Abstract
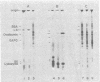
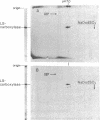
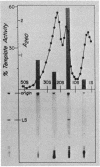
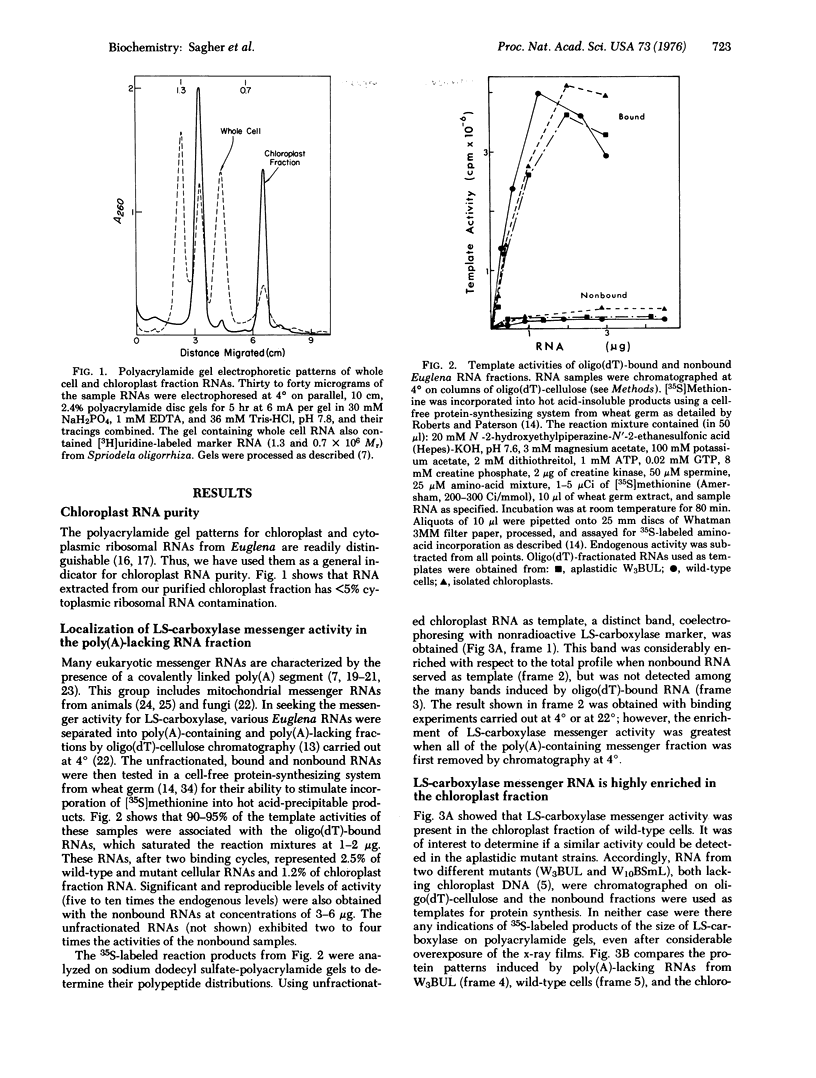
RNA from wild-type Euglena, aplastidic mutant cells, and purified chloroplasts was chromatographed on oligo(dT)-cellulose at 4 degrees. The poly(A)-containing and poly(A)-lacking fractions from each were then tested in a cell-free protein-synthesizing system from wheat germ for their abilities to specifically stimulate synthesis of large subunit ribulosebisphosphate carboxylase [EC 4.1.1.39; 3-phospho-D-glycerate carboxy-lyase (dimerizing)]. The large subunit polypeptide (59,000 molecular weight) was identified in the in vitro reaction by two-dimensional electrophoresis involving isoelectric focusing and size filtration on polyacrylamide gels. Template activity for the large subunit was detected in wild-type cells but not in aplastidic mutant strains; it was highly enriched in the isolated chloroplasts. This messenger was present only in the poly(A)-lacking RNA fraction, where it constituted the most prominent template species of chloroplast RNA. The large subunit message was freed of considerable non-messenger RNA contamination and localized to the 10-20S fraction by sucrose gradient centrifugation.
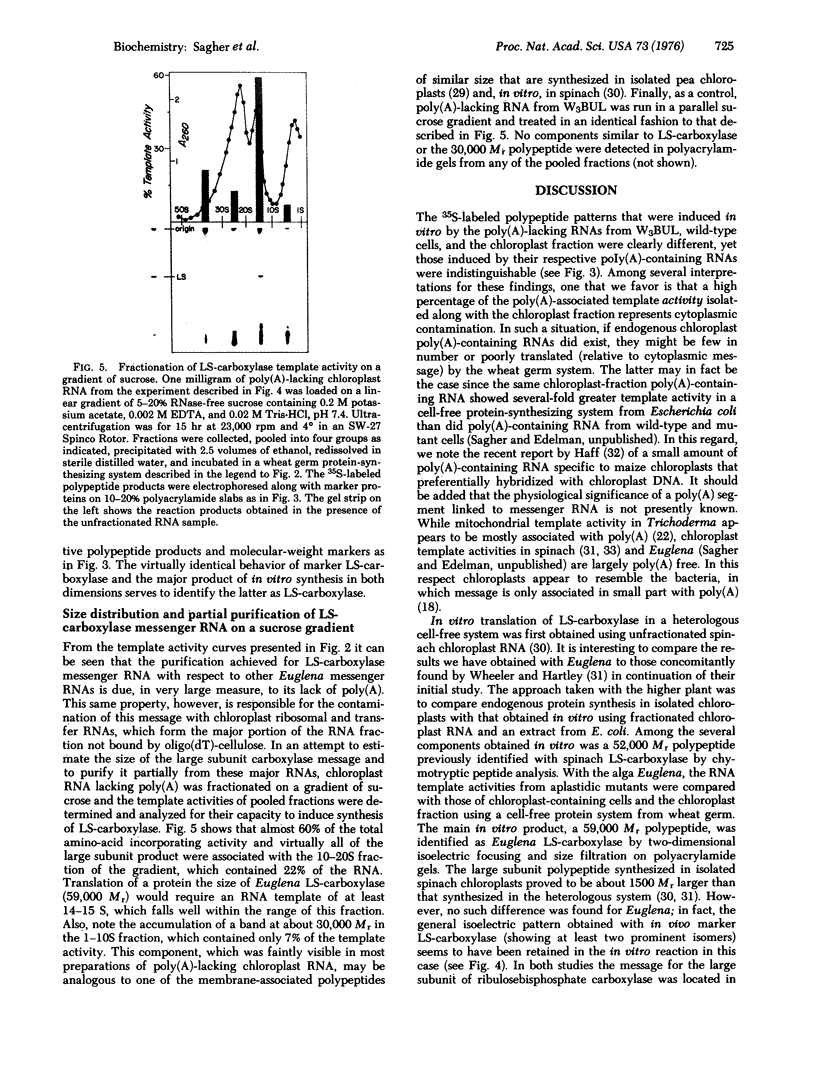
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN M., SCHIFF J. A., EPSTEIN H. T. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA. XII. TWO TYPES OF SATELLITE DNA. J Mol Biol. 1965 Apr;11:769–774. doi: 10.1016/s0022-2836(65)80034-1. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite J. J., Bogorad L. A one-step method for the isolation and determination of leaf ribulose-1,5-diphosphate carboxylase. Anal Biochem. 1971 May;41(1):57–66. doi: 10.1016/0003-2697(71)90191-6. [DOI] [PubMed] [Google Scholar]
- Grierson D., Loening U. E. Distinct transcription products of ribosomal genes in two different tissues. Nat New Biol. 1972 Jan 19;235(55):80–82. doi: 10.1038/newbio235080a0. [DOI] [PubMed] [Google Scholar]
- Hartley M. R., Wheeler A., Ellis R. J. Protein synthesis in chloroplasts. V. Translation of messenger RNA for the large subunit of fraction I protein in a heterologous cell-free system. J Mol Biol. 1975 Jan 5;91(1):67–77. doi: 10.1016/0022-2836(75)90372-1. [DOI] [PubMed] [Google Scholar]
- Heizmann P. Propriétés des ribosomes et des RNA ribosomiques d'Euglena gracilis. Biochim Biophys Acta. 1970 Nov 12;224(1):144–154. [PubMed] [Google Scholar]
- Hirsch M., Penman S. Mitochondrial polyadenylic acid-containing RNA: localization and characterization. J Mol Biol. 1973 Nov 5;80(3):379–391. doi: 10.1016/0022-2836(73)90410-5. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A., Luginbill B., Feeley J. Polysome formation with tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1243–1250. doi: 10.1073/pnas.59.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A. Autotrophic CO2 assimilation and the evolution of ribulose diphosphate carboxylase. Bacteriol Rev. 1973 Sep;37(3):289–319. doi: 10.1128/br.37.3.289-319.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato H., Venkatesan S., Edmonds M. Polyadenylic acid sequences in E. coli messenger RNA. Nature. 1975 Jul 10;256(5513):144–146. doi: 10.1038/256144a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Identification and partial characterization of multiple discrete polyadenylic acid containing RNA components coded for by HeLa cell mitochondrial DNA. J Mol Biol. 1974 Sep 5;88(1):205–219. doi: 10.1016/0022-2836(74)90305-2. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Rabinowitz H., Reisfeld A., Sagher D., Edelman M. Ribulose Diphosphate Carboxylase from Autotrophic Euglena gracilis. Plant Physiol. 1975 Sep;56(3):345–350. doi: 10.1104/pp.56.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson J. R., Stutz E. Isolation and characterization of Euglena gracilis cytoplasmic and chloroplast ribosomes and their ribosomal RNA components. Biochim Biophys Acta. 1969 Oct 22;190(2):368–380. doi: 10.1016/0005-2787(69)90087-2. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D., Edelman M., Galun E. Characterization of polyadenylate from the fungus Trichoderma viride. J Bacteriol. 1975 Aug;123(2):765–767. doi: 10.1128/jb.123.2.765-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagher D., Edelman M., Jakob K. M. Poly(A)-associated RNA in plants. Biochim Biophys Acta. 1974 Apr 27;349(1):32–38. doi: 10.1016/0005-2787(74)90005-7. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Avron M. High biological activity in chloroplasts from Euglena gracilis prepared with a new gas pressure device. FEBS Lett. 1970 Jun 1;8(3):164–166. doi: 10.1016/0014-5793(70)80253-8. [DOI] [PubMed] [Google Scholar]
- Wheeler A. M., Hartley M. R. Major mRNA species from spinach chloroplasts do not contain poly(A). Nature. 1975 Sep 4;257(5521):66–67. doi: 10.1038/257066a0. [DOI] [PubMed] [Google Scholar]