 | 55/346 | 1,109/5,669 | 14.9(0.6) [12.0] |
15.3(2.1) [12.6] |
20.8(0.6) [19.1] |
1.05(0.77–1.42) P=76 |
1.66(1.47–1.86) P<.001 |
0.66(0.49–0.90) P=.007 |
| Amlodipine | 347/2,578 | 18/153 | 652/3,422 | 13.7(0.7) [11.3] |
12.6(2.9) [13.6] |
20.3(0.8) [20.7] |
1.22(0.74–1.99) P=.43 |
1.93(1.65–2.26) P<.001 |
0.63(0.39–1.04) P=.07 |
| Lisinopril | 373/2,520 | 19/114 | 637/3,334 | 15.0(0.8) [11.5] |
18.0(4.1) [14.4] |
19.9(0.8) [20.1] |
1.27(0.77–2.11) P=.35 |
1.84(1.57–2.14) P<.001 |
0.69(0.42–1.14) P=.14 |
| All-Cause Mortality | |||||||||
| Total | 2,477/9,380 | 182/613 | 4,564/12,425 | 24.8(0.5) [20.0] |
27.4(1.8) [23.0] |
35.0(0.4) [33.6] |
1.17(0.99–1.38) P=.07 |
1. 83(1.73–1.94) P<.001 |
0.65(0.55–0.77) P<.001 |
| Chlorthalidone | 1,142/4,282 | 98/346 | 2,126/5,669 | 25.1(0.7) [20.5] |
25.3(2.4) [21.1] |
35.9(0.7) [33.4] |
1.04(0.82–1.30) P=76 |
1.78(1.63–1.93) P<.001 |
0.60(0.48–0.75) P<.001 |
| Amlodipine | 671/2,578 | 47/153 | 1,250/3,422 | 24.4(0.9) [19.9] |
29.3(3.7) [26.7] |
34.6(0.9) [33.8] |
1.40(1.01–1.95) P=.045 |
1.86(1.66–2.08) P<.001 |
0.77(0.55–1.07) P=.12 |
| Lisinopril | 664/2,520 | 37/114 | 1,188/3,334 | 24.6(0.9) [19.3] |
31.5(4.6) [24.0] |
33.9(0.9) [33.5] |
1.28(0.88–1.86) P=.19 |
1.91(1.70–2.14) P<.001 |
0.66(0.45–0.96) P=.03 |
| Non-fatal MI + Fatal CHD | |||||||||
| Total | 655/5,803 | 63/359 | 1,230/8,003 | 12.6(0.5) [10.0] |
20.2(2.4) [14.3] |
18.2(0.5) [16.8] |
1.46(1.09–1.96) P=.01 |
1.75(1.56–1.95) P<.001 |
0.85(0.63–1.13) P=.26 |
| Chlorthalidone | 292/2,656 | 29/203 | 592/3,670 | 12.2(0.7) [9.9] |
16.5(3.0) [11.4] |
19.3(0.8) [17.2] |
1.18(0.77–1.81) P=.45 |
1.84(1.56–2.18) P<.001 |
0.65(0.42–0.99) P=.047 |
| Amlodipine | 193/1,600 | 19/98 | 347/2,205 | 13.3(1.0) [10.6] |
24.2(5.1) [14.4] |
18.3(1.0) [16.7] |
1.39(0.79–2.45) P=.26 |
1.63(1.32–2.01) P<.001 |
0.89(0.50–1.58) P=69 |
| Lisinopril | 170/1,547 | 15/58 | 291/2,128 | 12.4(1.0) [9.5] |
26.2(6.1) [22.7] |
16.2(0.9) [15.6] |
2.57(1.45–4.54) P=.001 |
1.68(1.34–2.10) P<.001 |
1.50(0.85–2.64) P=.17 |
| CVD | |||||||||
| Total | 1,434/5,717 | 112/351 | 2,655/7,732 | 27.2(0.7) [23.0] |
35.7(2.9) [28.3] |
39.2(0.6) [37.2] |
1.28(1.03–1.59) P=.03 |
1.81(1.68–1.96) P<.001 |
0.72(0.58–0.89) P=.003 |
| Chlorthalidone | 663/2,622 | 61/198 | 1,246/3,562 | 27.4(1.0) [22.8] |
34.2(3.8) [26.6] |
40.4(1.0) [37.5] |
1.22(0.91–1.64) P=.19 |
1.84(1.65–2.06) P<.001 |
0.67(0.50–0.90) P=.007 |
| Amlodipine | 413/1,577 | 31/97 | 746/2,113 | 27.9(1.3) [24.3] |
35.3(5.3) [28.9] |
38.9(1.2) [36.6] |
1.22(0.81–1.86) P=.34 |
1.69(1.46–1.95) P<.001 |
0.78(0.51–1.18) P=.24 |
| Lisinopril | 358/1,518 | 20/56 | 663/2,057 | 26.0(1.3) [22.0] |
40.9(7.4) [31.4] |
37.2(1.2) [37.2] |
1.52(0.92–2.51) P=.11 |
1.90(1.63–2.21) P<.001 |
0.81(0.49–1.34) P=.41 |
| Stroke | |||||||||
| Total | 458/5,875 | 38/360 | 888/8,051 | 9.1(0.4) [7.7] |
12.9(2.1) [9.3] |
13.9(0.5) [13.0] |
1.23(0.83–1.81) P=.31 |
1.74(1.52–2.00) P<.001 |
0.71(0.48–1.05) P=.09 |
| Chlorthalidone | 210/2,695 | 17/203 | 406/3,698 | 8.9(0.6) [7.0] |
9.5(2.3) [6.4] |
13.8(0.7) [12.6] |
0.91(0.49–1.67) P=76 |
1.85(1.51–2.26) P<.001 |
0.49(0.27–0.91) P=.02 |
| Amlodipine | 128/1,623 | 15/97 | 243/2,201 | 9.5(0.8) [8.0] |
18.1(4.5) [15.1] |
13.6(0.9) [12.2] |
1.95(1.04–3.65) P=04 |
1.62(1.25–2.10) P<.001 |
1.33(0.71–2.49) P=.37 |
| Lisinopril | 120/1,557 | 6/60 | 239/2,152 | 8.8(0.8) [8.0] |
15.7(6.2) [10.0] |
14.4(0.9) [13.7] |
1.27(0.52–3.11) P=.61 |
1.71(1.33–2.22) P<.001 |
0.69(0.28–1.69) P=.41 |
| Heart failure | |||||||||
| Total | 498/5,879 | 38/361 | 1,136/8,012 | 9.8(0.5) [7.9] |
13.0(2.2) [9.3] |
18.0(0.5) [15.4] |
1.24(0.86–1.80) P=.26 |
2.14(1.89–2.42) P<.001 |
0.58(0.40–0.84) P=.004 |
| Chlorthalidone | 230/2,700 | 27/206 | 518/3,691 | 9.7(0.7) [7.7] |
15.9(3.1) [10.2] |
18.3(0.8) [14.5] |
1.46(0.93–2.29) P=.10 |
2.03(1.69–2.43) P<.001 |
0.70(0.45–1.09) P=.11 |
| Amlodipine | 148/1,618 | 6/98 | 324/2,181 | 10.3(0.9) [8.1] |
7.3(2.9) [5.8] |
17.8(1.0) [15.1] |
0.71(0.29–1.75) P=46 |
2.07(1.63–2.61) P<.001 |
0.36(0.15–0.88) P=.03 |
| Lisinopril | 120/1,561 | 5/57 | 294/2,140 | 9.4(0.9) [7.5] |
10.9(4.7) [8.7] |
17.6(1.0) [17.3] |
1.17(0.43–3.20) P=.75 |
2.54(1.97–3.27) P<.001 |
0.47(0.17–1.27) P=.14 |
| ESRD | |||||||||
| Total | 92/9,380 | 10/613 | 580/12,425 | 0.7(0.1) [0.6] |
1.1(0.4) [1.0] |
3.8(0.2) [2.7] |
1.60(0.80–3.19) P=.18 |
4.48(3.47–5.77) P<.001 |
0.37(0.19–0.72) P=.003 |
| Chlorthalidone | 40/4,282 | 8/346 | 256/5,669 | 0.7(0.1) [0.6] |
1.6(0.7) [1.3] |
3.9(0.3) [2.5] |
2.13(0.94–4.82) P=07 |
4.28(2.92–6.26) P<.001 |
0.53(0.25–1.14) P=.10 |
| Amlodipine | 28/2,578 | 0/153 | 163/3,422 | 0.6(0.2) [0.6] |
--- (no cases) | 4.0(0.4) [2.9] |
--- | 4.52(2.84–7.21) P<.001 |
--- |
| Lisinopril | 24/2,520 | 2/114 | 161/3,334 | 0.7(0.2) [0.5] |
0.9(0.9) [1.1] |
3.7(0.4) [2.3] |
2.09(0.49–9.00) P=32 |
4.68(2.84–7.72) P<.001 |
0.46(0.11–1.89) P=.28 |
Diab = diabetes, BL = baseline, IDM = incident diabetes
Adjusted for age, gender, race, cigarette smoking, ASCVD, LVH, baseline SBP, baseline DBP, baseline BMI, HDL-cholesterol, and LDL-cholesterol at ALLHAT baseline.
Nonfatal MI + CHD death: HR for IDM compared with NDB is significantly different in the lisinopril group compared with the HR in the chlorthalidone group.
Nonfatal MI + CHD death: HR for IDM compared with DBL is significantly different in the lisinopril group compared with the HR in the chlorthalidone group.
For all treatment groups combined and in each treatment group, participants with baseline DM had significantly higher risks for all outcomes than those with no DM at baseline or at 2 years. For all treatment groups combined, participants with incident DM at 2 years were also at higher risk than participants with no DM for nonfatal MI plus CHD death (Hazard ratio [HR] 1.46, 95% CI 1.09 – 1.96) and total CVD (1.28, 95% CI 1.03 – 1.59). For most endpoints, participants with incident DM had an intermediate risk between participants with no DM and baseline DM.
For the primary outcome of CVD mortality, there were no significant differences in HRs between treatment groups in those who developed incident DM versus those with no DM (p=0.24, 0.46 for interaction, see Appendix, Figure 1). However, given the number of events observed, there was less than 20% power to detect an interaction effect (ratio of hazard ratios) on the order of what was actually seen (1.28–1.41).8 There were differences, however, in HRs across treatment groups for several secondary outcomes. For fatal/nonfatal CHD (see Appendix, Figure 1), the comparison of incident DM with baseline DM within the lisinopril group (HR 1.50 (95% CI 0.85–2.64)) was significantly different from the same comparison in the chlorthalidone group (0.65 (95% CI 0.42–0.99)) (p=0.01 for interaction). Participants assigned to lisinopril who developed incident DM had significantly elevated risk for fatal MI/nonfatal CHD relative to those with no DM (2.57, 95% CI 1.45–4.54), whereas the HR for chlorthalidone was not significant (1.18 [95% CI 0.77–1.81] [p=.04 for interaction]). Participants treated with amlodipine who developed incident DM had significantly elevated risks for all-cause mortality (1.40 [1.01–1.95]) and stroke (1.95 [1.04–3.65]) relative to those who had no DM, but interaction terms were not significant (versus chlorthalidone and lisinopril). Among the other incident DM/no DM comparisons, HRs were lowest in the chlorthalidone group for all-cause mortality, CVD mortality, non-CVD mortality, and stroke, but the differences were not statistically significant (non-significant interaction terms). The proportional hazard assumption was tested for all treatment and diabetes status hazard ratios and there were no violations.
Mortality and morbidity outcomes among those with no DM at baseline or after 2 years follow-up, those with incident DM at year 2, or those whose status at year 2 was unknown were calculated (Appendix Tables 2a and 2b). Notwithstanding the limitations of analyses using subgroups based on a post-randomization definition,9 neither amlodipine nor lisinopril was significantly superior to chlorthalidone in preventing occurrence of outcomes before and after 2 years, regardless of DM status. The one exception was heart failure among those with incident DM. However, there were few events (n=4) before 2 years and post 2 years there were no significant differences among the treatment groups for this outcome (p=.17).
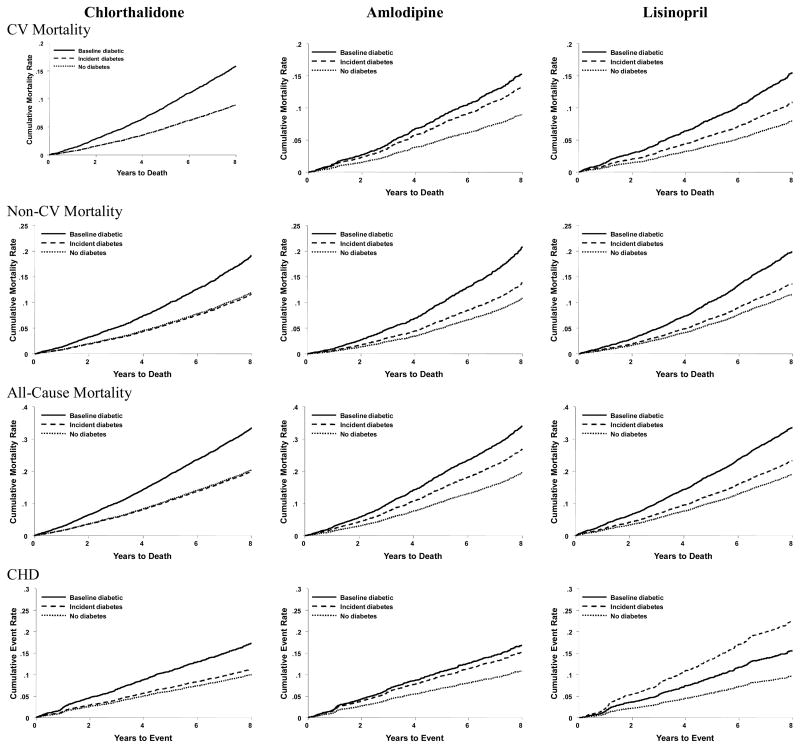
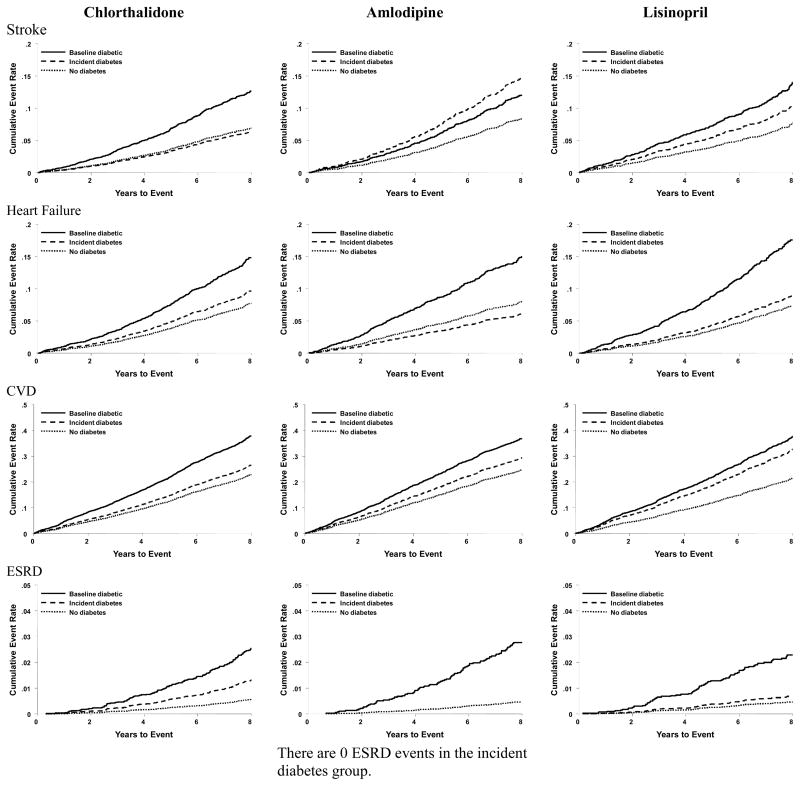
Adjusted Kaplan-Meier plots of outcomes by DM status are shown in Figure 2. Participants randomized to chlorthalidone who developed incident DM had outcomes similar to chlorthalidone participants with no DM; amlodipine and lisinopril participants who developed incident DM had outcomes intermediate to those with baseline DM. These patterns were especially noticeable for CVD mortality, non-CVD mortality, and fatal/nonfatal CHD. They were less so for fatal/nonfatal total CVD.
Figure 2.
Adjusted Kaplan-Meier Plots
DISCUSSION
In this extension of ALLHAT, analysis by assigned primary antihypertensive medication showed that for participants who developed incident DM versus those with no DM, chlorthalidone had the lowest hazard ratio for CVD mortality. Those treated with chlorthalidone also had the lowest hazard ratio for total mortality, non-CV mortality, CHD, and stroke. For no outcome did incident DM have a significant adverse effect on risk in the chlorthalidone group, despite the much larger sample size compared to the amlodipine and lisinopril groups.
Our findings are consistent with those of our previous ALLHAT report10 and 2 long term post hoc analyses of trials that examined the impact of diuretic-associated DM on CVD mortality. In the 14-year follow-up of the Systolic Hypertension in Elderly Program (SHEP) study, DM that developed during chlorthalidone therapy did not have a statistically significant impact on CVD mortality (RR 1.04 [0.75, 1.46]; similar to our own result) or on all cause mortality (1.15 [0.92, 1.43]) in contrast to DM that developed while assigned to placebo.11 In a second study, a 14-year follow up of 686 middle-aged adults with hypertension treated with diuretics, incident DM did not have a significant effect on CVD mortality, whereas baseline DM did.12
With regard to fatal/nonfatal stroke, HF and ESRD, randomization to chlorthalidone was associated with a mixed picture. The hazard ratio for stroke was lowest, while for ESRD it was highest, compared to amlodipine or lisinopril. There were fewer ESRD outcomes as compared to stroke outcomes, introducing uncertainty to this comparison and mitigating the absolute impact associated with chlorthalidone for ESRD outcomes. With regard to HF, incident DM in association with chlorthalidone use was associated with a higher risk compared to amlodipine or lisinopril, but interaction terms were not significant, suggesting that the DM-associated risks of HF in the three groups were not statistically different.
Other findings should be noted. First, in almost all instances, participants with baseline DM had a worse outcome than either those with no DM or those with incident DM. Within diabetes subgroups, there were (in general) no differences in hazard ratios whether the participant was treated with chlorthalidone, amlodipine, or lisinopril. Second, our starting point for follow-up was based on incident DM detected at 2 years of in-trial treatment. A higher percent of the incident DM associated with chlorthalidone use was captured during this time period than that associated with amlodipine or lisinopril use. This makes our estimates of the long-term impact of incident DM associated with amlodipine or lisinopril conservative relative to those reported for chlorthalidone.
Third, among chlorthalidone participants, CVD mortality and fatal and non-fatal CHD associated with incident DM closely resembled those with no DM, whereas among amlodipine or lisinopril participants outcomes resembled participants with baseline DM. These findings suggest differences in the nature of amlodipine- and lisinopril-associated DM compared to chlorthalidone-associated DM. We previously hypothesized10 that depletion of potassium plays a role in the excess incidence of DM that is associated with chlorthalidone use (above and beyond the DM that is expected with having hypertension). Depletion of potassium inhibits insulin release from pancreatic beta cells; potassium restoration reverses this effect.13–15 In contrast, DM associated with the use of amlodipine or lisinopril is likely due to progression of insulin resistance that is present despite the glucose-neutral or glucose-protective effects of these agents.
Compared to all other studies, ours has the largest number of participants with incident DM and compares results of randomization to chlorthalidone against other HTN medications. This point is important, since people with HTN are prone to develop DM irrespective of treatment type. Assuming that calcium blockers are metabolically neutral, comparison of DM incidence rates at 2 years of in-trial follow-up for chlorthalidone (7.5%) versus amlodipine (5.6%) suggests that 74.7% of the new-onset DM in the chlorthalidone group is not “caused by” the diuretic, i.e. only about ¼ of cases was diuretic-induced.
This study has several limitations. First, the randomized treatments were discontinued at the conclusion of the trial in 2002. The administrative data used to document extended follow-up do not provide information about medication use during the period of passive observation. Second, glucose levels fluctuate and repeated testing is recommended to confirm glycemic status. This was not done in ALLHAT, a common practice in large trials owing to the inconvenience and cost of re-calling large numbers of participants. Misclassification of DM status would, however, have occurred randomly across treatment groups and should not have impacted our findings. Third, our results present data stratified by post-randomization characteristics (incident DM and no DM), which is the only way of studying the post-randomization effects of randomly assigned drugs, and many participants were excluded. Therefore, the treatment groups may no longer have been balanced on observed and unobserved variables.9 Finally, many participants from the trial were not included in post-trial follow-up due to lack of access to relevant databases.
In conclusion, our findings suggest that thiazide-associated incident DM is associated with lower CVD mortality and morbidity relative to amlodipine- or lisinopril-associated incident DM over an average of 6.9 years. . Therefore concerns regarding potential adverse diabetic effects associated with thiazide-type diuretic therapy should not inhibit its use. In this regard, a recent pooled analysis of 5 statin studies16 showed that incident DM was more common in people treated with intensive-dose therapy versus moderate-dose therapy. Nonetheless, the benefits of reduced cholesterol were deemed to outweigh any possible deleterious effects of incident diabetes on CVD outcomes. Similarly, thiazide-like diuretics have been shown to be highly effective for preventing CVD outcomes through decades of rigorously controlled clinical trials.
Supplementary Material
WHAT IS KNOWN
The use of chlorthalidone therapy for the treatment of hypertension has been questioned due to its associated increased risk of elevated glucose levels and diabetes as compared to other blood pressure lowering medications.
In a prior report from ALLHAT, the increased risk of diabetes with chlorthalidone therapy versus ACE inhibitor or calcium channel blocker therapy was modest and did not translate into more cardiovascular disease as compared to diabetes during an average of 2.9 years of follow-up for post-development of new-onset diabetes during the first 2 years of ALLHAT.
WHAT THIS ARTICLE ADDS
ALLHAT participant follow-up was extended up to an average of 6.9 years through querying national data bases and confirms our prior findings.
While chlorthalidone therapy is associated with an increased risk of diabetes compared to other blood pressure lowering medications, diabetes associated with chlorthalidone use has lower long-term cardiovascular disease risk than diabetes associated with ACE inhibitor or calcium channel blocker use.
The risk of diabetes associated with chlorthalidone should not deter clinicians from using it long term.
Acknowledgments
This study was supported by contract N01-HC-35130 from the National Heart, Lung, and Blood Institute, (NHLBI). The ALLHAT Investigators acknowledge contributions of study medications supplied by Pfizer Inc (amlodipine), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
Footnotes
Clinical trial registration: www.clinicaltrials.gov NCT00000542
Disclosures: None of the authors reports a conflict of interest with regards to the contents of this paper. The authors have the following financial disclosures:
Joshua I. Barzilay has held a financial interest in Pfizer and Schering Plough.
Henry R. Black has consulted for Bayer Corporation, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, Daiichi Sankyo, Gilead, Merck, Mitsubishi, Novartis, Pfizer, Servier, and Takeda; has received honoraria from Bristol-Myers Squibb and has held a financial interest in Boehringer Ingelheim.
William C. Cushman has consulted for Daiichi Sankyo, Novartis, Noven, Sanofi Aventis, Takeda, and Theravance; has received honoraria from Bristol-Myers Squibb, Daiichi Sankyo, Novartis, and Sanofi Aventis; and has had research grants/contracts with GlaxoSmithKline, Merck, and Novartis.
Barry R. Davis has consulted for Amgen and Takeda.
Karen L. Margolis has received research grants from Bristol-Myers Squibb.
Dr. Oparil has consulted for Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Forest Laboratories, Forest Pharmaceuticals, NicOx, Novartis, Omron Healthcare, Pfizer, and Schering Plough; has received research grants from Amgen, Daiichi Sankyo, Gilead, Merck, and Takeda.
Mary Ann Sweeney has received research grants from GlaxoSmithKline, Merck, and Novartis.
Nathan D. Wong has received research grants/contracts from Forest Pharmaceuticals, and Novartis.
Jackson T. Wright, Jr. has consulted for CVRx, Daiichi Sankyo, Novartis, and Sanofi Aventis; and has received honoraria from Sanofi Aventis.
Jeffrey A. Cutler, Paula T. Einhorn, Charles E. Ford, Jamaluddin Moloo, Sara L. Pressel, Linda B. Piller, Debra L. Simmons, and Paul K. Whelton have no financial interests to report.
References
- 1.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC-7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Carter BL, Einhorn PT, Brands M, He J, Cutler JA, Whelton PK, Bakris GL, Brancati FL, Cushman WC, Oparil S, Wright JT, Jr Working Group from the National Heart, Lung, and Blood Institute. Thiazide-induced dysglycemia: call for research from a working group from the National Heart, Lung, and Blood Institute. Hypertension. 2008;52:30–36. doi: 10.1161/HYPERTENSIONAHA.108.114389. [DOI] [PubMed] [Google Scholar]
- 4.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. . Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 5.Barzilay JI, Cutler JA, Davis BR. Antihypertensive medications and risk of diabetes mellitus. Curr Opin Nephrol Hypertens. 2007;16:256–260. doi: 10.1097/MNH.0b013e328057dea2. [DOI] [PubMed] [Google Scholar]
- 6.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003;42:239–246. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Two-way translator for the Ninth and Tenth Revisions. Geneva: 1997. [Google Scholar]
- 8.Peterson B, George SL. Sample size requirements and length of study for testing interaction in a 2 x k factorial design when time-to-failure is the outcome [corrected. Control Clin Trials. 1993;14:511–522. doi: 10.1016/0197-2456(93)90031-8. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA. 1991;266:93–98. [PubMed] [Google Scholar]
- 10.Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, Margolis KL, Ong ST, Sadler LS, Summerson J ALLHAT Collaborative Research Group. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2006;166:2191–2201. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 11.Kostis JB, Wilson AC, Freudenberger RS, Cosgrove NM, Pressel SL, Davis BR SHEP Collaborative Research Group. Long-term effect of diuretic-based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005;95:29–35. doi: 10.1016/j.amjcard.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 12.Samuelsson O, Hedner T, Berglund G, Persson B, Andersson OK, Wilhelmsen L. Diabetes mellitus in treated hypertension: incidence, predictive factors and the impact of non-selective beta-blockers and thiazide diuretics during 15 years treatment of middle-aged hypertensive men in the Primary Prevention Trial Goteborg, Sweden. J Hum Hypertens. 1994;8:257–263. [PubMed] [Google Scholar]
- 13.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48:219–224. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 14.Shafi T, Appel LJ, Miller ER, 3rd, Klag MJ, Parekh RS. Changes in serum potassium mediate thiazide-induced diabetes. Hypertension. 2008;52:1022–1029. doi: 10.1161/HYPERTENSIONAHA.108.119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutler JA. Thiazide-associated glucose abnormalities: prognosis, etiology, and prevention: is potassium balance the key? Hypertension. 2006;48:198–200. doi: 10.1161/01.HYP.0000231339.51310.b3. [DOI] [PubMed] [Google Scholar]
- 16.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.