Abstract
We assessed the safety and efficacy of combined intravenous and aerosolized antioxidant administration to attenuate chlorine gas–induced airway alterations when administered after exposure. Adult male Sprague-Dawley rats were exposed to air or 400 parts per million (ppm) chlorine (a concentration likely to be encountered in the vicinity of industrial accidents) in environmental chambers for 30 minutes, and returned to room air, and they then received a single intravenous injection of ascorbic acid and deferoxamine or saline. At 1 hour and 15 hours after chlorine exposure, the rats were treated with aerosolized ascorbate and deferoxamine or vehicle. Lung antioxidant profiles, plasma ascorbate concentrations, airway morphology, and airway reactivity were evaluated at 24 hours and 7 days after chlorine exposure. At 24 hours after exposure, chlorine-exposed rats had significantly lower pulmonary ascorbate and reduced glutathione concentrations. Treatment with antioxidants restored depleted ascorbate in lungs and plasma. At 7 days after exposure, in chlorine-exposed, vehicle-treated rats, the thickness of the proximal airways was 60% greater than in control rats, with twice the amount of mucosubstances. Airway resistance in response to methacholine challenge was also significantly elevated. Combined treatment with intravenous and aerosolized antioxidants restored airway morphology, the amount of airway mucosubstances, and airway reactivity to control levels by 7 days after chlorine exposure. Our results demonstrate for the first time, to the best of our knowledge, that severe injury to major airways in rats exposed to chlorine, as characterized by epithelial hyperplasia, mucus accumulation, and airway hyperreactivity, can be reversed in a safe and efficacious manner by the post-exposure administration of ascorbate and deferoxamine.
Keywords: epithelial injury, epithelial repair, mucosubstances, ascorbate, deferoxamine, aerosol
Clinical Relevance
Accidental exposures to chlorine gas pose a significant public health concern, and can result in severe pulmonary injury. No chlorine-specific post-exposure therapy currently exists. Our results demonstrate for the first time, to the best of our knowledge, that the severe injury to major airways in rats exposed to chlorine, as characterized by epithelial hyperplasia, mucus accumulation, and airway hyperreactivity, can be reversed in a safe and efficacious manner by the post-exposure administration of ascorbate and deferoxamine.
Chlorine is essential to global industry and to global public health. According to the World Chlorine Council (http://www.worldchlorine.org), 14.4 million metric tons were produced in North America in 2008, and 62.8 million metric tons were produced globally. Water treatment makes up only 5% of the world's use of chlorine. The majority of chlorine is used in the production of plastics such as polyvinyl chloride (1). It is also used as a bleaching agent for pulp and paper production, as feedstock in the production of chlorinated solvents used in metalworking, in dry cleaning, in electronics, and in pharmaceutical production (1–6).
Only 20 American states contain facilities that produce chlorine, but every American state has facilities that use chlorine. This results in the shipping of chlorine by railcar, and increases the potential for large-scale accidents. According to the Environmental Protection Agency, chlorine gas was related to 518 serious accidents over a 5-year period during the 1990s (1). Multiple-casualty exposures to chlorine have resulted from industrial accidents involving chlorine tank ruptures on railcars here in the United States, and from the deliberate release of chlorine gas by terrorists as a chemical weapon in Iraq (7, 8). In addition, exposures to high concentrations of chlorine gas have resulted from accidental releases during recreational swimming pool water treatment (9–11) and from household accidents when mixing bleach with acidic cleaners (12–15).
Clinically, at low concentrations, the effects of inhaled chlorine can range from local irritation to increasing inflammation, bronchospasm, cough, and dyspnea. At higher concentrations, severe airway epithelial cell injury, pulmonary edema, and death may occur (16, 17). No specific treatment for chlorine exposure exists; symptoms are treated as they present. For the more severe symptoms such as a significant cough, difficulty breathing, or bronchospasm, 100% humidified oxygen in addition to β2-agonists and corticosteroids may be used (14). The delivery of 100% oxygen may contribute additional oxidative stress to the lung. Exposure to chlorine may result in the development of chronic respiratory problems such as reactive airway disease in some patients (7, 18–20).
Previously, we showed that the post-exposure treatment of mice with the β2-agonist arformoterol decreased chlorine-induced airway hyperreactivity (21), and that both prophylactic and post-exposure treatment with systemically administered and aerosolized low-molecular weight antioxidants (22, 23) decreased chlorine-induced lung injury and mortality in both mice and rats. The administration of inhaled antioxidants is necessary for direct delivery to major airways and distal lung spaces, the major sites of injury from inhaled chlorine in rodents.
According to some indications, acute, high-concentration exposures to chlorine may result in long-term pulmonary alterations in rats (24). However, the long-term sequelae of lung epithelial cell injury because of chlorine exposures, and the potential protective effects of post-exposure administrations of antioxidants, have not been documented. Here, we exposed rats to 400 parts per million (ppm) chlorine for 30 minutes, returned them to room air, and assessed the extent of airway injury using biochemical, physiological, and quantitative morphological techniques up to 7 days after exposure. After establishing the existence of significant airway alterations at 7 days after exposure, we assessed the safety and efficacy of the post-exposure administration of an intravenous mixture of ascorbate and deferoxamine, followed by two aerosolized administrations of ascorbate at 1 hour and 15 hours after exposure, to decrease the extent of injury, most likely by enhancing repair. Some of the results from these studies were previously reported in the form of an abstract (25).
Materials and Methods
In compliance with the regulations and approval of the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham, adult male Sprague-Dawley rats (200–250 g body weight; Harlan, Inc., Indianapolis, IN) were housed under standard conditions, with food and water provided ad libitum. Rats were exposed to air or 400 ppm chlorine in air for 30 minutes, as previously described (22, 23).
Peripheral oxygen saturations and respiratory rates were measured before and after chlorine gas (Cl2) exposure and aerosol administrations (26). Saline or freshly prepared ascorbic acid (80 mg/kg) and deferoxamine mesylate (15 mg/kg), at 0.2-ml volume, were administered intravenously within 5 minutes after chlorine exposure. At 1 hour and 15 hours after chlorine exposure, rats received an aerosol of saline or ascorbic acid (150 mg/ml) and deferoxamine mesylate (0.357 mg/ml) by nose-only inhalation for 60 minutes (23), with some modifications (as described in the online supplement).
At 24 hours or 7 days after chlorine exposure, the rats were deeply anesthetized via an intraperitoneal injection of 0.4 ml diazepam (5 mg/ml) and 0.4 ml ketamine HCl (5 mg/ml). For biochemical analysis, blood was drawn from the left ventricle of the heart with heparin to prevent agglutination, and centrifuged at 700 × g for 10 minutes at 4°C. The plasma was stabilized with an equal volume of 10% metaphosphoric acid (MPA), and stored at −80°C for measurements of ascorbate and reduced glutathione by HPLC (22). Lungs were flushed free of remaining blood, removed, weighed, homogenized in an equal volume to tissue weight of 10% MPA, and stored at −80°C for the determination of ascorbate and reduced glutathione concentrations (22). Seven days after exposure, we measured airway resistance before and after the methacholine challenge in all groups, using the flexiVent system (SCIREQ, Montreal, PQ, Canada). For details, see the online supplement.
For histology, lungs were inflated via a tracheal cannula with 10% formalin at 25 cm H2O pressure for 1 hour, removed en bloc, and immersed in 10% formalin for an additional 48 hours. The left lung was microdissected to ensure that the proximal airways were between intrapulmonary airway generations 2–3, cut perpendicular to the long axis. Three sections were paraffin-processed and stained with hematoxylin and eosin or Alcian blue/period acid–Schiff (AB/PAS) stain (27). Images (×40 magnification; 5-μm-thick sections) were recorded on an Olympus BX41 microscope with a Q-Color3 digital camera attached to a computer, using QCapture software (QImaging, Surrey, BC, Canada), and composed in Adobe Photoshop. Morphometric analyses of midlevel airway epithelium (intrapulmonary generations 2–3) and terminal bronchioles followed standard procedures (28). Details are presented in the online supplement.
Data are presented as means ± standard errors (biochemical assays) or standard deviations (morphometric data). Statistical analyses were performed with Graph Pad InStat software (GraphPad Software, Inc., San Diego, CA). Significance was determined using one-way ANOVA and the Bonferroni multiple comparisons test.
Results
When returned to room air, the chlorine-exposed rats were cyanotic, with labored breathing and significantly depressed respiratory rates and peripheral oxygen saturations (Tables 1 and 2). Respiratory rates and oxygen saturations improved gradually over time, and returned to their control values by 24 hours after exposure (Tables 1 and 2). No significant differences were evident among chlorine-exposed rats treated with vehicle or antioxidants.
TABLE 1.
RESPIRATORY RATES OF RATS EXPOSED TO AIR OR 400 PARTS PER MILLION CHLORINE*
| Respiratory Rates | ||||
| Air |
Chlorine (400 ppm) |
|||
| Number of Hours after Air or Chlorine | Vehicle | Antioxidants | Vehicle | Antioxidants |
| 0 | 127 ± 3 (16) | 124 ± 5 (16) | 131 ± 2 (24) | 125 ± 3 (21) |
| 0.1 | N/A | N/A | 70 ± 3 (24)† | 75 ± 2 (21)† |
| 1 | 129 ± (16) | 124 ± 4 (16) | 78.33 ± 4 (24)† | 78.29 ± 4 (21)† |
| 2 | 127 ± 4 (16) | 127 ± 5 (16) | 101 ± 4 (24)† | 101 ± 4 (21)† |
| 15 | 125 ± 4 (16) | 123 ± 4 (16) | 116 ± 3 (24)† | 114 ± 2 (21)† |
| 24 | 126 ± 4 (16) | 125 ± 3 (16) | 121 ± 3 (24) | 117 ± 3 (21) |
| 48 | N/A | N/A | 115 ± 2 (8) | 113 ± 2 (8) |
| 168 | N/A | N/A | 118 ± 3 (8) | 113 ± 3 (8) |
| 192 | N/A | N/A | 117 ± 2 (8) | 119 ± 3 (8) |
Definition of abbreviations: N/A, measurements were not performed at this time point; ppm, parts per million.
Data are expressed as mean ± SEM (n).
P < 0.01, compared with the corresponding 0 hour value in the same group (one-way ANOVA followed by selective comparisons of means with the Bonferroni modification of the t test, adjusted for multiple comparisons).
TABLE 2.
PERIPHERAL OXYGEN SATURATIONS IN RATS EXPOSED TO AIR OR 400 PPM CHLORINE*
| O2 Saturations | ||||
| Air |
Chlorine (400 ppm) |
|||
| Number of Hours after Air or Chlorine | Vehicle | Antioxidant | Vehicle | Antioxidant |
| 0 | 95.46 ± 0.27 (16) | 95.29 ± 0.28 (16) | 95.20 ± 0.20 (24) | 94.74 ± 0.19 (21) |
| 0.1 | N/A | N/A | 78.43 ± 0.68 (24)† | 78.12 ± 0.85 (21)† |
| 1 | 94.29 ± 0.53 (16) | 94.68 ± 0.24 (16) | 82.24 ± 0.64 (24)† | 83.64 ± 0.86 (21)† |
| 2 | 93.41 ± 0.68 (16) | 93.74 ± 0.21 (16) | 88.08 ± 1.09 (24)† | 91.16 ± 0.41 (21)† |
| 15 | 95.16 ± 0.20 (16) | 94.09 ± 0.36 (16) | 91.09 ± 0.85 (24)† | 91.56 ± 0.72 (21)† |
| 24 | 94.72 ± 0.28 (16) | 94.98 ± 0.28 (16) | 93.47 ± 0.34 (24) | 93.99 ± 0.29 (21) |
| 48 | N/A | N/A | 93.04 ± 0.38 (8) | 93.50 ± 0.26 (8) |
| 168 | N/A | N/A | 93.47 ± 0.38 (8) | 92.74 ± 0.27 (8) |
| 192 | N/A | N/A | 93.11 ± 0.17 (8) | 94.27 ± 0.30 (8) |
Definition of abbreviations: N/A, measurements were not performed at this time point; ppm, parts per million.
Data are expressed as mean ± SEM (n).
P < 0.01, compared with the corresponding 0-hour value in the same group (one-way ANOVA followed by selective comparisons of means with the Bonferroni modification of the t test, adjusted for multiple comparisons).
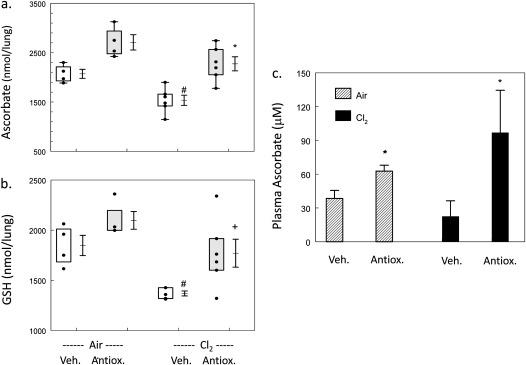
Rats exposed to chlorine and returned to room air for 24 hours had significantly lower values of ascorbate and reduced glutathione in their lungs (Figures 1a and 1b). Treatment with intravenous and aerosolized ascorbate and deferoxamine restored depleted ascorbate and glutathione (GSH) concentrations in lungs and plasma (Figures 1a–1c). At 7 days after exposure, ascorbate concentrations in the lung tissue of rats exposed to chlorine and treated with antioxidants were 50% higher than those in chlorine-exposed rats receiving vehicle treatment (data not shown). We calculated that each rat inhaled approximately 30 mg of ascorbate per treatment and 0.07 mg deferoxamine. Based on the mean geometric diameter of the aerosolized particles (3 μm) (23) (see online supplement for further details) and on the findings of previous studies (29), we calculated that approximately 5–10% of the inhaled ascorbate (1.5–3 mg) and deferoxamine (0.004–0.007 mg) reached the pulmonary airways. The rest was deposited in either the nose or upper airways.
Figure 1.
Systemic and aerosolized antioxidant administration after chlorine exposure increases ascorbate and reduced glutathione (GSH) values in rat lungs and in plasma. Rats were exposed to air or 400 parts per million (ppm) chlorine for 30 minutes and treated with vehicle or intravenous and aerosolized antioxidants, as described in Materials and Methods, and evaluated 24 hours after chlorine exposure. (a) Ascorbate concentrations in lung tissue: box–whisker plots show individual points, boxes (25th to 75th percentiles of the data), and means ± 1 SE. Exposure to chlorine decreased lung ascorbate concentrations, and the administration of antioxidants restored values to control concentrations. #P < 0.05, compared with the corresponding air value. *P < 0.01, compared with the corresponding vehicle value in the same group. One-way ANOVA was followed by the Tukey t test, adjusted for multiple comparisons. (b) GSH concentrations in lung tissue: box–whisker plots show individual points, boxes (25th to 75th percentiles of the data), and means ± 1 SE. Exposure to chlorine decreased lung GSH concentrations, and the administration of antioxidants (Antiox.) restored values to control levels. #P < 0.05, compared with the corresponding air value. *P < 0.05, compared with the corresponding vehicle (Veh.) value in the same group. One-way ANOVA was followed by the Tukey t test, adjusted for multiple comparisons. (c) Concentrations of ascorbate in plasma: values represent means ± 1 SE. Numbers of samples: air, n = 4 each; chlorine/vehicle, n = 6; chlorine/antioxidant, n = 5. Plasma ascorbate concentrations were significantly increased in all rats treated with antioxidants, regardless of chlorine exposure. *Significantly different from exposure-matched, vehicle-treated control rats, P < 0.05. One-way ANOVA was followed by the Tukey t test, adjusted for multiple comparisons.
Histological Evaluation of the Airways at 24 Hours after Chlorine Exposure
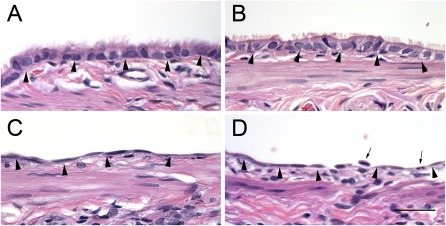
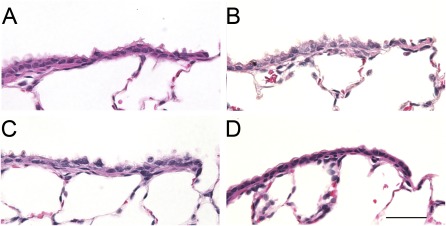
Figures 2 and 3 show the typical appearances of proximal and terminal bronchial airway epithelia of air control rats and chlorine-exposed rats. Three cell types were evaluated: (1) nonciliated cells (identified by their lack of cilia, and nuclei located away from the basal lamina); (2) ciliated cells (identified by cilia on the luminal sides, and nuclei located near the basal lamina); and (3) basal cells (identified by their triangular shape, and nuclei near the basal lamina with cytoplasm that did not reach the lumen). Cells that were not identifiable as one of the three defined cells types were defined as “other.” Proximal airways consisted of a majority of ciliated cells, with a smaller number of nonciliated cells and basal cells, whereas terminal bronchioles consisted of a majority of nonciliated cells, with fewer ciliated cells (Figures 2 and 3 and Table 3). Proximal and terminal airways from air-exposed rats treated with intravenous and aerosolized antioxidants (Figures 2B and 3B) looked very similar to airways of rats treated with vehicle (Figures 2A and 3A). Quantitative assessment indicated that antioxidant treatment alone did not significantly alter the epithelial composition of airways, compared with the airways of vehicle-treated rats (Table 3). Histological examination of proximal airways from rats 24 hours after exposure to 400 ppm chlorine indicated severe epithelial injury. The majority of the proximal airways are lined with flattened, squamous epithelium (Figure 2C). Quantitative assessment indicated a significant decrease in the ciliated cell volume, basal cell volume, and overall thickness of the epithelium (Table 3). Proximal airways from rats exposed to 400 ppm chlorine and treated with aerosolized antioxidant looked similar to those in rats exposed to 400 ppm chlorine and treated with aerosolized vehicle (Figure 2C). Histologically, the terminal bronchioles of rats exposed to 400 ppm chlorine and treated with either vehicle or antioxidants looked similar to those in air-exposed rats (Figures 3C and 3D), although focal areas of injury in the gas-exchange region could be identified. Quantitatively, a significant decrease was evident in the ciliated cell volume of both chlorine-exposed groups compared with control rats, but no significant difference in the cell volume of nonciliated cells was observed at this airway level. The epithelial thickness of the terminal bronchioles was also not significantly different from that in control rats, because of the variability in response at this airway level (Table 3).
Figure 2.
Histopathological effects of chlorine inhalation in proximal airways 24 hours after exposure. Rats were exposed to air or 400 ppm chlorine for 30 minutes and treated with vehicle or intravenous and aerosolized antioxidants, as described in Materials and Methods. Tissue was collected 24 hours after exposure, embedded in paraffin, and stained with hematoxylin and eosin. Representative light micrographs are of proximal airways from air-exposed rats treated with vehicle (A) or antioxidants (B), or from rats exposed to 400 ppm chlorine for 30 minutes and treated with vehicle (C) or antioxidants (D). Arrowheads, basal lamina; arrows, neutrophils. Magnification bar = 50 μm.
Figure 3.
Histopathological effects of chlorine inhalation in terminal bronchioles, 24 hours after exposure. Rats were exposed to air or 400 ppm chlorine for 30 minutes and treated with vehicle or intravenous and aerosolized antioxidants, as described in Materials and Methods. Tissues were collected 24 hours after exposure, embedded in paraffin, and stained with hematoxylin and eosin. Representative light micrographs are of proximal airways from air-exposed rats treated with vehicle (A) or antioxidants (B), or from rats exposed to 400 ppm chlorine for 30 minutes and treated with vehicle (C) or antioxidants (D). Magnification bar = 50 μm.
TABLE 3.
QUANTITATIVE ASSESSMENT OF CHLORINE-INDUCED ALTERATIONS IN EPITHELIAL CELL VOLUME AND EPITHELIAL THICKNESS IN AIRWAYS OF RATS, 24 HOURS AFTER CHLORINE EXPOSURE
| Air |
Chlorine (400 ppm) |
|||
| Vehicle | Antioxidants | Vehicle | Antioxidants | |
| Proximal airways | ||||
| Ciliated cell volume* | 7.63 ± 1.11 | 5.94 ± 1.08 | 0.51 ± 0.46‡ | 0.26 ± 0.34‡§ |
| Nonciliated cell volume* | 1.70 ± 0.42 | 1.58 ± 0.40 | 3.07 ± 1.35 | 2.43 ± 1.08 |
| Basal cell volume* | 1.05 ± 0.30 | 0.82 ± 0.29 | 0.03 ± 0.03‡ | 0.08 ± 0.09‡§ |
| Epithelial thickness† | 11.30 ± 2.34 | 8.60 ± 1.43 | 3.73 ± 1.41‡ | 2.82 ± 1.22‡§ |
| Terminal bronchioles | ||||
| Ciliated cell volume* | 2.76 ± 1.02 | 3.07. ± 1.32 | 1.20 ± 0.57‡ | 1.01 ± 0.48‡§ |
| Nonciliated cell volume* | 5.77 ± 0.72 | 5.53 ± 0.84 | 5.88 ± 0.96 | 6.04 ± 2.48 |
| Epithelial thickness† | 8.58 ± 0.58 | 8.37 ± 0.70 | 7.15 ± 1.37 | 7.07 ± 2.12 |
Definition of abbreviation: ppm, parts per million.
Data are expressed as μm3/μm2 basal lamina, mean ± SD, n = 4–6 per group.
Data are expressed as microns, mean ± SD, n = 4–6 per group.
Significantly different from air/saline control values, P < 0.05 (ANOVA, Holm-Sidak multiple comparisons test).
Significantly different from air/antioxidant control values, P < 0.05 (ANOVA, Holm-Sidak multiple comparisons test).
Histological Evaluation of the Airways at 7 Days after Chlorine Exposure
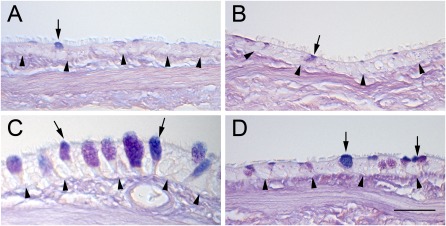
Normal AB/PAS-stained positive mucous cells in the proximal airways of control rats are shown in Figure 4A. Proximal airways from air-exposed rats and treated with aerosolized antioxidants looked very similar to control airways (Figure 4B). Histological examination of proximal airways from rats 7 days after exposure to 400 ppm chlorine produced results consistent with the presence of significant mucous cell hyperplasia, as shown by the increase in AB/PAS-positive cells and a significant increase in airway epithelial thickness (Figure 4C and Table 4). Proximal airways from rats exposed to 400 ppm chlorine and treated with aerosolized antioxidant had fewer AB/PAS-positive mucous cells and thinner epithelia than rats exposed to 400 ppm chlorine followed by aerosolized vehicle (Figure 4D and Table 4). In the terminal bronchioles, quantitative assessment indicated no difference between air-exposed, vehicle-treated rats and any of the other exposure or treatment groups at this time point (Table 4).
Figure 4.
Histopathological effects of chlorine inhalation in proximal airways, 7 days after exposure. Rats were exposed to air or 400 ppm chlorine for 30 minutes and treated with vehicle or intravenous and aerosolized antioxidants, as described in Materials and Methods. Tissue was collected 7 days after exposure, embedded in paraffin, and stained with Alcian blue and period acid–Schiff stain. Representative light micrographs are of proximal airways from air-exposed rats treated with vehicle (A) or antioxidants (B), or from rats exposed to 400 ppm chlorine for 30 minutes and treated with vehicle (C) or antioxidants (D). Arrows, mucous cells; arrowheads, basal lamina. Magnification bar = 50 μm.
TABLE 4.
QUANTITATIVE ASSESSMENT OF CHLORINE-INDUCED ALTERATIONS IN MUCOSUBSTANCES AND EPITHELIAL THICKNESS IN AIRWAYS OF RATS, 7 DAYS AFTER CHLORINE EXPOSURE
| Air |
Chlorine (400 ppm) |
|||
| Vehicle | Antioxidants | Vehicle | Antioxidants | |
| Proximal airways | ||||
| Mucosubstances* | 1.67 ± 0.61 | 1.82 ± 1.15 | 3.55 ± 1.89¶ | 1.35 ± 0.49# |
| Ciliated cell volume† | 7.40 ± 1.41 | 7.38 ± 3.40 | 12.84 ± 4.43 | 6.53 ± 2.74# |
| Nonciliated cell volume†‡ | 2.36 ± 0.10 | 2.18 ± 0.63 | 4.07 ± 1.52 | 2.32 ± 0.90 |
| Basal cell volume† | 0.92 ± 0.25 | 0.90 ± 0.44 | 0.96 ± 0.43 | 0.78 ± 0.17 |
| Epithelial thickness§ | 10.07 ± 1.58 | 10.47 ± 3.60 | 17.89 ± 5.93¶ | 9.66 ± 3.05§ |
| Terminal bronchioles | ||||
| Ciliated cell volume† | 1.96 ± 0.83 | 2.02 ± 0.45 | 2.76 ± 0.60 | 1.69 ± 0.50 |
| Nonciliated cell volume† | 6.40 ± 0.83 | 4.17 ± 0.61 | 5.08 ± 0.83 | 5.03 ± 0.89 |
| Epithelial thickness§ | 8.39 ± 1.50 | 6.19 ± 0.86¶ | 7.87 ± 1.27 | 6.74 ± 0.71 |
Definition of abbreviation: ppm, parts per million.
Data are expressed as nl/mm2 basal lamina, mean ± SD, n = 6 per group.
Data are expressed as μm3/μm2, mean ± SD, n = 6 per group.
Nonciliated group includes mucous, nonmucous, and nonciliated cells.
Data are expressed as microns, mean ± SD, n = 6 per group.
Significantly different from air/saline control values, P < 0.05 (ANOVA, Dunnett multiple comparisons test).
Significantly different from 400 ppm/saline control values, P < 0.05 (ANOVA, Holm-Sidak multiple comparisons test).
Measurement of Airway Reactivity 7 Days after Chlorine Exposure
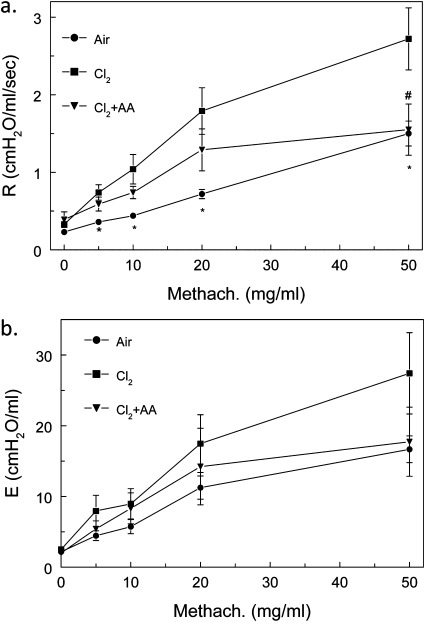
To correlate changes in structure with function, we measured airway resistance (Figure 5a) and elastance (Figure 5b) before and after challenge with aerosolized methacholine in rats exposed to chlorine and returned to room air for 7 days. As shown in Figure 5A, rats exposed to chlorine developed significant airway hyperreactivity when challenged with methacholine. These data agree with our previous measurements in mice exposed to 400 ppm chlorine for 30 minutes and returned to room air for 7 days (21), although the levels of airway resistance in rats were considerably smaller than in mice. Rats exposed to air and treated with antioxidants had similar baseline and methacholine-treated airway resistances as nontreated rats. For this reason, we combined the air-exposed, nontreated, and antioxidant-treated rats into a single group (labeled “air”). These data indicate that the post-exposure administration of antioxidants causes no deleterious effects. In addition, the post-exposure administration of antioxidants in chlorine-treated rats decreased airway reactivity during methacholine challenge. The values were similar to those in control rats for a methacholine dose of 50 mg/ml. A similar trend was observed for elastance. However, because of the large variance, the results did not reach statistical significance.
Figure 5.
Measurements of airway resistance (R) and elastance (E) in chlorine-exposed rats, 7 days after exposure. Rats were exposed to air or 400 ppm chlorine for 30 minutes and treated with vehicle or intravenous and aerosolized antioxidants (AA), as described in Materials and Methods. At 7 days after exposure, the rats were connected to a flexiVent for measurements of airway resistance (a) and elastance (b), as described in Materials and Methods. Rats exposed to air and treated with antioxidants had baseline and methacholine (Methach.)–treated airway resistances similar to those of nontreated rats. For this reason, we combined the air-exposed nontreated and antioxidant-treated rats into a single group (labeled air). Values represent means ± 1 SEM. Numbers of data: air, n = 21; chlorine, n = 14; chlorine + antioxidants, n = 7. *,#P < 0.05, compared with corresponding chlorine values (ANOVA followed by the Bonferroni t test).
Discussion
Chlorine gas exposures pose a significant public health concern because of the amount of chlorine that is transported throughout the United States (1). In addition to the mortalities resulting from industrial accidents involving chlorine tank ruptures on railcars here in the United States, as well as from deliberate release by terrorists as a chemical weapon in Iraq, many individuals who survive the exposure will develop delayed respiratory complications (7). The importance of developing a rescue treatment to help severely injured people while not harming those less severely injured cannot be overstated.
We hypothesized and verified (6) that exposure to high concentrations of chlorine gas (> 100 ppm) will result in the depletion of low molecular weight lung antioxidants. Using a competition kinetics analysis, we showed that inhaled chlorine gas will first react with low molecular weight oxidant scavengers in the epithelial lining fluid (such as ascorbate, uric acid, and reduced glutathione) before undergoing hydrolysis or reaction with cellular targets (6). Previous studies by our group showed that a 30-minute exposure to 400 ppm of chlorine results in a significant and sustained depletion of lung ascorbate (22). We therefore reasoned that the administration of antioxidants (in particular, ascorbate) that are not produced by humans and must be obtained from the diet will help restore basal pulmonary levels and facilitate recovery when administered after exposure to chlorine. Similarly, because focal hemorrhagic damage was observed in the distal lung regions (22), we reasoned that including deferoxamine in the intravenous administration will help by chelating Fe3+ released at hemorrhagic sites and thereby prevent damage attributable to Fenton reactions. In addition, deferoxamine will help maintain ascorbate in the reduced state, thus allowing it to act as an antioxidant. The combined administration of aerosolized and intravenous antioxidants was used for two reasons: first, based on the results of previous morphological, biochemical, and physiological studies, we showed that inhaled chlorine and its reactive intermediates will deplete antioxidants in the lung epithelial lining fluid, and then damage primarily airway and alveolar epithelial cells, resulting in airway hyperreactivity, mucous hyperplasia, increased permeability to plasma proteins, and compromised ion transport (21–23, 26, 30). The administration of aerosols with radii of 2–4 μm constitutes the most effective way of replenishing airway and distal lung antioxidants. Second, we showed that the exposure of rats to chlorine causes systemic injury, characterized by inflammation and endothelial dysfunction because of the inactivation of endothelial nitric oxide synthase, an event linked to atherosclerosis and hypertension (31). We thus argued that to augment antioxidant defenses in the plasma would be important.
Ascorbate supplementation has been shown in both animals and humans to decrease oxidant lung and systemic injury. For example, the oral and intravenous administration of ascorbate decreased the severity of acute lung injury in patients with severe burns (32) and the incidence of organ failure in septic surgical patients, when coadministered with vitamin E (33). Similarly, the combined administration of ascorbate and vitamin E improved 28-day survival in patients with sepsis (34). Ascorbate may also have a role in down-regulating hypoxia-inducible transcription factor (HIF)-1α protein levels (35). Ascorbate inhibits endothelial cell nicotinamide adenine dinucleotide phosphate-reduced oxidase (36), and decreases the lipid peroxidation that leads to the initiation of local and systemic inflammation (37–39). Deferoxamine alone is used clinically in chronic iron overload (37) and as an antidote in acute iron poisoning (40), and it was found to decrease protein tyrosine nitration (41).
The coadministration of ascorbate and deferoxamine reversed chlorine gas–induced lipid peroxidation in the bronchoalveolar lavage fluid (BALF) of mice (23), underscoring the importance of these antioxidants in scavenging various reactive species that are formed after chlorine exposures. They may also be beneficial by modulating and decreasing post-exposure inflammation. Mice exposed to high concentrations of chlorine (400–800 ppm for 5–30 minutes) demonstrated alveolar damage, the oxidation of lung proteins, proteinaceous exudates, and inflammatory cells in alveoli and lavage fluid, as well increased airway hyperreactivity to methacholine. This response was postulated to be caused by oxidative stress (21, 42). A 3-month observation of rats exposed to 1,500 ppm chlorine for 5 minutes showed epithelial flattening, necrosis, smooth muscle mass elevation, and an increase in the number of neutrophils in BALF (43). Isolated rabbit lungs that were exposed to 500 ppm chlorine demonstrated that alveolar edema could be explained in terms of epithelial injury (44). Moreover, chlorine was suggested to compound lung injury and edema by increasing microvascular permeability (44). Previous studies showed that the post-exposure administration of a β2-agonist decreases chlorine-induced airway hyperreactivity in mice (21) by increasing lung cyclic adenosine monophosphate content, depleted after exposure to chlorine. Systemically administered dexamethasone after exposure to chlorine decreases chlorine-induced airway hyperreactivity in rats and decreases some indices of lung injury (45), and the post-exposure systemic administration of ascorbic acid and deferoxamine decreases chlorine-induced mortality and some indices of lung injury (23). In clinical studies, the post-exposure administration of ascorbate and vitamin E to patients with severe burns decreased the severity of acute lung injury (32). Previous studies indicate that rats also develop significant chlorine-induced hyperreactivity at 7 days after exposure, although hyperreactivity in rats was considerably lower compared with that of mice (21). The return of airway hyperreactivity to baseline levels via the post-exposure administration of antioxidants is highly significant, and shows that oxidants may be responsible, at least in part, for the pathogenesis of airway hyperreactivity.
At 24 hours after chlorine exposure, we did not see any histological differences in the airways of chlorine-exposed, saline-treated rats and chlorine-exposed, antioxidant-treated rats. This is not unexpected. It would be difficult to alter the early course of chlorine-induced epithelial injury, or induce a rapid repair process within 24 hours by treating after the injury has been initiated. However, at 7 days after chlorine exposure, a significant difference was evident in the airway epithelium of rats exposed to chlorine and treated with antioxidants, compared with rats exposed to chlorine and treated with saline vehicle. Chlorine-exposed rats treated with saline have thickened airway epithelium with mucous cell hyperplasia, similar to the response reportedly induced by 1,500 ppm chlorine for 5 minutes (43, 46). At 7 days after exposure, the airways of chlorine-exposed rats treated with aerosolized antioxidants look very similar to the airways of air-exposed rats. Toxicant-induced mucous cell hyperplasia is a potential concern in long-term outcomes of chlorine toxicity. Mucous cell hyperplasia was shown to occur in response to bacterial endotoxin (47), and is potentiated by additional exposures to ozone (27, 48, 49). The increase in mucous cells is not associated with an increase in epithelial cell density, and may be attributable to the loss of serous cells. Lactoferrin and lysozyme are localized in the secretory granules of serous cells (50, 51), and both lactoferrin and lysozyme are considered to be antimicrobial peptides (52). An increase in mucus within an airway, coupled with a loss of serous cells (and potentially some inmate immunity), could result in increased susceptibility to bacterial infections. The results of the present study demonstrate for the first time, to the best of our knowledge, that the severe injury to major airways in rodents exposed acutely to high levels of chlorine, characterized by epithelial hyperplasia, mucus accumulation, and airway reactivity, can be reversed in a safe and efficacious manner by the post-exposure administration of ascorbate and deferoxamine. Previously, we also showed that the post-exposure administration of intramuscular and aerosolized ascorbate and deferoxamine in mice exposed to lethal concentrations of chlorine (600 ppm for 45 minutes) decreased mortality and lung injury (23). Thus, our data point out the beneficial effects of aerosolized and intravenous ascorbate and deferoxamine as potential therapy in chlorine-exposed individuals. The beneficial effects of ascorbate may be more pronounced in humans exposed to chlorine, because humans are unable to synthesize ascorbate.
Supplementary Material
Footnotes
This work was supported by National Institute of Environmental Health Sciences grants U54ES017218 and U01ES15676 (S.M.) from the National Institutes of Health.
Andreas Bracher is currently at the Division of Skin and Endothelium Research, Department of Dermatology, Medical University of Vienna, Vienna, Austria.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0196OC on December 8, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Evans RB. Chlorine: state of the art. Lung 2005;183:151–167 [DOI] [PubMed] [Google Scholar]
- 2.Nieuwenhuijsen MJ, Toledano MB, Eaton NE, Fawell J, Elliott P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup Environ Med 2000;57:73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons JE, Richardson SD, Speth TF, Miltner RJ, Rice G, Schenck KM, Hunter ES, III, Teuschler LK. Development of a research strategy for integrated technology–based toxicological and chemical evaluation of complex mixtures of drinking water disinfection byproducts. Environ Health Perspect 2002;110 (Suppl 6):1013–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toren K, Blanc PD. The history of pulp and paper bleaching: respiratory-health effects. Lancet 1997;349:1316–1318 [DOI] [PubMed] [Google Scholar]
- 5.Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc 2010;7:278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 2010;299:L289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones R, Wills B, Kang C. Chlorine gas: an evolving hazardous material threat and unconventional weapon. West J Emerg Med 2010;11:151–156 [PMC free article] [PubMed] [Google Scholar]
- 8.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, et al. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 2009;27:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babu RV, Cardenas V, Sharma G. Acute respiratory distress syndrome from chlorine inhalation during a swimming pool accident: a case report and review of the literature. J Intensive Care Med 2008;23:275–280 [DOI] [PubMed] [Google Scholar]
- 10.Bonetto G, Corradi M, Carraro S, Zanconato S, Alinovi R, Folesani G, Da Dalt L, Mutti A, Baraldi E. Longitudinal monitoring of lung injury in children after acute chlorine exposure in a swimming pool. Am J Respir Crit Care Med 2006;174:545–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngo A, Ponampalam R, Leong M, Han LS. Chlorine and its impact on an emergency department. Prehosp Disaster Med 2007;22:136–139 [DOI] [PubMed] [Google Scholar]
- 12.Becker M, Forrester M. Pattern of chlorine gas exposures reported to Texas poison control centers, 2000 through 2005. Tex Med 2008;104:52–57 [PubMed] [Google Scholar]
- 13.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Heard SE. 2007 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th annual report. Clin Toxicol (Phila) 2008;46:927–1057 [DOI] [PubMed] [Google Scholar]
- 14.Howard C, Ducre B, Burda AM, Kubic A. Management of chlorine gas exposure. J Emerg Nurs 2007;33:402–404 [DOI] [PubMed] [Google Scholar]
- 15.LoVecchio F, Blackwell S, Stevens D. Outcomes of chlorine exposure: a 5-year poison center experience in 598 patients. Eur J Emerg Med 2005;12:109–110 [DOI] [PubMed] [Google Scholar]
- 16.Urbanetti JS. Toxic inhalational injury. : Sidell FR, Takafuji ET, Franz DR, Medical aspects of chemical and biological warfare. Washington, DC: Office of the Surgeon General at TMM Publications; 1997 [Google Scholar]
- 17.Winder C. The toxicology of chlorine. Environ Res 2001;85:105–114 [DOI] [PubMed] [Google Scholar]
- 18.Parimon T, Kanne JP, Pierson DJ. Acute inhalation injury with evidence of diffuse bronchiolitis following chlorine gas exposure at a swimming pool. Respir Care 2004;49:291–294 [PubMed] [Google Scholar]
- 19.Lemiere C, Malo JL, Boutet M. Reactive airways dysfunction syndrome due to chlorine: sequential bronchial biopsies and functional assessment. Eur Respir J 1997;10:241–244 [DOI] [PubMed] [Google Scholar]
- 20.Moore BB, Sherman M. Chronic reactive airway disease following acute chlorine gas exposure in an asymptomatic atopic patient. Chest 1991;100:855–856 [DOI] [PubMed] [Google Scholar]
- 21.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Post exposure administration of a {beta}2-agonist decreases chlorine induced airway hyper-reactivity in mice. Am J Respir Cell Mol Biol 2011;45: 88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 2008;295:L733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and deferoxamine administration post chlorine exposure decrease mortality and lung injury in mice. Am J Respir Cell Mol Biol 2011;45:386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yildirim C, Kocoglu H, Goksu S, Cengiz B, Sari I, Bagci C. Long-term pulmonary histopathologic changes in rats following acute experimental exposure to chlorine gas. Inhal Toxicol 2004;16:911–915 [DOI] [PubMed] [Google Scholar]
- 25.Yadav AK, Doran S, Sharma R, Squadrito GL, Fanucchi MV, Postlethwait E, Matalon S. Antioxidants attenuate acute lung injury post chlorine gas exposure in rats. Am J Respir Crit Care Med 2010;181:A6783 [Google Scholar]
- 26.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, Jr, Patel RP, et al. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol 2011;300:L362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanucchi MV, Hotchkiss JA, Harkema JR. Endotoxin potentiates ozone-induced mucous cell metaplasia in rat nasal epithelium. Toxicology and Applied Pharmacology 1998;152:1–9 [DOI] [PubMed] [Google Scholar]
- 28.Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 2010;181:394–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health 1985;15:197–214 [DOI] [PubMed] [Google Scholar]
- 30.Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem 2010;285:9716–9728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honavar J, Samal AA, Bradley KM, Brandon A, Balanay J, Squadrito GL, MohanKumar K, Maheshwari A, Postlethwait EM, Matalon S, et al. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am J Respir Cell Mol Biol 2011;45:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg 2000;135:326–331 [DOI] [PubMed] [Google Scholar]
- 33.Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002;236:814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crimi E, Liguori A, Condorelli M, Cioffi M, Astuto M, Bontempo P, Pignalosa O, Vietri MT, Molinari AM, Sica V, et al. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial. Anesth Analg 2004;99:857–863 [DOI] [PubMed] [Google Scholar]
- 35.Flashman E, Davies SL, Yeoh KK, Schofield CJ. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem J 2010;427:135–142 [DOI] [PubMed] [Google Scholar]
- 36.Wu F, Schuster DP, Tyml K, Wilson JX. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic Biol Med 2007;42:124–131 [DOI] [PubMed] [Google Scholar]
- 37.Hoyle GW, Hoyle CI, Chen J, Chang W, Williams RW, Rando RJ. Identification of triptolide, a natural diterpenoid compound, as an inhibitor of lung inflammation. Am J Physiol Lung Cell Mol Physiol 2010;298:L830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57Bl/6 and FVB/N mice. Inhal Toxicol 2008;20:783–793 [DOI] [PubMed] [Google Scholar]
- 39.Aguirre R, May JM. Inflammation in the vascular bed: importance of vitamin C. Pharmacol Ther 2008;119:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother 2001;55:333–339 [DOI] [PubMed] [Google Scholar]
- 41.McGuigan MA. Acute iron poisoning. Pediatr Ann 1996;25:33–38 [DOI] [PubMed] [Google Scholar]
- 42.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 2003;168:568–574 [DOI] [PubMed] [Google Scholar]
- 43.Demnati R, Fraser R, Ghezzo H, Martin JG, Plaa G, Malo JL. Time-course of functional and pathological changes after a single high acute inhalation of chlorine in rats. Eur Respir J 1998;11:922–928 [DOI] [PubMed] [Google Scholar]
- 44.Menaouar A, Anglade D, Baussand P, Pelloux A, Corboz M, Lantuejoul S, Benchetrit G, Grimbert FA. Chlorine gas induced acute lung injury in isolated rabbit lung. Eur Respir J 1997;10:1100–1107 [DOI] [PubMed] [Google Scholar]
- 45.Demnati R, Fraser R, Martin JG, Plaa G, Malo JL. Effects of dexamethasone on functional and pathological changes in rat bronchi caused by high acute exposure to chlorine. Toxicol Sci 1998;45:242–246 [DOI] [PubMed] [Google Scholar]
- 46.Demnati R, Fraser R, Plaa G, Malo JL. Histopathological effects of acute exposure to chlorine gas on Sprague-Dawley rat lungs. J Environ Pathol Toxicol Oncol 1995;14:15–19 [PubMed] [Google Scholar]
- 47.Harkema JR, Hotchkiss JA. In vivo effects of endotoxin on intraepithelial mucosubstances in rat pulmonary airways: quantitative histochemistry. Am J Pathol 1992;141:307–317 [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner JG, Hotchkiss JA, Harkema JR. Effects of ozone and endotoxin coexposure on rat airway epithelium: potentiation of toxicant-induced alterations. Environ Health Perspect 2001;109 Suppl 4:591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner JG, Van Dyken SJ, Wierenga JR, Hotchkiss JA, Harkema JR. Ozone exposure enhances endotoxin-induced mucous cell metaplasia in rat pulmonary airways. Toxicol Sci 2003;74:437–446 [DOI] [PubMed] [Google Scholar]
- 50.Bowes D, Corrin B. Ultrastructural immunocytochemical localisation of lysozyme in human bronchial glands. Thorax 1977;32:163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowes D, Clark AE, Corrin B. Ultrastructural localisation of lactoferrin and glycoprotein in human bronchial glands. Thorax 1981;36:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence 2010;1:440–464 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.