Abstract
Supplemental oxygen is frequently prescribed. However, prolonged exposure to high concentrations of oxygen causes hyperoxic acute lung injury (HALI), which manifests as acute respiratory distress syndrome in adults and leads to bronchopulmonary dysplasia in newborns (NBs). Nitric oxide (NO), NO synthases (NOSs), and angiopoietin (Ang) 2 have been implicated in the pathogenesis of HALI. However, the mechanisms of the contributions of NOS/NO and the relationship(s) between NOS/NO and Ang2 have not been addressed. In addition, the relevance of these moieties in adults and NBs has not been evaluated. To address these issues, we compared the responses in hyperoxia of wild-type (NOS [+/+]) and NOS null (−/−) young adult and NB mice. When compared with NOS2+/+ adult controls, NOS2−/− animals manifest exaggerated alveolar–capillary protein leak and premature death. These responses were associated with enhanced levels of structural cell death, enhanced expression of proapoptotic regulatory proteins, and Ang2. Importantly, silencing RNA knockdown of Ang2 decreased the levels of cell death and the expression of proapoptotic mediators. These effects were at least partially NOS2 specific, and were development dependent, because survival was similar in adult NOS3+/+ and NOS3−/− mice and NB NOS2+/+ and NOS2−/− mice, respectively. These studies demonstrate that NOS2 plays an important protective role in HALI in adult animals. They also demonstrate that this response is mediated, at least in part, by the ability of NOS2 to inhibit hyperoxia-induced Ang2 production and thereby decrease Ang2-induced tissue injury.
Keywords: cytokines, hyperoxia, lung
Clinical Relevance
The article reports novel data that increased hyperoxia-induced lung injury in NOS2 null mutant mice is mediated via angiopoietin-2, and highlights the developmental regulation of this reponse. This has potential clinical implications for hyperoxia-mediated pulmonary disorders in the newborn (bronchopulmonary dysplasia) and adult (acute lung injury).
Supplemental oxygen is commonly employed to counteract tissue hypoxemia. Although this is lifesaving in most circumstances, prolonged exposure to high concentrations of oxygen can lead to untoward effects. In the lung, exposure to hyperoxia is characterized by an inflammatory response (mediated by cytokines) and breakdown of the alveolar–capillary barrier, leading to pulmonary edema and endothelial and epithelial cell injury/death (1). In adults, hyperoxic acute lung injury (HALI) causes acute respiratory distress syndrome (2). In neonates, HALI has been shown to lead to a pulmonary phenotype suggestive of bronchopulmonary dysplasia (3). Hence, HALI is a major cause of morbidity and mortality in both adult (2) and neonatal (3, 4) populations.
In addition, nitric oxide (NO) is known to have oxidant effects (5), and has been implicated in the pathogenesis of HALI. NO is generated via the action of NO synthases (NOSs). The inflammatory response in HALI is initiated and propagated, to a large extent, by the release of cytokines (1), which are known to induce NOS (6, 7). Most data suggest that hyperoxia exposure up-regulates inducible NOS (NOS2) and endothelial NOS (NOS3) expression in adult (8–16) and neonatal (7, 16–19) lungs. In ALI and cell death, the endogenous production of NO is said to be mostly contributed by up-regulation of NOS2 in the lung (20–22).
Previous work from our laboratory has reported on the significant contribution of angiopoietin (Ang) 2 to HALI in adults and newborns (NBs) (23, 24). The interactive role and mechanism(s) of NOS and Ang2 in HALI have not been explored to date.
Hence, we hypothesized that endogenous NOS2 is an important mediator of Ang2-regulated HALI. To test this hypothesis, we studied the tissue responses that were induced by hyperoxia in mice with wild-type and null (−/−) NOS2 loci. These studies demonstrate that, in the absence of NOS2, hyperoxia-induced alveolar protein leak, DNA injury, cell death, expression of cell death regulators, and mortality were increased. In addition, we noted increased expression of Ang2. Use of Ang2 silencing (si) RNA significantly attenuated the HALI response in the young adult NOS2−/− mice. Interestingly, there were significant developmental differences as the NB NOS2−/− mice had similar survival as NB NOS2+/+ mice on exposure to hyperoxia. No differences in mortality were noted in the adult NOS3−/− compared with NOS3+/+ controls on exposure to hyperoxia.
Materials and Methods
Animals
NOS2 null (NOS2−/−) and NOS3 null (NOS3−/−) mice (both C57Bl/6 strain) were obtained from Jackson Laboratories (Bar Harbor, ME). Wild-type (NOS2+/+ or NOS3+/+) animals were used as controls. Young adult mice were 4–6 weeks of age, whereas NB animals were exposed to hyperoxia (100% O2) from Postnatal Day (PN) 1. All animal work was approved by the Institutional Animal Care and Use Committee at the Yale University School of Medicine (New Haven, CT).
Oxygen Exposure
Young adult mice (NOS2−/− or NOS3−/− and respective NOS2+/+ or NOS3+/+control animals) were placed in cages in an airtight Plexiglass chamber (55 × 40 × 50 cm), as described previously (25–27). For the NB (NOS2−/− and NOS2+/+ controls) exposure to hyperoxia, the NB mice (along with their mothers) were placed in cages in the same chamber. Exposure to oxygen was initiated on PN1. Two lactating dams were used. They were alternated in hyperoxia and room air every 24 hours. The litter size was kept limited to 12 pups to control for the effects of litter size on nutrition and growth.
Throughout the experiment, mice were given free access to food and water. Oxygen levels were constantly monitored by an oxygen sensor that was connected to a relay switch incorporated into the oxygen supply circuit. The inside of the chamber was kept at atmospheric pressure, and mice were exposed to a 12-hour light–dark cycle.
In separate experiments, young adult NOS2+/+ or NOS2−/− mice were killed 60 hours after oxygen exposure and lungs removed for RNA analysis.
Bronchoalveolar Lavage
Young adult mice were killed 60 hours after oxygen exposure, the trachea was isolated by blunt dissection, and a small-caliber tube was inserted into the airway and secured. Two volumes of 1 ml of PBS with 0.1% BSA were instilled and gently aspirated and pooled (bronchoalveolar lavage [BAL] fluid). Samples were then centrifuged at 1,250 × g for 5 minutes to recover cells, and the supernatants were collected and stored at −70°C for further analysis. Total protein was measured using the DC protein assay (Bio-Rad, Hercules, CA), per the manufacturer's recommendations, as previously described (28). Cell pellets were resuspended in PBS and total cell counts determined using a hemocytometer.
Histology, Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling/Ang2/Surfactant Protein C Staining, Analysis of RNA, and Western Blotting
Details have been noted in Materials and Methods in the online supplement.
Ang2 siRNA Generation and Delivery
We generated Ang2 siRNA (and control scrambled siRNA), as previously described (23). We delivered the siRNA intranasally to the young adult mice prior to starting hyperoxia exposure and repeating the doses at 36 hours, as previously described (23). We delivered “high-dose” siRNA, which we have previously documented to decrease the expression of Ang2 in NOS2+/+ mouse lungs by 60–70% (23).
Statistical Analysis
Values are expressed as means (±SEM). Groups were compared with the Student's two-tailed, unpaired t test, one-way ANOVA or the log-rank test using GraphPad Prism 3.0 (GraphPad Software, Inc., San Diego, CA), as appropriate. A P value of 0.05 or less was considered statistically significant.
Results
Role of NOS2 in HALI
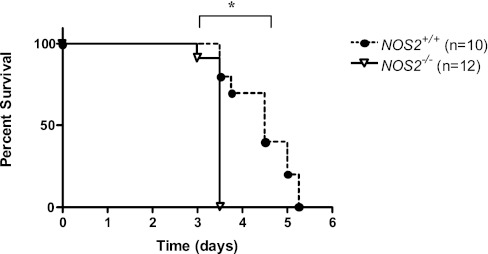
To address the role of NOS2 in the pathogenesis of HALI, we compared the survival of NOS2+/+ and NOS2−/− young adult mice in 100% O2. In accord with studies from our laboratory and others, NOS2+/+ young adult mice died after 3–5 days of exposure to 100% O2 (25–27, 29, 30). In this setting, approximately 50% of NOS2+/+ young adult mice were dead after 3.5 days and 100% had expired by day 5.25 (Figure 1). In contrast, this response was accelerated in young adult mice that were deficient in NOS2. In these experiments, 100% of the NOS2−/− young adult mice were dead after 3.5 days of exposure to 100% O2 (Figure 1). These studies demonstrate that endogenous NOS2 plays a protective role in HALI.
Figure 1.
Role of nitric oxide (NO) synthase (NOS) 2 on survival in hyperoxia. NOS2+/+ (n = 10) and NOS2−/− (n = 12) young adult mice were exposed to 100% O2 and survival was assessed. *P = 0.0002.
Role of NOS2 in Hyperoxia-Induced Inflammation
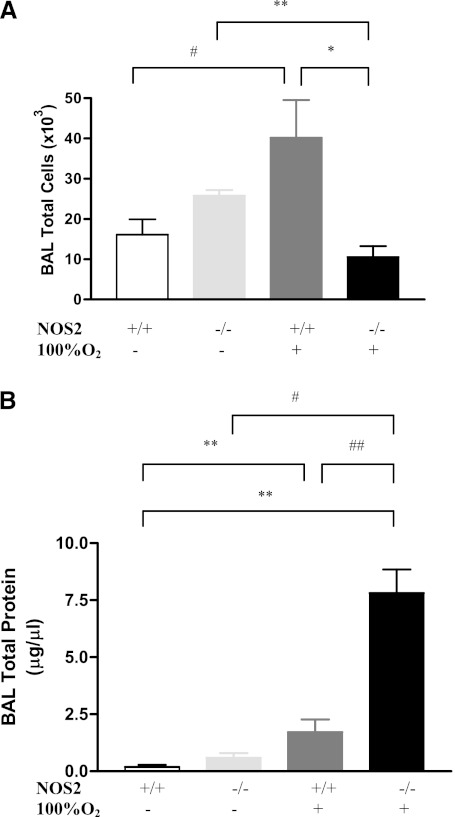
Studies were next undertaken to define the role(s) of NOS2 in the hyperoxia-induced inflammatory response. In NOS2+/+ young adult mice, 100% O2 caused significant injury (see Figures E1A and E1B in the online supplement) and inflammatory cell accumulation with enhanced BAL total cell recovery (Figure 2A) after 60 hours of exposure. NOS2 played a significant role in these responses, because, in the absence of NOS2, the injury was evident with septal polymorphonuclear infiltrate, slightly thickened alveoli, proteinaceous alveolar exudates and scattered areas of hemorrhage (Figures E1A and E1B); however, BAL total cell was significantly decreased (Figure 2A). Thus, presence of NOS2 appears to have an important role in the pathogenesis of hyperoxia-induced pulmonary injury and inflammation.
Figure 2.
Role of NOS2 on bronchoalveolar lavage (BAL) cellularity and total protein in hyperoxia. NOS2+/+ and NOS2−/− young adult mice were exposed to 100% O2 for 60 hours. (A) BAL total cell recovery. The noted values represent assessments in a minimum of four animals in each group. *P < 0.001, **P < 0.01, #P = 0.03. (B) BAL total protein levels. The values noted represent assessments in a minimum of four animals in each group. **P < 0.001, #P < 0.01, ##P = 0.02.
Role of Endogenous NOS2 in Hyperoxia-Induced Alveolar Permeability
HALI is characterized by endothelial cell damage and disruption of the alveolar capillary barrier, which is manifest as an increased protein leak. In accord with this conceptualization, there was increased BAL total protein recovery in the NOS2+/+ young adult mice exposed to hyperoxia (Figure 2B). This protein leak was significantly enhanced in the NOS2−/− null young adult mice exposed to 100% O2 (Figure 2B) for 60 hours. These data suggest that endogenous NOS2 has a protective role in the maintenance of the alveolar–capillary barrier in the presence of hyperoxia.
Role of Endogenous NOS2 in Hyperoxia-Induced Cell Death
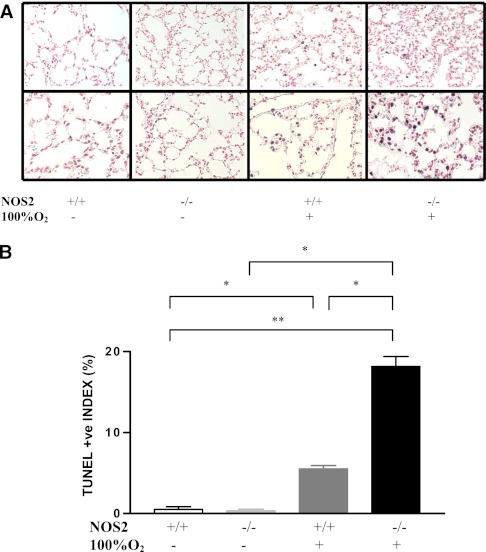
Because oxidant-induced DNA injury and cell death are felt to play important roles in the pathogenesis of HALI (26, 30, 31), the role(s) of NOS2 in these responses was evaluated. In accord with the above concept, 100% O2 caused DNA injury and cell death that manifested as enhanced pulmonary tissue terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining in NOS2+/+ young adult mice (Figure 3A) after 60 hours of exposure. Endogenous NOS2 appeared to be a critical regulator of this response, because the levels of TUNEL staining were significantly increased in comparisons of hyperoxia-challenged NOS2−/− and NOS2+/+ young adult animals (Figure 3B). These studies demonstrate that endogenous NOS2 is a critical inhibitor of hyperoxia-induced DNA injury and cell death.
Figure 3.
Role of NOS2 in hyperoxia-induced DNA injury and cell death. NOS2+/+ and NOS2−/− young adult mice were exposed to room air or 100% O2 for 60 hours and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) evaluations were undertaken. (A) A nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt blue stain and a nuclear red counterstain of lungs from animals exposed to room air or 100% O2 for 60 hours. The percentage of TUNEL (+) cells is illustrated in (B). The noted values represent assessments in a minimum of four animals in each group. *P < 0.0001, **P < 0.001.
Endogenous NOS2 Modulation of Cell Death Regulators
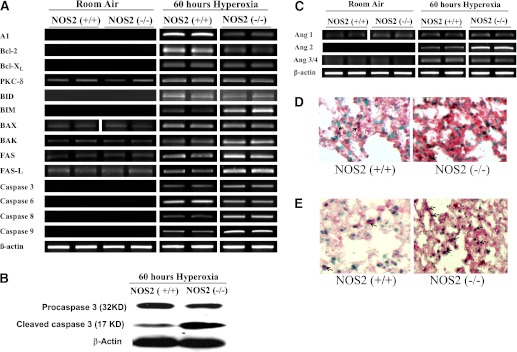
To gain insights into the mechanisms that NOS2 might use to regulate tissue cell death responses, we compared the expression of cell death regulators in lungs from NOS2+/+ and NOS2−/− young adult mice in room air and 100% O2. In the NOS2+/+ and NOS2−/− young adult mice in room air, mRNA encoding protein kinase C (PKC)-δ, Bcl-2–associated X protein (Bax), Bcl-2 antagonist/killer (Bak), Fas, and Fas ligand (Fas-L) were readily apparent, whereas the levels of mRNA encoding A1, B cell lymphoma protein (Bcl)-2, Bcl-xl, BH3-interacting domain death agonist (Bid), Bcl2-interacting mediator of cell death (Bim), and caspases were below the limits of detection of our assays (Figure 4A). In NOS2+/+ young adult mice, hyperoxia increased the levels of mRNA encoding mediators of cell death (Figure 4A). In the absence of NOS2, the induction of Bcl-xl,, PKC-δ, Bid, Bax, and caspase-6 by hyperoxia were not altered compared with NOS2+/+ young adult mice in hyperoxia (Figure 4A). However, the levels of mRNA encoding A1 and Bcl-2 decreased, whereas Bim, Bak, Fas, Fas-L, and caspase-3, -8, and -9 were increased compared with NOS2+/+ young adult mice experiencing a similar hyperoxic challenge (Figure 4A). Figure 4B illustrates the protein expression of pro- and cleaved caspase 3 in NOS2+/+ and NOS2−/− young adult mice exposed to 100% O2 for 60 hours. The level of procaspase 3 decreased, with concomitant increase in cleaved caspase 3 in NOS2−/− mouse lungs. These studies demonstrate that, in hyperoxia, lack of endogenous NOS2 shifts the expression of mediators toward increased cell death.
Figure 4.
Role of NOS2 in hyperoxia-induced alterations in cell death regulators and angiopoietins (Angs). (A) Levels of mRNA encoding A1, B cell lymphoma protein (Bcl)-2, Bcl-XL, protein kinase C-δ, BH3-interacting domain death agonist (Bid), Bcl2-interacting mediator of cell death (Bim), Bcl-2–associated X protein (Bax), Bcl-2 antagonist/killer (Bak), Fas ligand (Fas-L), and caspase-3, -6, -8, and -9 in NOS2+/+ and NOS2−/− young adult mice exposed to room air or 100% O2 for 60 hours. Each lane in the gel is representative of the condition described, with the corresponding β-actin as control. This figure is illustrative of a minimum of four experiments. (B) Protein expression of pro- and cleaved caspase-3 in NOS2+/+ and NOS2−/− young adult mice exposed to 100% O2 for 60 hours. Each lane in the gel is representative of the condition described, with β-actin controls. (C) Levels of mRNA encoding Ang1, -2, and -3/4 in NOS2+/+ and NOS2−/− young adult mice exposed to room air or 100% O2 for 60 hours. Each lane in the gel is representative of the condition described, with β-actin controls as in A. This figure is illustrative of a minimum of four experiments. (D) Localization of Ang2 protein with TUNEL staining; (E) the localization of surfactant protein C protein with TUNEL staining in hyperoxia-exposed NOS2+/+ and NOS2−/− young adult mouse lung samples. Original magnification, 40×. The arrows point to the localization of type II pneumocytes.
Endogenous NOS2 Modulation of Angs
Because Angs have been implicated in HALI, to gain further insights into the mechanisms that NOS2 might use to regulate tissue cell death responses, we compared the expression of Ang in lungs from NOS2+/+ and NOS2−/− young adult mice in room air and 100% O2. In the NOS2+/+ and NOS2−/− young adult mice in room air, mRNA encoding Ang1 and Ang3/4 were apparent, whereas the levels of mRNA encoding Ang2 were below the limits of detection of our assays (Figure 4C). In contrast, hyperoxia increased the levels of mRNA encoding Ang2 and 3/4, whereas it decreased that of Ang1 (Figure 4C). Although there was no difference in the hyperoxia-induced mRNA expression of Ang1 and Ang3/4 between NOS2+/+ and NOS2−/− young adult mouse lungs, Ang2 levels were markedly increased compared with those in NOS2+/+ young adult mice (Figure 4C). Next, we localized Ang2 protein and surfactant protein C protein with TUNEL staining (Figures 4D and 4E, respectively; Figures E2A and E2B). These studies demonstrate that, at baseline, endogenous NOS2 inhibits the expression of Ang2 in the murine lung, and regulates its increase on hyperoxia exposure. This increased Ang2 is mostly found in the TUNEL-positive type II pneumocytes.
Regulation of Endogenous NOS2-Induced HALI by Ang2 siRNA
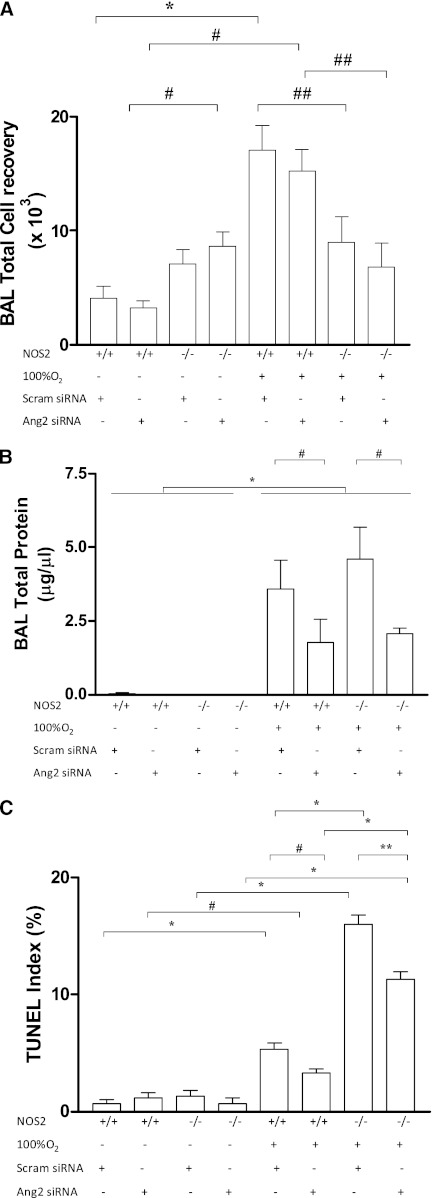
To assess the role of Ang2, if any, in endogenous NOS2–induced HALI, we delivered Ang2 (and scrambled) siRNA to NOS2+/+ and NOS2−/− young adult mice in room air and 100% O2 for 60 hours. Ang2 siRNA significantly increased total BAL cell recovery when given to NOS2−/− adult mice in room air compared with NOS2+/+ controls (Figure 5A). Hyperoxia exposure significantly decreased total BAL cell recovery in NOS2−/− young adult mice administered Ang2 or scrambled siRNA compared with matched NOS2+/+ controls (Figure 5A). There was increased BAL total protein recovery in the NOS2+/+ and NOS2−/− null young adult mice exposed to hyperoxia and administered scrambled or Ang2 siRNA compared with those exposed to room air (Figure 5C). This protein leak was significantly decreased in the NOS2+/+ and NOS2−/− null young adult mice administered Ang2 siRNA compared with their respective control mice administered scrambled siRNA and exposed to hyperoxia for 60 hours (Figure 5C). Examination of TUNEL index revealed no significant difference in the room air–exposed groups (Figure 5B). However, in hyperoxia-exposed groups, NOS2+/+ and NOS2−/− young adult mice given Ang2 siRNA had significantly decreased TUNEL index scores (Figure 5B). Taken together, our data demonstrate that endogenous NOS2-induced HALI inflammatory cellular, protein leak, DNA injury, and cell death responses are dependent on Ang2.
Figure 5.
Role of NOS2 in hyperoxia and Ang2 silencing (si) RNA administered alterations in BAL cellularity and DNA injury and cell death. NOS2+/+ and NOS2−/− young adult mice were exposed to room air or 100% O2 for 60 hours and administered Ang2 siRNA or scrambled siRNA. (A) BAL total cell recovery was assessed. The noted values represent assessments in a minimum of four animals in each group. *P < 0.001, #P < 0.01, ##P < 0.05. (B) BAL total protein levels. The noted values represent assessments in a minimum of three animals in each group. *P < 0.0001, #P < 0.05. The percentage of TUNEL (+) cells is illustrated in (C). The noted values represent assessments in a minimum of four animals in each group. *P < 0.0001, **P = 0.001, #P ≤ 0.01. HYP, hyperoxia (100% O2); Scram siRNA, scrambled siRNA.
Regulation of Endogenous NOS2-Induced Cell Death Regulators in HALI by Ang2 siRNA
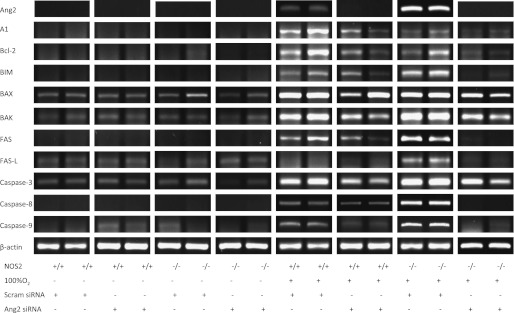
We next evaluated the expression of cell death regulators in NOS2+/+ and NOS2−/− young adult mice given Ang2 (or scrambled) siRNA. There was little difference noted in the room air–exposed groups (Figure 6). NOS2+/+ and NOS2−/− young adult mice given Ang2 siRNA and exposed to hyperoxia for 60 hours had markedly decreased expression of A1, Bcl-2, Bim, Bax, Bak, Fas, and casapse-3, -8, and -9 compared with control animals administered scrambled siRNA. Compared with hyperoxia-exposed NOS2+/+ young adult mice given Ang2 siRNA, expression of A1, Bcl-2, Fas, Fas-L, and caspase-8 and -9 were further suppressed. Thus, our data show that expression of key cell death regulators are diminished markedly by inhibiting Ang2 on hyperoxia exposure in young adult mice lacking endogenous NOS2.
Figure 6.
Role of NOS2 in hyperoxia and Ang2 siRNA administered alterations in cell death regulators. The levels of mRNA encoding A1, Bcl-2, Bim, Bax, Bak, Fas, Fas-L, and caspase-3, -8, and -9 in NOS2+/+ and NOS2−/− young adult mice exposed to room air or 100% O2 for 60 hours and administered Ang2 or scrambled siRNA are shown. This figure is illustrative of a minimum of four experiments. HYP, hyperoxia (100% O2); Scram siRNA, scrambled siRNA.
Developmental Regulation of NOS2 in HALI
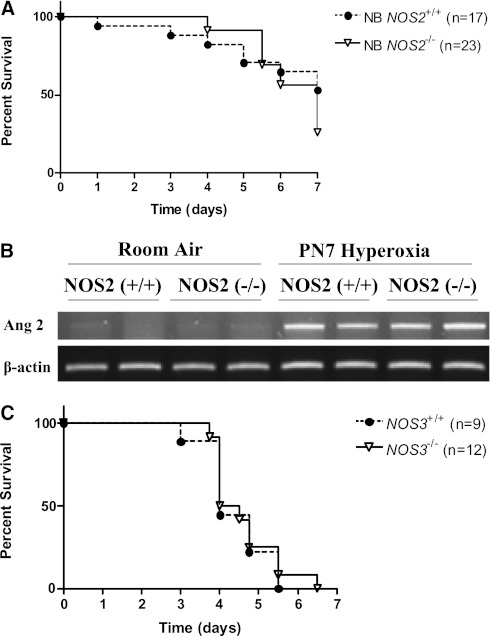
To address the developmental role of inducible NOS in the pathogenesis of HALI, we compared the survival of NB NOS2+/+ and NOS2−/− mice in 100% O2. In contrast to the effects on survival noted in the young adult NOS2−/− mice, there were no differences in survival when comparing the survival in hyperoxia of the NB NOS2+/+ and NOS2−/− mice (Figure 7A). There was no significant difference in the Ang2 mRNA expression levels at PN7 in the NB NOS2+/+ and NOS2−/− mice in room air or hyperoxia (Figure 7B). These studies demonstrate that there is developmental regulation of the response to hyperoxia in the NOS2-deficient state in mice, which impacts on their survival in hyperoxia.
Figure 7.
Developmental regulation of the role of NOS2 on survival and Ang2 expression in hyperoxia and role of NOS3 on survival in hyperoxia. NOS2+/+ (n = 17) and NOS2−/− (n = 23) newborn (NB) mice were exposed to 100% O2 and survival was assessed at Postnatal Day (PN) 7 (A). The levels of mRNA encoding Ang2 in NOS2+/+ and NOS2−/− NB mice exposed to room air or 100% O2 until PN7 is illustrated. (B) Each lane in the gel is representative of the condition described, with β-actin controls. NOS3+/+ (n = 9) and NOS3−/− (n = 12) young adult mice were exposed to 100% O2 and survival was assessed.
Role of NOS3 in HALI
To address the role of NOS3 in the pathogenesis of HALI, we compared the survival of NOS3+/+ and NOS3−/− young adult mice in 100% O2. In contrast to the effects on survival noted in the young adult NOS2−/− mice, but similar to the neonatal response, there were no differences in survival when comparing the survival in hyperoxia of the young adult NOS3+/+ and NOS3−/− mice (Figure 7C). These studies demonstrate that endogenous NOS3 does not appear to play a critical role in HALI at this age.
Discussion
Our studies reveal that, in the absence of NOS2, exposure to hyperoxia significantly increased mortality in the young adult murine model. In addition, HALI, as measured by alveolar protein leak, DNA injury, cell death, and expression of key cell death regulators, was increased in the NOS2 null mutant young adult mice.
The role of endogenous NOS2 in HALI has been controversial. Adult rats exposed to hyperoxia, in the presence of l-NAME (nonspecific NOS inhibitor) and aminoguanidine (specific NOS2 inhibitor), had significantly increased mortality and HALI (32, 33). In 6- to 8-week-old mice (FVBN strain), hyperoxia exposure did not impact on NOS2 expression or activity, but decreased NOS3 activity (6). Interestingly, l-NAME significantly increased the mortality in hyperoxia-exposed mice (6). We found both protective (12, 34, 35) and damaging (11) roles being ascribed to adult NOS2-deficient mice in relation to HALI. Investigators have reported that NOS2 expression increased in response to hyperoxia, and there was worse HALI in NOS2-deficient, 4- to 6-week old mice (C57Bl/6J and SV129 strain) (11). In NOS2-deficient mice (∼8–12 wk, based on the weights mentioned), hyperoxia exposure was associated with decreased amounts of total surfactant when compared with normoxia, but the values were not different from those in wild-type control animals exposed to hyperoxia (36). In 8-week-old mice treated with an inhibitor of NOS2, there was reduction in HALI (37). Similar protective responses in older (8–16 wk) NOS2-deficient mice have been reported by other investigators (12, 34, 35).
Our data of increased mortality and HALI in young adult (4–6 wk of age) NOS2 null mutant mice is in accord with the above observations in that there appears to be an age-related dependence on whether NOS2 is protective or harmful in the context of HALI. Our results are also supported in another form of oxidant injury (ozone-induced), in which NOS2 null mutant mice (∼6 wk of age) were noted to be significantly more susceptible to lung injury than NOS2+/+ control mice (38). We speculate that, as mice age, the increased endogenous NOS2 levels (39), in combination with hyperoxia, result in enhanced lung injury, as has been reported for an LPS-induced injury model (39). Such effects may be augmented by a concomitant interactive decrease in antioxidant inducibility, as has been reported in other lung injury models (40–42).
Inflammation and lung injury are often seen together in animal models of HALI. This observation has led to studies investigating the mechanisms of hyperoxia-induced inflammation and the relationship between injury and inflammation in this disorder (31, 43–45). In our present study, we demonstrate evidence of HALI in the absence of a significant cellular inflammatory response (total BAL cell recovery). We have previously reported a similar response in IL-13 null mutant mice on hyperoxia exposure (28), and such has also been noted in peptide substance P receptor (NK1R) null mutant mice on hyperoxia exposure (46). It is plausible that there may be defects in inflammatory cell transmigration to and/or from the parenchymal compartment to the bronchial tree, leading to low BAL total cell counts, despite evidence of increased cell injury and death in the IL-13, NOS2, and NK1R null mutant mouse lungs exposed to hyperoxia. This dissociation demonstrates that HALI cannot always be attributed to local tissue inflammation. In addition, although structural cell apoptosis (such as that seen in HALI) can stimulate tissue inflammation (47, 48), these studies also demonstrate that hyperoxia-induced inflammation cannot be attributed solely to the nearby cell death response.
Cell death response on exposure to hyperoxia is modulated by the balance of pro– and anti–cell death signals. The involvement of key cell death regulators in the process of HALI has been reported by us (23, 27, 28, 49) and others (50). Aside from the membrane (extrinsic) pathway that triggers cell surface “death receptors,” such as Fas, which binds Fas-L and activates caspase 8 (51, 52), many other stimuli use mitochondrial dysfunction to signal the cell death response. In this intrinsic pathway, BH3 only domain family members, such as Bid, are activated to truncated Bid and interact with Bax-type proteins to form and interact with mitochondrial pores, release cytochrome C, activate caspase 9, and induce cell death (30, 53–55). In accord with prior studies of HALI (23, 27, 28, 30, 49, 56, 57), our studies demonstrate that hyperoxia stimulates both of the above cell death pathways in the murine lung.
Our studies highlight the fact that loss of endogenous NOS2 in young adult mice, in the presence of hyperoxia, diminishes the induction of the antiapoptotic proteins A1 and Bcl-2, while enhancing the expression of Fas, Fas-L, Bim, Bak, and caspase-3, -8, and -9. Previous studies from our laboratory and others have demonstrated that these cell death mediators are critical regulators of HALI (30, 50, 56). To understand the mechanism of increased HALI in NOS2 null mutant young adult mice, we documented increased expression of Ang2, a molecule previously shown by us to be a critical regulator of cell death in HALI (23). We were also able to localize Ang2 protein with TUNEL staining, and confirmed the location in type II pneumocytes, as previously reported (23). Inhibition of Ang2 significantly attenuated the cell death response and mediators (A1, Bcl-2, Fas, Fas-L, and caspase-8 and -9), when compared with NOS2+/+ young adult mice, on exposure to HALI. When viewed in combination, these studies demonstrate that endogenous NOS2 suppresses the ability of hyperoxia to activate the Ang2-dependent death receptor and mitochondrial cell death pathways. We also show increased cleaved caspase 3 protein expression in NOS2−/− mouse lungs, suggesting that the mRNA expression of cell death regulators were translated.
Our studies also reveal the interesting observation that there is developmental regulation of the response of NOS2-null mutant mice to hyperoxia. In contrast to the increased mortality of the young adult mice (4–6 wk of age), there was no difference in survival of the neonatal mice exposed to hyperoxia. In 3-day-old premature neonatal rats, hyperoxia exposure significantly increased NOS2 and NOS3 expression, along with evidence of lung injury (19). However, nonspecific inhibition of NOS (using l-NAME) worsened HALI (19). Interestingly, the protein expression of pulmonary NOS2 increased by PN7, and then decreased by PN14 in room air in NB rats (7). Hyperoxia-exposed NB lungs had similar levels at PN7, but significantly decreased levels at PN14, compared with age-matched room air control lungs (7). In NB rats, NOS inhibition (using l-NAME) started 7 days before birth and continued until PN14, with hyperoxia exposure from PN1 to PN14, significantly increased their mortality (58). In rat pups exposed to hyperoxia from PN21 to PN29, hyperoxia induced a fivefold and twofold increase in NOS2 and NOS3 levels, respectively (17). Interestingly, using specific inhibitors of NOS2 and NOS3 did not reverse the hyperoxia-induced pathologic changes in the lung (17). Interestingly, the lack of difference in Ang2 mRNA expression at PN7 in the NB NOS2+/+ and NOS2−/− mice in hyperoxia (Figure 7B) could be contributing to the lack of difference in survival of these mice in hyperoxia until PN7.
Thus, there appears to be a biphasic response to exposure to hyperoxia in terms of the endogenous NOS2. Inhibition of endogenous NOS2 in the premature lung worsened HALI, with no significant impact in the neonatal lung (birth to 4 wk), but worsening in the young adult lung. Hence, overall, endogenous NOS2 has a protective role to play in cell death responses in HALI in rodent models until approximately 6 weeks of age. In striking contrast, older mice (>8 wk of age) appear to be somewhat protected from HALI in the NOS2-deficient state (12, 34, 35, 37), which may be dependent on other factors (15). We speculate that the developmentally regulated response of endogenous NOS2 in HALI could be enhanced due to age-related differences in antioxidant inducibility (40–42).
In mouse lungs, NOS2 is expressed constitutively in bronchial epithelial cells, alveolar type II pneumocytes, and interstitial cells (11). Earlier reports have suggested that hyperoxia can up-regulate NOS2 in type II cells (11, 19), airway epithelium (15), and macrophages (12). In rat lungs, NOS2 up-regulation in hyperoxia has been noted in alveolar epithelial cells and macrophages (9, 18, 59). Possible differences in alveolar macrophages obtained from adult (2 mo old) and NB (PN7) rats in response to hyperoxia, with the latter having increased production of NO, have been suggested (16). Cell source–specific source of up-regulation of NOS2 has been proposed to explain the differential effects of NOS2 inhibition in HALI (20, 60). We would like to suggest that a critical mediator of the effect of endogenous NOS2 in HALI is the developmental stage/age of the lung, as we have reported in additional murine models of HALI (61, 62).
We noted no difference in survival in NOS3 null mutant young adult mice compared with NOS3+/+ control animals on exposure to hyperoxia. Our data is concordant with the results of indices of HALI in NOS3−/− mice exposed to hyperoxia for 72 hours (35).
Oxidative injury is a key element in the pathogenesis of a variety of diseases and disorders. In neonates, for example, it is a key element in the pathogenesis of bronchopulmonary dysplasia (3), whereas, in adults, it is said to significantly contribute to acute respiratory distress syndrome/ALI (63). Our studies add to the paradigm of the contribution of NOS in adult and neonatal lungs. These observations point to specific pathways that can be potentially modified to decrease cell death and HALI. They also highlight the significant impact of developmental regulation of such responses. An important caveat to remember is that, whereas rodent inflammatory cells, on up-regulation of NOS2, can generate significant amounts of NO, the same is not true of human immune cells (64, 65). Hence, caution must be exercised in the translation of data from animal studies to human disease.
Supplementary Material
Footnotes
This work was supported by in part by American Heart Association grant 0755843T (V.B.), American Thoracic Society grant ATS-07-005 (V.B.), and National Heart, Lung, and Blood Institute/National Institutes of Health grants HL-74195, HL-85103 (V.B.), HL-64642, HL-61904, and HL-56389 (J.A.E.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0074OC on January 6, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med 2006;41:4–18 [DOI] [PubMed] [Google Scholar]
- 2.Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 2007;13:73–78 [DOI] [PubMed] [Google Scholar]
- 3.Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med 2010;15:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 2009;123:1562–1573 [DOI] [PubMed] [Google Scholar]
- 5.Turanlahti M, Pesonen E, Lassus P, Andersson S. Nitric oxide and hyperoxia in oxidative lung injury. Acta Paediatr 2000;89:966–970 [DOI] [PubMed] [Google Scholar]
- 6.Arkovitz MS, Szabo C, Garcia VF, Wong HR, Wispe JR. Differential effects of hyperoxia on the inducible and constitutive isoforms of nitric oxide synthase in the lung. Shock 1997;7:345–350 [DOI] [PubMed] [Google Scholar]
- 7.Radomski A, Sawicki G, Olson DM, Radomski MW. The role of nitric oxide and metalloproteinases in the pathogenesis of hyperoxia-induced lung injury in newborn rats. Br J Pharmacol 1998;125:1455–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steudel W, Watanabe M, Dikranian K, Jacobson M, Jones RC. Expression of nitric oxide synthase isoforms (NOS II and NOS III) in adult rat lung in hyperoxic pulmonary hypertension. Cell Tissue Res 1999;295:317–329 [DOI] [PubMed] [Google Scholar]
- 9.Cucchiaro G, Tatum AH, Brown MC, Camporesi EM, Daucher JW, Hakim TS. Inducible nitric oxide synthase in the lung and exhaled nitric oxide after hyperoxia. Am J Physiol 1999;277:L636–L644 [DOI] [PubMed] [Google Scholar]
- 10.Comhair SA, Thomassen MJ, Erzurum SC. Differential induction of extracellular glutathione peroxidase and nitric oxide synthase 2 in airways of healthy individuals exposed to 100% O2 or cigarette smoke. Am J Respir Cell Mol Biol 2000;23:350–354 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Hataishi R, Mitsufuji H, Tanaka M, Jacobson M, Tomita T, Zapol WM, Jones RC. Antiinflammatory properties of inducible nitric oxide synthase in acute hyperoxic lung injury. Am J Respir Cell Mol Biol 2001;24:390–397 [DOI] [PubMed] [Google Scholar]
- 12.Hardiman KM, Lindsey JR, Matalon S. Lack of amiloride-sensitive transport across alveolar and respiratory epithelium of iNOS(−/−) mice in vivo. Am J Physiol Lung Cell Mol Physiol 2001;281:L722–L731 [DOI] [PubMed] [Google Scholar]
- 13.Yeh DY, Kao SJ, Feng NH, Chen HI, Wang D. Increased nitric oxide production accompanies blunted hypoxic pulmonary vasoconstriction in hyperoxic rat lung. Chin J Physiol 2006;49:305–312 [PubMed] [Google Scholar]
- 14.Kobayashi H, Sakashita N, Okuma T, Terasaki Y, Tsujita K, Suzuki H, Kodama T, Nomori H, Kawasuji M, Takeya M. Class A scavenger receptor (CD204) attenuates hyperoxia-induced lung injury by reducing oxidative stress. J Pathol 2007;212:38–46 [DOI] [PubMed] [Google Scholar]
- 15.Zhang XF, Liu S, Zhou YJ, Zhu GF, Foda HD. Osteopontin protects against hyperoxia-induced lung injury by inhibiting nitric oxide synthases. Chin Med J (Engl) 2010;123:929–935 [PubMed] [Google Scholar]
- 16.Shang LH, Luo ZQ, Deng XD, Wang MJ, Huang FR, Feng DD, Yue SJ. Expression of N-methyl-D-aspartate receptor and its effect on nitric oxide production of rat alveolar macrophages. Nitric Oxide 2010;23:327–331 [DOI] [PubMed] [Google Scholar]
- 17.Potter CF, Kuo NT, Farver CF, McMahon JT, Chang CH, Agani FH, Haxhiu MA, Martin RJ. Effects of hyperoxia on nitric oxide synthase expression, nitric oxide activity, and lung injury in rat pups. Pediatr Res 1999;45:8–13 [DOI] [PubMed] [Google Scholar]
- 18.Bhandari V, Johnson L, Smith-Kirwin S, Vigliotta G, Funanage V, Chander A. Hyperoxia and nitric oxide reduce surfactant components (DSPC and surfactant proteins) and increase apoptosis in adult and fetal rat type II pneumocytes. Lung 2002;180:301–317 [DOI] [PubMed] [Google Scholar]
- 19.Chang L, Ma L, Zhang X, Chen Y. The role of nitric oxide in hyperoxic lung injury in premature rats. J Tongji Med Univ 2001;21:78–81 [DOI] [PubMed] [Google Scholar]
- 20.Mehta S. The effects of nitric oxide in acute lung injury. Vascul Pharmacol 2005;43:390–403 [DOI] [PubMed] [Google Scholar]
- 21.Borutaite V, Brown G. What else has to happen for nitric oxide to induce cell death? Biochem Soc Trans 2005;33:1394–1396 [DOI] [PubMed] [Google Scholar]
- 22.Klings ES, Lowry MH, Li G, Jean JC, Fernandez BO, Garcia-Saura MF, Feelisch M, Joyce-Brady M. Hyperoxia-induced lung injury in gamma-glutamyl transferase deficiency is associated with alterations in nitrosative and nitrative stress. Am J Pathol 2009;175:2309–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, et al. Hyperoxia causes angiopoietin 2–mediated acute lung injury and necrotic cell death. Nat Med 2006;12:1286–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aghai ZH, Faqiri S, Saslow JG, Nakhla T, Farhath S, Kumar A, Eydelman R, Strande L, Stahl G, Leone P, et al. Angiopoietin 2 concentrations in infants developing bronchopulmonary dysplasia: attenuation by dexamethasone. J Perinatol 2008;28:149–155 [DOI] [PubMed] [Google Scholar]
- 25.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin-6–induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol 2000;22:535–542 [DOI] [PubMed] [Google Scholar]
- 26.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest 1998;101:1970–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Chen Q, Corne J, Zhu Z, Lee CG, Bhandari V, Homer RJ, Elias JA. Pulmonary expression of leukemia inhibitory factor induces B cell hyperplasia and confers protection in hyperoxia. J Biol Chem 2003;278:31226–31232 [DOI] [PubMed] [Google Scholar]
- 28.Bhandari V, Choo-Wing R, Homer RJ, Elias JA. Increased hyperoxia-induced mortality and acute lung injury in IL-13 null mice. J Immunol 2007;178:4993–5000 [DOI] [PubMed] [Google Scholar]
- 29.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000;106:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, Chen Q, Zhu Z, Zhong M, Nakayama K, et al. Bcl-2–related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest 2005;115:1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantell LL, Horowitz S, Davis JM, Kazzaz JA. Hyperoxia-induced cell death in the lung–the correlation of apoptosis, necrosis, and inflammation. Ann N Y Acad Sci 1999;887:171–180 [DOI] [PubMed] [Google Scholar]
- 32.Capellier G, Maupoil V, Boillot A, Kantelip JP, Rochette L, Regnard J, Barale F. l-NAME aggravates pulmonary oxygen toxicity in rats. Eur Respir J 1996;9:2531–2536 [DOI] [PubMed] [Google Scholar]
- 33.Garat C, Jayr C, Eddahibi S, Laffon M, Meignan M, Adnot S. Effects of inhaled nitric oxide or inhibition of endogenous nitric oxide formation on hyperoxic lung injury. Am J Respir Crit Care Med 1997;155:1957–1964 [DOI] [PubMed] [Google Scholar]
- 34.Hesse AK, Dorger M, Kupatt C, Krombach F. Proinflammatory role of inducible nitric oxide synthase in acute hyperoxic lung injury. Respir Res 2004;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demchenko IT, Atochin DN, Gutsaeva DR, Godfrey RR, Huang PL, Piantadosi CA, Allen BW. Contributions of nitric oxide synthase isoforms to pulmonary oxygen toxicity, local vs. mediated effects. Am J Physiol Lung Cell Mol Physiol 2008;294:L984–L990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey TC, Cavanagh C, Mehta S, Lewis JF, Veldhuizen RA. Sepsis and hyperoxia effects on the pulmonary surfactant system in wild-type and iNOS knockout mice. Eur Respir J 2002;20:177–182 [DOI] [PubMed] [Google Scholar]
- 37.Yuba T, Nagata K, Yamada T, Osugi S, Kuwahara H, Iwasaki Y, Handa O, Naito Y, Fushiki S, Yoshikawa T, et al. A novel potent inhibitor of inducible nitric oxide synthase, ONO-1714, reduces hyperoxic lung injury in mice. Respir Med 2007;101:793–799 [DOI] [PubMed] [Google Scholar]
- 38.Kenyon NJ, van der Vliet A, Schock BC, Okamoto T, McGrew GM, Last JA. Susceptibility to ozone-induced acute lung injury in iNOS-deficient mice. Am J Physiol Lung Cell Mol Physiol 2002;282:L540–L545 [DOI] [PubMed] [Google Scholar]
- 39.Escames G, Lopez LC, Ortiz F, Ros E, Acuna-Castroviejo D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: effects of melatonin treatment. Exp Gerontol 2006;41:1165–1173 [DOI] [PubMed] [Google Scholar]
- 40.Starr ME, Ueda J, Yamamoto S, Evers BM, Saito H. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free Radic Biol Med 2011;50:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brady TC, Chang LY, Day BJ, Crapo JD. Extracellular superoxide dismutase is upregulated with inducible nitric oxide synthase after NF-kappa B activation. Am J Physiol 1997;273:L1002–L1006 [DOI] [PubMed] [Google Scholar]
- 42.Feng NH, Chu SJ, Wang D, Hsu K, Lin CH, Lin HI. Effects of various antioxidants on endotoxin-induced lung injury and gene expression: mRNA expressions of MnSOD, interleukin-1beta and iNOS. Chin J Physiol 2004;47:111–120 [PubMed] [Google Scholar]
- 43.Bustani P, Kotecha S. Role of cytokines in hyperoxia mediated inflammation in the developing lung. Front Biosci 2003;8:s694–s704 [DOI] [PubMed] [Google Scholar]
- 44.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, et al. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 2005;174:7250–7256 [DOI] [PubMed] [Google Scholar]
- 45.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol 2005;3:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dib M, Zsengeller Z, Mitsialis A, Lu B, Craig S, Gerard C, Gerard NP. A paradoxical protective role for the proinflammatory peptide substance P receptor (NK1R) in acute hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 2009;297:L687–L697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henson PM. Dampening inflammation. Nat Immunol 2005;6:1179–1181 [DOI] [PubMed] [Google Scholar]
- 48.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005;6:1191–1197 [DOI] [PubMed] [Google Scholar]
- 49.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Chen NY, Lee CG, Elias JA. The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am J Respir Crit Care Med 2010;182:918–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budinger GR, Mutlu GM, Urich D, Soberanes S, Buccellato LJ, Hawkins K, Chiarella SE, Radigan KA, Eisenbart J, Agrawal H, et al. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am J Respir Crit Care Med 2011;183:1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat Immunol 2002;3:1013–1018 [DOI] [PubMed] [Google Scholar]
- 52.De Paepe ME, Mao Q, Chao Y, Powell JL, Rubin LP, Sharma S. Hyperoxia-induced apoptosis and Fas/FasL expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2005;289:L647–L659 [DOI] [PubMed] [Google Scholar]
- 53.Joza N, Kroemer G, Penninger JM. Genetic analysis of the mammalian cell death machinery. Trends Genet 2002;18:142–149 [DOI] [PubMed] [Google Scholar]
- 54.Werner AB, de Vries E, Tait SW, Bontjer I, Borst J. Bcl-2 family member Bfl-1/A1 sequesters truncated bid to inhibit is collaboration with pro-apoptotic Bak or Bax. J Biol Chem 2002;277:22781–22788 [DOI] [PubMed] [Google Scholar]
- 55.Schuchmann M, Galle PR. Apoptosis in liver disease. Eur J Gastroenterol Hepatol 2001;13:785–790 [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Ryter SW, Dai C, Tang ZL, Watkins SC, Yin XM, Song R, Choi AM. Necrotic cell death in response to oxidant stress involves the activation of the apoptogenic caspase-8/bid pathway. J Biol Chem 2003;278:29184–29191 [DOI] [PubMed] [Google Scholar]
- 57.Budinger GR, Tso M, McClintock DS, Dean DA, Sznajder JI, Chandel NS. Hyperoxia-induced apoptosis does not require mitochondrial reactive oxygen species and is regulated by Bcl-2 proteins. J Biol Chem 2002;277:15654–15660 [DOI] [PubMed] [Google Scholar]
- 58.Pierce MR, Voelker CA, Sosenko IR, Bustamante S, Olister SM, Zhang XJ, Clark DA, Miller MJ. Nitric oxide synthase inhibition decreases tolerance to hyperoxia in newborn rats. Mediators Inflamm 1995;4:431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Klaveren RJ, Roelant C, Boogaerts M, Pype JL, Demedts M, Nemery B. Protective effects of the lazaroid U-74389G against hyperoxia in rat type II pneumocytes. Pulm Pharmacol Ther 1998;11:23–30 [DOI] [PubMed] [Google Scholar]
- 60.Allen BW, Demchenko IT, Piantadosi CA. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J Appl Physiol 2009;106:662–667 [DOI] [PubMed] [Google Scholar]
- 61.Choo-Wing R, Nedrelow JH, Homer RJ, Elias JA, Bhandari V. Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2007;293:L142–L150 [DOI] [PubMed] [Google Scholar]
- 62.Bhandari V, Choo-Wing R, Lee CG, Yusuf K, Nedrelow JH, Ambalavanan N, Malkus H, Homer RJ, Elias JA. Developmental regulation of NO-mediated VEGF-induced effects in the lung. Am J Respir Cell Mol Biol 2008;39:420–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 2005;33:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379–L399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans 2009;37:886–891 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.