Abstract
Background
Acute myocardial infarction (AMI) may contribute to health status declines including independence loss and physical function decline. Despite the importance of these outcomes for prognosis and quality of life, their incidence and predictors have not been well described.
Methods
We studied 2002 patients with AMI enrolled across 24 sites in the TRIUMPH registry who completed assessments of independence and physical function at the time of AMI and one year later. Independence was evaluated by the EQ-5D (mobility, self care, usual activities), and physical function was assessed with the SF-12 Physical Component Summary (PCS). A decline in ≥1 level on EQ-5D and >5 points in PCS were considered clinically significant changes. Hierarchical, multivariable modified Poisson regression models accounting for within-site variability were used to identify predictors of independence loss and physical function decline.
Results
One year post-AMI, 43.0% of patients experienced health status declines: 12.8% independence loss alone, 15.2% physical function decline alone, and 15.0% both. After adjustment, variables that predicted independence loss included female sex, non-Caucasian race, unmarried status, uninsured status, end-stage renal disease, and depression. Variables that predicted physical function decline were uninsured status, lack of cardiac rehabilitation referral, and absence of pre-AMI angina. Age was not predictive of either outcome after adjustment.
Conclusions
More than 40% of patients experience independence loss or physical function decline one year following AMI. These changes are distinct but can occur simultaneously. While some risk factors are not modifiable, others suggest potential targets for strategies to preserve patients’ health status.
INTRODUCTION
Ten years ago, the Institute of Medicine recommended refocusing the healthcare system to more successfully achieve the provision of patient-centered care (1). From patients’ perspectives, this warrants the preservation and optimization of their health status, symptoms, function, and quality of life (2). Acute myocardial infarction (AMI) represents a common condition that is a harbinger of potential independence loss and physical function decline among survivors (3–6). Despite the importance of these patient-centered outcomes, both in terms of prognosis (7, 8) and quality of life (9), the incidence and predictors of independence loss and physical function decline after AMI have not been well described. Furthermore, although these outcomes are related concepts, the degree to which their predictors and trajectories are similar is unknown.
To address these gaps in knowledge, we sought to assess the incidence of independence loss and physical function decline, separately and together, one year after hospitalization for AMI. We further evaluated potential clinical and sociodemographic risk factors associated with each adverse outcome to identify patients vulnerable to health status declines after AMI.
METHODS
Study Design and Participants
Details of the Translational Research Investigating Underlying disparities in recovery from acute Myocardial infarction: Patients’ Health status (TRIUMPH) registry have been previously described (10). Briefly, TRIUMPH is a large, prospective, multi-center registry of AMI patients from 24 study sites across the United States. Patients were ≥18 years of age and met objective criteria for AMI (biomarker evidence of myocardial injury and clinical features of ischemia), and presented to the enrolling institution within 24 hours of original presentation. Between April 2005 and December 2008, 4340 patients were enrolled out of 31,567 screened. All participants provided written informed consent, and the study protocols were approved by the institutional review board at each participating site.
Patients underwent detailed interviews by trained research personnel within 24 to 72 hours of initial presentation, with additional information obtained by chart abstraction. Data were collected on an array of variables, including sociodemographic characteristics, medical comorbidities, AMI severity, interventions and events during hospitalization, and discharge medications. Angina was assessed with the Seattle Angina Questionnaire (SAQ) (11), a 19-item disease-specific health status measure for patients with coronary artery disease (CAD). A follow-up telephone assessment was attempted on all participants at one year.
The TRIUMPH registry received funding from the National Heart, Lung, and Blood Institute (P50 HL077113) and this study was funded in part by CV Outcomes, Inc., Kansas City, MO. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Analytic Sample
Of the 4340 patients in the TRIUMPH registry, we excluded those who died during the initial hospitalization (n=24), left against medical advice (n=19), or were discharged to hospice or a nursing home (N=64). Because our primary outcomes of interest were independence loss and physical function decline, we excluded patients who were already debilitated, which was defined as EuroQol-5D (EQ-5D) maximum disability in 2 of 3 independence items (n=121) or a Short Form-12 (SF-12) physical component score (PCS) <25 (n=622). After one year, 151 patients were deceased, 154 refused, 42 were too ill to participate in an interview, 266 had incomplete assessments, and 875 were lost to follow-up. Excluding deceased patients, those missing one-year assessments were more likely to be younger (mean age 56.5 vs. 59.3, P<0.001), nonwhite (40.6% vs. 25.7%, P<0.001), and uninsured (26.8% vs. 17.8%, P<0.001). Our final analytic sample included 2002 patients.
Measures of Independence and Physical Function
Measures of independence and physical function were obtained by the EQ-5D (12) and SF-12 (13) surveys, respectively, during the index hospitalization and at 12 months.
The EQ-5D is a two-page survey of physical and mental health that has previously been used in longitudinal analysis of AMI survivors (14). Page one includes 5 questions assessing different dimensions of health (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) and page two consists of a self-reported visual analog scale (VAS) (12). Each question is on page one is answered on a three-point scale (no problems, some problems, extreme problems); prior studies have defined problems in any domain as abnormal (15, 16). The visual analog scale (VAS) is reported on a scale of 0 to 100 with 100 being “best imaginable health state”. Since our interest was in independent functioning, we focused our analyses on the three questions assessing independent living capability: mobility (“I have no problems in walking about”, “I have some problems in walking about”, or “I am confined to bed”), self-care (“I have no problems with self-care”, “I have some problems washing or dressing myself”, or “I am unable to wash or dress myself”), and usual activities (“I have no problems performing my usual activities”, “I have some problems performing my usual activities”, or “I am unable to perform my usual activities”). Based on clinical judgment, we defined “independence loss” as worsening of ≥1 independent capability (for example, changing from “I have some problems washing or dressing myself” to “I am unable to wash or dress myself”) between baseline and one-year follow-up. To investigate the sensitivity of this threshold, we analyzed VAS scores at one year between patients with worsening of ≥1 capability vs. those with no worsening; VAS among patients with any worsening was significantly lower (70.1 vs. 82.8, P<0.001).
The SF-12 is a generic health status measure that has demonstrated reliability and validity in patients with CAD (12). The physical component score (PCS), a summary measure of physical well-being that is calculated from the 12-item questionnaire, was created using norm-based methods that standardize the scores to a mean of 50 and a standard deviation of 10, with higher scores indicating better physical functioning. We defined “physical function decline” as a >5-point decrease in PCS (moderate effect size). This level of decline was shown in a previous study of AMI patients to be associated with worse CAD-related quality of life, depressive symptoms, and mortality, indicating the clinical relevance of this threshold (17).
Statistical Analysis
We divided the population into four groups: independence loss alone, physical function decline alone, both, and neither. Baseline clinical and sociodemographic characteristics were described using mean and standard deviation for continuous variables and percentages for dichotomous variables.
Multivariable modified Poisson regression hierarchical models that accounted for within-site variability were used to identify the factors independently associated with independence loss and physical function decline, respectively. Typical analyses utilize logistic regression to estimate adjusted odds ratios, which are then generally interpreted as relative risks. However, in this study the event being modeled (independence loss or physical function decline) was not rare, in which case odds ratios are poor estimates of relative risks. To address this issue, we estimated adjusted relative risks directly using a modified Poisson regression model with robust error variance (18). Missing baseline covariate data were minimal (12% missing ejection fraction (EF), 5% missing Patient Health Questionnaire (PHQ), 4 covariates missing < 2%) and, hence, were imputed in IVEware (19).
Potential risk factors evaluated in the multivariable model were selected a priori and included sociodemographic characteristics (age, gender, race, marital status, insurance, living alone), medical history (CAD, congestive heart failure (CHF), angina, hypertension (HTN), cerebrovascular accident (CVA), chronic kidney disease (CKD), peripheral arterial disease (PAD), diabetes, chronic lung disease), and details of patients’ AMI presentation and treatment (STEMI, Killip class, revascularization, EF, depression, referral to cardiac rehabilitation).
All tests for statistical significance were two-tailed with an alpha level of 0.05. Statistical analyses were conducted using SAS software, release 9.2 (SAS Institute, Cary, North Carolina) and R version 2.11.1 (20).
RESULTS
Patient Characteristics
Baseline characteristics of the study sample are shown in Table I. The mean age of the population was 59.3 years, 31.8% were female, and 25.7% were nonwhite. At the time of AMI admission, 27.4% had a prior history of CAD, 13.3% had a history of angina, and 26.3% were diabetic. Most were managed invasively, with 94.9% proceeding to cardiac catheterization and 77.2% undergoing revascularization (67.8% PCI, 9.4% CABG).
Table I.
Baseline characteristics by health status declines at one year (N=2002)
| Overall | A | B | C | D | ||
|---|---|---|---|---|---|---|
| Total N= 2002 |
Independence Loss Only N = 257 (12.8%) |
Physical Function Decline Only N = 305 (15.2%) |
Both N = 300 (15.0%) |
Neither N = 1140 (56.9%) |
P* | |
| Demographics | ||||||
| Age (mean ± std. dev.) | 59.3 ± 11.7 | 60.8 ± 12.9 | 59.0±11.3 | 57.9±11.7 | 59.3±11.5 | 0.033 |
| Age distribution (%) | 0.004 | |||||
| <65 | 69.5 | 63.8 | 71.5 | 76.0 | 68.5 | |
| 65–74 | 19.0 | 20.2 | 18.0 | 12.3 | 20.7 | |
| 75–84 | 10.2 | 12.5 | 9.5 | 10.7 | 9.7 | |
| ≥85 | 1.3 | 3.5 | 1.0 | 1.0 | 1.1 | |
| Female (%) | 31.8 | 40.5 | 29.8 | 42.3 | 27.5 | <0.001 |
| Race (%) | <0.001 | |||||
| White | 74.4 | 58.4 | 71.8 | 63.7 | 81.5 | |
| Black | 20.7 | 36.2 | 21.3 | 30.0 | 14.5 | |
| Other | 5.0 | 5.4 | 6.9 | 6.3 | 4.0 | |
| Married (%) | 58.5 | 43.2 | 56.7 | 49.3 | 64.8 | <0.001 |
| Insured (%) | 82.2 | 78.1 | 80.3 | 66.3 | 87.8 | <0.001 |
| Living alone (%) | 22.1 | 28.0 | 20.8 | 20.1 | 21.6 | 0.093 |
| Medical History | ||||||
| Prior coronary artery disease (%) | 27.4 | 33.9 | 28.5 | 28.0 | 25.4 | 0.050 |
| Congestive heart failure (%) | 5.2 | 9.3 | 6.9 | 5.0 | 3.9 | 0.003 |
| Angina (%) | 13.3 | 18.7 | 10.5 | 15.7 | 12.2 | 0.011 |
| Hypertension (%) | 63.3 | 72.4 | 63.9 | 65.7 | 60.5 | 0.003 |
| Cerebrovascular accident (%) | 3.8 | 5.4 | 3.9 | 4.0 | 3.4 | 0.500 |
| Peripheral arterial disease (%) | 3.6 | 6.2 | 3.0 | 3.3 | 3.2 | 0.114 |
| Diabetes (%) | 26.3 | 35.4 | 28.2 | 27.3 | 23.5 | <0.001 |
| Chronic lung disease (%) | 5.5 | 8.6 | 4.6 | 7.0 | 4.7 | 0.054 |
| Presentation Characteristics | ||||||
| STEMI (%) | 45.9 | 35.8 | 47.5 | 43.7 | 48.2 | 0.003 |
| Killip class ≥2 (%) | 8.5 | 12.2 | 9.6 | 8.8 | 7.2 | 0.066 |
| Revascularization (%) | <0.001 | |||||
| None | 22.8 | 33.1 | 24.9 | 27.7 | 18.7 | |
| PCI | 67.8 | 58.4 | 66.9 | 65.3 | 70.7 | |
| CABG | 9.4 | 8.6 | 8.2 | 7.0 | 10.6 | |
| Preserved ejection fraction (%) | 65.9 | 67.7 | 60.7 | 63.2 | 67.5 | 0.092 |
| Other Hospitalization characteristics | ||||||
| GFR† (%) | 0.035 | |||||
| <30 | 2.4 | 4.0 | 2.3 | 4.4 | 1.6 | |
| 30–59 | 15.4 | 17.5 | 17.5 | 13.1 | 14.9 | |
| 60–89 | 46.9 | 42.9 | 41.6 | 47.7 | 49.1 | |
| ≥90 | 34.3 | 35.7 | 38.6 | 34.9 | 34.4 | |
| Depression (PHQ score >9)‡ (%) | 13.5 | 20.8 | 11.4 | 16.5 | 11.7 | <0.001 |
| Referral to cardiac rehab (%) | 43.9 | 35.8 | 39.7 | 37.3 | 48.5 | <0.001 |
| Aspirin on discharge (%) | 95.5 | 93.4 | 97.4 | 94.3 | 95.7 | 0.104 |
| Beta blocker on discharge (%) | 91.0 | 89.9 | 89.8 | 89.3 | 92.0 | 0.340 |
| Statin on discharge (%) | 88.3 | 85.3 | 89.9 | 86.4 | 86.9 | 0.074 |
| Thienopyridine on discharge (%) | 78.2 | 73.2 | 80.0 | 74.7 | 79.7 | 0.042 |
P value represents global test between four sub-categories (A-D)
GFR = Glomerular Filtration Rate (mg/dL), calculated by Modification of Diet in Renal Disease (MDRD) formula
PHQ = Patient Health Questionnaire
In regards to patients’ baseline independence, as assessed by EQ-5D, 72.9% of subjects had no problems with mobility, 90.3% had no problems with self-care, and 66.0% had no problems with usual activities. Overall 56.3% had no problems with any of the three domains. Regarding patients’ baseline physical function, the mean SF-12 PCS score for the population was 45.7 (standard deviation, 9.9).
Independence Loss and Physical Function Decline
Over the follow-up period, 43.0% of the study population experienced either independence loss or physical function decline: 12.8% independence loss alone, 15.2% physical function decline alone, and 15.0% both (Table I). Patients with independence loss alone were slightly older than those in other groups. Patients with independence loss (either alone or with physical function decline) were also more likely to be female, non-Caucasian, single, uninsured, and to have more depressive symptoms. Comorbidities (both cardiac and non-cardiac) were generally highest among those with independence loss alone, and were lowest among those with no declines.
Among the EQ-5D dimensions that constituted independence loss (mobility, self-care, and usual activities), declines in mobility (58.2%) and usual activities (65.5%) were more than twice as common as declines in self-care (21.4%). Within each EQ-5D dimension, the majority of declines were by one level (mobility: 97.8%, self-care: 90.8%, usual activities: 90.4%), while the remainder were by two levels. Among patients with physical function decline, SF-12 PCS decreased in the following distribution: 5–9 points: 39.3%, 10–14 points: 26.9%, ≥15 points: 33.7%.
Factors Associated with Independence Loss and Physical Function Decline
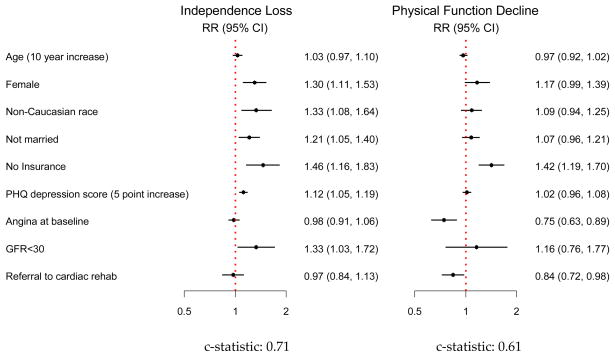
The results of the multivariable models for independence loss and physical function decline are shown in Figure 1. After multivariable adjustment, only medical insurance status (uninsured) remained independently associated with both outcomes. Variables that were associated with independence loss included female sex, non-Caucasian race, unmarried status, end-stage renal disease (GFR <30), and depression. In the model for physical function decline, patients without a cardiac rehabilitation referral or without baseline angina were more likely to have physical function declines at one year. Patient age was not independently associated with either outcome.
Figure 1.
Multivariable models of Independence Loss and Physical Function Decline (N=2002)
*Included in models but not significant: history of CAD, CVA, CHF, PVD, lung disease, diabetes; ejection fraction <40%; presentation with STEMI; in-hospital revascularization
Angina and Physical Function
In the multivariable model for physical function decline, patients with angina at baseline were less likely to experience physical function decline one year following AMI. To further examine this association, we performed an unadjusted exploratory analysis evaluating change in angina status between baseline and one year among groups (groups were divided based on angina status at these time points). In patients with an improvement in angina (present at baseline/absent at follow-up, N=578), 21% experienced physical function decline, while in patients with angina that developed (absent at baseline/present at follow-up, N=180), 59% experienced this outcome. Rates of physical function decline among patients with no angina (absent at baseline and follow-up, N=997), or persistent angina (present at baseline and follow-up, N=239), were 29% and 34%, respectively.
DISCUSSION
More than four in ten survivors of an AMI hospitalization experience clinically important declines in independence or physical functioning after one year. These declines are as likely to occur separately as they are to occur together, underscoring the distinct nature of these outcomes. Demographic characteristics and depression were associated with independence loss, while symptom status and referral to cardiac rehabilitation were associated with physical function decline. The only common risk factor for both outcomes was a lack of medical insurance. These observations emphasize the importance of clarifying the unique predictors of health status outcomes after AMI.
Comparison to Prior Studies
The relationship between AMI and physical function decline or independence loss has been reported in prior studies (3, 4, 6, 17, 21), although estimates of AMI-related declines using a single measure have ranged from 10% (4) to 30% (17). This variation is likely due to heterogeneous patient populations, differing definitions of disability, and varying intervals of follow-up. The Framingham Disability Study, which represented an early evaluation of health status after AMI, used three questions from the Rosow and Breslau Functional Health scale: ability to do heavy work around the house, ability to walk up and down stairs, and ability to walk half a mile (22). Subsequent studies have evaluated self-report of basic upper and lower extremity tasks (crouching, kneeling, extending arms above shoulder level) (23), explicit activities of daily living (e.g. dressing, bathing, eating) (4, 23, 24), the EQ-5D (25), SF-12 PCS (3, 17), and performance-based measures such as the 6-minute walk test (26). For the current study we used the EQ-5D and SF-12 PCS, which have the advantage of being obtainable by self-report, and are extensively validated patient-centered outcomes. Furthermore, we used one-year follow-up, a clinically meaningful period following hospitalization to assess changes in health status.
Implications for Preserving Independence
Among AMI survivors discharged home, female gender, non-Caucasian race, unmarried status, lack of insurance, renal impairment, and depression were each associated with independence loss. While some of these factors are not modifiable, their identification is important in raising questions as to whether there are systematic disparities in the post-hospitalization care of these patients that may account for worse outcomes, or prevention strategies that may be specifically targeted to these higher-risk groups. Other factors that are modifiable, including depression and lack of medical insurance, suggest that identifying and addressing these at the time of index hospitalization may prevent future disability. For example, among uninsured AMI survivors, 54.6% reported difficulty in obtaining medical care (vs. 12.6% with insurance). Being uninsured may therefore serve as a barrier to obtaining appropriate post-AMI care including medications, outpatient follow-up, and rehabilitation, all of which may increase the likelihood of independence loss.
Implications for Preserving Physical Function
Among AMI survivors discharged home, referral to cardiac rehabilitation and the presence of baseline angina were associated with preservation of physical function over time. The finding that referral to cardiac rehabilitation is associated with reduced physical function decline supports prior studies linking structured exercise programs with improvement in gait speed (27) and the prevention of disability (28). Specifically, patients undergoing rehabilitation have reported improvements in activities of daily living (29) and decreases in angina frequency (28, 30). Our results are observational and we cannot determine causality, and in addition we do not have data on the frequency or intensity of participation in rehabilitation. However, as it is known that a significant number of eligible AMI patients are not referred for rehabilitation (31) this deserves further study as a potentially modifiable risk factor.
The presence of angina has been linked to disability (21, 22, 32), and angina-related functional limitation has been independently associated with mortality (33, 34). In our study, baseline angina was associated with less physical function decline one year later, which in our exploratory analysis appeared to be mostly due to patients in whom angina resolved at follow-up. These patients had a considerably lower rate of physical function decline compared with those in whom angina developed between baseline and one year assessments. We did not have information on the etiology of resolved angina; this may have been due to multiple factors including revascularization, appropriate medical therapy, or infarction of previously ischemic myocardium. Although further study is necessary to better elucidate this relationship, our results suggest that angina treated at the time of incident AMI may preserve physical function, if angina relief is sustained.
Limitations
There are several limitations to our study that deserve consideration. We relied on self-reported health status measures rather than objective assessments, although prior studies have found a reasonable correlation between the SF-12 and EQ-5D and objective in-person functional measurements (35–37). However, it is possible that some patients (for example, those with higher social support) did not perceive the onset of disability, since new health status declines may have been compensated for by their social contacts. In addition, the SF-12 and EQ-5D are not specific to cardiac disease and we were unable to attribute declines to specific cardiac limitations (for example, angina or exertional dyspnea); therefore our ability to extrapolate whether cardiac interventions would be beneficial in preventing declines is limited. Secondly, as with any observational study, there is the potential for unmeasured confounding – for example, patients may not have been referred for cardiac rehabilitation at the time of index hospitalization because they were too ill (and could not participate), or there may have been inadequate community referral programs available at specific study hospitals. Third, our findings are only applicable to the AMI survivors who completed assessments at one year; we could not evaluate functional status in the subset of enrolled patients lost to follow-up, who were more likely to be younger, nonwhite, and uninsured. Finally, the numbers of “oldest old” in our study (≥85 years of age) were low and our conclusions may underestimate the risk of health status loss in this population. Further investigations are needed to elucidate the association between very advanced age and health status changes post-AMI, as well as the efficacy of prevention efforts across the age spectrum.
CONCLUSIONS
A significant proportion of patients experience health status declines in independence or physical function one year following AMI. While there is some degree of overlap, these health outcomes appear to be distinct: occurring in isolation as well as together, and influenced by different predictors. These findings underscore the importance of considering each outcome separately when evaluating health status after a cardiac event. By understanding the pathways for these declines, better avenues for preserving health status following AMI may be identified.
Acknowledgments
Funding Sources:
Dr. Dodson is supported by a training grant in Geriatric Clinical Epidemiology from the NIH/NIA (T32 AG019134).
The TRIUMPH Registry received support from the National Heart, Lung and Blood Institute (P50 HL077113) and CV Outcomes, Inc., Kansas City, MO.
Footnotes
Disclosures:
John A. Dodson: none
Suzanne V. Arnold: none
Kimberly J. Reid: none
Thomas M. Gill: none
Michael W. Rich: none
Frederick A. Masoudi: none
John A. Spertus: Dr. Spertus owns the copyright to the Seattle Angina Questionnaire (SAQ).
Harlan M. Krumholz: none
Karen P. Alexander: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute of Medicine. Crossing the quality chasm: A new health system for the 21st century. Washington, D.C.: National Academy Press; 2001. [PubMed] [Google Scholar]
- 2.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103–10. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 3.Ades PA, Savage PD, Tischler MD, et al. Determinants of disability in older coronary patients. Am Heart J. 2002;143:151–6. doi: 10.1067/mhj.2002.119379. [DOI] [PubMed] [Google Scholar]
- 4.Kamper AM, Stott DJ, Hyland M, et al. Predictors of functional decline in elderly people with vascular risk factors or disease. Age Ageing. 2005;34:450–5. doi: 10.1093/ageing/afi137. [DOI] [PubMed] [Google Scholar]
- 5.Alexander KP, Newby LK, Cannon CP, et al. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: A scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: In collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–69. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 6.Mendes de Leon CF, Krumholz HM, Vaccarino V, et al. A population-based perspective of changes in health-related quality of life after myocardial infarction in older men and women. J Clin Epidemiol. 1998;51:609–16. doi: 10.1016/s0895-4356(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 7.Domínguez H, Torp-Pedersen C, Koeber L, et al. Prognostic value of exercise testing in a cohort of patients followed for 15 years after acute myocardial infarction. Eur Heart J. 2001;22:300–6. doi: 10.1053/euhj.2000.2281. [DOI] [PubMed] [Google Scholar]
- 8.McAuley P, Myers J, Abella J, et al. Evaluation of a specific activity questionnaire to predict mortality in men referred for exercise testing. Am Heart J. 2006;151:890.e1–890.e7. doi: 10.1016/j.ahj.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Masoudi FA, Rumsfeld JS, Havranek EP, et al. Age, functional capacity, and health-related quality of life in patients with heart failure. J Card Fail. 2004;10:368–73. doi: 10.1016/j.cardfail.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Arnold SV, Chan PS, Jones PG, et al. Translational research investigating underlying disparities in acute myocardial infarction patients’ health status (TRIUMPH): Design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–76. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 12.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Lacey EA, Walters SJ. Continuing inequality: Gender and social class influences on self perceived health after a heart attack. J Epidemiol Community Health. 2003;57:622–7. doi: 10.1136/jech.57.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tukker A, Visscher T, Picavet H. Overweight and health problems of the lower extremities: Osteoarthritis, pain and disability. Pub Health Nutr. 2009;12:359–68. doi: 10.1017/S1368980008002103. [DOI] [PubMed] [Google Scholar]
- 16.Kind P, Dolan P, Gudex C, et al. Variations in population health status: Results from a United Kingdom National Questionnaire Survey. BMJ. 1998;316:736–41. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold SV, Alexander KP, Masoudi FA, et al. The effect of age on functional and mortality outcomes after acute myocardial infarction. J Am Geriatr Soc. 2009;57:209–17. doi: 10.1111/j.1532-5415.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 18.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 19.Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation and variance estimation software - user guide. Michigan: Survey Research Center, Institute for Social Research, University of Michigan; 2002. [Google Scholar]
- 20.R Development Core Team. R: A language and environment for statistical computing. 2011;2011(5/6) [Google Scholar]
- 21.Kattainen A, Koskinen S, Reunanen A, et al. Impact of cardiovascular diseases on activity limitations and need for help among older persons. J Clin Epidemiol. 2004;57:82–8. doi: 10.1016/S0895-4356(03)00252-X. [DOI] [PubMed] [Google Scholar]
- 22.Pinsky JL, Jette AM, Branch LG, et al. The Framingham Disability Study: Relationship of various coronary heart disease manifestations to disability in older persons living in the community. Am J Public Health. 1990;80:1363–7. doi: 10.2105/ajph.80.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendes de Leon CF, Bang W, Bienias JL, et al. Changes in disability before and after myocardial infarction in older adults. Arch Intern Med. 2005;165:763–8. doi: 10.1001/archinte.165.7.763. [DOI] [PubMed] [Google Scholar]
- 24.Vaccarino V, Berkman LF, de Leon CFM, et al. Functional disability before myocardial infarction in the elderly as a determinant of infarction severity and postinfarction mortality. Arch Intern Med. 1997;157:2196–204. [PubMed] [Google Scholar]
- 25.Nowels D, McGloin J, Westfall JM, et al. Validation of the EQ-5D quality of life instrument in patients after myocardial infarction. Qual Life Res. 2005;14:95–105. doi: 10.1007/s11136-004-0614-4. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan M, Levy WC, Russo JE, et al. Depression and health status in patients with advanced heart failure: A prospective study in tertiary care. J Card Fail. 2004;10:390–6. doi: 10.1016/j.cardfail.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Lopopolo RB, Greco M, Sullivan D, et al. Effect of therapeutic exercise on gait speed in community-dwelling elderly people: A meta-analysis. Phys Ther. 2006;86:520–40. [PubMed] [Google Scholar]
- 28.Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med. 2001;345:892–902. doi: 10.1056/NEJMra001529. [DOI] [PubMed] [Google Scholar]
- 29.Ades PA, Maloney A, Savage P, et al. Determinants of physical functioning in coronary patients: Response to cardiac rehabilitation. Arch Intern Med. 1999;159:2357–60. doi: 10.1001/archinte.159.19.2357. [DOI] [PubMed] [Google Scholar]
- 30.Jones DA, West RR. Psychological rehabilitation after myocardial infarction: Multicentre randomised controlled trial. BMJ. 1996;313:1517–21. doi: 10.1136/bmj.313.7071.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortés O, Arthur HM. Determinants of referral to cardiac rehabilitation programs in patients with coronary artery disease: A systematic review. Am Heart J. 2006;151:249–56. doi: 10.1016/j.ahj.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Vetter NJ, Ford D. Angina among elderly people relationship with disability. Age Ageing. 1990;19:159–63. doi: 10.1093/ageing/19.3.159. [DOI] [PubMed] [Google Scholar]
- 33.Spertus JA, Jones P, McDonell M, et al. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–9. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Bryson CL, Spertus JA, et al. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–22. doi: 10.1016/S0002-8703(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 35.Tiedemann A, Sherrington C, Lord SR. Physiological and psychological predictors of walking speed in older community-dwelling people. Gerontology. 2005;51:390–5. doi: 10.1159/000088703. [DOI] [PubMed] [Google Scholar]
- 36.Lord SR, Murray SM, Chapman K, et al. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57:M539–43. doi: 10.1093/gerona/57.8.m539. [DOI] [PubMed] [Google Scholar]
- 37.Moseley AM, Sherrington C, Lord SR, et al. Mobility training after hip fracture: A randomised controlled trial. Age Ageing. 2009;38:74–80. doi: 10.1093/ageing/afn217. [DOI] [PubMed] [Google Scholar]