Abstract
Maternal smoking during pregnancy has been associated with adverse effects on respiratory health. Whereas the epidemiologic link is incontrovertible, the mechanisms responsible for this association are still poorly understood. Although cigarette smoke has many toxic constituents, nicotine, the major addictive component in cigarette smoke, may play a more significant role than previously realized. The objectives of this study were to determine whether exposure to nicotine prenatally leads to alterations in pulmonary function and airway geometry in offspring, and whether α7 nicotinic acetylcholine receptors (nAChRs) mediate these effects. In a murine model of in utero nicotine exposure, pulmonary function, airway size and number, methacholine response, and collagen deposition were examined. Exposure periods included Gestation Days 7–21, Gestation Day 14 to Postnatal Day 7, and Postnatal Days 3–15. Prenatal nicotine exposure decreases forced expiratory flows in offspring through α7 nAChR–mediated signals, and the critical period of nicotine exposure was between Prenatal Day 14 and Postnatal Day 7. These physiologic changes were associated with increased airway length and decreased diameter. In addition, adult mice exposed to prenatal nicotine exhibit an increased response to methacholine challenge, even in the absence of allergic sensitization. Collagen expression was increased between adjacent airways and vessels, which was absent in α7 nAChR knockout mice. These observations provide a unified mechanism of how maternal smoking during pregnancy may lead to lifelong alterations in offspring pulmonary function and increased risk of asthma, and suggest potential targets to counteract those effects.
Keywords: lung development, nicotine, branching
Clinical Relevance
Although there is a significant body of literature addressing the adverse effects of cigarette smoke on respiratory health, there is little known about the effects of nicotine, the major addictive component of cigarette smoke, on the developing lung. This study establishes that exposure to nicotine prenatally leads to permanent changes in airway geometry and physiology.
In 1986, the U.S. Surgeon General's report stated that there was sufficient evidence that involuntary tobacco smoke exposure was associated with adverse respiratory health effects in children (1). Subsequently, scientific literature has more robustly shown the effects of involuntary or environmental tobacco smoke exposure on respiratory health, particularly on infants and children. Environmental tobacco smoke (ETS) exposure can occur in three exposure periods: prenatal, postnatal, and pre- and postnatal. The most direct method of prenatal ETS exposure is through maternal smoking. Maternal smoking during pregnancy has been associated with increased risk of asthma, lower respiratory tract infections, as well as decrements in lung function in healthy children (2–15). The decreases in lung function of offspring of smokers appear permanent, as they can be measured in adolescents and adults (16, 17).
Tobacco smoke contains thousands of compounds, and identifying whether one or more components play a key role in the pathogenesis of smoking-related lung diseases is difficult. Although there are possibly several chemicals involved, nicotine is of particular interest in prenatal exposure, because it can easily cross the placental barrier. Levels of nicotine and its metabolite, cotinine, are measurable in the fetus, and closely mimic maternal levels (18, 19). Nicotine acts by binding to nicotinic acetylcholine receptors (nAChRs), which are ligand-gated ion channels that allow entry of Na+ or Ca++ into the cells. nAChRs are pentamers composed of five identical α subunits or heteromers composed of α and β subunits. To date, 10 α and 4 β subunits have been described (20). In addition to being expressed in the brain, where nAChR mediates the addictive properties of nicotine, nAChRs are widely expressed in peripheral organs, with high concentrations in developing lung. The (α7)5 nAChR is the most abundant nAChR subtype in lung (21).
Previous studies in a nonhuman primate model of in utero nicotine exposure revealed that prenatal nicotine exposure leads to altered alveolar surface area, increased collagen expression around airways and blood vessels, decreased flows on pulmonary function tests, and increased airway resistance (21–23). We and others have also shown that nicotine stimulates lung cell proliferation (24, 25). Nicotine also stimulates lung branching morphogenesis in fetal murine lungs exposed to nicotine during the pseudoglandular stage of development, and these effects are mediated in part by α7 nAChR (26, 27). However, despite this previous knowledge, significant gaps still remain in our understanding of the effects of in utero nicotine exposure and the role that nicotinic acetylcholine receptors play. In this article, we attempt to improve knowledge by translating our previous in vitro findings to in vivo models in order to: (1) address the critical period of nicotine exposure during fetal development; (2) address the role that α7 nAChR plays in mediating nicotine's effects; (3) identify the lifelong effects of in utero nicotine exposure on airway growth and function; and (4) examine how airway growth may affect airway hyperresponsiveness. We hypothesized that, by acting on α7 nAChRs, prenatal nicotine exposure may lead to lifelong changes in pulmonary function by altering airway geometry in a murine model of prenatal and postnatal nicotine exposure.
Materials and Methods
All animal and experimental protocols were approved by the Emory University and Oregon Health and Science University Institutional Animal Care and Use Committees and their Offices of Biosafety.
Murine Model of In Utero Nicotine Exposure
Nicotine was given to C57BL/6 mice and α7 knockout mice on a C57BL/6 background (α7 nAChR−/−, Chrna7tm1Bay; Jackson Laboratories, Bar Harbor, ME), either by subcutaneous osmotic minipump at a dose of 2 mg/kg/d or by 100 μg/ml of nicotine in the drinking water (26). To determine the critical period of exposure, nicotine was given by osmotic minipump to quickly reach steady-state levels for the following time intervals during gestation and postnatally: Gestational Day 7 to Gestational Day 21, Gestational Day 14 to Postnatal Day 7, and Postnatal Day 3 to Postnatal Day 15. To avoid multiple minipump changes in growing animals, nicotine water was administered to dams 6 weeks before pregnancy, and pups were weaned onto nicotine water in experiments requiring adult animals (28).
Stereologic Analysis
Whole lungs from 8-week-old mice were inflated to 20 cm H2O with 4% paraformaldehyde for fixation overnight, paraffin embedded, oriented in an isotropic, uniform random manner, and fractionated into 1-mm slabs to sample the entire lung. Random 5-μm sections were examined. Total airway length (L) was estimated by multiplying the length density by lung volume. Length density is defined in isotropic structures as:
where Lv is length density, Q is the number of airway transects (lobar to terminal bronchiole), and A is area of lung. Lung volume is estimated by Cavalieri's method (29, 30); simple mathematical rearrangements yield the following equations:
where VL is lung volume and t is thickness of the slab.
Assessment of Airway Size
The 5-μm sections used for stereological analysis were scanned using an Olympus VS110 slide scanner at 20× (Olympus, Center Valley, PA). Airways with cartilage and pseudostratified columnar epithelium were classified as bronchi, whereas those lacking cartilage and with cuboidal epithelium were classified as bronchioles. Luminal diameters were estimated from the maximal diameter measured orthogonal to the longest diameter of the cut airway.
Pulmonary Function Testing
Respiratory mechanics were measured by forced oscillation technique by fitting the constant phase model using the integrated software in the flexiVent System (SCIREQ, Montreal, PQ, Canada) in anesthetized, tracheostomized, 8-week-old mice (31–33). Increasing doses of inhaled methacholine were administered. Forced expiratory flows were measured using Buxco BioSystem for Maneuvers hardware and software (Buxco Electronics Inc., Wilmington, NC) in anesthetized, tracheostomized mice, as previously described for monkeys except using scaled-down apparatus (34).
Histologic Analysis
A total of 10 random 5-μm sections were obtained from each specimen for Masson trichrome staining. A total of 10 random fields from each section were selected for image analysis using ImageJ (National Institutes of Health, Bethesda, MD) to determine the area staining for collagen (blue staining) relative to the total tissue area of the section.
Real-Time PCR Analysis
Real-time PCR was used to quantify collagen I mRNA levels, as previously described (35).
Statistical Analysis
Statistical analysis was performed using Student's t test and ANOVA with Bonferroni's post test analysis with GraphPad Prism 4.0 software (GraphPad Software Inc., La Jolla, CA). A P value less than 0.05 was considered statistically significant.
Results
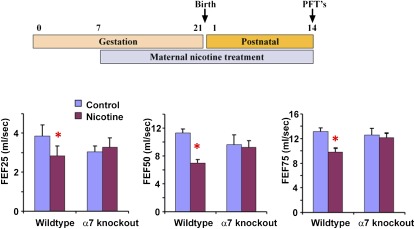
Human infants whose mothers smoked during pregnancy have persistent decreases in pulmonary function, as measured by forced expiratory flows and volumes (8–11). Mice exposed to similar doses of nicotine during gestation and early postnatal life show similar decreases in forced expiratory flows (Figure 1). Mice lacking the α7 nAChR did not have decreased forced expiratory flows in the setting of nicotine exposure, showing that the α7 nAChR mediates the ability of nicotine to impair lung function (Figure 1).
Figure 1.
Mice deficient in α7 nicotinic acetylcholine receptor (nAChR) with prenatal nicotine exposure do not exhibit decreased forced expiratory flows (FEFs). Heterozygous Chrna7tm1Bay mice were mated and pregnant/lactating females treated with nicotine (2 mg/kg/d) from Gestation Day 7 to Postnatal Day 14. On Postnatal Day 14, mice were subjected to pulmonary function testing as described in Materials and Methods. The combination of pre- and postnatal nicotine exposure significantly decreased FEFs (FEF25, FEF50, and FEF75) in wild-type mice, but not in α7 knockout mice. Error bars denote SD. *P < 0.05 compared with control (n = 6–8 animals in each group).
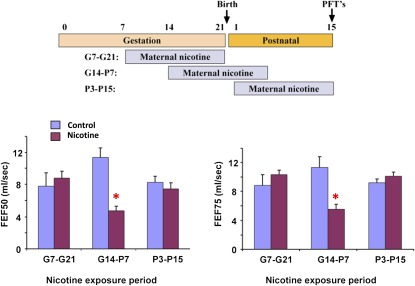
Many human epidemiologic studies looking at the effect of maternal smoking on lung function have been unable to detect the crucial period of exposure. To determine the critical period for the effects of nicotine, mice were exposed to three 2-week windows of nicotine exposure: Gestation Days 7–21, Gestation Day 14 to Postnatal Day 7, and Postnatal Days 3–15. Only nicotine exposure from Gestation Day 14 to Postnatal Day 7 affected pulmonary function (Figure 2). This establishes, for the first time, the critical period for nicotine to affect pulmonary function.
Figure 2.
Decrease in FEFs with prenatal nicotine exposure is most significant when occurring between Gestation Day 15 to Postnatal Day 7. Wild-type and homozygous Chrna7tm1Bay (α7 knockout) mice were mated and pregnant/lactating females treated with nicotine (2 mg/kg/d) for 2-week windows of exposure, from Gestation Day 7 to 21, Gestation Day 14 to Postnatal Day 7, and from Postnatal Day 3 to Postnatal Day 15. On Postnatal Day 15, mice were subjected to pulmonary function testing as described in Materials and Methods. Only wild-type mice treated with nicotine from Gestation Day 14 to Postnatal Day 7 showed significant decreases in FEFs. Error bars denote SD. *P < 0.05 compared with control (n = 6–8 animals in each group).
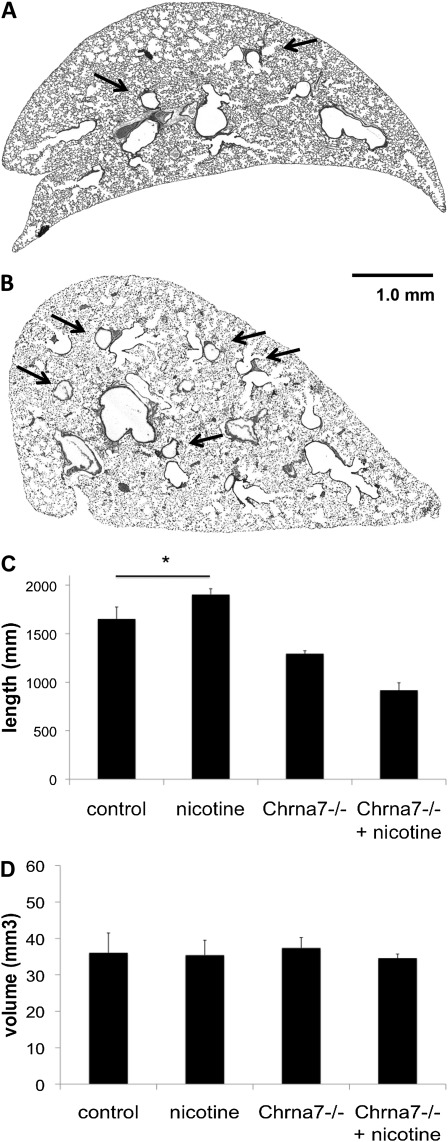
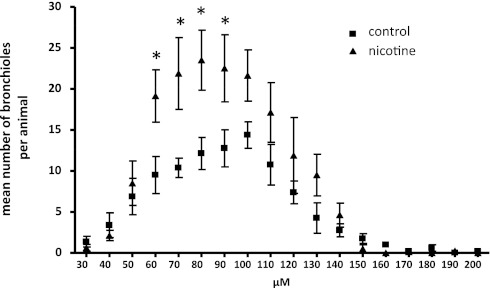
To determine the mechanism by which nicotine decreases forced expiratory flows, we next examined the effects of nicotine on airway geometry. We have previously shown that nicotine can increase branching during the period of lung branching morphogenesis, the developmental program responsible for creating the conducting airways, in a lung explant model (26). To determine if the ability of nicotine to alter lung branching and growth in the fetal period translated into structural changes in the adult lung, we assessed airway formation and branching in adult lungs by unbiased stereology. As described in Materials and Methods, lungs were sectioned in an isotropic, uniform random manner to sample the entire airway anatomy for analysis. On gross examination, fewer airways were present in unexposed adult lungs compared with lungs exposed to prenatal nicotine (Figures 3A–3B). Using standard unbiased stereologic methods, we found that 8-week-old adult mice exposed to prenatal nicotine had increased total airway length compared with offspring from untreated mothers (Figure 3C). Nicotine exposure did not increase airway length in α7 nAChR animals. In addition, lung volumes were similar between the exposed and unexposed groups using Cavalieri's method (Figure 3D). All lungs were sampled in toto. Because there was no change in volume, greater airway length must occur either because of increased branching or increased airway tortuosity, consistent with the gross image in Figure 3. To evaluate the change in distribution of airway sizes, airway size was measured in isotropic, uniform random sections and counted. Mice exposed to prenatal nicotine had both a greater total number of bronchi and a greater number of bronchioles when compared with the unexposed control group. Average bronchial diameter (229.77 ± 135.37 versus 237.86 ± 139.70 μm) and bronchiolar diameter (84.77 ± 28.44 versus 84.84 ± 25.36 μm) did not differ between the control group or the nicotine group (Table 1). However, lungs from mice exposed to prenatal nicotine had a statistically increased number of bronchioles that were 60–90 μm in diameter (Table 1 and Figure 4).
Figure 3.
Adult mice exposed to nicotine prenatally have greater airway length without a concomitant change in volume. C57BL6/J female mice were administered either untreated water or water containing 100 μg/ml nicotine before timed breeding and through gestation. Lungs were obtained from age-matched offspring at 8 weeks of age. Unexposed lungs (A) have less airway branching (arrows highlight selected airways) compared with lungs exposed to nicotine in the prenatal period (B) on gross examination. Using standard stereologic techniques, total airway length and volumes were estimated. Mice exposed to prenatal nicotine have greater total airway length (C) without any change in volume (D) when compared with unexposed wild-type control animals. Animals deficient in α7 nAChR (Chrna7−/−) exposed to prenatal nicotine do not demonstrate greater total airway length when compared with unexposed animals deficient in α7 nAChR (C) (n = 3–8 per group). Error bars denote SD (*P < 0.05 compared with control). Scale bar, 1 mm.
TABLE 1.
AIRWAY CHARACTERISTICS BETWEEN UNEXPOSED MICE AND MICE WITH PRENATAL NICOTINE EXPOSURE
| Control | Nicotine | |
| Characteristics | (n = 8) | (n = 8) |
| Total no. of airways | 1,259 | 2,034* |
| Bronchi | 468 | 723* |
| Bronchioles | 791 | 1311* |
| Average airway diameter, μm | ||
| Bronchi | 229.77 ± 135.37 | 237.86 ± 139.70 |
| Bronchioles | 84.77 ± 28.44 | 84.84 ± 25.36 |
C57BL6/J female mice were administered either untreated water or water containing 100 μg/ml nicotine before timed breeding and through gestation. Lungs were obtained from age-matched offspring at 8 weeks of age. The mice exposed to prenatal nicotine have a significantly greater number of bronchi and bronchioles compared with the unexposed control animals. Average airway diameter was not significantly different between groups (n = 8–9).
P < 0.05 compared to control.
Figure 4.
Adult mice exposed to prenatal nicotine have significantly greater numbers of small bronchioles compared with unexposed control animals. C57BL6/J female mice were administered either untreated water or water containing 100 μg/ml nicotine before timed breeding and through gestation. Lungs were obtained from age-matched offspring at 8 weeks of age. Whole lungs were embedded and sectioned in an isotropic, uniform random manner to create unbiased samples for examination. Airway diameter was measured for each visible bronchus and bronchiole. Mice exposed to prenatal nicotine had significantly greater numbers of small bronchioles, with diameters between 60–90 μm (n = 8 per group). Error bars denote SD. *P < 0.05 compared with control.
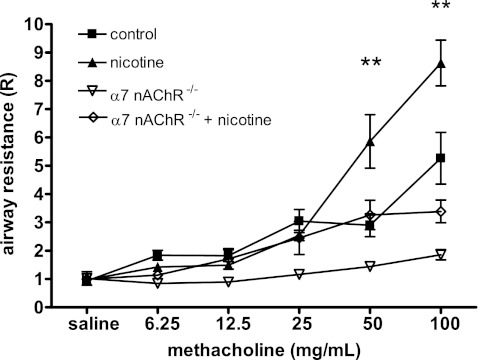
After seeing reduced forced expiratory flows in nicotine-exposed mice, we examined whether prenatal nicotine exposure also affected bronchial reactivity using the forced oscillation technique to measure input impedance to obtain airway resistance from the constant phase model. Mice with prenatal nicotine exposure showed increased airway resistance at lower doses of inhaled methacholine when compared with unexposed control animals (Figure 5). This was consistent with increased airway hyperresponsiveness in the absence of allergic sensitization.
Figure 5.
Mediated through α7 nAChR–dependent signals, prenatal nicotine exposure leads to increased airway resistance in response to methacholine challenge. Adult mice (8 wk old) exposed to nicotine in utero, as previously described, underwent pulmonary function testing with forced oscillation technique using the flexiVent system. Airway resistance (R) was determined using the constant phase model. After baseline measurements were obtained, mice were administered aerosolized methacholine in increasing doses. Mice with prenatal nicotine exposure had significantly increased airway resistance compared with unexposed mice during the latter portion of the inhaled methacholine challenge. Prenatal nicotine exposure in mice deficient in the α7 nAChR did not increase airway resistance during inhaled methacholine challenge (n = 5–8 per group). Error bars denote SEM. **P < 0.01 compared with control.
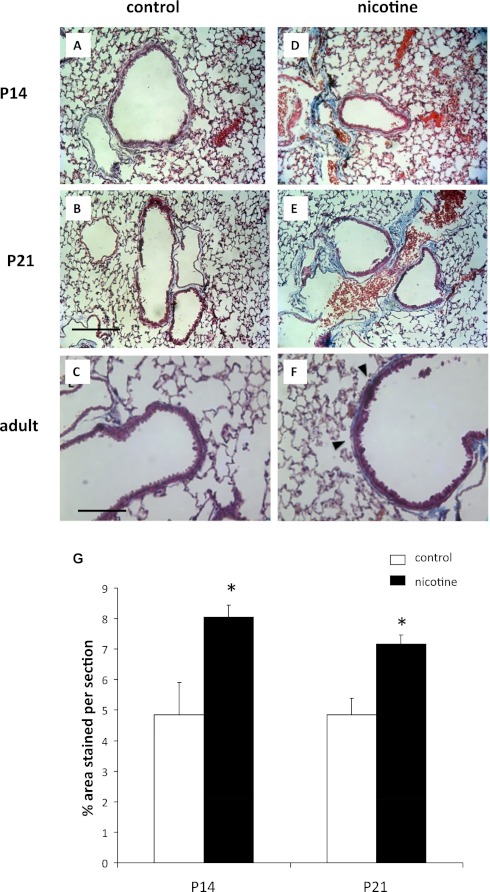
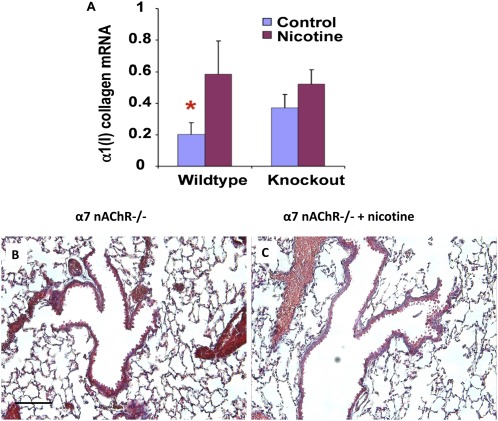
Next, we measured collagen expression by mRNA and Masson trichrome stain. Collagen has previously been reported to be increased around airways and vessels in near-term fetuses with prenatal nicotine exposure (21, 23). We examined unexposed mice and nicotine-exposed mice at Postnatal Days 14 and 21, as well as at 8 weeks. We found that collagen was increased around the confluence of airways and vessels in mice exposed to prenatal nicotine, and that this persisted into adulthood (Figure 6). However, α7 nAChR–deficient mice exposed to prenatal nicotine did not exhibit the increase in collagen 1 mRNA levels or collagen deposition by Masson trichrome staining that was seen in wild-type mice exposed to prenatal nicotine (Figure 7). Our results demonstrate that nicotine-induced collagen expression is dependent on nicotine binding α7 nAChR.
Figure 6.
Increased collagen staining around airways and vessels is seen in mice exposed to nicotine prenatally. Lungs from mice were obtained at Postnatal Days 14 and 21, and adult mice exposed to nicotine in utero. Collagen was identified using Masson trichrome stain. In areas where airways and vessels were in close proximity, increased collagen staining was observed in postnatal and adult lungs exposed to prenatal nicotine (D–F, G) when compared with unexposed control animals (A–C). Scale bars, 100 μm (A, B, D, E) and 200 μM (C, F). Error bars denote SEM. *P < 0.05 compared with control.
Figure 7.
Increased collagen deposition after prenatal nicotine exposure is dependent on α7 nAChR signaling. Lungs from both wild-type and α7 nAChR–deficient adult mice (α7 nAChR−/−) were obtained after prenatal nicotine exposure for RNA isolation. Using quantitative real-time PCR, collagen 1 mRNA levels were measured and results represented are normalized to 18S as the housekeeping gene. Unlike wild-type mice, mice deficient in α7 nAChR did not have up-regulation of collagen 1 mRNA levels after prenatal nicotine exposure (A). Collagen deposition, as identified by Masson trichrome stain, was not different in α7 nAChR−/− adult mice after prenatal nicotine exposure (B–C). Scale bar, 100 μm. Error bars denote SEM. *P < 0.05 compared with nicotine.
Discussion
The mechanism by which maternal smoking during pregnancy causes lifelong changes in offspring pulmonary function remains poorly understood, and is likely multifactorial. We have previously shown that one key tobacco component is nicotine, which crosses the placenta to interact with nAChR in the developing lung (21). Although not all results from a model of nicotine exposure can be extrapolated to cigarette smoke exposure, understanding the effects of nicotine on the fetal lung is crucial, because the fetus and the mother have similar exposure to nicotine, whereas many of the other components in tobacco smoke will not enter the fetal compartment. Thus, the effects of maternal smoking are likely very different between mother and fetus. Interestingly, prenatal nicotine exposure alone in our murine model causes the same decreases in expiratory flows in newborn monkeys that are also seen in the offspring of maternal human smokers (22). In this article, we establish mouse models to study the effects of nicotine on lung development, and show that combined prenatal and early postnatal nicotine exposure leads to decreases in pulmonary function in mice, and that these effects are mediated by the α7 nAChR. In addition, these changes are found to be associated with changes in airway structure and persistent changes in collagen expression. Finally, prenatal nicotine exposure also causes increased airway hyperresponsiveness. This hyperresponsiveness occurs without allergen sensitization, suggesting that nicotine is an agent responsible for ETS-induced airway hyperresponsiveness.
Murine lungs at birth are less mature than human lungs, so, to model the effects of prenatal nicotine exposure, mice were exposed to nicotine from Day 7 to Day 21 of pregnancy and Postnatal Days 1–14. As shown in Figure 1, this caused a decrease in forced expiratory flows very similar to what is observed in human infants whose mothers smoked during pregnancy (6, 8, 11, 36, 37). This confirms our observation in nonhuman primates that nicotine is the primary component in cigarette smoke that alters lung development, leading to decreased pulmonary function (21–23). The establishment of this model now allows us to take advantage of mouse genetics to characterize the mechanism by which nicotine alters lung development.
Given its high affinity for nicotine, the α7 nAChR is a likely candidate to mediate the effects of prenatal nicotine exposure on pulmonary function. α7 nAChRs have already been shown to mediate the ability of nicotine to stimulate lung branching morphogenesis in lung explant models, as well as play a role in nicotine-stimulated proliferation of airway epithelial cells (24, 26, 27, 38, 39). Our studies reveal that the ability of prenatal nicotine exposure to affect offspring pulmonary function is blocked in α7 nAChR knockout mice. This clearly establishes that α7 nAChRs mediate the effects of nicotine on lung development. This is also consistent with the high levels of α7 in developing lung and the ability of prenatal nicotine exposure to increase these levels (21). When compared with wild-type mice, our data for expiratory flows, collagen mRNA expression, and airway length in the α7 nAChR knockout mice suggest that loss of α7 nAChR may influence baseline airway development, but these findings were not statistically significant. Although a type II error could potentially explain the lack of statistical significance, the experimental sample size was adequately powered to detect β = 0.2. Nonetheless, although there may be inherent differences between wild-type and α7 nAChR knockout mice that could potentially influence the outcome of these studies, when our current findings are taken together with those in the literature, they provide robust support for the importance of α7 nAChRs in mediating the effects of smoking on lung development.
Although there are numerous studies describing the effects of maternal smoking on lung function in children and adults, most of them are unable to discern the exact period of exposure that is most relevant (40, 41). Therefore, to identify the critical period for nicotine to affect lung development, mice were exposed to nicotine from Gestation Day 7 to 21, from Gestation Day 14 to Postnatal Day 7, and from Postnatal Day 3 to 15. As shown in Figure 2, nicotine only affected offspring pulmonary function if given from Gestation Day 14 to Postnatal Day 7. This is the time period corresponding to the last half of lung branching morphogenesis, and the entirety of the canalicular and saccular phases of mouse lung development, during which the airways are growing and differentiating, but after the majority of lung branching has been completed and before extensive alveolarization (42–44). This suggests that nicotine is affecting the development of the conducting airways, and that the main target is not alveolarization.
In the group exposed to nicotine from Gestation Day 14 to Postnatal Day 7, effects on pulmonary function were present at Postnatal Day 14. Because alveolarization is still ongoing until Postnatal Day 21, the effects on pulmonary function are more likely due to airway-dependent events. Because nicotine is known to affect branching morphogenesis, the effects on pulmonary function at Postnatal Day 14 are most likely related to changes in branching and airway growth (26, 27). Our data on pulmonary function are consistent with those of others who have used models that include perinatal exposure (i.e., exposure into the late postnatal period). Liu and colleagues (45) found that rats with nicotine exposure between Embryonic Day 6 and Postnatal Day 21 also exhibited increased airway hyperresponsiveness on Postnatal Day 21.There may be additional effects of nicotine during later gestational development that influence the airways and pulmonary function. Sandberg and colleagues (46) found that newborn lambs with maternal nicotine exposure in the last trimester of pregnancy had reduced bronchiolar diameter and increased airway smooth muscle mass when compared with unexposed lambs.
To determine the long-term effects on airway growth from prenatal nicotine exposure, we performed detailed whole-lung stereology. As shown in Figure 3, total airway length is significantly increased in nicotine-exposed animals, without a concomitant change in total lung volume. Because total lung volume is an important variable in the calculation for airway length, all lungs were treated equally and inflated to equal pressure (see Materials and Methods). Although rigorous methods are followed, small, regional variability in the level of inflation may occur and lead to areas of the lungs that are more or less inflated. However, if present, these small areas have little influence on the total lung volume measurement. Because our results suggest that the change in airway length is not from the lungs simply being larger, then there are either more airway branches to contribute to total airway length, or airways in nicotine-exposed animals are more twisted and tortuous. Although animals deficient in α7 nAChR have similar total airway length to wild-type control animals at baseline, prenatal nicotine exposure does not increase total airway length in animals deficient in α7 nAChR. The slightly lower total airway length seen in nicotine-exposed α7 nAChR−/− animals suggests that the α7 nAChR is an important mediator of conducting airway formation in the setting of prenatal nicotine exposure.
To analyze further the increased length, we counted the numbers of airways and categorized them by airway diameters. Figure 4 shows an increased total number of airways and, specifically, an increased number of bronchioles in the nicotine-exposed animals. Our results provide in vivo confirmation of previously published work showing that nicotine increases branching morphogenesis in lung explants in vitro and also increases airway tortuosity (26, 27, 47).
The nicotine-exposed animals have a statistically significant increase in airways between 60–90 μm. Although the average diameters of the bronchi and bronchioles were not statistically different between the control animals and the nicotine animals, the increased number of smaller bronchioles in the nicotine-exposed animals provides a geometric explanation for the physiologic decreases in forced expiratory flows and increased airway resistance with a methacholine challenge. If one assumes the basic geometric properties of an airway are similar to that of a circular tube, based on Poiseuille's law, flow is inversely proportional to length and directly proportional to diameter (i.e., flow is decreased in a longer and narrower tube). Admittedly, the calculations for airflow in the tracheobronchial tree are more complex than for a simple tube, but, in our model, the animals exposed to nicotine have increased length and increased number of small diameter airways, both of which can affect airflow. In addition, the smaller cross-sectional area of the bronchioles can contribute to increased turbulence and drag, which reduces airflow.
Our results also compliment previously published data in other animal models of prenatal nicotine exposure. In a study by Sandberg and colleagues, young lambs exposed to nicotine prenatally had significantly decreased specific airway conductance (48). Decreased specific airway conductance is consistent with the decreased forced expiratory flows at baseline that we found in our model. Although we did not find a difference in airway resistance at baseline, they found a trend toward increased airway resistance. However, this may be related to the difference in animal species and ages when tested. In the young lambs exposed to prenatal nicotine, there was also more efficient gas mixing in the distal airways, as assessed by multiple breath nitrogen washout. Our findings that prenatal nicotine exposure leads to significantly greater number of airways, specifically small airways, provide an explanation for why nitrogen mixing is more efficient in animals exposed to nicotine prenatally, because more distal airway branches facilitate gas mixing (48).
Overall, our findings support the concept of dysanaptic growth (e.g., disassociation between growth of the conducting airways and growth of the lung parenchyma) (49). Dysanaptic growth has been associated with decreased expiratory flows in the absence of respiratory symptoms in models comparing sex and hypoxia exposure (50–52). Dysanaptic growth has also been described in compensatory growth models (53–55). Our results, showing that chronic nicotine exposure leads to greater airway length and numbers without changing overall lung volume, is further evidence that dysanaptic growth occurs and can affect lung function.
In addition to changes in airway structure, we also found that structural changes early in life from nicotine exposure persist into adulthood. Previous studies have shown increased collagen around airways and vessels in near-term fetuses exposed to nicotine in utero (21, 23). In our study, we found increased collagen around airways in early postnatal and adult lungs exposed to chronic nicotine beginning in utero. The source of the deposited collagen is unknown, but fibroblasts are a likely source, particularly, because expression of α7 nAChR is high in these cells with prenatal nicotine exposure (21). These findings suggest that in utero nicotine exposure promotes airway remodeling, which persists into childhood and adulthood with continued nicotine exposure. This early airway remodeling resembles changes seen in airway disorders, such as asthma and chronic obstructive pulmonary disease. The higher incidence of asthma with prenatal nicotine exposure may be because the airways are already primed for further remodeling changes.
Nicotine exposure beginning in the prenatal period also affects the physiologic response to bronchoprovocational challenge. We found that mice exposed to prenatal nicotine are more hyperresponsive after a methacholine challenge, even in the absence of allergic stimulation. This may help explain why offspring of maternal smokers are more likely to develop asthma (9, 56–58). Although many other genetic and environmental factors are important in determining whether a child develops asthma, having more hyperresponsive airways creates a more favorable environment for developing the full clinical manifestations of asthma. The exact mechanisms by which nicotine increases bronchoreactivity remain to be determined, but may reflect the altered airway geometry that may lead to increased airway resistance for a given contractile stimulus.
In conclusion, although there are differences between cigarette smoke exposure and our nicotine exposure model that may limit extrapolation of findings, our results strongly suggest that nicotine is a significant component in tobacco smoke responsible for the detrimental effects of tobacco in the developing lung The effect on expiratory flows in our murine model is consistent with a nonhuman primate model of prenatal nicotine exposure, and mimics changes in expiratory flow seen in offspring of maternal smokers (22). A primary mechanism for this effect appears to be the ability of nicotine to interact with α7 nAChR expressed in lung cells, which promotes abnormal patterns of airway growth and airway collagen deposition. Our findings also emphasize that fetal exposure to nicotine or ETS has a significant impact on respiratory health and function that remains with age. Indeed, multiple studies have demonstrated that pulmonary function and respiratory health as an adult may be predetermined by pulmonary function in early life (10, 16, 17, 59). Understanding the mechanisms by which maternal smoking affects fetal lung development may be helpful in optimizing adult respiratory health. Although nicotine replacement therapy is generally considered a much better option than smoking during pregnancy, our results suggest that nicotine itself has significant developmental effects. The nicotine exposure model allows for a simpler approach to explore possible targeted interventions, because therapeutics targeting α7 nAChR are currently available, and the technology exists to find safer and more effective alternatives. More work is necessary to understand fully the ramifications of nicotine exposure during pregnancy and to identify alternative methods of smoking cessation.
Supplementary Material
Acknowledgments
The authors sincerely thank Susanne Roser-Page and Kora Grooms for excellent technical assistance, specifically immunohistochemistry and animal care, Judy St. George for assisting in airway measurements, and Xiaowei Yang for assisting in the statistical analysis. They also thank Art Beaudet for assistance with the α7 nicotinic acetylcholine receptor knockout mice.
Footnotes
This work was supported by National Institutes of Health (NIH) grants HL080293 (C.W.), NIH RR00163 (E.R.S.), NIH HL066118 (E.R.S.), and by the American Thoracic Society Research Program (C.W.) and a Veterans Affairs Merit Review (J.R.).
Author Contributions: All authors substantially contributed to (1) the study conception and design; (2) acquisition, analysis, and interpretation of data; (3) drafting and revising the manuscript; and (4) final approval of the submitted manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0028OC on January 12, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.CDC The health consequences of involuntary exposure to tobacco smoke exposure: a report from the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control; 1986. DHHS publication no. [CDC] 87–8398 [Google Scholar]
- 2.Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, Olsen MA, Fraser VJ. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. Pediatrics 2003;112:1283–1289 [DOI] [PubMed] [Google Scholar]
- 3.Cook DG, Strachan DP. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax 1997;52:1081–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook DG, Strachan DP, Carey IM. Health effects of passive smoking. 9. Parental smoking and spirometric indices in children. Thorax 1998;53:884–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold DR. Environmental tobacco smoke, indoor allergens, and childhood asthma. Environ Health Perspect 2000;108:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, Hruba F, Pattenden S, Rudnai P, Slachtova H, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med 2006;173:1255–1263 [DOI] [PubMed] [Google Scholar]
- 7.Adler A, Tager IB, Brown RW, Ngo L, Hanrahan JP. Relationship between an index of tidal flow and lower respiratory illness in the first year of life. Pediatr Pulmonol 1995;20:137–144 [DOI] [PubMed] [Google Scholar]
- 8.Cunningham J, Dockery DW, Speizer FE. Maternal smoking during pregnancy as a predictor of lung function in children. Am J Epidemiol 1994;139:1139–1152 [DOI] [PubMed] [Google Scholar]
- 9.Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med 1999;159:403–410 [DOI] [PubMed] [Google Scholar]
- 10.Haland G, Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kass MC, Pettersen M, Carlsen KH. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med 2006;355:1682–1689 [DOI] [PubMed] [Google Scholar]
- 11.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, Weiss ST, Speizer FE. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis 1992;145:1129–1135 [DOI] [PubMed] [Google Scholar]
- 12.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med 1988;319:1112–1117 [DOI] [PubMed] [Google Scholar]
- 13.Tager IB, Hanrahan JP, Tosteson TD, Castile RG, Brown RW, Weiss ST, Speizer FE. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. Am Rev Respir Dis 1993;147:811–817 [DOI] [PubMed] [Google Scholar]
- 14.Young S, Arnott J, O'Keeffe PT, Le Souef PN, Landau LI. The association between early life lung function and wheezing during the first 2 yrs of life. Eur Respir J 2000;15:151–157 [DOI] [PubMed] [Google Scholar]
- 15.Yuksel B, Greenough A, Giffin F, Nicolaides KH. Tidal breathing parameters in the first week of life and subsequent cough and wheeze. Thorax 1996;51:815–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet 2007;370:758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O'Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax 2009;64:810–814 [DOI] [PubMed] [Google Scholar]
- 18.Luck W, Nau H. Exposure of the fetus, neonate, and nursed infant to nicotine and cotinine from maternal smoking. N Engl J Med 1984;311:672. [DOI] [PubMed] [Google Scholar]
- 19.Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther 1985;8:384–395 [DOI] [PubMed] [Google Scholar]
- 20.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 2009;78:703–711 [DOI] [PubMed] [Google Scholar]
- 21.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 1999;103:637–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med 2001;164:989–994 [DOI] [PubMed] [Google Scholar]
- 23.Sekhon HS, Proskocil BJ, Clark JA, Spindel ER. Prenatal nicotine exposure increases connective tissue expression in foetal monkey pulmonary vessels. Eur Respir J 2004;23:906–915 [DOI] [PubMed] [Google Scholar]
- 24.Fu XW, Lindstrom J, Spindel ER. Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells. Am J Respir Cell Mol Biol 2009;41:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, Proskocil BJ, Gravett C, Lindstrom J, Mark GP, Saha S, Spindel ER. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res 2008;68:4693–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wongtrakool C, Roser-Page S, Rivera HN, Roman J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am J Physiol Lung Cell Mol Physiol 2007;293:L611–L618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuenschell CW, Zhao J, Tefft JD, Warburton D. Nicotine stimulates branching and expression of SP-A and SP-C mRNAs in embryonic mouse lung culture. Am J Physiol 1998;274:L165–L170 [DOI] [PubMed] [Google Scholar]
- 28.Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci 1997;17:9165–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard CV. Unbiased stereology MGR. Three-dimensional measurement in microscopy, 2nd ed. Abingdon, Oxon, UK: Taylor & Francis; 2005 [Google Scholar]
- 30.Smith CS, Guttman L. Measurement of internal boundaries in three-dimensional structures by random sectioning. Trans AIME 1953;197:81–93 [Google Scholar]
- 31.Hantos Z, Collins RA, Turner DJ, Janosi TZ, Sly PD. Tracking of airway and tissue mechanics during TLC maneuvers in mice. J Appl Physiol 2003;95:1695–1705 [DOI] [PubMed] [Google Scholar]
- 32.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178 [DOI] [PubMed] [Google Scholar]
- 33.Lutchen KR, Suki B, Zhang Q, Petak F, Daroczy B, Hantos Z. Airway and tissue mechanics during physiological breathing and bronchoconstriction in dogs. J Appl Physiol 1994;77:373–385 [DOI] [PubMed] [Google Scholar]
- 34.Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Jia Y, Hull WM, Whitsett JA, Starcher BC, Spindel ER. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med 2005;171:1032–1039 [DOI] [PubMed] [Google Scholar]
- 35.Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung: association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 2002;26:31–41 [DOI] [PubMed] [Google Scholar]
- 36.Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, Avol E, Peters JM. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax 2000;55:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy: effects on lung function during the first 18 months of life. Am J Respir Crit Care Med 1995;152:977–983 [DOI] [PubMed] [Google Scholar]
- 38.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin–mediated activation of Src and Rb–Raf-1 pathways. J Clin Invest 2006;116:2208–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Y, Ritzenthaler JD, Roman J, Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am J Respir Cell Mol Biol 2007;37:681–690 [DOI] [PubMed] [Google Scholar]
- 40.Upton MN. Effects of parental smoking on the respiratory health of adults. Thorax 2004;59:274–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upton MN, Smith GD, McConnachie A, Hart CL, Watt GC. Maternal and personal cigarette smoking synergize to increase airflow limitation in adults. Am J Respir Crit Care Med 2004;169:479–487 [DOI] [PubMed] [Google Scholar]
- 42.Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatr Pulmonol 1996;21:383–397 [DOI] [PubMed] [Google Scholar]
- 43.Schittny JC, Burri PH. Morphogenesis of the mammalian lung: aspects of structure and extracellular matrix components. : Massaro D, Massaro G, Chambon P, Lung development and regeneration. New York: Dekker Inc.; 2003. pp. 275–318 [Google Scholar]
- 44.Ten Have-Opbroek AA. The development of the lung in mammals: an analysis of concepts and findings. Am J Anat 1981;162:201–219 [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Sakurai R, O'Roark EM, Kenyon NJ, Torday JS, Rehan VK. PPARgamma agonist rosiglitazone prevents perinatal nicotine exposure-induced asthma in rat offspring. Am J Physiol Lung Cell Mol Physiol 2011;300:L710–L717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandberg KL, Pinkerton KE, Poole SD, Minton PA, Sundell HW. Fetal nicotine exposure increases airway responsiveness and alters airway wall composition in young lambs. Respir Physiol Neurobiol 2011;176:57–67 [DOI] [PubMed] [Google Scholar]
- 47.Wang NS, Chen MF, Schraufnagel DE, Yao YT. The cumulative scanning electron microscopic changes in baby mouse lungs following prenatal and postnatal exposures to nicotine. J Pathol 1984;144:89–100 [DOI] [PubMed] [Google Scholar]
- 48.Sandberg K, Poole SD, Hamdan A, Arbogast P, Sundell HW. Altered lung development after prenatal nicotine exposure in young lambs. Pediatr Res 2004;56:432–439 [DOI] [PubMed] [Google Scholar]
- 49.Green M, Mead J, Turner JM. Variability of maximum expiratory flow–volume curves. J Appl Physiol 1974;37:67–74 [DOI] [PubMed] [Google Scholar]
- 50.Kosch PC, Gillespie JR, Berry JD. Flow–volume curves and total pulmonary resistance in normal bonnet and rhesus monkeys. J Appl Physiol 1979;46:176–183 [DOI] [PubMed] [Google Scholar]
- 51.Merkus PJ, Borsboom GJ, Van Pelt W, Schrader PC, Van Houwelingen HC, Kerrebijn KF, Quanjer PH. Growth of airways and air spaces in teenagers is related to sex but not to symptoms. J Appl Physiol 1993;75:2045–2053 [DOI] [PubMed] [Google Scholar]
- 52.Sekhon HS, Thurlbeck WM. Lung growth in hypobaric normoxia, normobaric hypoxia, and hypobaric hypoxia in growing rats. I. Biochemistry. J Appl Physiol 1995;78:124–131 [DOI] [PubMed] [Google Scholar]
- 53.Dane DM, Johnson RL, Jr, Hsia CC. Dysanaptic growth of conducting airways after pneumonectomy assessed by CT scan. J Appl Physiol 2002;93:1235–1242 [DOI] [PubMed] [Google Scholar]
- 54.Hsia CC, Zhou XS, Bellotto DJ, Hagler HK. Regenerative growth of respiratory bronchioles in dogs. Am J Physiol Lung Cell Mol Physiol 2000;279:L136–L142 [DOI] [PubMed] [Google Scholar]
- 55.McBride JT, Wohl ME, Strieder DJ, Jackson AC, Morton JR, Zwerdling RG, Griscom NT, Treves S, Williams AJ, Schuster S. Lung growth and airway function after lobectomy in infancy for congenital lobar emphysema. J Clin Invest 1980;66:962–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2001;163:429–436 [DOI] [PubMed] [Google Scholar]
- 57.Lannero E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res 2006;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magnusson LL, Olesen AB, Wennborg H, Olsen J. Wheezing, asthma, hayfever, and atopic eczema in childhood following exposure to tobacco smoke in fetal life. Clin Exp Allergy 2005;35:1550–1556 [DOI] [PubMed] [Google Scholar]
- 59.Guerra S, Sherrill DL, Kurzius-Spencer M, Venker C, Halonen M, Quan SF, Martinez FD. The course of persistent airflow limitation in subjects with and without asthma. Respir Med 2008;102:1473–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.