Abstract
Pain is a unified experience composed of interacting discriminative, affective-motivational, and cognitive components, each of which is mediated and modulated through forebrain mechanisms acting at spinal, brainstem, and cerebral levels. The size of the human forebrain in relation to the spinal cord gives anatomical emphasis to forebrain control over nociceptive processing. Human forebrain pathology can cause pain without the activation of nociceptors. Functional imaging of the normal human brain with positron emission tomography (PET) shows synaptically induced increases in regional cerebral blood flow (rCBF) in several regions specifically during pain. We have examined the variables of gender, type of noxious stimulus, and the origin of nociceptive input as potential determinants of the pattern and intensity of rCBF responses. The structures most consistently activated across genders and during contact heat pain, cold pain, cutaneous laser pain or intramuscular pain were the contralateral insula and anterior cingulate cortex, the bilateral thalamus and premotor cortex, and the cerebellar vermis. These regions are commonly activated in PET studies of pain conducted by other investigators, and the intensity of the brain rCBF response correlates parametrically with perceived pain intensity. To complement the human studies, we developed an animal model for investigating stimulus-induced rCBF responses in the rat. In accord with behavioral measures and the results of human PET, there is a progressive and selective activation of somatosensory and limbic system structures in the brain and brainstem following the subcutaneous injection of formalin. The animal model and human PET studies should be mutually reinforcing and thus facilitate progress in understanding forebrain mechanisms of normal and pathological pain.
Forebrain Mediation of Pain.
Pain is a conscious experience that includes discriminative, affective-motivational, and cognitive components that produce the unified sensation of pain. These components are each mediated through separate, interactive forebrain mechanisms (1). For example, the ability to localize somatic stimuli in time, space, and along a continuum of intensities is greatly impaired following lesions limited to the primary somatosensory (S1) cortex or the ventral posterolateral thalamus. These lesions do not produce analgesia, however, because the aversive nature of noxious stimuli, although poorly localized, is still evident in the behavior of animals and the verbal reports of humans (2). Neurons in the S1 cortex and ventral posterolateral thalamus, including those responding primarily to noxious stimuli, have small, contralateral receptive fields consistent with the mediation of spatial stimulus localization (3). In contrast, lesions within the anterior cingulate cortex have no effect on innocuous or nociceptive somesthetic discriminative functions, but impair the recognition of the noxious or aversive quality of the stimulus in animals and the perceived affective quality of pain in humans (4, 5). Anterior cingulate neurons that respond to noxious stimuli have large, often bilateral receptive fields, consistent with a limited role in spatial discriminative capacity (6). There is no comparable information about the neuronal substrate for the cognitive dimension of pain, but there are numerous studies and observations showing the profound influences of attention, suggestion, and emotional state on the perception of pain (7). The broad range of environmental influences, such as attention, fear, and the placebo effect on the perception of pain suggests that cortical association areas and their subcortical connections are critical participants in mediating the cognitive aspects of pain.
The Forebrain Modulation of Pain.
The processing of nociceptive stimuli is modulated by the forebrain at spinal, brainstem, and diencephalic levels. Stimulation of the cerebral cortex or thalamus can facilitate or suppress the responses of spinothalamic or trigeminothalamic tract neurons (8, 9). In the awake monkey, the response of trigeminothalamic cells to noxious heat depends on behavioral state (10, 11). Corticobulbar and corticothalamic neurons have marked effects on the excitability of brainstem and thalamic cells that receive nociceptive input (12–16).
Because of the large volume of the human forebrain in relation to that of the spinal cord (77% vs. 2% of central nervous system volume), these descending modulatory influences may assume greater importance in humans than in other species, such as the laboratory rat, where the forebrain is less anatomically dominant (31% vs. 35% of central nervous system volume) (17). The human spinothalamic tract, for example, contains an estimated 2,000 to 5,000 fibers whereas the corticospinal tract, which includes fibers terminating in the superficial layers of the dorsal horn (18, 19), is estimated to contain from 5 × 105 to 1 × 106 fibers (20, 21). Corticothalamic influences are also likely to be dominant in the human; in the cat, approximately 50% of the estimated 5,000 to 9,000 synapses on thalamocortical projection neurons are presumed to be of cortical origin, whereas only 15% are formed by ascending afferent fibers (22).
The Physiological Rationale of Positron Emission Tomography (PET).
Synaptic activity generates increases in cerebral blood flow (CBF). This physiological fact is the basis for both PET and functional magnetic resonance imaging (fMRI). The most commonly used fMRI method relies on local shifts in the magnetic field that accompany the shift from deoxyhemoglobin to oxyhemoglobin within activated perfused tissue (23). PET and fMRI are complementary methods of assessing brain activity. This article will be limited to a discussion of PET.
The first indication that brain activity increases global CBF was reported by Roy and Sherrington over 100 years ago (24). Subsequent radioactive tracer techniques revealed increases in regional CBF (rCBF) during sensory stimulation or the performance of motor tasks (25). Recent studies that use the technique of optical imaging have demonstrated that cortical blood flow responses occur within 3 sec of sensory stimulation and are initially restricted to the 300- to 500-μm dimensions of cortical columns before spreading to involve the surrounding 3 mm to 5 mm of cortical tissue (26–28). The biochemical coupling of rCBF and synaptic activity is unknown and is still an area of active investigation. Studies of regional cerebral glucose utilization show that it and rCBF are normally tightly coupled and that the coupling occurs within the synaptic neuropil. The degree of coupling may vary among regions and under special experimental conditions (29), but it is reliably present in the normal mammal. Blocking the production of nitric oxide has no effect on synaptically induced rCBF responses in the rat somatosensory system (30). There is evidence that adenosine may be a critical link in this process, but it is likely that the action of several mediators may be important (31).
A reduction of rCBF should occur when synaptic activity is suppressed below some resting or background level. Indeed, reductions in rCBF are observed in many PET studies of rCBF (32). However, the physiological significance of reduced rCBF is uncertain; it does not necessarily indicate the presence of inhibitory synaptic activity, because both inhibitory and excitatory synaptic activity can contribute to increases in synaptic metabolism (33). It is possible that some of the observed reductions in rCBF reflect autoregulatory mechanisms for global CBF, these reductions may not affect neuronal function; others may signal the removal of synaptic excitation (disfacilitation) by an inhibitory process located outside the area of rCBF decrease. In any event, it is not now possible to establish the valence of synaptic activity by rCBF estimation methods.
PET Methodology.
In most current studies, water (as H215O) or [15O]butanol is injected intravenously, or carbon dioxide (as C15O2) is inhaled and converted in the lungs to H215O. The 15O has a half-life of 122 sec. This length of time is sufficient for CBF measurements, because a bolus injection (e.g., 50 mCi) of this compound is nearly completely diffused into brain tissue on the first arterial pass (34). The count of emissions from a given volume of brain tissue is therefore a good estimate of the perfusion of that brain region during the counting period (approximately 60 sec for a typical scan).
With the analytical methods that we use, we find that there are approximately 95,000 voxels in the gray matter of the average human brain. At our facility, a three-dimensional voxel is a cube 2.25 mm on each side. However, the spatial resolution of PET is limited by the smoothing introduced by image reconstruction filters and by the ability of the radiation detectors to differentiate the radiation emitted from two separate point sources. For PET, this distance, the full width at half maximum, is between 6 and 9 mm. However, the spatial accuracy in the localization of an activation focus is improved (to less than half the full width at half maximum) when subtraction images are made.
Each image set is normalized to whole brain counts, and mean radioactivity concentration images are created by estimating rCBF across all subjects with stereotactic anatomical standardization techniques. Image voxel intensities are normalized to global cerebral activity with the use of a linear proportional model to remove baseline differences in global CBF between scans and subjects (35). In our facility, CBF images are aligned onto the coordinates of a standard stereotactic atlas (36), by using anatomical landmarks identified within the PET images of each individual so that the CBF differences are compared within the same brain regions (37–39). To determine whether a task or a stimulus has produced an increase in rCBF, the rCBF computed during a control condition is subtracted from that computed during the test condition. The resulting subtraction image, then, shows those brain regions with differences in CBF between the two conditions.
A voxel-by-voxel statistical subtraction analysis (Z-score) with adjustment for multiple comparisons is performed by estimating the smoothness of subtraction images (40) following three-dimensional Gaussian filtering to enhance signal-to-noise ratio and compensate for residual anatomical variance. Typically, only those voxels with normalized CBF values larger than 60% of the global value are analyzed, because these voxels represent the gray matter of the brain. Voxels showing a significantly increased CBF compared with the average noise variance computed across all voxels (pooled variance) are identified (41). The critical level of significance is determined by using this information to adjust P = 0.05 (42). With this method, the results of interest are revealed primarily through the data analysis. However, it is also possible to perform correlations between the intensity of the rCBF responses throughout the brain and some behavioral parameter of interest, such as the perceived intensity of stimulation (43).
In addition, volumes of interest (VOI) may be established within brain structures selected because of a priori hypotheses and the results of previously published PET studies. The size and shape of each VOI may be standardized across studies or determined separately according to functional criteria. We presently use a method similar to that described by Burton (44), in which voxels showing significant peak increases in CBF between comparison conditions are identified within the brain structure of interest; the voxels are progressively expanded in three dimensions to include contiguous voxels that meet the statistical criterion established by the voxel-by-voxel Z-score analysis. To determine the statistical significance of rCBF increases, a paired t statistic is computed for each VOI from the average percentage increase in CBF across all subjects. Levels of significance are established, based on the Bonferroni correction for multiple comparisons among VOI.
Important Variables in the Conduct and Interpretation of PET Studies of Pain.
There are now numerous studies from various facilities that have used PET during the application of experimental pain. The results are difficult to compare because they are affected by intersubject variability, type of stimulus, method of scanning, and data analysis methods. To assess the effect of some of these variables, we have conducted several investigations in normal subjects with a variety of stimulation methods. The variables we have considered thus far include gender, the physical characteristics of the stimulus, and the sources of nociceptive afferent input.
Gender.
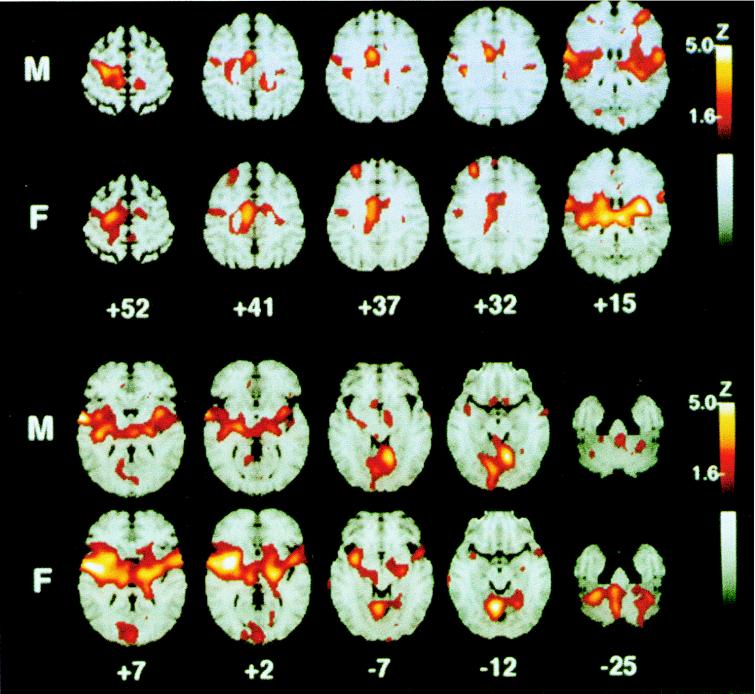
The prevailing evidence suggests that although there is no reliable gender difference in pain thresholds, pain tolerance is generally higher in male than in female subjects (45). PET studies find gender differences in resting rCBF (46) or in the cerebral metabolic rate of glucose utilization (47–49). These findings suggest that there may be underlying gender differences in the neural mechanisms that mediate pain perception. Accordingly, we performed PET studies in normal right-handed male (n = 10) and female (n = 10) subjects (18 to 39 years old) as they discriminated differences in the intensity of innocuous and noxious heat stimuli applied to the left forearm (50). Thermal stimuli were 40°C or 50°C heat, applied with a thermode as repetitive 5-sec contacts to the left volar forearm. Both male and female subjects rated the 40°C stimuli as warm but not painful and the 50°C stimuli as painful, but females rated the 50°C stimuli as significantly more intense than did the males (P = 0.0052). Both genders showed a bilateral activation of premotor cortex during heat pain in addition to the activation of a number of contralateral structures, including the posterior insula, anterior cingulate cortex, and the cerebellar vermis (Fig. 1). Overall, a nearly complete overlap of the activation patterns occurred between genders. However, direct image subtraction showed that females had significantly greater activation of the contralateral prefrontal cortex compared with males. A VOI comparison (t-statistic) also showed greater activation of the contralateral insula and thalamus in females compared with males (P < 0.05). These pain-related differences in brain activation may be attributed to gender, perceived pain intensity, or both factors. These results show that gender differences are important considerations in the investigation of forebrain responses to noxious stimuli.
Figure 1.
Statistical map of rCBF responses of 10 males (M) and 10 females (F) to repetitive noxious heat stimulation (50°C) of the left volar forearm. Color coding of Z scores as indicated by flame bar at right. The right hemisphere of the MRI stereotactic template is on the reader’s left. The numbers below columns of images indicate millimeters above a plane connecting the anterior and posterior commissures. In both genders, there is significant activation of the contralateral anterior cingulate cortex (+41, +37), premotor, and insular cortex (+15, +7), ipsilateral insula (+7, +15), and bilateral cerebellar vermis (−12). Voxel-by-voxel analysis indicated that some structures were significantly activated (Z > 4.0) only in males (contralateral sensorimotor cortex, +52; contralateral lenticular nucleus, +2; ipsilateral prefrontal cortex, +15) and others only in females (contralateral prefrontal cortex, +32; anterior insula, +2; thalamus, +15; ipsilateral lenticular nucleus, +2; contralateral cerebellum, −25). Direct comparisons of percent increase in rCBF, however, revealed that the only difference is that the contralateral thalamus, anterior insula, and prefrontal cortex show a greater response in females compared with males. Reproduced from ref. 50 with permission from the International Association for the Study of Pain.
Physical Characteristics of the Noxious Stimulus.
Subtraction images have been interpreted as revealing those cerebral structures that have increased synaptic activity related specifically to the central processing of the neuronal signals produced by noxious heat. In our earlier report (51), we controlled for the cerebral processes mediating the discrimination of heat intensity within the innocuous range. By cooling the skin, we were able to produce innocuous, easily perceptible differences in the degree of skin temperature increase and to duplicate the intensity differences that were applied within the noxious range. However, it is possible that by cooling the skin, we reduced the perceived difference between the innocuous stimuli below that which would exist normally. The resulting rCBF responses may have been reduced below the sensitivity of our PET analysis. To test this possibility, we performed a series of PET studies on subjects who were asked to discriminate the differences between innocuous warm stimuli delivered to the volar forearm at normal baseline skin temperature. We wished to determine whether this procedure would lead to a demonstration of rCBF increases that could be compared with those elicited by noxious heat stimulation. An equally important and related issue is whether other methods of producing pain result in the same intensity and pattern of rCBF increases as the increases elicited by repetitive noxious heat applied to the skin. To examine this question, we performed PET studies on normal subjects as they experienced the deep, aching pain produced by immersion of one hand in 6°C water for 105 sec. We were then able to compare the PET rCBF results obtained during warm discrimination and during tonic, noxious cold immersion with the results previously obtained during repetitive cutaneous noxious heat (51).
We studied three groups of nine normal, healthy subjects, 18 to 39 years old, all of whom were given instruction and practice in the use of the visual analog scale for the estimation of stimulus intensity and unpleasantness. One group was assigned a warm discrimination task, another group rated innocuous and noxious heat intensity and unpleasantness, and the third group participated in the ice-water immersion study. In the warm discrimination study, two intensities of innocuous heat (36°C and 43°C) were applied with a thermode as repetitive 5-sec contacts to the volar forearm throughout the scan. Neither stimulus was rated as painful. All subjects discriminated the 43°C stimulus from the 36°C stimulus (P < 0.0001). Significant increases in rCBF to the 43°C stimuli were found in the contralateral ventral posterior thalamus, lenticular nucleus, medial prefrontal cortex (Brodmann’s areas 10 and 32), and the cerebellar vermis. In the study of noxious and innocuous heat, all subjects rated the 50°C stimuli as painful and the 40°C stimuli as warm, but not painful. Significant rCBF increases to 50°C stimuli were found contralaterally in the lenticular nucleus, thalamus, anterior cingulate cortex, premotor cortex, and the secondary somatosensory (S2) and insular cortices. The ipsilateral premotor cortex and thalamus as well as the medial dorsal midbrain and cerebellar vermis showed significant rCBF increases. CBF increases just below the threshold for statistical significance were seen in the contralateral sensorimotor cortex (S1/M1).
In the ice-water immersion study, the left hand was immersed to the wrist throughout each of six scans in water kept at an average temperature of either 20.5°C ± 1.15°C or 6.02°C ± 1.18°C on alternate scans. All subjects rated the intensity of the stimuli on a scale in which 0 = “no pain” and 10 = “barely tolerable pain.” Subjects rated the 20°C water immersion as painless (average rating ± SD of 0.18 ± 0.48), but gave ratings indicating intense pain during immersion in 6°C water (7.89 ± 1.45). All subjects expressed the perception of the pain as very cold, steady, and deep. Highly significant increases in rCBF were found contralaterally in the sensorimotor cortex (M1/S1), premotor cortex, anterior cingulate cortex, and in the region of the anterior insula and lenticular nucleus. Ipsilateral increases in rCBF were seen in the lateral prefrontal cortex (Brodmann’s areas 10 and 46), anterior cingulate cortex, the region of the insular and opercular precentral cortices, and the thalamus. The cerebellar vermis also showed a significant increase in rCBF. CBF increases just below the threshold for statistical significance were seen in the contralateral thalamus.
Comparisons of rCBF response magnitude were made among the five stereotactically concordant brain regions that showed significant responses in both the heat pain and cold pain conditions: the cerebellar vermis, ipsilateral thalamus, contralateral premotor cortex, contralateral anterior cingulate cortex, and the region of the contralateral anterior insula and lenticular nucleus. Each region showed a higher increase in rCBF during the cold pain study (3.26% ± 0.061%) than during the heat pain study (2.85% ± 0.124%; paired t4 = 3.60; P < 0.022).
The results show that in conscious humans, two forms of noxious stimulation that are different in temporal pattern, afferent fiber activation, and perceived spatiotemporal and qualitative characteristics produce similar, but not identical, patterns of brain rCBF increases. These pain-related response patterns are each quite different from the brain responses observed during the discrimination between two intensities of innocuous heat stimuli. The results suggest that the increased rCBF responses observed during noxious stimulation reflect physiological differences in neuronal activity that are related to both nociceptive processing and the perception of pain. The overlap in the spatial distribution of rCBF increases during noxious cutaneous heat and noxious deep-cold stimulation suggests that a reproducible pattern of rCBF responses may occur that is common to the perceptions of pain produced by different stimuli. Differences in the intensity and spatial patterns of these pain-related rCBF increases may reflect physiological differences in neuronal nociceptive processing that are linked with these two forms of pain perception.
The Source of Nociceptive Input.
In comparing the rCBF changes induced by cutaneous-contact heat pain with that induced by deep-cold pain, we found that the brain activation patterns showed a considerable overlap; the contralateral anterior cingulate, anterior insula/lenticular nucleus, premotor cortex, and the ipsilateral thalamus and cerebellar vermis were activated by both forms of noxious stimulation (52). Because cold noxious stimulation activates cutaneous, subcutaneous, muscle, periosteal, and venous nociceptors (53), we wished to compare two forms of noxious stimulation that more selectively activate nociceptive afferents from different sources.
For each of eight PET scans, 11 normal subjects rated the intensity of cutaneous and intramuscular stimuli delivered to the nondominant (left) forearm on a visual analog scale; stimulus intensity was adjusted to approximate pain threshold levels. Cutaneous pain was produced with a high-energy CO2 laser stimulator. Muscle pain was elicited with high-intensity intramuscular electrical stimulation. The pain intensity ratings and the differences between noxious and innocuous ratings were similar for cutaneous and intramuscular stimuli (P > 0.05). After stereotactic registration, statistical pixel-by-pixel summation (Z-score) and VOI analyses of subtraction images were performed. Direct statistical comparisons between cutaneous and intramuscular stimulations showed no reliable differences between these two forms of noxious stimulation, indicating that a substantial overlap occurred in brain activation patterns. These activated cerebral structures may represent those recruited early in nociceptive processing, because both forms of stimuli were near pain threshold.
Increases in rCBF of 3.5% or more were seen in the contralateral S2, anterior insular, anterior cingulate, prefrontal, and inferior parietal cortices; and in the contralateral thalamus, lenticular nucleus, ipsilateral premotor cortex, and cerebellum. Cutaneous laser stimulation was relatively ineffective in evoking rCBF responses in the contralateral anterior cingulate or in the lenticular nucleus. Intramuscular stimulation was similarly ineffective in activating the contralateral prefrontal and ipsilateral premotor cortex. However, each form of stimulation evoked responses of sufficient magnitude in each structure, but a direct statistical comparison failed to differentiate significantly between them. The similar cerebral activation patterns suggest that the perceived differences between acute skin and muscle pain are mediated by differences in intensity and temporospatial patterns of neuronal activity within similar sets of forebrain structures.
An Emerging Pattern.
In summarizing the data obtained from right-handed subjects in our facility, we found that certain structures were activated by noxious stimuli across a wide range of conditions. The pattern of activation is best represented by inspection of the results of our study of gender (Fig. 1). Among the most prominent activation sites are the contralateral insular cortex, primarily the anterior portion, and the cerebellar vermis. These structures responded to each form of noxious stimulation in all groups of subjects. Bilateral insular activation is seen, but it often does not reach statistical significance in voxel-by-voxel analysis. The contralateral thalamic responses to noxious stimuli are equally robust, but are more frequently bilateral. The contralateral anterior cingulate cortex responded to all noxious stimuli except cutaneous laser pulses; clear bilateral responses were observed only during deep cold pain. Finally, the premotor cortex (Brodmann’s area 6) responded bilaterally in all studies, except the study performed with a cutaneous laser, where the response was ipsilateral only; and the study performed with intramuscular stimulation, where the response was not significant. Other structures, including the S1 and S2, lateral prefrontal (Brodmann’s areas 10 and 46), and inferior parietal (Brodmann’s area 40) cortices were significantly activated in a minority of our studies.
The insular cortex has been considered a component of cerebral pain mechanisms based primarily on clinical information (54–57). Because of its anatomical connections, this region is likely to mediate affective, mnemonic, and autonomic features of pain (58). The insula receives input from the ventral medial posterior thalamus, a region that receives direct nociceptive input from the superficial dorsal horn (59, 60). The spinothalamic tract projects also to the medial and intralaminar thalamus, the origin of thalamocortical fibers to the anterior cingulate gyrus; nociceptive neurons are found in both structures (6, 61–63). Furthermore, clinical and experimental evidence shows that the anterior cingulate cortex is critical for normal pain-related behaviors (64–66). The activation of these thalamocortical pathways to the insular and cingulate cortices is therefore consistent with other information about nociceptive processing. How the cerebellum and premotor cortices fit into this picture is currently unclear. Based on evidence from other PET studies, it is possible that cortical and subcortical motor mechanisms become activated in anticipation of movements intended to escape the noxious stimulation (67).
The contralateral S1 cortex responds significantly in a minority of our studies. Often there is a peak of activation within S1, but it fails to reach statistical significance in most of our studies. The activation of S1 across other PET studies of pain also seems quite variable. This observation raises again the question of the role of S1 in the cerebral processing of pain. Neurophysiological studies leave little doubt that nociceptive information reaches the S1 cortex (3, 68, 69). Clinical observations and PET studies show that S1 is critical for somesthetic discriminative performance, but that surgical extirpation of S1 does little to relieve pain (70, 71). The conditions that require S1 activity for nociceptive processing have yet to be determined and present an interesting challenge for the future.
Each of the five structures identified above (six, counting each hemithalmus separately) is known to participate in cerebral functions other than nociceptive processing and pain. It is premature to consider that this particular pattern of activation is unique for pain. Nonetheless, in nearly all other PET studies of experimentally applied pain, the structures named here have been activated by using a wide variety of stimuli and different data processing methods. There are other important variables to investigate. The issue of cerebral asymmetry in pain processing has yet to be addressed systematically; it is probably an important factor in pathological pain states in humans (72). It is likely that further experience with whole-brain imaging methods will allow us to identify a pain-specific pattern of cerebral activation.
Meanwhile we have the opportunity to test specific hypotheses about the participation of each of these regions in nociceptive processing and pain. Introducing conditions that perturb the cerebral activation pattern can test hypotheses about the mechanisms of pain and analgesia. For example, Rainville et al. (73), by using hypnotic suggestion, were able to uncouple perceived intensity from perceived unpleasantness to demonstrate a strong correlation between the ratings of unpleasantness and the degree of rCBF increase within a portion of the anterior cingulate gyrus. And Derbyshire et al. (43) have shown that several cerebral regions, including those named above, show a significant correlation of rCBF response magnitude with perceived stimulus intensity. Information relevant to perceived stimulus intensity thus appears to be distributed widely, not simply to structures such as the S1 cortex that are known to mediate discriminative function. The ability of PET to provide information about nociceptive processing in the awake human brain offers an opportunity to study the effects of neuropathic pain, central nervous system damage, and the unique effects of analgesics. For example, although opioid analgesia specifically attenuates pain-activated, but not vibration-activated, cerebral responses, it strongly activates the anterior cingulate cortex (K.L.C., P. Svenssen, T. J. Morrow, J. Raz, C. S. Jone, and S. Minoshima, unpublished data). Such a result suggests the involvement of both spinal and supraspinal sites of analgesic action, including the participation of descending inhibitory modulation.
Future progress in the analysis of the physiology of this pain-related network will require the development of an animal model for invasive studies that cannot be performed in humans. We have recently developed a model for studies of nocifensive behaviors in the rat.
An Animal Model for Future rCBF Studies.
We used rCBF in an animal model to identify the patterns of forebrain nociceptive processing that occur during early and late phases of the well-established formalin test of inflammatory pain in the rat (74). During the early phase, immediately after the injection of formalin into the dorsal hindpaw, pain behaviors are frequently elicited that are most intense. This phase continues for approximately 5 min, after which nociception is considerably reduced. The late phase is marked by the return of moderate to high levels of pain-related behaviors, beginning 10 to 15 minutes after formalin injection and continuing for ≤1 h. The early phase is thought to be caused by the direct activation of peripheral nociceptors by formalin, whereas the late phase is believed to be related to the development of inflammation and sensitization of central nociceptive neurons (75–78).
We measured normalized rCBF increases by an autoradiographic method that uses the radiotracer [99m]Tc-exametazime. Rats were restraint-adapted to a soft towel for 2 to 3 weeks. To examine changes in rCBF during the early acute pain phase of the formalin test, we injected the left hindfoot of the restrained rat with 0.05 ml of a 2.5% solution of formalin. After 2 min, we injected each animal with an intravenous bolus of radiotracer. The same procedure was followed in the late phase of the test, but we injected the radiotracer 20 minutes later. After the first injection, these adapted rats showed little or no movement while in the restraint. Two to five minutes after the radiotracer injection, the rat was overdosed with anesthetic (chloral hydrate, 300 mg/kg i.v.) and decapitated; slides of the frozen brain were prepared for routine histological staining and quantitative autoradiography. Eighteen regions of interest (ROIs) were selected, representing various structures within the limbic and somatosensory systems. Densitometric analysis of autoradiograms was performed with microcomputer-assisted video imaging. Anatomic location of selected ROIs was accomplished by overlaying matching transparencies from a standard stereotactic atlas. We converted the film densities to apparent tissue radioactivity concentrations (nCi/mg) by comparing them with the film optical densities of 14C-labeled standards, allowing ROI comparisons across different films and animals. An index of activation was then calculated from individual ROI activities as a percentage of the average total activity of the entire brain. Significant differences in activation for each ROI were detected between experimental groups by using ANOVA with post-hoc t tests (P ≤ 0.05).
During the early phase of the formalin test, a highly significant (31%) increase in rCBF occurred in the contralateral hindlimb cortex. At the same time the retrosplenial portion of the cingulate cortex and the midbrain periaqueductal gray were activated bilaterally (31% and 7.8%, respectively). During the late phase, these structures remained active but the hindlimb activation became bilateral. In addition, the intensity of periaqueductal gray activation increased to 20% and was joined by significant rCBF increases in the interpeduncular and paraventricular nuclei (66% and 30%, respectively), in the habenular complex (58%), anterior dorsal nucleus of the thalamus (30%), and the parietal cortex (30%) adjacent to the hindlimb cortex. The somatotopic organization of the somatosensory thalamus and the small number of neurons excited by hindlimb stimulation probably resulted in an underestimation of specific thalamic nuclei activity. Nonetheless, we detected blood flow increases in the ventral posterolateral thalamus (8.7%) and in the medial thalamus (9.0%) that did not reach statistical significance but did tend to be greater in the late phase compared with the early phase of the formalin test.
These results show that specific structures known to be important in nociceptive processing and modulation are selectively activated in the awake rat during the formalin test. Activation of a structure may be related to nociception, antinociception, or both. The contralateral hindlimb cortex and midbrain periaqueductal gray received nociceptive input and were active during the early phase. In the late phase, bilateral activity was seen throughout the forebrain, with the recruitment of limbic system components, each of which has been shown to participate in mediating or modulating nocifensive behaviors. In addition to the well-known analgesic effect of periaqueductal gray stimulation, interpeduncular nucleus stimulation modulates antinociceptive circuitry in the medullary raphe nuclei (79), and stimulation of the paraventricular nucleus produces analgesia (80). Analgesia also follows the microinjection of morphine and electrical stimulation within the habenular complex (81, 82). Activation of the cingulate cortex is consistent with the activation of one of its major inputs, the anterior dorsal thalamic nuclei, and is in accord with the limbic cortical activation seen in human PET studies. Overall, the bilateral activation of somatosensory and limbic structures agrees with 2-deoxyglucose studies of glucose uptake in rats with chronic constriction injures of the sciatic nerve (83). Here we show that rCBF analysis is useful in studying central responses to acute and chronic stimuli.
The Future of Pain Imaging.
This developing technology may undergo significant improvements in both spatial and temporal resolution. Currently, PET provides a quantitative, statistically reliable method for assessing the activity of large brain and brainstem regions. Hypotheses can then focus on the conditions necessary and sufficient to activate one or more regions in a group of subjects. Although it is now possible to obtain reliable and quantitative information from single subjects with PET, fMRI has the ability to focus with great precision on rCBF responses in specific regions. Working together in a complementary manner, the two procedures should help develop a more precise understanding of the functional organization of pain and nociceptive processing . This progress will be facilitated by the parallel use of animal models, allowing questions about dynamics and functional connectivity to be addressed by selective stimulation, lesion, and drug microinjection studies.
The clinical impact of this effort will be apparent as we develop an understanding of how the central nervous system adapts to chronic nociceptive input and injury. The changes in nociceptive processing demonstrated at the spinal cord level in experimental animals are likely to affect nociceptive processing and hence pain at higher levels. Such studies may have an important impact on descending modulatory influences, especially in forebrain-dominated animals such as humans. Evidence has accumulated showing that peripheral injury can profoundly affect thalamic and cortical sensory processes over long periods of time (84–86). In some cases, these plastic changes can be correlated with pain (87). A significant minority of patients with injury or disease of the central nervous system also suffer chronic, often unremitting pain as a consequence of the central lesion(s) (88). The pathophysiology of this condition is unknown, but the methods discussed here hold the promise for better solutions to the treatment and prevention of these chronic pain conditions.
ABBREVIATIONS
- CBF
cerebral blood flow
- rCBF
regional CBF
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
- ROI
region of interest
- S1
primary somatosensory (cortex)
- S2
secondary somatosensory (cortex)
- VOI
volumes of interest.
References
- 1.Melzack R, Casey K L. In: The Skin Senses. Kenshalo D R, Thomas C C, editors. Springfield, IL: Thomas; 1968. pp. 423–439. [Google Scholar]
- 2.Head H, Holmes G. Brain. 1911;34:102–254. [Google Scholar]
- 3.Kenshalo D R, Jr, Isensee O. J Neurophysiol. 1983;50:1479–1496. doi: 10.1152/jn.1983.50.6.1479. [DOI] [PubMed] [Google Scholar]
- 4.Foltz E L, White L E. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 5.Hurt R W, Ballantine H T. Clin Neurosurg. 1973;21:334–351. doi: 10.1093/neurosurgery/21.cn_suppl_1.334. [DOI] [PubMed] [Google Scholar]
- 6.Sikes R W, Vogt B A. J Neurophysiol. 1992;68:1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- 7.Price D D. Psychological and Neural Mechanisms of Pain. New York: Raven; 1988. pp. 1–241. [Google Scholar]
- 8.Gerhart K D, Yezierski R P, Fang Z R, Willis W D. J Neurophysiol. 1983;49:406–423. doi: 10.1152/jn.1983.49.2.406. [DOI] [PubMed] [Google Scholar]
- 9.Yezierski R P, Gerhart K D, Schrock B J, Willis W D. J Neurophysiol. 1983;49:424–441. doi: 10.1152/jn.1983.49.2.424. [DOI] [PubMed] [Google Scholar]
- 10.Dubner R, Hoffman D S, Hayes R L. J Neurophysiol. 1981;46:444–464. doi: 10.1152/jn.1981.46.3.444. [DOI] [PubMed] [Google Scholar]
- 11.Hayes R L, Dubner R, Hoffman D S. J Neurophysiol. 1981;46:428–443. doi: 10.1152/jn.1981.46.3.428. [DOI] [PubMed] [Google Scholar]
- 12.Basbaum A I, Fields H L. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 13.Yuan B, Morrow T J, Casey K L. J Neurosci. 1986;6:3611–3617. doi: 10.1523/JNEUROSCI.06-12-03611.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin H C, Chapin J K. Somatosens Mot Res. 1990;7:421–434. doi: 10.3109/08990229009144717. [DOI] [PubMed] [Google Scholar]
- 15.Alloway K D, Johnson M J, Wallace M B. J Neurophysiol. 1993;70:892–908. doi: 10.1152/jn.1993.70.3.892. [DOI] [PubMed] [Google Scholar]
- 16.Liu X B, Honda C N, Jones E G. J Comp Neurol. 1995;352:69–91. doi: 10.1002/cne.903520106. [DOI] [PubMed] [Google Scholar]
- 17.Swanson L W. Trends Neurosci. 1995;18:471–474. doi: 10.1016/0166-2236(95)92766-j. [DOI] [PubMed] [Google Scholar]
- 18.Cheema S S, Rustioni A, Whitsel B L. J Comp Neurol. 1984;225:276–290. doi: 10.1002/cne.902250211. [DOI] [PubMed] [Google Scholar]
- 19.Ralston D D, Ralston H J., III J Comp Neurol. 1985;242:325–337. doi: 10.1002/cne.902420303. [DOI] [PubMed] [Google Scholar]
- 20.Blinkov S M, Glezer I I. The Human Brain in Figures and Tables. New York: Plenum; 1968. [Google Scholar]
- 21.Towe A L. J Brain Res. 1995;36:393–398. [Google Scholar]
- 22.Liu X-B, Honda C N, Jones E G. J Comp Neurol. 1995;352:69–91. doi: 10.1002/cne.903520106. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa S, Lee T-M, Kay A R, Tank D. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy C S, Sherrington C S. J Physiol (London) 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roland P E. Brain Activation. New York: Wiley-Liss; 1993. [Google Scholar]
- 26.Malonek D, Grinvald A. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Tanaka K, Tanifuji M. Science. 1996;272:1665–1668. doi: 10.1126/science.272.5268.1665. [DOI] [PubMed] [Google Scholar]
- 28.MacVicar B A. Neuroscientist. 1997;3:381–388. [Google Scholar]
- 29.Mraovitch S, Calando Y, Pinard E, Pearce W J, Seylaz J. Neuroscience. 1992;49:451–466. doi: 10.1016/0306-4522(92)90110-n. [DOI] [PubMed] [Google Scholar]
- 30.Adachi K, Takahashi S, Melzer P, Campos K L, Nelson T, Kennedy C, Sokoloff L. Am J Physiol. 1994;267:H2155–H2162. doi: 10.1152/ajpheart.1994.267.6.H2155. [DOI] [PubMed] [Google Scholar]
- 31.Dirnagl U, Niwa K, Lindauer U, Villringer A. Am J Physiol. 1994;267:H296–H301. doi: 10.1152/ajpheart.1994.267.1.H296. [DOI] [PubMed] [Google Scholar]
- 32.Seitz R J, Roland P E. Acta Neurol Scand. 1992;86:60–67. doi: 10.1111/j.1600-0404.1992.tb08055.x. [DOI] [PubMed] [Google Scholar]
- 33.Auker C R, Meszler R M, Carpenter D O. J Neurophysiol. 1983;49:1504–1516. doi: 10.1152/jn.1983.49.6.1504. [DOI] [PubMed] [Google Scholar]
- 34.Ter-Pogossian M M, Eichling J O, Davis D O. Radiology. 1969;93:31–40. doi: 10.1148/93.1.31. [DOI] [PubMed] [Google Scholar]
- 35.Fox P T, Raichle M E. J Neurophysiol. 1984;51:1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- 36.Talairach J, Tournoux A. A Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 37.Minoshima S, Berger K L, Lee K S, Mintun M A. J Nucl Med. 1992;33:1579–1585. [PubMed] [Google Scholar]
- 38.Minoshima S, Koeppe R A, Mintun M A, Berger K L, Taylor S F, Frey K A, Kuhl D E. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- 39.Minoshima S, Koeppe R A, Frey K A, Kuhl D E. J Nucl Med. 1994;35:1528–1536. [PubMed] [Google Scholar]
- 40.Friston K J, Frith C D, Liddle P F, Frackowiak R S. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 41.Worsley K J, Evans A C, Marrett S, Neelin P. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 42.Adler R J, Hasofer A M. Ann Probabil. 1976;4:1–12. [Google Scholar]
- 43.Derbyshire S W, Jones A K, Gyulai F, Clark S, Townsend D, Firestone L L. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 44.Burton H, Videen T O, Raichle M E. Somatosens Mot Res. 1993;10:297–308. doi: 10.3109/08990229309028839. [DOI] [PubMed] [Google Scholar]
- 45.Goolkasian P. Psychol Women Q. 1985;9:15–28. [Google Scholar]
- 46.Gur R C, Gur R E, Obrist W D, Hungerbuhler J P, Younkin D, Rosen A D, Skolnick B E, Reivich M. Science. 1982;217:659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 47.Gur R C, Mozley L H, Mozley P D, Resnick S M, Karp J S, Alavi A, Arnold S E, Gur R E. Science. 1995;267:528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- 48.Andreason P J, Zametkin A J, Guo A C, Baldwin P, Cohen R M. Psychiatry Res. 1994;51:175–183. doi: 10.1016/0165-1781(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 49.Azari N P, Rapoport S I, Grady C L, DeCarli C, Haxby J V, Schapiro M B, Horwitz B. Brain Res. 1992;574:198–208. doi: 10.1016/0006-8993(92)90817-s. [DOI] [PubMed] [Google Scholar]
- 50.Paulson P E, Minoshima S, Morrow T J, Casey K L. Pain. 1998;76:223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casey K L, Minoshima S, Berger K L, Koeppe R A, Morrow T J, Frey K A. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 52.Casey K L, Minoshima S, Morrow T J, Koeppe R A. J Neurophysiol. 1996;76:571–581. doi: 10.1152/jn.1996.76.1.571. [DOI] [PubMed] [Google Scholar]
- 53.Klement W, Arndt J O. J Physiol (London) 1992;449:73–83. doi: 10.1113/jphysiol.1992.sp019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biemond A. A M A Arch Neurol Psychiatry. 1956;75:231–244. doi: 10.1001/archneurpsyc.1956.02330210011001. [DOI] [PubMed] [Google Scholar]
- 55.Schmahmann J D, Leifer D. Arch Neurol. 1992;49:1032–1037. doi: 10.1001/archneur.1992.00530340048017. [DOI] [PubMed] [Google Scholar]
- 56.Bassetti C, Bogousslavsky J, Regli F. Neurology. 1993;43:1942–1949. doi: 10.1212/wnl.43.10.1942. [DOI] [PubMed] [Google Scholar]
- 57.Greenspan J D, Winfield J A. Pain. 1992;50:29–39. doi: 10.1016/0304-3959(92)90109-O. [DOI] [PubMed] [Google Scholar]
- 58.Saper C B. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- 59.Craig A D, Bushnell M C, Zhang E-T, Blomqvist A. Nature (London) 1994;372:770–773. doi: 10.1038/372770a0. [DOI] [PubMed] [Google Scholar]
- 60.Craig A D, Bushnell M C. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- 61.Casey K L. J Neurophysiol. 1966;29:727–750. doi: 10.1152/jn.1966.29.4.727. [DOI] [PubMed] [Google Scholar]
- 62.Dong W K, Ryu H, Wagman I H. J Neurophysiol. 1978;41:1592–1613. doi: 10.1152/jn.1978.41.6.1592. [DOI] [PubMed] [Google Scholar]
- 63.Bushnell M C, Duncan G H. Exp Brain Res. 1989;78:415–418. doi: 10.1007/BF00228914. [DOI] [PubMed] [Google Scholar]
- 64.Vogt B A, Sikes R W, Vogt L J. In: Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Vogt B A, Gabriel M, editors. Boston: Birkhauser; 1993. [Google Scholar]
- 65.Devinsky O, Morrell M J, Vogt B A. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 66.Gabriel M, Poremba A. In: The Role of Pain in Cingulate Cortical and Limbic Thalamic Mediation of Avoidance Learning. Besson J-M, Guilbaud G, Ollat H, editors. Eurotext, Paris: John Libbey; 1995. pp. 197–212. [Google Scholar]
- 67.Hsieh J-C, Hagermark O, Stahle-Backdahl M, Ericson K, Eriksson L, Stone-Elander S, Ingvar M. J Neurophysiol. 1994;72:3004–3008. doi: 10.1152/jn.1994.72.6.3004. [DOI] [PubMed] [Google Scholar]
- 68.Kenshalo D R, Chudler E H, Anton F, Dubner R. Brain Res. 1988;454:378–382. doi: 10.1016/0006-8993(88)90841-4. [DOI] [PubMed] [Google Scholar]
- 69.Chudler E H, Anton F, Dubner R, Kenshalo D R., Jr J Neurophysiol. 1990;63:559–569. doi: 10.1152/jn.1990.63.3.559. [DOI] [PubMed] [Google Scholar]
- 70.White J C, Sweet W H. Pain and the Neurosurgeon. A Forty-Year Experience. Springfield, IL: Thomas; 1969. [Google Scholar]
- 71.Sweet W H. In: New Perspectives in Cerebral Localization. Thompson R A, Green J R, editors. New York: Raven; 1982. pp. 205–242. [Google Scholar]
- 72.Nasreddine Z S, Saver J L. Neurology. 1997;48:1196–1199. doi: 10.1212/wnl.48.5.1196. [DOI] [PubMed] [Google Scholar]
- 73.Rainville P, Duncan G H, Price D D, Carrier M, Bushnell M C. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 74.Morrow T J, Paulson P E, Danneman P J, Casey K L. Pain. 1998;75:355–365. doi: 10.1016/s0304-3959(98)00016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coderre T J, Vaccarino A L, Melzack R. Brain Res. 1990;535:155–158. doi: 10.1016/0006-8993(90)91835-5. [DOI] [PubMed] [Google Scholar]
- 76.Coderre T J, Katz J, Vaccarino A L, Melzack R. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 77.Wiebe J P, Kavaliers M. Brain Res. 1988;461:150–157. doi: 10.1016/0006-8993(88)90733-0. [DOI] [PubMed] [Google Scholar]
- 78.Brown A G. Organization in the Spinal Cord. New York: Springer; 1981. [Google Scholar]
- 79.Hentall I D, Budhrani V M. Brain Res. 1990;522:322–324. doi: 10.1016/0006-8993(90)91476-w. [DOI] [PubMed] [Google Scholar]
- 80.Yirmiya R, Ben-Eliyahu S, Shavit Y, Marek P, Liebeskind J C. Brain Res. 1990;537:169–174. doi: 10.1016/0006-8993(90)90354-e. [DOI] [PubMed] [Google Scholar]
- 81.Cohen S R, Melzack R. Brain Res. 1985;359:131–139. doi: 10.1016/0006-8993(85)91420-9. [DOI] [PubMed] [Google Scholar]
- 82.Cohen S R, Melzack R. Neurosci Lett. 1986;70:165–169. doi: 10.1016/0304-3940(86)90457-x. [DOI] [PubMed] [Google Scholar]
- 83.Mao J, Mayer D J, Price D D. J Neurosci. 1993;13:2689–2702. doi: 10.1523/JNEUROSCI.13-06-02689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaas J H. Annu Rev Neurosci. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- 85.Dubner R, Ruda M A. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 86.Kaas J H, Florence S L, Neeraj J. Neuroscientist. 1997;3:123–130. [Google Scholar]
- 87.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Nature (London) 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 88.Casey K L. Pain and Central Nervous System Disease: The Central Pain Syndromes. New York: Raven; 1991. pp. 1–280. [Google Scholar]