Abstract
Rationale: Drug-resistant tuberculosis transmission in hospitals threatens staff and patient health. Surgical face masks used by patients with tuberculosis (TB) are believed to reduce transmission but have not been rigorously tested.
Objectives: We sought to quantify the efficacy of surgical face masks when worn by patients with multidrug-resistant TB (MDR-TB).
Methods: Over 3 months, 17 patients with pulmonary MDR-TB occupied an MDR-TB ward in South Africa and wore face masks on alternate days. Ward air was exhausted to two identical chambers, each housing 90 pathogen-free guinea pigs that breathed ward air either when patients wore surgical face masks (intervention group) or when patients did not wear masks (control group). Efficacy was based on differences in guinea pig infections in each chamber.
Measurements and Main Results: Sixty-nine of 90 control guinea pigs (76.6%; 95% confidence interval [CI], 68–85%) became infected, compared with 36 of 90 intervention guinea pigs (40%; 95% CI, 31–51%), representing a 56% (95% CI, 33–70.5%) decreased risk of TB transmission when patients used masks.
Conclusions: Surgical face masks on patients with MDR-TB significantly reduced transmission and offer an adjunct measure for reducing TB transmission from infectious patients.
Keywords: infection control, multidrug-resistant tuberculosis, transmission, surgical mask
At a Glance Commentary
Scientific Knowledge on the Subject
Tuberculosis (TB) transmission in hospitals, clinics, and other congregate settings threatens the health of health care workers and patients and remains an important obstacle to achieving TB control. In TB-endemic, resource-poor settings, infection control strategies such as mechanical/natural ventilation, air disinfection, personal respiratory protection, and patient isolation may be difficult to access, implement, or sustain. Simple face masks worn by patients, however, may offer an additional approach to reduce TB transmission. Unfortunately, there is no prior field evidence to support their efficacy in actual clinical settings.
What This Study Adds to the Field
This study suggests that surgical masks worn by infectious patients with multidrug-resistant TB on a hospital ward reduced transmission from these patients by 56% compared with periods when masks were not worn.
Of an estimated 9 million new cases of tuberculosis (TB) in 2008 globally (1), 440,000 were multidrug-resistant TB (MDR-TB) (2), and more than half of those are believed to have occurred in previously untreated patients, the result of transmission of already drug-resistant strains (2). Recent reports of infection with highly drug-resistant strains of Mycobacterium tuberculosis among patients and health care workers illustrate the dire consequences of nosocomial transmission, especially in areas where HIV is endemic (3, 4). Although once believed to arise primarily from unsupervised or erratic treatment of drug-susceptible TB, MDR-TB and extensively drug-resistant TB (XDR-TB) are now known to be transmissible and have emerged as important threats to patients who enter hospitals for drug-susceptible TB (reinfection) or other illnesses, to the clinical staff caring for them, and to occupants of other congregate settings, such as correctional facilities and shelters. One study in Russia found that hospitalization, rather than treatment nonadherence, conferred a sixfold greater relative risk for the acquisition of MDR-TB by patients (5), whereas another study in Latvia revealed that previous hospitalization was a highly significant risk factor for MDR-TB (odds ratio, 18.33; P < 0.002) (6). In addition, health care workers in diverse settings have been shown to be disproportionately exposed to and infected with drug-susceptible and drug-resistant TB (4, 7). TB among health care workers erodes the already limited supply of hospital personnel in many resource-constrained settings, both directly through illness and indirectly through fear of working in such high-risk environments (8, 9).
Although the most important means of TB infection control involves prompt and effective treatment of the patient, it is contingent on timely and accurate diagnosis. Passive case finding, delayed/incorrect diagnosis, and initiation of ineffective treatment regimens when unsuspected drug resistance exists all contribute to prolonged infectiousness. Measures to reduce the spread of TB (e.g., administrative controls, environmental or engineering controls, and personal respiratory protection) within hospitals, clinics, and other congregate settings reduce transmission by modifying the environment in which TB transmission could occur, mitigating the risk from unsuspected cases and protecting persons at risk of acquiring TB within those environments. Many of these measures, however, have not been tested rigorously in field or clinical trials.
A common low-technology approach to source control, intended to reduce the emission of transmissible aerosols by infectious patients, is the wearing of surgical face masks. Here, we make a clear distinction between surgical masks designed to protect an operating field or health care environment from microbial contamination, versus respirators, a special category of masks specifically designed to protect the wearer. The barrier properties of surgical masks and respirators are different, reflecting their different functions (10, 11). The tight face seal of a respirator, for example, although essential for protecting the wearer, is not required for surgical masks designed simply to impact relatively large respiratory droplets. It is easy to demonstrate that even a well-fitted respirator is unlikely to contain the considerable force of cough, and that face-seal leak occurs. This leakage, in theory, defeats one of the main advantages of respirators over masks. With this rationale, and because respirators are much more costly than surgical masks, respirators were not evaluated in this study.
According to Barry, surgical-type masks for infectious source control were first used during the 1918 to 1919 influenza pandemic (12). Like effective treatment or isolation, surgical masks may provide effective source control, but also like treatment and isolation, some suspicion for TB is required. However, the threshold for asking a coughing patient to use a mask may be considerably lower, especially in resource-limited settings. Short-term use of surgical masks may be optimal, for example, in an emergency room or clinic for a coughing patient awaiting a clinical diagnosis, isolation, and presumptive treatment for TB.
The handful of published studies on surgical face masks in the literature have all focused on masks worn by at-risk individuals to protect them from acquiring infection, rather than on masks for source control to protect the environment (10, 13, 14). The rationale for using a simple surgical mask on infectious patients is based on the theory of droplet nuclei, wherein masks may stop some portion of larger respiratory droplets expelled from the mouth or nose by breathing, coughing, sneezing, or talking from becoming infectious droplet nuclei (15, 16). Given the lack of data on this potentially useful, commonly recommended, and low-risk method of TB infectious source control (17), we conducted a prospective clinical study to evaluate the efficacy of surgical face masks worn by patients with MDR-TB in reducing transmission. Some of the results of this study have been previously reported in the form of an abstract (18).
Methods
Airborne Infections Research Facility
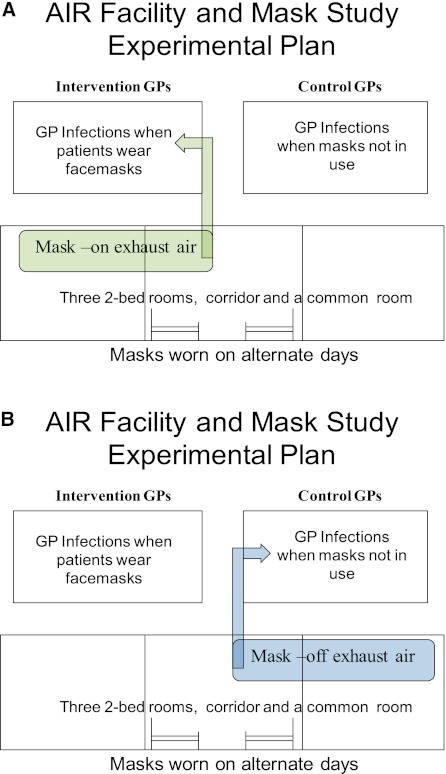
This study was performed at the Airborne Infections Research (AIR) Facility (in eMalahleni, formerly known as Witbank, South Africa). Details regarding the facility have been previously published (19) and can also be found in the online supplement. Briefly, the AIR facility consists of a six-bed inpatient MDR-TB ward from which all exhaust air containing patient-generated infectious droplet nuclei can be delivered entirely to one or the other of two guinea pig exposure chambers (Figure 1). In the current study, guinea pigs in one chamber sampled ward air only when patients wore masks (intervention group), whereas guinea pigs in the other chamber did so only when patients did not wear masks (control). Air from the patient ward was only exhausted to the guinea pig chambers between 7:00 a.m. and 7:00 p.m. On days when a given chamber did not receive ward air, it received a separate supply of high-efficiency particulate air–filtered, conditioned air that bypassed the patient ward. Additional description regarding the pattern and timing of guinea pig exposure is in the online supplement.
Figure 1.
Diagram of AIR facility and exhaust system. Guinea pigs receive and breathe ward air for 12-hour periods on either (A) intervention (masks on), or (B) control (masks off) days. On days when a chamber does not receive ward air, its air comes directly from a central ventilation source (not shown). AIR = airborne infections research. GP = guinea pigs.
Patient Enrollment and Selection
Seventeen patients (eight men, nine women) occupied the AIR Facility ward during the study. They were sequentially selected from among patients newly admitted to the adjacent hospital for MDR-TB treatment initiation in accordance with South African guidelines (20) between May and August 2010. They were approached for enrollment only if they were ambulatory, could tolerate mask wearing for extended periods of time, were coughing, and had no contraindications to mask usage (listed in online supplement). Informed consent was obtained from each patient before enrollment. Clinical and laboratory characteristics of the patient cohort are summarized in Table 1. Patients contributed equal amounts of time under control and intervention conditions to each guinea pig cohort.
TABLE 1.
CLINICAL CHARACTERISTICS OF THE 17 STUDY PATIENTS
| Characteristic | Subjects |
| Male | 8 (47) |
| AFB culture positive | 17 (100) |
| AFB smear positive | 14 (82) |
| Age, yr | 37 (23–59) |
| HIV positive | 11 (65) |
| Length of stay on AIR facility ward, days | 29 (1–69) |
Definition of abbreviations: AFB = acid fast bacilli; AIR = airborne infections research.
Data are presented as No. (%) or average (range).
Study Protocol
On admission, patients were instructed on how to wear the surgical mask over their nose and mouth. Masks could be removed for meals and medications and were not worn during sleep or between the hours of 7:00 p.m. and 7:00 a.m. Mask use occurred on alternate days during the entire study period of 3 months (12 wk) and was monitored by nursing staff through 10 daily unannounced spot checks. Patients were eligible for a small nonmonetary incentive for demonstrating the highest consistency in mask use at the conclusion of the study.
Guinea Pigs and Tuberculin Skin Testing
Outbred male and female, specific pathogen-free, Dunkin-Hartley guinea pigs (National Health Laboratories Services, South Africa; n = 180; 90 per exposure chamber) were used and evaluated for infection using tuberculin skin tests (TST) as previously published (19) and as described in detail in the online supplement. For this study, any TST induration was considered indicative of infection. A detailed explanation of this TST criterion is available in the online supplement. Animals exhibiting a reactive TST were removed from the exposure chambers. As described in the online supplement, 12 additional guinea pigs (not in the AIR Facility) were used as positive and negative controls for responses to purified protein derivative (PPD).
Human Subjects and Animal Ethics Approval
This study was approved by the human studies committees of the South African Medical Research Council, the U.S. Centers for Disease Control and Prevention, the Harvard School of Public Health, and the Brigham and Women's Hospital. Animal care and husbandry was overseen by a licensed laboratory veterinarian, and all protocols were approved by the Animal Use committees of the South African Medical Research Council, the U.S. Centers for Disease Control and Prevention, and Harvard Medical School.
Estimation of Infectious Quanta Produced by Patients during First Month of Study
The number of infectious particles generated by the patients on the ward (q) during the first month of the study with and without the use of surgical masks was estimated by the Wells-Riley method (21, 22). This estimate of patient infectiousness permitted comparison with other published values for q among TB patient cohorts in prior studies. Using known or measured values for the numbers of source patients generating infectious particles, the number of susceptible guinea pigs, and ventilation in the facility, calculations were only made for the first group of six patients during the first month of the study to satisfy the assumptions of the Wells-Riley equation (described elsewhere) (21, 22).
Statistical Analyses
The number of guinea pigs exhibiting TST conversions at each testing time point in each group (intervention vs. control) over the course of the study was compared using Kaplan-Meier analysis. We also compared the cumulative proportions of guinea pigs infected in each group by the end of the study using χ2 test. Statistical analyses were performed using STATA (23), with significance level set at P < 0.05.
Mask Type/Brand
Green ear-loop face masks (Dental Warehouse, Pretoria, South Africa; Figure 2) were freely available on the ward at all times on mask use days. Patients could obtain a new mask anytime the mask they were wearing became soiled, moist, deformed, or otherwise unwearable or uncomfortable.
Figure 2.
Picture of ear-loop face masks used in study.
Results
Guinea Pig Infections
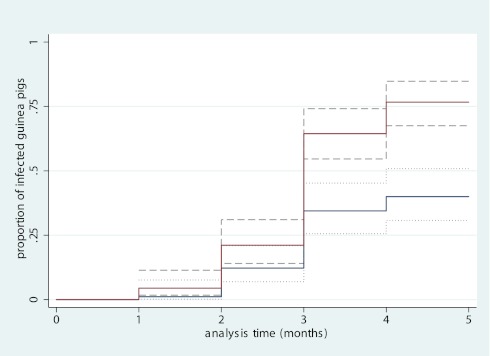
Before any ward air exposure, all guinea pigs tested nonreactive to PPD (TST 0). By the end of the study, there were 36 infections (40%; 95% CI, 31–51%) in the intervention group and 69 infections (76.6%; 95% CI, 68–85%) in the control group. Figure 3 illustrates the risk of TB infection in each guinea pig cohort and reveals a 2.3-fold (95% CI, 1.5–3.4; P < 0.0005) lower risk among guinea pigs exposed to air exhausted from the ward when patients wore face masks. Mask usage provided a 56% (95% CI, 33–71%) risk reduction in transmission. Among the guinea pigs infected with M. tuberculosis H37Rv intranasally (not in the AIR Facility), all developed TST reactions (results not shown), whereas none of the uninfected guinea pigs did.
Figure 3.
Kaplan-Meier graph of guinea pig infections. Intervention group (blue line) represents guinea pigs sampling ward air when masks were worn by patients. Control group (red line) represents guinea pigs sampling ward air when masks were not worn by patients. The dashed lines denote the 95% confidence intervals for each line. X-axis shows timing of each monthly tuberculin skin test. Y-axis shows the cumulative proportion of infected guinea pigs at each testing interval.
Estimation of the Infectious Quanta Generated by Patients during First Month of Study
Using the Wells-Riley mathematical model, the six patients entering the AIR ward together emitted approximately 1,659 infectious quanta per 12-hour sampling period (138 quanta/h) during the first month of the study. When masks were worn, they emitted approximately 408 infectious quanta per 12-hour period (34 quanta/h) during the first month of the study. It is important to note that the values for q in the first month are not necessarily representative of the quanta produced (or magnitude of reduction with masks) in subsequent months of the study. Table 2 summarizes the calculated values for q under control and mask-wearing conditions for the patient cohort that occupied the AIR ward during the first month and also provides values of q for other historic and recent TB patient cohorts.
TABLE 2.
SUMMARY OF CALCULATED VALUES OF THE NUMBER OF INFECTIOUS QUANTA PRODUCED BY PATIENTS ON THE AIRBORNE INFECTIONS RESEARCH WARD WITH AND WITHOUT SURGICAL MASKS, AND REFERENCE VALUES IN OTHER PUBLISHED STUDIES, USING THE WELL-RILEY MATHEMATICAL AIRBORNE INFECTION MODEL (22)
| Number of Patients Used for Calculating q | Patient Characteristics | q/h Emitted on the Ward | Reference |
| 6 | MDR-TB; mix of HIV-infected and HIV-uninfected; no mask use | 138 | Current study |
| 6 | MDR-TB; mix of HIV-infected and HIV-uninfected; masks in use | 34 | Current study |
| 6 | Drug-susceptible TB | 0.62–0.82 | Riley and colleagues, 1959 (24) |
| 5 | Drug-susceptible and drug-resistant TB; some untreated initially | 1.25 | Riley and colleagues, 1962 (25) |
| 1 | Drug-susceptible and MDR-TB; all HIV infected | 8.2 | Escombe and colleagues, 2007 (26) |
Definition of abbreviations: MDR = multidrug resistant; q = number of infectious quanta; TB = tuberculosis.
Mask Use by Patients
During this 12-week study, of which half the days (42 d) were designated as mask-use days for the 12 hours between 7:00 a.m. and 7:00 p.m., patients generally used two to three masks per 12-hour period. Approximately 650 masks were used for the entire study. With few exceptions, patients wore masks for the entire 12-hour mask-wearing period, with removal for meals and medication administration. The nursing spot checks revealed that patients used masks consistently during daytime hours on mask-use days. The most common reasons for mask change in a given day were excessive moisture accumulation within the mask.
Discussion
Using a quantitative biological air sampling and transmission model in which susceptible guinea pigs serving as surrogate hosts were naturally exposed to the aerosols produced by infectious patients with MDR-TB, we found that surgical masks worn by patients reduced the risk of MDR-TB transmission by 56% (2.3-fold). The need for evidence on easy-to-use methods to reduce TB transmission is great, given the infectiousness of patients like the ones in this study. For example, compared with a 2-year TB transmission study by Riley and colleagues done in the 1950s, in which six patients together generated 0.62 to 0.83 quanta/h (24), a second 2-year study by Riley and colleagues done thereafter in which five patients together generated 1.25 quanta/h (25), and more recent transmission studies with patients with TB in Peru in which each patient generated 8.2 quanta/h (26), the patients with MDR-TB occupying the AIR ward during the first month of the current study were presumably more infectious, together producing approximately 138 infectious quanta per hour at baseline.
In high TB prevalence settings, such as South Africa, masks are likely to offer modest levels of protection to health care personnel and other patients exposed to patients with TB. However, it has been well recognized that the risk of TB transmission from unsuspected or unrecognized patients is likely far greater than the risk from known patients with TB, in large part because patients who are not yet diagnosed may more readily emit infectious aerosols than patients under effective treatment (27). Although there have been few comprehensive studies on the exact prevalence of unsuspected TB, a few surveys among general medical wards or emergency departments have revealed that approximately one-third of patients with TB may pass through the early stages of entry into hospital unsuspected (28, 29). In high-burden settings with limited existing infection control resources, masks may be a relatively inexpensive yet effective initial measure through which to limit TB transmission, especially if offered and used short term by all patients presenting with prolonged cough or other symptoms suggestive of TB, for example. More work is needed to understand the economic costs and benefits of mask use relative to other early interventions.
The barrier properties of masks have long been used to reduce contamination of operating fields or clinical environments by respiratory droplets emitted by the wearer. A recent study by Chatterjee and colleagues even demonstrated their ability to effectively reduce transmission of pertussis from patients wearing masks to hospital personnel, when combined with an educational campaign in a tertiary health care center (30). Unlike many other respiratory pathogens, including Bordetella pertussis, however, M. tuberculosis is almost exclusively transmitted through inhalation of aerosolized droplet nuclei into alveolar air spaces rather than through direct proximal mucous membrane contact via larger droplets. Large droplets are more likely to settle and less likely to reach the alveoli to establish TB infection, but before settling, some fraction of large droplets desiccates to become droplet nuclei capable of reaching the alveoli (31–34). Coughing, normal breathing, and sneezing are known to produce particles in both the large droplet and droplet nuclei size range (32, 35, 36). Therefore, the mechanism by which surgical masks limit TB transmission is probably by trapping the larger respiratory droplets that would otherwise evaporate into respirable droplet nuclei. During close person-to-person contact, not simulated in this experiment, masks may also work through additional mechanisms, such as reducing the distance, changing the trajectory, or impacting a proportion of the smaller respirable droplets that are generated by infectious patients.
There are several important factors that must be considered in the interpretation of this study and the use of masks in TB transmission control. Face masks are unlikely to adequately protect those who wear them from acquiring TB infection because they almost always have significant leaks at the mask–skin interface. These face seal leaks are a low-resistance pathway through which aerosolized droplet nuclei may be inhaled (37). For individuals at risk for TB exposure and infection, N95 respirators or their equivalent provide a better face seal and better filtration properties. When worn by patients with TB, on the other hand, we believe that simple surgical masks, rather than the much more expensive N95 respirators, are sufficient to reduce the extent to which patients emit infectious particles. We do not believe that higher-level respirator use by patients with TB is warranted for this purpose due to cost and discomfort (38). Moreover, whereas a better face seal can reduce leakage around the filtration piece of a respirator during inspiration, it is unlikely to substantially resist the air pressure generated by cough, and air is likely to escape around either a surgical mask or respirator, as illustrated by others using real-time optical imaging of air flow patterns during cough (39). The purpose of a surgical mask, like a hand or tissue, is not to contain the cough but to stop the exit of a portion of the larger droplets otherwise destined to become droplet nuclei. As noted, a surgical mask undoubtedly also stops or deflects a portion of smaller respirable particles.
Although mask use by patients in the current study was well tolerated, even for periods up to 12 hours and with interruptions for meals, the results of this study must be considered in the context of several important limitations. First, this study required a moderate level of nursing surveillance to ensure that patients wore their masks for more than 80% of the time during designated times. Second, patients in this study were provided small incentives for demonstrating adherence to mask use. In the absence of incentives, perceived benefits to self, or reminders, real-life mask use may fall short of the use needed to reduce TB transmission to the levels achieved in this study. For these reason, mask use may be more feasible in clinics, waiting rooms, or other triage patient care areas, where supervised use by individual patients who may harbor TB would be of limited duration and where the risk of transmission from patients not yet on treatment may be high. For this study, we enrolled hospitalized patients without respiratory distress. Mask use by more severely ill, hypoxemic, or moribund patients with TB may be less well tolerated; whether such patients are more or less infectious than less sick patients is unknown. In addition, it is unclear whether the effectiveness of the masks was driven by their impact on the aerosols emitted by one, a few, or the whole group of patients. Moreover, different types of masks may exhibit differences in the extent of face leakage or gross filtration of large droplets (40). Finally, greater mask use should also consider the potential stigma associated with mask use as well as the impact mask use has on the desire for unencumbered communication. In many parts of sub-Saharan Africa, TB is often a strong predictor of HIV coinfection, and this otherwise simple intervention may have significant social, economic, and psychological implications. Research to better understand the full implications of mask use on patients as well as societal perceptions of such use is needed.
In summary, our study shows that surgical face masks provide significant, but incomplete, source control when used under conditions in which daytime adherence was monitored and encouraged by modest incentives. Under ordinary clinical conditions without monitoring and incentives, adherence and effectiveness may be lower than what we found. It would undoubtedly be lower at night during sleep, which we did not study. Alternatively, the actual effectiveness could have been higher than we found, since patients removed masks for meals and medications even on mask use days. For those reasons, we believe that surgical masks are optimally used short term, for symptomatic patients in waiting rooms, during transport, and in other temporary situations. In addition, the implementation of mask use on patients for transmission control should as always be just one component of a broader infection control strategy. Although the principles of mask use are applicable to TB control in general, their use may be prioritized in settings in which drug-resistant TB is prevalent and/or other methods to reduce MDR/XDR-TB transmission (e.g., availability of effective treatment regimens for patients, adequate mechanical ventilation, ultraviolet air disinfection, physical separation, personal respiratory protection for health care workers) are scarce. They may also be of use as a source-control measure for other airborne or partially airborne diseases, such as influenza, if supported by further research.
Supplementary Material
Acknowledgments
The authors thank Chandresh Ladva for assistance provided during study preparation. They also thank Dr. Ian Orme and his laboratory for providing PPD reagent. They also thank the clinical and nonclinical staff as well as the patients of the Mpumalanga Provincial MDR-TB Hospital, and the Mpumalanga Ministry of Health, for allowing this study to be carried out.
Footnotes
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201107-1190OC on February 9, 2012
Author Contributions: A.S.D., M.M., K.V., M.F., P.A.J., and E.A.N. designed the study and prepared the protocol. M.M., A.S., K.V., R.M., T.M., W.L., and M.V.D.W. enrolled patients, supervised and performed study procedures, and assisted with data collection. M.P. performed statistical analyses and prepared figures. A.S.D., P.A.J., M.F., M.V.D.W., and E.A.N. worked on data interpretation. A.S.D., M.M., K.V., M.F., P.A.J., A.S., M.V.D.W., and E.A.N. wrote, revised, and edited the manuscript.
Supported by the National Institute for Occupational Safety and Health.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization WHO report 2009 global tuberculosis control: epidemiology, strategy, financing. Geneva, Switzerland: World Health Organization; 2009 [Google Scholar]
- 2.World Health Organization WHO report 2010: multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response [Internet; accessed 2011 Jun 2]. Available from: http://www.Who.Int/tb/publications/2010/en/index.Html [Google Scholar]
- 3.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368:1575–1580 [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell MR, Jarand J, Loveday M, Padayatchi N, Zelnick J, Werner L, Naidoo K, Master I, Osburn G, Kvasnovsky C, et al. High incidence of hospital admissions with multidrug-resistant and extensively drug-resistant tuberculosis among South African health care workers. Ann Intern Med 2010;153:516–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelmanova IY, Keshavjee S, Golubchikova VT, Berezina VI, Strelis AK, Yanova GV, Atwood S, Murray M. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ 2007;85:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nodieva A, Jansone I, Broka L, Pole I, Skenders G, Baumanis V. Recent nosocomial transmission and genotypes of multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2010;14:427–433 [PubMed] [Google Scholar]
- 7.Escombe AR, Huaroto L, Ticona E, Burgos M, Sanchez I, Carrasco L, Farfan E, Flores F, Moore DA. Tuberculosis transmission risk and infection control in a hospital emergency department in Lima, Peru. Int J Tuberc Lung Dis 2010;14:1120–1126 [PubMed] [Google Scholar]
- 8.Padayatchi N, Daftary A, Moodley T, Madansein R, Ramjee A. Case series of the long-term psychosocial impact of drug-resistant tuberculosis in HIV-negative medical doctors. Int J Tuberc Lung Dis 2010;14:960–966 [PubMed] [Google Scholar]
- 9.Harrington M. Health systems failing health workers: time for a change. Int J Tuberc Lung Dis 2010;14:935–936 [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141. [PubMed]
- 11.Brosseau L, Ann RB. N95 respirators and surgical masks [Internet]. c2009 [accessed 2011 Jun 10]. Available from: http://www.cdc.gov/niosh/blog/nsb101409_respirator.html
- 12.Barry JM. The great influenza. The story of the deadliest pandemic in history. New York: Viking Adult; 2004 [Google Scholar]
- 13.Baig AS, Knapp C, Eagan AE, Radonovich LJ., Jr Health care workers’ views about respirator use and features that should be included in the next generation of respirators. Am J Infect Control 2010;38:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maciel EL, Viana MC, Zeitoune RC, Ferreira I, Fregona G, Dietze R. Prevalence and incidence of Mycobacterium tuberculosis infection in nursing students in Vitoria, Espirito Santo. Rev Soc Bras Med Trop 2005;38:469–472 [DOI] [PubMed] [Google Scholar]
- 15.Riley RL. Airborne infection. Am J Med 1974;57:466–475 [DOI] [PubMed] [Google Scholar]
- 16.Wells WF. On air-borne infection: II. Droplets and droplet nuclei. Am J Hyg 1934;20:611–618 [Google Scholar]
- 17.World Health Organization. WHO report 2009: WHO policy on TB infection control in health-care facilities, congregate settings and households [Internet; accessed 2011 May 29]. Available from: http://whqlibdoc.who.int/publications/2009/9789241598323_eng.pdf.
- 18.Dharmadhikari A, Mphahlele M, Stoltz A, Venter K, Mathebula R, Masotla T, Jensen PA, First M, Pagano M, van der Walt M, et al. Surgical face masks reduce multidrug-resistant tuberculosis transmission from patients on a hospital ward [abstract]. Am J Respir Crit Care Med 2011;183:A5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dharmadhikari AS, Basaraba RJ, Van Der Walt ML, Weyer K, Mphahlele M, Venter K, Jensen PA, First MW, Parsons S, McMurray DN, et al. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb) 2011;91:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.South Africa Department of Health: Management of drug-resistant tuberculosis - draft policy guidelines. Pretoria, South Africa: South Africa Department of Health; 2009 [Google Scholar]
- 21.Dharmadhikari A, Nardell E. Transmission of Mycobacterium tuberculosis. : Schaaf H, Zumla A, Tuberculosis: a comprehensive clinical reference. UK: Elsevier; 2009. pp. 8–16 [Google Scholar]
- 22.Riley RL, Nardell EA. Clearing the air. The theory and application of ultraviolet air disinfection. Am Rev Respir Dis 1989;139:1286–1294 [DOI] [PubMed] [Google Scholar]
- 23.StataCorp Stata statistical software: Release 9. College Station, TX: StataCorp LP; 2005 [Google Scholar]
- 24.Riley RL, Mills CC, Nyka W, Weinstock N, Storey PB, Sultan LU, Riley MC, Wells WF. Aerial dissemination of pulmonary tuberculosis: a two-year study of contagion in a tuberculosis ward. Am J Hyg 1959;70:2. [DOI] [PubMed] [Google Scholar]
- 25.Riley RL, Mills CC, O'Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis 1962;85:511–525 [DOI] [PubMed] [Google Scholar]
- 26.Escombe AR, Oeser C, Gilman RH, Navincopa M, Ticona E, Martinez C, Caviedes L, Sheen P, Gonzalez A, Noakes C, et al. The detection of airborne transmission of tuberculosis from HIV-infected patients, using an in vivo air sampling model. Clin Infect Dis 2007;44:1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005;54:1–141 [PubMed] [Google Scholar]
- 28.Moran GJ, McCabe F, Morgan MT, Talan DA. Delayed recognition and infection control for tuberculosis patients in the emergency department. Ann Emerg Med 1995;26:290–295 [DOI] [PubMed] [Google Scholar]
- 29.Willingham FF, Schmitz TL, Contreras M, Kalangi SE, Vivar AM, Caviedes L, Schiantarelli E, Neumann PM, Bern C, Gilman RH. Hospital control and multidrug-resistant pulmonary tuberculosis in female patients, Lima, Peru. Emerg Infect Dis 2001;7:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee A, Plummer S, Heybrock B, Bardon T, Eischen K, Hall M, Lazoritz S. A modified “cover your cough” campaign prevents exposures of employees to pertussis at a children's hospital. Am J Infect Control 2007;35:489–491 [DOI] [PubMed] [Google Scholar]
- 31.Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 2006;16:335–347 [DOI] [PubMed] [Google Scholar]
- 32.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med 1997;10:105–116 [DOI] [PubMed] [Google Scholar]
- 33.Xie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets can move in indoor environments–revisiting the Wells evaporation-falling curve. Indoor Air 2007;17:211–225 [DOI] [PubMed] [Google Scholar]
- 34.Wells WF. Airborne contagion and air hygiene. Cambridge, MA: Harvard University Press; 1955 [Google Scholar]
- 35.Fairchild CI, Stampfer JF. Particle concentration in exhaled breath. Am Ind Hyg Assoc J 1987;48:948–949 [DOI] [PubMed] [Google Scholar]
- 36.Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S, Chao CYH, Li Y, Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci 2009;40:256–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charney W, Fisher J, Ishida C. The inefficiency of surgical masks for protection against droplet nuclei tuberculosis. J Occup Med 1991;33:943–944 [PubMed] [Google Scholar]
- 38.Li Y, Tokura H, Guo YP, Wong AS, Wong T, Chung J, Newton E. Effects of wearing N95 and surgical facemasks on heart rate, thermal stress and subjective sensations. Int Arch Occup Environ Health 2005;78:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang JW, Liebner TJ, Craven BA, Settles GS. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface 2009;6:S727–S736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CC, Willeke K. Aerosol penetration through surgical masks. Am J Infect Control 1992;20:177–184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.