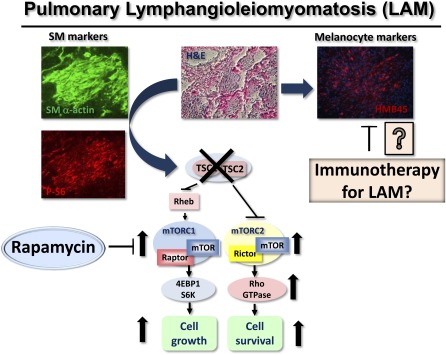
Pulmonary lymphangioleiomyomatosis (LAM) is a rare progressive cystic lung disease affecting primarily women of childbearing age (1, 2). LAM occurs sporadically (LAM-S) with prevalence of 2.6 per 1 million women or in 34% of women with tuberous sclerosis (TS) (LAM-TS) (3), an autosomal dominant hamartoma syndrome that occurs in 1 of 5,800 live births (2). Clinical manifestations of LAM are pneumothorax from cyst rupture, chylothorax from obstruction of lymphatics, and progressive decline of pulmonary function (1, 2). About 40% of patients with LAM-S and about 80% of patients with LAM-TS also develop in kidney an angiomyolipoma (AML), a benign tumor of smooth muscle (SM), blood vessels, and fat cells (1, 2). Pathological changes in the LAM lung are associated with growth throughout the lung parenchyma of LAM nodules that consist of SM-like spindle-shaped cells and epithelioid-like polygonal cells positive for melanocytic cell marker HMB45 (human melanoma black 45) (4, 5) (Figure 1). SM-like LAM cells show high immunoreactivity for PCNA (proliferating cell nuclear antigen), a marker of DNA synthesis and cell proliferation, compared with the epithelioid-like HMB45-positive cells (2), suggesting that SM-like LAM cells represent the proliferative component of the LAM nodules. The role of melanocyte-specific markers in LAM and whether they could be targeted therapeutically have been explored (6) and were reviewed in the January issue (7).
Figure 1.
Therapeutic targeting of mTORC1 signaling with rapamycin analog sirolimus in smooth muscle–like lymphangioleiomyomatosis (LAM) cells shows encouraging results in clinical trial. The experimental targeting of novel molecules deregulated by tuberous sclerosis complex (TSC)1/TSC2 loss in LAM, including melanoma-associated antigens, holds a promise for innovative strategies to harness the disease.
Major advances in understanding LAM occurred with identifying in the proliferative SM-like LAM cells a loss of heterozygosity in the tumor suppressor tuberous sclerosis complex 2 (TSC2) gene (8), and linking the mutational inactivation of TSC2 to abnormal SM-like LAM cell growth (9) and the constitutive activation of the mammalian target of rapamycin complex 1 (mTORC1) (9, 10) (Figure 1), an integrator of growth factor, nutrient, energy, and stress signaling (11). TSC2 forms a tumor suppressor complex with TSC1 and regulates mTORC1 by directly controlling the activity of the small GTPase Rheb via the GTPase-activating protein (GAP) domain of TSC2 (12) (Figure 1). Rheb binds to raptor and controls the activity of the mTOR that phosphorylates p70 S6 kinase (S6K1) and 4E-BP1 (11). Importantly, TSC2-dependent S6K1 activation suppresses phosphatidylinositol 3-kinase (PI3K) signaling, named a negative feedback loop, that may explain the benign tumorigenesis (13) in LAM and has implications for the therapeutic targeting of mTORC1 (see below). Activity of mTORC1 is sensitive to the inhibition by bacterial microlide rapamycin (14), which by binding with FKBP12 (FK506-binding protein of 12 kD) interacts with FKBP12-binding domain of mTOR and inhibits mTORC1 activity (15). Importantly, rapamycin inhibits SM-like LAM cell proliferation at concentrations that have little effect on human airway and vascular SM cells (9, 16–18). The discovery of the TSC2 as a negative regulator of the mTORC1 (9, 19) and inhibitory effects of rapamycin in preclinical studies (9, 16, 20, 21) provided a rationale for use of rapamycin analogs in the clinic.
Importantly, Frank McCormack and colleagues (22) described results of the first double-blinded placebo-controlled sirolimus (rapamycin analog) clinical trial involving patients with LAM. The sirolimus trial was conducted in two stages, including a 12-month treatment stage and a 12-month observation stage, with the difference between the groups in the rate of change (slope) in FEV1 as a primary endpoint. After 12 months of treatment, sirolimus stabilized lung function, reduced symptoms, and improved quality of life as compared with the placebo group. After discontinuation of sirolimus, however, the decline of the lung function resumed and paralleled that in the placebo group (22). Further, tolerance and safety concerns are also serious limits to the long-term treatment of patients with sirolimus.
Why did rapamycin fail to have a long-lasting effect in LAM? One of the major limitations of rapamycin as a drug is that in many tumors it has a cytostatic but not cytotoxic effect (11). Rapamycin only partially inhibits mTORC1 because it induces allosteric inhibition of mTOR without affecting ATP binding site of mTOR. Further, rapamycin inhibition of S6K1 releases a negative feedback loop on PI3K signaling that induces activation of the pathway and supports cell survival (14). Rapamycin also transiently and partially inhibits phosphorylation of 4E-BP1, thus having only modest inhibitory effect on protein translation (11) (see Figure 1). These limitations of rapamycin have motivated the search for novel or additional therapeutic targets for LAM. To overcome the limitation of rapamycin, the second generation of mTOR inhibitors targeting the catalytic activity of mTOR have been developed and are currently being tested in preclinical studies and clinical trials in the treatment of cancer (14). This group of drugs has not been preclinically tested for LAM (1, 2). The study by Joel Moss and colleagues found a correlation between a positive response to bronchodilators with more airflow obstruction and a predominantly solid pattern of LAM lesions in the lung biopsy (23). Further, there is no evidence that corticosteroids and hormonal therapy are beneficial for LAM (2, 24). Based on the prevailing hypothesis that the cystic lung destruction in LAM occurs due to up-regulation of matrix metalloproteases (MMPs), a clinical case of one patient was reported in which doxycycline, a nonspecific MMP inhibitor, reduced urinary MMP level that was associated with improved FEV1 (1–3). However, this is a single case that needs further preclinical and clinical investigation.
The finding that RhoA GTPase is activated in LAM (25, 26) and is required for LAM-derived cell survival (27) (Figure 1) led to preclinical testing of statins, 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase inhibitors, and pleiotropic agents that might contribute to the prevention of human cancers (28). Statins, which modulate the lipid metabolism, regulate the geranylgeranylation of Rho GTPases that is required for their membrane anchoring and activation. Initial preclinical studies using synthetic atorvastatin (Lipitor) did not improve the outcome of syngeneic growth of TSC2-null tumors in nude mice formed by mouse embryonic fibroblast immortalized by deletion of the tumor suppressor p53 (29) and renal and liver tumors in TSC2+/− mice (30) that do not develop lung tumors. In contrast, a simvastatin (Zocor) not only inhibited xenographic tumor growth of TSC2-null SM-like cells derived from uterine leiomyoma by promoting apoptosis, but also prevented tumor recurrence after treatment withdrawal (31). Despite the difference in experimental approaches and animal models, these studies suggest that simvastatin (Zocor) and atorvastatin (Lipitor) have differential effects on TSC2-null tumors. Current or retrospective analysis (32) of clinical cases to evaluate whether simvastatin and atorvastatin have differential effects in the clinic are needed. Among other concepts now being tested that could have a potential applicability to LAM are pre-clinical studies in TS showing that glucose deprivation (33) and autophagy (34) may have an impact on growth of TS-related tumors.
A major limitation in developing new strategies for treatment of LAM and performing preclinical studies, however, is the lack of a LAM animal model (35). Attempts to create xenographic human LAM cell tumors in the lungs of immunodeficient mice have generally not been successful. Homozygous TSC1−/− and TSC2−/− mice are embryonically lethal. The major features of heterozygous TSC1+/− and TSC2+/− mice are development of cystadenomas of kidney and liver hemangiomas due to loss of heterozygosity at 6 to 12 months (35–37). By 15 to 18 months of age, some animals develop malignant renal carcinoma and lung adenoma (37, 38). In the Eker rat, which carries naturally occurring TSC2 mutations, the natural occurrence of lung metastasis of TSC2-null cells from primary renal carcinomas and uterine leiomyosarcomas is extremely rare and only occurs late in the animal's life (39, 40). Thus, existing animal models are challenging and incongruous for the study of human lung disease. It appears that TSC2-null cells from the Eker rat can form small clusters in the lung when injected into SCID mice (41). Whether these cell clusters can induce cystic airspace enlargement has not been reported in the study (41) and needs further experimental validation. Thus, an animal model of LAM is needed to perform preclinical studies before new therapies can be translated into the clinic. Despite these limitations facing the LAM community, LAM researchers and clinicians strive to outpace them with innovative strategies to harness the disease.
In the January issue of the Journal, Le Poole and colleagues (pp. 1–5) explore the translational hypothesis about immunotherapeutic options in LAM focusing on potential benefits of melanosomal antigens (7). The melanocytic cell markers have been identified in LAM. Spindle-shaped LAM cells expressing SM-specific proteins SM α-actin, desmin, and vimentin form the core of the nodule surrounded by epithelioid-like cells immunopositive for HMB45, which binds glycoprotein gp100, a marker of melanoma cells and immature melanocytes (5). Interestingly, some of the SM-positive LAM cells, which form small nodules, also express HMB45, suggesting that some SM-positive LAM cells have melanocytic differentiation (42). LAM cells also express another two melanocyte-specific proteins: CD63, a melanoma-associated protein, and PNL2, an uncharacterized melanocytic protein. Le Poole and colleagues investigated the expression of melanoma-associated antigens gp100 and melanoma antigen recognized by T cells (MART-1) (6). Using tissue samples from subjects with LAM, the authors identified the expression of tyrosinase-related proteins (TRPs) 1 and 2 involved in melanogenesis in LAM samples in comparison to normal lung. Interestingly, the LAM nodules were densely infiltrated by macrophages but not dendritic cells or T cell subsets, demonstrating that LAM cell growth was not accompanied by enhanced immune infiltration (6). Further, cells dissociated from the LAM lung were susceptible to cytotoxic, gp100-reactive, and major histocompatibility complex class I restricted CD8+ T cells, suggesting that immunotargeting gp100 provides beneficial cytotoxic effects on LAM cell growth. Vaccines for malignant melanoma have been developed and show promise in phase III clinical trials (43). Although stimulating an immune response with vaccine might be challenging, targeting melanocytic markers in LAM provides a novel potential approach.
Supplementary Material
Acknowledgments
The author gratefully acknowledges Dr. Reynold Panettieri, Jr. and Dr. Elena Goncharova for advice and information; and Ms. Mary McNichol and Ms. Beatrice Delamerced for assistance.
Footnotes
Dr. Krymskaya receives support from the National Institutes of Health/National Heart, Lung, and Blood Institute 2RO1 HL071106 and RO1 HL090829 grants.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Johnson SR. Lymphangioleiomyomatosis. Eur Respir J 2006;27:1056–1065 [DOI] [PubMed] [Google Scholar]
- 2.Taveira-DaSilva AM, Moss J. Lymphangioleiomyomatosis. Cancer Contr 2006;13:276–285 [DOI] [PubMed] [Google Scholar]
- 3.Juvet SC, McCormack FX, Kwiatkowski DJ, Downey GP. Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. Am J Respir Cell Mol Biol 2006;36:398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darling TN, Pacheco-Rodriguez G, Gorio A, Lesma E, Walker C, Moss J. Lymphangioleiomyomatosis and TSC−/− cells. Lymphat Res Biol 2010;8:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krymskaya VP. Smooth muscle-like cells in lymphangioleiomyomatosis. Proc Am Thorac Soc 2008;5:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klarquist J, Barfuss A, Kandala S, Reust MJ, Braun RK, Hu J, Dilling DF, McKee MD, Boissy RE, Love RB, et al. Melanoma-associated antigen expression in lymphangioleiomyomatosis renders tumor cells susceptible to cytotoxic T cells. Am J Pathol 2009;175:2463–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dilling DF, Gilbert ER, Picken MM, Eby J, Love RB, Le Poole IC. A current viewpoint of lymphangioleiomyomatosis supporting immunotherapeutic treatment options. Am J Respir Cell Mol Biol 2012;46:1–5 [DOI] [PubMed] [Google Scholar]
- 8.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2000;97:6085–6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. J Biol Chem 2002;277:30958–30967 [DOI] [PubMed] [Google Scholar]
- 10.McManus EJ, Alessi DR. TSC1-TSC2: A complex tale of PKB-mediated S6K regulation. Nat Cell Biol 2002;4:E214–E216 [DOI] [PubMed] [Google Scholar]
- 11.Zoncu R, Efeyan A, Sabatini DM. Mtor: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011;12:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoki K, Corradetti MN, Guan K-L. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 2005;37:19–24 [DOI] [PubMed] [Google Scholar]
- 13.Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of akt signaling limits the growth of tumors lacking TSC2. Genes Dev 2005;19:1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal 2009;2:1–6 [DOI] [PubMed] [Google Scholar]
- 15.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9–22 [DOI] [PubMed] [Google Scholar]
- 16.Goncharova EA, Goncharov DA, Spaits M, Noonan D, Talovskaya E, Eszterhas A, Krymskaya VP. Abnormal smooth muscle cell growth in lymphangioleiomyomatosis (LAM): role for tumor suppressor TSC2. Am J Respir Cell Mol Biol 2006;34:561–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krymskaya VP, Penn RB, Orsini MJ, Scott PH, Plevin RJ, Walker TR, Eszterhas AJ, Amrani Y, Chilvers ER, Panettieri RA. Phosphatidylinositol 3-kinase mediates mitogen-induced human airway smooth muscle cell proliferation. Am J Physiol 1999;277:L65–L78 [DOI] [PubMed] [Google Scholar]
- 18.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol 2002;283:L354–L363 [DOI] [PubMed] [Google Scholar]
- 19.Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res 2002;62:5645–5650 [PubMed] [Google Scholar]
- 20.Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V, Sun Y, Scgmidt K, Albert MS, El-Hashemite N, et al. Efficacy of a rapamycin analog (CCI-779) and IFN-γ in tuberous sclerosis mouse models. Genes Chromosomes Cancer 2005;42:213–227 [DOI] [PubMed] [Google Scholar]
- 21.Krymskaya VP, Goncharova EA. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle 2009;8:403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 2011;364:1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taveira-DaSilva AM, Hedin C, Stylianou MP, Travis WD, Matsui K, Ferrans VJ, Moss J. Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome of lymphangioleiomyomatosis. Am J Respir Crit Care Med 2001;164:1072–1076 [DOI] [PubMed] [Google Scholar]
- 24.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest 2004;126:1867–1874 [DOI] [PubMed] [Google Scholar]
- 25.Goncharova E, Goncharov D, Noonan D, Krymskaya VP. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 gtpase. J Cell Biol 2004;167:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goncharova EA, Goncharov DA, Lim PN, Noonan D, Krymskaya VP. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 2006;34:473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncharova EA, Goncharov DA, Li H, Pimtong W, Lu S, Khavin I, Krymskaya VP. mTORC2 is required for proliferation and survival of TSC2-null cells. Mol Cell Biol 2011;31:2484–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demierre M-F, Higgins PDR, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer 2005;5:930–942 [DOI] [PubMed] [Google Scholar]
- 29.Lee N, Woodrum C, Nobil A, Rauktys A, Messina M, Dabora S. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finlay G, Malhowski AJ, Polizzi K, Malinowska-Kolodziej I, Kwiatkowski DJ. Renal and liver tumors in TSC2(+/−) mice, a model of tuberous sclerosis complex, do not respond to treatment with atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor. Mol Cancer Ther 2009;8:1799–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howe SR, Gottardis MM, Everitt JI, Goldsworthy TL, Wolf DC, Walker CL. Rodent model of reproductive tract leiomyomata: establishment and characterization of tumor-derived cell lines. Am J Pathol 1995;146:1568–1579 [PMC free article] [PubMed] [Google Scholar]
- 32.El-Chemaly S, Taveira-DaSilva A, Stylianou MP, Moss J. Statins in lymphangioleiomyomatosis: a word of caution. Eur Respir J 2009;34:513–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X, Kenerson HL, Yeung RS. Glucose deprivation in tuberous sclerosis complex-related tumors. Cell Biosci 2011;1:34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkhitko A, Myachina F, Morrison TA, Hindi KM, Auricchio N, Karbowniczek M, Wu JJ, Finkel T, Kwiatkowski DJ, Yu JJ, et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (sqstm1)-dependent. Proc Natl Acad Sci USA 2011;108:12455–12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwiatkowski DJ. Animal models of lymphangioleiomyomatosis (LAM) and tuberous sclerosis complex (TSC). Lymphat Res Biol 2010;8:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi E, Minowa O, Kuno J, Mitani H, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line TSC2 mutation in mice. Cancer Res 1999;59:1206–1211 [PubMed] [Google Scholar]
- 37.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. TSC2+/− mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest 1999;104:687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson C, Idziaszczyk S, Parry L, Guy C, Griffiths DFR, Lazda E, Bayne RAL, Smith AJH, Sampson JR, Cheadle JP. A mouse model of tuberous sclerosis 1 showing background specific early post-natal mortality and metastatic renal cell carcinoma. Hum Mol Genet 2005;14:1839–1850 [DOI] [PubMed] [Google Scholar]
- 39.Eker R, Mossige J, Johannessen JV, Aars H. Hereditary renal adenomas and adenocarcinomas in rats. Diagn Histopathol 1981;4:99–110 [PubMed] [Google Scholar]
- 40.Everitt J, Wolf D, Howe S, Goldsworthy T, Walker C. Rodent model of reproductive tract leiomyomata: clinical and pathological features. Am J Pathol 1995;146:1556–1567 [PMC free article] [PubMed] [Google Scholar]
- 41.Yu JJ, Robb VA, Morrison TA, Ariazi EA, Karbowniczek M, Astrinidis A, Wang C, Hernandez-Cuebas L, Seeholzer LF, Nicolas E, et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci USA 2009;106:2635–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhe X, Schuger L. Combined smooth muscle and melanocytic differentiation in lymphangioleiomyomatosis. J Histochem Cytochem 2004;52:1537–1542 [DOI] [PubMed] [Google Scholar]
- 43.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. Gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011;364:2119–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.