Abstract
Background and Aims
The classification and phylogeny of Eurasian (EA) Aster (Asterinae, Astereae, Asteraceae) remain poorly resolved. Some taxonomists adopt a broad definition of EA Aster, whereas others favour a narrow generic concept. The present study aims to delimit EA Aster sensu stricto (s.s.), elucidate the phylogenetic relationships of EA Aster s.s. and segregate genera.
Methods
The internal and external transcribed spacers of nuclear ribosomal DNA and the plastid DNA trnL-F region were used to reconstruct the phylogeny of EA Aster through maximum parsimony and Bayesian analyses.
Key Results
The analyses strongly support an Aster clade including the genera Sheareria, Rhynchospermum, Kalimeris (excluding Kalimeris longipetiolata), Heteropappus, Miyamayomena, Turczaninowia, Rhinactinidia, eastern Asian Doellingeria, Asterothamnus and Arctogeron. Many well-recognized species of Chinese Aster s.s. lie outside of the Aster clade.
Conclusions
The results reveal that EA Aster s.s. is both paraphyletic and polyphyletic. Sheareria, Rhynchospermum, Kalimeris (excluding K. longipetiolata), Heteropappus, Miyamayomena, Turczaninowia, Rhinactinidia, eastern Asian Doellingeria, Asterothamnus and Arctogeron should be included in Aster, whereas many species of Chinese Aster s.s. should be excluded. The recircumscribed Aster should be divided into two subgenera and nine sections. Kalimeris longipetiolata, Aster batangensis, A. ser. Albescentes, A. series Hersileoides, a two-species group composed of A. senecioides and A. fuscescens, and a six-species group including A. asteroides, should be elevated to generic level. With the Aster clade, they belong to the Australasian lineages. The generic status of Callistephus should be maintained. Whether Galatella (including Crinitina) and Tripolium should remain as genera or be merged into a single genus remains to be determined. In addition, the taxonomic status of A. auriculatus and the A. pycnophyllus–A. panduratus clade remains unresolved, and the systematic position of some segregates of EA Aster requires further study.
Keywords: Asteraceae, Astereae, ETS, Eurasian Aster, generic delimitation, infrageneric classification, ITS, molecular phylogeny, trnL-F
INTRODUCTION
Aster sensu lato (s.l.; Asterinae, Astereae, Asteraceae) has been a taxonomic dumping ground for large numbers of morphologically similar but distantly related taxa (Noyes and Rieseberg, 1999; Dorn, 2003). Aster s.l. occurs mainly in the Northern Hemisphere in both Eurasia (EA) and North America (NA) and is estimated to comprise 250–1000 species (Ling et al., 1985; Nesom, 1994b; Ito and Soejima, 1995; Noyes and Rieseberg, 1999). Based primarily on achene morphology and cytology, Nesom (1994b) segregated NA Aster species from Aster s.l. and redistributed them among generic segregates Symphyotrichum, Doellingeria, Eucephalus, etc. At the same time, he kept the remainder, about 180 species, as Aster sensu stricto (s.s.), typified by A. amellus. Consequently, Aster, containing approx. 180 species, is restricted to the Northern Hemisphere of the Old World. Internal transcribed spacer (ITS) sequence phylogenetic data (Noyes and Rieseberg, 1999) support the viewpoint of Nesom (1994b) that a fundamental difference exists between NA and EA Aster. Furthermore, ITS data indicate that EA Aster is nested in the Southern Hemisphere grade and does not form a monophyletic group (Noyes and Rieseberg, 1999, Brouillet et al., 2001, 2009b; Fiz et al., 2002), and African Aster should be separated from Aster s.s. (Brouillet et al., 2009b). The classification and phylogeny of EA Aster have remained poorly resolved, however, because of insufficient sampling in these studies.
The circumscription of EA Aster has confused botanists for several decades. Many taxonomists have adopted a broad definition of EA Aster. In Flora Europaea, Merxmüller et al. (1976) maintained Doellingeria, Galatella, Crinitaria (the name Crinitaria is a synonym of Galatella and species that are considered part of Crinitaria should be included in Crinitina) and Tripolium in Aster. Similarly, Grieson (1975) accepted Aster s.l. in Flora of Turkey and the East Aegean Islands because he did not recognize Kemulariella and Tripolium as segregate genera. In Flora of Japan, Ito and Soejima (1995) merged Tripolium as section Tripolium into Aster, placed Heteropappus in section Pseudocalimeris, included Kalimeris within section Asteromoea, and associated Doellingeria and Miyamayomena into section Teretiachaenium.
Other taxonomists have favoured a narrow generic concept of EA Aster and have recognized small genera endemic to eastern Asia. Tamamschyan (1959) segregated two new genera (Kemulariella and Conyzanthus) from Aster and recognized many small genera such as Doellingeria, Kalimeris, Asterothamnus, Krylovia, Turczaninowia, Galatella, Linosyris (= Crinitina) and Tripolium. Czerepanov (1995) followed Tamamschyan (1959) except that he placed Galatella and Linosyris under the genus name Crinitaria (= Crinitina). Nesom (1994a, b) made Aster largely equal to EA Aster s.s. and EA Aster s.l. almost equal to sub-tribe Asterinae Dumort.
Ling et al. (1985) treated Chinese Asterinae in the narrow sense of Aster, recognizing generic status for Gymnaster (= Miyamayomena), Kalimeris, Callistephus, Heteropappus, Doellingeria, Turczaninowia, Krylovia (= Rhinactinidia), Asterothamnus, Galatella, Linosyris (= Crinitina), Arctogeron and Tripolium. These treatments were followed completely for floras of Chinese provinces (e.g. Zhuang, 2004; Lin, 2007). Despite this, Chinese Aster s.s. remains a large genus with approx. 100 species, of which 75 are endemic to China (Fu, 1983; Ling et al., 1985; Chen, 1988, 1990; Zhu and Min, 1990; Li and Liu, 2002; Li and Zhang, 2004). Therefore, China, especially south-western China (the Qinghai–Tibetan and Yunnan–Guizhou Plateaux and Sichuan Province), is the diversity centre of Aster, as it is for many genera (Huang, 2011).
Molecular markers, especially ITS and the external transcribed spacer (ETS) of 35S ribosomal DNA, have frequently been used to investigate phylogenetic relationships in Astereae (e.g. Noyes and Rieseberg, 1999; Lowrey et al., 2001; Markos and Baldwin, 2001; Cross et al., 2002; Fiz et al., 2002; Roberts, 2002; Lowell et al., 2003; Urbatsch and Roberts, 2003; Urbatsch et al., 2003; Roberts and Urbatsch, 2004; Karaman, 2006; Selliah and Brouillet, 2008; Andrus et al., 2009; Brouillet et al., 2009a,b; Karaman-Castro and Urbatsch, 2009; Vaezi and Brouillet, 2009). Molecular evidence implies that neither EA Aster s.l. nor EA Aster s.s. is monophyletic (Gu et al., 1994; Ito et al., 1995, 1998; Xiang and Semple, 1996; Noyes and Rieseberg, 1999; Fiz et al., 2002), but only a few species of EA Aster have been included in previous analyses. Although molecular data support a close relationship among Kalimeris, Heteropappus, Miyamayomena, Sheareria, Rhynchospermum and Aster s.s. (Ito et al., 1995, 1998; Noyes and Rieseberg, 1999; Fiz et al., 2002; Gao et al., 2009), the phylogenetic relationships among these genera are unresolved owing to limited taxon sampling of EA Aster s.s. Recently, 27 species of EA Aster s.l. were included in a phylogenetic analysis of Aster s.l. (Brouillet et al. 2009b), but no statistical support was presented for the clades of the ITS phylogenetic tree. To date, no molecular data have been provided for Turczaninowia, Krylovia, Asterothamnus and Arctogeron, and, in particular, Chinese Aster s.s. has not been phylogenetically studied using DNA sequences even though it represents the overwhelming majority of EA Aster s.s. Thus, a reliable phylogenetic analysis based on extensive taxon sampling is essential to determine the inter- and intrageneric relationships of EA Aster.
Principally based on nuclear ribosomal DNA (nrDNA) ITS, ETS and plastid trnL-F sequence data of Sheareria nana, Rhynchospermum verticillatum and 62 species of EA Aster s.l., the present study aims to (1) reconstruct the phylogeny of EA Aster s.l.; (2) redelimit the genus Aster and discuss its infrageneric classification; and (3) discuss the systematic position of EA Aster segregates.
MATERIALS AND METHODS
Generic circumscriptions and nomenclature of Astereae follow Nesom and Robinson (2007) except for Turczaninowia, which follows Ling et al. (1985), and Crinitina, which is substituted for Crinitaria (a synonym of Galatella). The name Aster setchuenensis follows the International Plant Names Index. The division of phylogenetic lineages of Astereae refers to Brouillet et al. (2009b). Voucher DBY9206 was deposited in the Wenzhou University Herbarium (WZU) and the others in the Hunan Normal University Herbarium (HNNU; see the Appendix).
Taxon sampling
Seventy-six species of Astereae and three outgroup species were collected from China and Bulgaria and examined for sequence variations in nrDNA ITS, ETS and plastid DNA trnL-F (GenBank accession numbers are given in the Appendix). The vouchers of all accessions were identified using published keys and compared with herbarium specimens in the Institute of Botany, Chinese Academy of Sciences Herbarium (PE), Northwest Agriculture and Forestry University Herbarium (WUK), Sichuan University Herbarium (SZ), Chengdu Institute of Biology Herbarium (CDBI), HNNU, Herbarium of Kunming Institute of Botany, the Chinese Academy of Sciences (KUN), the Herbarium of the South China Botanical Garden, Chinese Academy of Sciences (IBSC), Guangxi Institute of Botany Herbarium (IBK), Institute of Botany, Jiangsu Province and Chinese Academy of Sciences Herbarium (NAS), Guizhou Academy of Sciences Herbarium (HGAS), Central China Normal University Herbarium (CCNU), Wuhan Botanical Garden, Chinese Academy of Sciences Herbarium (HIB), Inner Mongolia University Herbarium (HIMC) and Fudan University Herbarium (FUS). Of the 76 species included in this study (see the Appendix), 41 represent three sections and 20 series of EA Aster s.s. (Ling et al., 1985; Chen, 1988; Li and Liu, 2002), 21 represent 12 segregate genera of EA Aster s.l., four generic groups of Nesom's (1994b) Asterinae, and two recently recognized close relatives of EA Aster s.s. (S. nana and R. verticillatum; Fiz et al., 2002; Brouillet et al. 2009b; Gao et al., 2009). The data matrix for ITS comprises 110 accessions from 48 genera and 110 species of tribe Astereae (see the Appendix). Seventy-six accessions were newly sequenced, and the remaining 34 were obtained from GenBank (Appendix). Of the 110 accessions, 41 species belong to EA Aster s.s., 21 species are 12 separate genera of EA Aster s.l., one is Astereae incertae sedis, three are members of Bellidinae or Grangeinae, and 44 represent the six phylogenetic lineages of Astereae (Brouillet et al. 2009b). These phylogenetic lineages of Astereae are the early diverging lineages (e.g. Madagaster madagascariensis, Felicia filifolia and Printzia polifolia), the palaeo-South American clade (e.g. Chiliotrichum diffusum), the New Zealand clade (e.g. Olearia covenyi), the Australasian lineages, the South American lineages (e.g. Baccharis neglecta) and the NA lineage (e.g. Conyza sumatrensis and Symphyotrichum subulatum). Brouillet et al. (2009b) divided the Australasian lineages into seven genus or species groups, whereas ten genus or species groups are, in fact, included in the depiction of the grouping (fig. 37·1 C in Brouillet et al. 2009b). In the current analysis, 19 species (Appendix) were sampled to represent these ten groups. Because Brouillet et al. (2009b) consider Olearia s.s. to be a sister to EA Aster, five species were sampled to represent sub-clades of the Olearia s.s. clade.
In the combined matrix of ITS, ETS and trnL-F, 78 accessions from 25 genera and 78 species of tribe Astereae were included (Appendix). Seventy-six accessions were newly sequenced, and the remaining two were obtained from GenBank (Appendix). Of the 78 accessions (Appendix), 41 belong to EA Aster s.s., 21 belong to 12 segregate genera of EA Aster s.l., one is Astereae incertae sedis, and three are members of Bellidinae or Grangeinae. The remaining 12 accessions represent three phylogenetic lineages of tribe Astereae (Brouillet et al. 2009b), the palaeo-South American clade (e.g. C. diffusum), the Australasian lineages (e.g. two Myriactis spp.) and the NA lineage (seven species such as C. sumatrensis and S. subulatum). In all analyses, Chrysanthemum coronarium and Dendranthema indicum of tribe Anthemideae and Calendula officinalis of tribe Calenduleae were selected as outgroups for the rooting of the phylogenetic trees (Appendix) because in molecular phylogenetic analyses Anthemideae and Astereae are sisters, and Calenduleae is a sister to tribes Gnaphalieae, Anthemideae and Astereae (Panero and Funk, 2008; Garcia et al., 2010).
DNA extraction, polymerase chain reaction (PCR) and sequencing
Total genomic DNA was isolated from fresh leaf material or silica gel-dried leaves using a modified cetyltrimethylammonium bromide procedure (Doyle and Doyle, 1987). Amplification and sequencing were performed using the primers ITS1 and ITS4 (White et al., 1990) for the ITS region, Ast-8 (Markos and Baldwin, 2001) and 18S-ETS (Baldwin and Markos, 1998) for the ETS region, and c and f (Taberlet et al., 1991) for the plastid DNA trnL-F region (trnLUAA-trnLUAA-trnFGAA).
The PCR mixture contained 1 µL (50–100 ng) of sample DNA, 2 × 2 µL of primer (10 pmol), 5 µL of 10 × PCR buffer, 3 µL of Mg2+ (25 mm), 0·8 µL of deoxyribonucleotide triphosphate (each 25 mm), 0·5 µL of Taq DNA polymerase (5 U μL−1) and sterile water for a final volume of 50 µL. The PCR parameters were as follows: initial denaturation for 4 min at 95 °C followed by 30 cycles of denaturation (95 °C, 1 min), annealing (56 °C, 40 s) and extension (72 °C, 1 min), and a final extension of 10 min at 72 °C.
PCR products were purified using a UNIQ-10 Spin Column PCR Product Purification Kit (Sangon Biotech Co., Ltd, Shanghai, China) following the manufacturer's instructions. Sequencing reactions were performed in both directions by Sangon Biotech Co., Ltd.
Sequence alignment and phylogenetic analysis
Boundaries of the ITS, ETS and trnL-F regions were determined through comparison with previously published sequences of tribe Astereae (Noyes and Rieseberg, 1999; Liu et al., 2002; Urbatsch et al., 2003). All DNA sequences were aligned initially using Clustal X1·83 (Jeanmougin et al., 1998) and then adjusted manually in BioEdit (Hall, 1999). The ITS region was analysed separately and in a combined data set with the ETS and trnL-F regions. The incongruence length difference test (Farris et al. 1994) was carried out to test the homogeneity between data sets using PAUP* version 4·0b10 with 1000 replicates. Maximum parsimony (MP) and Bayesian inference (BI) methods were performed for the data sets using PAUP* version 4·0b10 (Swofford, 2001) and MrBayes version 3·1·2 (Ronquist and Huelsenbeck, 2003), respectively. In the MP analysis, characters were equally weighted and treated as unordered, gaps were treated as missing data, and a heuristic search was implemented with 1000 random additional sequence replicates and sub-tree pruning–regrafting branch swapping. Bootstrap analyses based on 1000 replicates with ten random additions per replicate were used to estimate the confidence of the clades. The MaxTrees setting in PAUP* was set to 5000 for the searches and bootstrap tests. For BI analysis of the ITS region and combined data set, the best-fitting model of each sequence partition (ITS1, ITS2, 5·8S, ETS, trnL-F intron, exon, the internal guide sequence) was determined using MrModeltest 2·2 (Nylander, 2004). The SYM + G model was chosen for the 5·8S region, and the GTR + I + G model for the ITS1, ITS2 and ETS regions. The GTR + G model was chosen for the intron and the internal guide sequence partitions of the trnL-F region and the K80 model for the exon partition. The Markov chain Monte Carlo algorithm was run for 1 000 000 generations, resulting in an overall sampling of 10 000 trees. The first 3000 trees were discarded as a conservation burn-in, and the remaining trees were used to construct the 50 % majority rule consensus tree.
RESULTS
Characterization of nucleotide data
The aligned ITS sequence matrix of 110 taxa contained 689 base pairs, of which 394 were variable and 315 were potentially parsimony informative. Pair-wise distance within ingroup varied from 0 to 18·7 % (average = 6·7 %). The incongruence length difference test indicated that the data sets were not significantly heterogeneous (P = 0·01). Therefore, a combined analysis of the three regions was performed using PAUP* and MrBayes. The combined data set of 78 taxa consisted of 2313 positions, with 641 potentially parsimony-informative characters and 283 phylogenetically uninformative variable characters. Pairwise distance between sequences varied from 0·1 to 11·7 % (average = 4·6 %).
Phylogenetic analyses
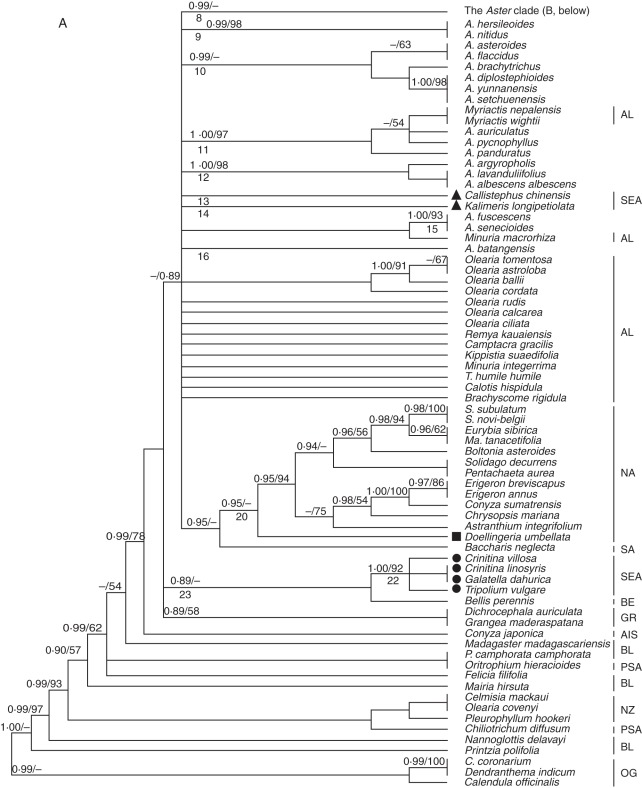
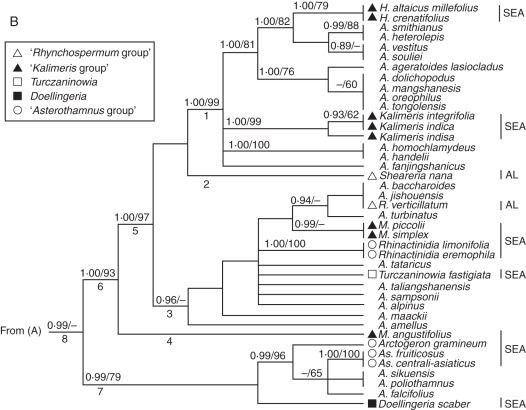
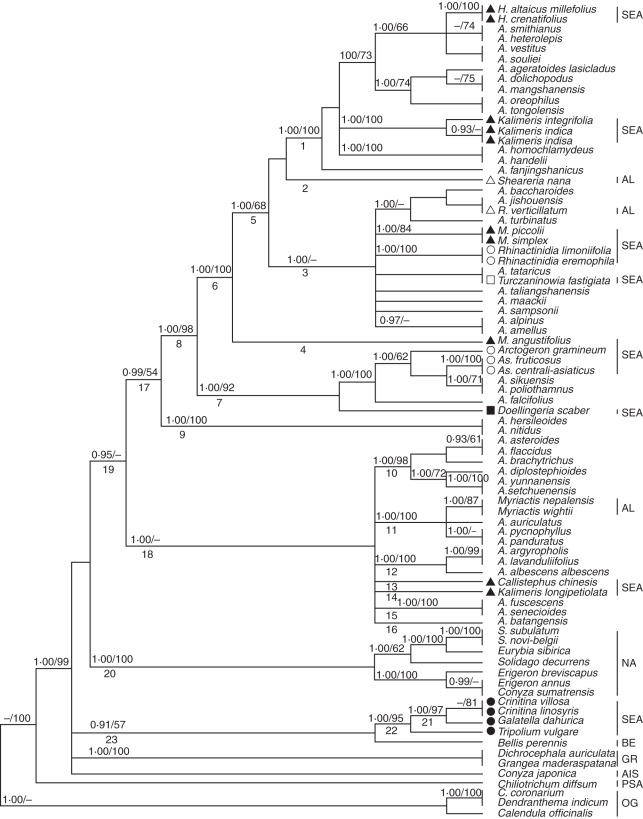
For convenience, some clades were numbered (Figs 1A, B and 2). Phylogenetic analyses using ITS and combined data sets yielded generally consistent phylogenetic trees (Bayesian trees; see Figs 1A, B and 2), although the BI and MP analyses based on the combined data generated trees with higher bootstrap support (BS) and Bayesian posterior probability (PP), and some clades (e.g. 17 and 18; Fig. 2) of the combined tree were unresolved in the ITS trees (Fig. 1A). The Aster clade (clade 8; Figs 1 and 2) with A. amellus (the type species of Aster) was strongly supported (PP = 0·99 in Fig. 1; PP = 1·00 and BS = 98 in Fig. 2) by the ITS and combined data set analyses. Sheareria, Rhynchospermum and some EA Aster segregates such as Heteropappus, Kalimeris (excluding section Cordifolium), Miyamayomena, Turczaninowia, Rhinactinidia, Arctogeron, Asterothamnus and eastern Asian Doellingeria were deeply nested within the Aster clade (clade 8), whereas other segregates (e.g. Callistephus, Galatella, Crinitina, Tripolium and K. longipetiolata) and 17 species of Aster s.s. (e.g. A. nitidus, A. asteroides and A. panduratus) occurred in other clades and showed close (clade 7; Figs 1A and 2), remote (e.g. clade 18 in Fig. 1A; clade 22 in Fig. 2) or unresolved (e.g. clade 9–16; Fig. 1A) relationships with the Aster clade. Callistephus, K. longipetiolata, two Myriactis spp. and 15 Aster spp. formed a moderately supported clade (clade 18; PP = 100; Fig. 2) that was unresolved in the ITS tree (Fig. 1A). Tripolium, Galatella and Crinitina constituted a well-supported clade (clade 22: PP = 1·00, BS = 92 in Fig. 1A; PP = 1·00, BS = 95 in Fig. 2) sister to Bellis perennis, but this relationship was weakly supported (clade 23: PP = 0·89 in Fig. 1A; PP = 0·91, BS = 57 in Fig. 2). The monophyly of the NA clade (clade 20) was moderately to strongly supported in both phylogenetic analyses (PP = 0·95 in Fig. 1; PP = 1·00, BS = 100 in Fig. 2).
Fig. 1.
The 50 % majority rule consensus tree from the Bayesian analysis of nuclear ribosomal DNA internal transcribed spacer sequences. (A) Bayesian posterior probabilities (≥0·89) and bootstrap values (≥50 %) are indicated above the branches; ‘–’ indicates that Bayesian posterior probabilities are <0·89 or bootstrap percentages are <50 %. Some clades are indicated by numbers below the branch. Abbreviations: A., Aster; C., Chrysanthemum; S., Symphyotrichum. Triangles, ‘Kalimeris group’; squares, Doellingeria; circles, ‘Galatella group’. (B) The Aster clade (continued part of A). Bayesian posterior probabilities (≥0·89) and bootstrap values (≥50 %) are indicated above the branches; ‘–’ indicates that Bayesian posterior probabilities are <0·89 or bootstrap percentages are <50 %. Some clades are indicated by numbers below the branch. Abbreviations: A., Aster; As., Asterothamnus; H., Heteropappus; M., Miyamayomena; R., Rhynchospermum. See key for symbols. Some clades are indicated by numbers below the branch. Abbreviations of the lineages are identical to those given in the Appendix and are shown on the right side of the taxa. The labelled species are discussed in groups in the text.
Fig. 2.
The 50 % majority rule consensus tree from the Bayesian analysis of the combined data set (nuclear ribosomal DNA internal and external transcribed spacer sequences and plastid genome DNA trnL-F sequences). Bayesian posterior probabilities (≥0·89) and bootstrap values (≥50 %) are indicated above the branches; ‘–’ indicates that Bayesian posterior probabilities are <0·89 or bootstrap percentages are <50 %. Some clades are indicated by numbers below the branch. Abbreviations: A., Aster; As., Asterothamnus; C., Chrysanthemum; H., Heteropappus; M., Miyamayomena; R., Rhynchospermum; S., Symphyotrichum. Abbreviations of the lineages are identical to those given in the Appendix and are shown on the right side of the taxa. Some species are labelled with the symbols shown in Fig. 1.
DISCUSSION
Relationship between EA Aster and NA asters
In this study, the ITS and combined data set analyses (Figs 1 and 2) clearly indicate that the Aster clade (clade 8 in Figs 1A and 2) is strongly supported (PP = 0·99 in Fig. 1; PP = 1·00, BS = 98 in Fig. 2) in an unresolved Astereae polytomy (Fig. 1A) or is embedded within clade 19 which includes Myriactis (sub-tribe Lagenophorinae) of the Australian lineages (see Fig. 2), whereas NA Astereae forms a moderately to strongly supported clade (clade 20: PP = 0·95 in Fig. 1; PP = 1·00, BS = 100 in Fig. 2). Therefore, EA Aster has no close relationship to NA asters. These results support the viewpoint of Nesom (1994b) that a fundamental difference exists between NA and EA Aster; they do not support the opinion of Xiang and Semple (1996) that Aster s.s. comprises not only EA taxa but also the segregate genus Eurybia and that EA Aster is derived from NA Aster. Aster alpinus, distributed in both EA and NA, is deeply nested within the EA Aster clade (clade 8; see Figs 1B and 2), which implies that this species originated in EA and dispersed to NA.
Relationship between EA Aster and Australasian lineages
According to Brouillet et al. (2009b), Australasian lineages are part of a large polytomy at the crown of Astereae. Although our data sets did not include a large sample of Australasian taxa, the ITS tree (Fig. 1) included 19 sampled species that represented the ten genus or species groups of Australasian lineages of Brouillet et al. (2009b). The ITS tree (Fig. 1A) showed that the Aster clade (clade 8) is a clade of the large polytomy of the crown of Astereae, but it does not group with any of the Australian (e.g. Olearia astroloba and Remya kauaiensis), Hawaiian (Tetramolopium humile) or Asian (Myriactis) species of the Australasian lineages. Brouillet et al. (2009b) have proposed that EA Aster s.s. was a sister to the Australasian Olearia s.s. and had Australasian ancestors. Three species (including the generic type Olearia tomentosa) of the Australasian Olearia s.s. (Brouillet et al. 2009b) constitute a clade (Fig. 1A) but not a sister to the Aster clade. The phylogenetic tree from the combined data set, which is more resolved, includes only a few species (Myriactis and Callistephus) of the Australasian lineages. In the combined tree (Fig. 2), clade 17 (PP = 0·99, BS = 54) with the Aster clade (clade 8) is a sister to clade 18 (PP = 1·00) that includes Myriactis and Callistephus (representatives of the Australasian lineages) and clades 17 and 18 group further into clade 19 (PP = 0·95) which might correspond to the Australasian lineages. Therefore, EA Aster (clade 8; Fig. 2) and some of its segregates (clades 9, 10, 12–16, and Aster spp. of clade 11; Fig. 2) belong to the Australasian lineages. A more extensive taxon sampling of Australasian Astereae for an analysis of combined DNA sequences is needed to study the origin of both EA Aster and its segregates.
Status of the ‘Rhynchospermum group’
According to Nesom (1994a) and Nesom and Robinson (2007), two monotypic genera, Sheareria and Rhynchospermum, belong to the Rhynchospermum group of sub-tribe Lagenophorinae. The present study shows that these genera are well nested within the Aster clade, however, and not closely related to each other (Figs 1B and 2).
Sheareria
Endemic to China, this was first placed in tribe Astereae and later transferred to tribe Heliantheae (Hoffmann, 1890). Chen (1979) recognized it as belonging to sub-tribe Milleriinae of Heliantheae. Robinson (1981) redelimited Heliantheae and considered Sheareria to be a member of Astereae. Li et al. (2008) provided micromorphological, anatomical and cytological evidence for moving the genus from Heliantheae to Astereae but did not determine its systematic position within tribe Astereae. Nesom (1994a) and Nesom and Robinson (2007) placed Sheareria in sub-tribe Lagenophorinae, but Nesom (1994a) doubted a natural alignment with Lagenophorinae. Gao et al. (2009) used an ITS data set to show that Sheareria formed a strongly supported clade with Kalimeris integrifolia and A. amellus rather than with Myriactis humilis, a species of Lagenophorinae, which implies that Sheareria should be transferred from Lagenophorinae to Asterinae. Both the ITS (Fig. 1B) and the combined (Fig. 2) trees show that Sheareria is well nested within the Aster clade. Sheareria nana differs noticeably from other species of the Aster clade owing to its somewhat reduced leaves (bract-like, linear) and assimilating branches, solitary head with only 5–8 florets, functionally staminate disc flowers and epappose and glabrous achenes. Sheareria nana forms a single-species sub-clade (clade 2) of clade 8 in all analyses (Figs 1B and 2), and it could be designated as a section of Aster.
Rhynchospermum
The monotypic genus Rhynchospermum is distributed in eastern and southeastern Asia (Nesom and Robinson, 2007). Ling et al. (1985) included the genus in sub-tribe Bellidinae, and Zhang and Bremer (1993) placed it into their ‘Bellis group’ with Bellis and Bellium, whereas Nesom (1994a) and Nesom and Robinson (2007) assigned it to the Rhynchospermum group of sub-tribe Lagenophorinae. A previous phylogenetic analysis of ITS (Fiz et al., 2002) suggested that Rhynchospermum is related to neither Bellis nor Myriactis (sub-tribe Lagenophorinae) but to A. amellus and K. integrifolia. Brouillet et al. (2009b) also showed Rhynchospermum nested within Aster s.s., which is supported by our ITS and combined data sets (see Figs 1B and 2). In the phylogenetic trees (Figs 1B and 2) R. verticillatum is nested within the Aster clade (clade 8) and belongs to a clade (PP = 0·94 in Fig. 1B; PP = 1·00 in Fig. 2) with three species of series Turbinati of section Aster (see the Appendix). Although Rhynchospermum has some unique characters, such as a caducous pappus and biseriate pistillate ray florets with a short ligule, our results (Figs 1B and 2) suggest that it should be merged in Aster s.s. and placed in series Turbinati.
Status of the ‘Kalimeris group’
Nesom (1994a, b, 2000) has suggested that the Kalimeris group is composed of five small genera: Boltonia, Callistephus, Heteropappus, Kalimeris and Miyamayomena. This arrangement is unsupported by previous reports and the present study.
Kalimeris
This is native to eastern Asia, and one of its diagnostic characters is short pappi. Its complex taxonomic history has been reviewed in detail by Gu and Hoch (1997). Kalimeris shares several floral and achene characters with the small NA genus Boltonia, which led Bentham (1861, 1873) to place Kalimeris in Boltonia as one of three sections. Tamamschyan (1959), Ling et al. (1985) and Nesom (1994b, 2000) retained Kalimeris as a segregate genus, however. Gu and Hoch (1997) made a detailed comparison of the achenes and pappi of Boltonia and Kalimeris and concluded that their similarity was rather superficial. Based on ITS data, Fiz et al. (2002) and Brouillet et al. (2009b) demonstrated that Kalimeris and Boltonia are in divergent clades. Our phylogenetic analyses show that Kalimeris and Boltonia belong to different, strongly supported clades (clades 8 and 20, respectively; Fig. 1), supporting the view that no close relationship exists between Kalimeris and Boltonia.
Kalimeris was sub-divided into two sections by Kitamura (1937): Kalimeris and Cordifolium. Section Cordifolium has cordiform leaves with long petioles, two or three series of sub-equal phyllaries and cylindrical achenes with 4–7 ribs. The section includes two species, K. miqueliana, endemic to Japan, and K. longipetiolata, endemic to China (Kitamura, 1937; Ling et al., 1985). Gu and Hoch (1997) excluded section Cordifolium from Kalimeris and left it as part of Aster, and Ito and Soejima (1995) merged the section within Aster section Aster, although restriction fragment length polymorphisms (RFLPs) of plastid DNA supported a close relationship between K. miqueliana and Doellingeria scaber (Ito et al., 1995, 1998). Nesom (1993) transferred K. longipetiolata to Doellingeria, as D. longipetiolata, but the present results show that it is related to neither NA Doellingeria species nor Asian Doellingeria species. In the ITS tree, K. longipetiolata occupies an unresolved position (clade 14 in Fig. 1B) within the big polytomy, and in the combined tree it belongs to a polytomy (clade 18 in Fig. 2) with two Myriactis spp. and many other species of the Australasian lineages. Kalimeris longipetiolata should be treated as a new monotypic genus and be placed with the Australasian lineages.
Kalimeris (excluding section Cordifolium) has been recognized as having a close relationship with EA Aster s.s. and Heteropappus according to morphological comparisons (Gu and Hoch, 1997), cytological studies (Huziwara, 1950; Tara, 1972, 1973), RFLPs of plastid DNA (Ito et al., 1995, 1998) and ITS data (Noyes and Rieseberg, 1999; Fiz et al., 2002; Brouillet et al. 2009b). The taxonomic status of Kalimeris remains to be determined, however (Gu and Hoch, 1997). In the two trees in our study, three Kalimeris spp. (excluding section Cordifolium) are well nested in the Aster clade and form a highly supported clade (PP = 1·00 and BS = 99 in Fig. 1B; PP = 1·00 and BS = 100 in Fig. 2). Kalimeris (excluding section Cordifolium) is characterized by laterally compressed achenes with short pappus bristles no longer than the length of the corolla tube (Gu and Hoch, 1997) and S-type chromosomes (Li, 2006). Thus, Kalimeris (excluding section Cordifolium) is monophyletic, and treating Kalimeris as series Kalimeris of Aster is reasonable.
In the phylogenetic trees (Figs 1 and 2) Kalimeris is nested in clade 1 with A. ageratoides and Heteropappus, whereas Miyamayomena belongs to clade 3 with A. amellus. Natural hybridizations between Kalimeris and A. ovatus (formerly A. ageratoides subsp. ovatus; Tara, 1972, 1989), between Kalimeris and A. ageratoides (Li, 2006), and between Kalimeris and Heteropappus (Tara, 1973) support a close relationship with the A. ageratoides complex and Heteropappus, as do morphological studies (Gu and Hoch, 1997). Hu (1967) transferred a few species of Aster, including A. smithianus, to Kalimeris based on their short pappi, whereas our analyses showed that A. smithianus is not closely related to Kalimeris (Figs 1 and 2).
Miyamayomena
This was separated from Kalimeris and initially named Gymnaster (Kitamura, 1937, 1982; Chen, 1986). It is characterized by a lack of pappi (Kitamura, 1937, 1982; Ling et al., 1985; Chen, 1986). Although there are only five species (Chen, 1986), Miyamayomena is as variable morphologically as the large genus Aster and may in fact be an artificial assemblage (Gu and Hoch, 1997). Ito and Soejima (1995) treated M. savatieri, the generic type, as a species of Aster section Teretiachaenium which also includes A. scaber (= D. scaber). In the phylogenetic trees based on RFLPs of plastid DNA, two species of Miyamayomena did not form a clade: M. koraiensis was nested in the Aster clade, and M. savatieri was a sister to the Aster clade. Therefore, Miyamayomena could be polyphyletic (Ito et al., 1994, 1998). Our analyses (Figs 1B and 2) show that three Chinese Miyamayomena spp., M. piccolii, M. simplex and M. angustifolius, are nested within the Aster clade (clade 8) and should be merged into Aster. These species belong to different clades, implying that a lack of pappi is not a homologous synapomorphy and that Miyamayomena is not monophyletic. Miyamayomena angustifolius (clade 4) is sister to clade 5 (Figs 1B and 2) and might be designed as a section of Aster. Miyamayomena piccolii and M. simplex form a strongly to weakly supported clade (PP = 0·99 in Fig. 1B; PP = 1·00 and BS = 84 in Fig. 2) embedded within the A. amellus clade (clade 3 in Figs 1B and 2) and might be treated as a series of section Aster, whereas the taxonomic positions of Miyamayomena koraiensis and M. savatieri, endemic to Japan and North Korea, respectively, remain to be determined.
Heteropappus
In 1832 the genus Heteropappus was established and the type species, H. hispidus, was transferred from Aster (Lessing, 1832). Heteropappus includes approx. 30 species distributed in eastern and central Asia and the Himalayan region (Ling et al., 1985). The genus is characterized by its two series of sub-equal herbaceous phyllaries and dimorphic pappi (shorter on the ray achenes and longer on the disc achenes; Ling et al., 1985; Gu and Hoch, 1997). Some species such as H. altaicus have a monomorphic pappus, so Grierson (1964) redefined Heteropappus by the unequal corolla lobes of the disc florets. Zygomorphic disc florets are also found in some species of Aster and Kalimeris, however. RFLPs of plastid DNA show that H. hispidus is embedded in Aster (Ito et al., 1998), implying that Heteropappus should be included in Aster. Our analyses (Figs 1 and 2) also strongly support the placement of Heteropappus in Aster. The two sampled species, representing two sections, form a highly to weakly supported (PP = 1·00 and BS = 79 in Fig. 1B; PP = 1·00 and BS = 100 in Fig. 2) sub-clade of the A. ageratoides clade (clade 1), which might indicate that Heteropappus should be treated as a series of section Ageratoides (corresponding to clade 1).
Callistephus
This is a monotypic genus native to China. Based on its double pappus and unique involucre (outer bracts foliaceous and innermost white scarious), it was distinguished from Aster in 1817 by Cassini (Ling et al., 1985; Nesom, 2000). Heteropappus hispidus was placed in Callistephus by de Candolle as Callistephus biennis (Nesom, 2000), implying that Callistephus and Heteropappus might be related to each other. Zhang and Bremer (1993) suggested that Callistephus, Gymnaster, Heteropappus and Kalimeris are closely related to each other and to Aster. Nesom (1994b) thought that Callistephus is similar to some species of Myriactis (sub-tribe Lagenophorinae) in habit and tendency toward pappus reduction, but he placed Callistephus within the Kalimeris group given the similar morphology of leaves, receptacles, disc corollas, and papillate collecting appendages of the style branches, the arrangement of the capitulum and the tendency toward pappus reduction (Nesom, 1994a, b, 2000). Our analyses (Figs 1 and 2) reveal that Callistephus has no close relationships with the other four genera of the Kalimeris group or with Myriactis. In the combined tree (Fig. 2), Callistephus and Myriactis occur in the same polytomy (clade 18) that is part of the Australasian lineages, which is concordant with the result of Brouillet et al. (2009b) that placed Callistephus in the large Australasian polytomy. We suggest that Callistephus maintain its generic status.
Status of Turczaninowia
Turczaninowia fastigiata is native to north-eastern Asia (Tamamschyan, 1959; Ling et al., 1985; Ito and Soejima, 1995) and is characterized by its dense vestiture and small heads (with flowers and fruits reduced correspondingly) in a compact capitulescence. Turczaninowia fastigiata was originally published as Aster fastigiatus in 1812 (Ling et al., 1985) and was segregated as the monotypic genus Turczaninowia by de Candolle in 1836 (Nesom, 1994b). Tamamschyan (1959), Ling et al. (1985) and Bremer (1994) followed de Candolle's treatment, whereas Nesom (1994b) and Nesom and Robinson (2007) supported the inclusion of the species in Aster, and Ito and Soejima (1995) placed this species in Aster section Aster. The ovarian sterility of some of the inner disc flowers of this species and the triangular collecting appendages of its style branches are considered hallmarks of a possible close relationship with Galatella (Ling et al., 1985; Nesom, 1994b). Our phylogenetic trees (Figs 1 and 2) suggest that T. fastigiata does not merit generic rank or have a close relationship to Galatella; rather it should be transferred to Aster section Aster.
Status of Doellingeria
Nees established Doellingeria in 1832, typified by D. umbellata. Bentham (1873) advocated a conglomerated Aster and included Doellingeria within a larger Aster. Some botanists continued to recognize Doellingeria as a distinct genus, however. Its phylogenetic position is equivocal. Zhang and Bremer (1993) placed Doellingeria in the Aster group. Nesom classified it first in sub-tribe Solidagininae (Nesom, 1993), then in sub-tribe Symphyotrichinae (Nesom, 1994a) or in sub-tribe Asterinae (Nesom, 1994b), and recently as an unplaced genus of Astereae (Nesom and Robinson, 2007). Doellingeria includes 11 species, of which three are NA and eight are eastern Asian species (Nesom, 1993, 1994b). RFLPs of plastid DNA show that eastern Asian Doellingeria is embedded in Aster s.s. (lto et al., 1994), and hybridization between eastern Asian Doellingeria and Aster has been reported (Saito et al., 2007), whereas ITS data support an early-branching position of NA Doellingeria (represented by D. umbellatus) in the NA Astereae clade (Noyes and Rieseberg, 1999; Brouillet et al., 2001). In our trees (Figs 1 and 2) NA Doellingeria belongs to the NA clade (clade 20; Fig. 1A), and eastern Asian Doellingeria (represented by Doellingeria scaber) is embedded in clade 8 (the Aster clade; Figs 1B and 2), which implies that Doellingeria is biphyletic and that eastern Asian Doellingeria should be moved from Doellingeria (which is typified by NA D. umbellatus) to Aster. Ito and Soejima (1995) placed eastern Asian Doellingeria and Miyamayomena together in Aster section Teretiachaenium. Our analyses (Figs 1B and 2) show that eastern Asian Doellingeria and Miyamayomena belong to different sub-clades (clades 6 and 7, respectively) of the Aster clade (clade 8), however. In clade 7 (Figs 1B and 2) eastern Asian Doellingeria is a sister to a clade with Arctogeron, Asterothamnus and three species of Aster s.s., showing that it diverged early in Aster evolution and suggesting that eastern Asian Doellingeria should be treated as an independent section of Aster.
Status of Aster segregates of the ‘Asterothamnus group’
Nesom (1994a, b) set up an Asterothamnus group consisting of five small genera, Asterothamnus, Krylovia (= Rhinactinidia), Arctogeron, Kemulariella and Psychrogeton, of which the first four are segregates of Aster. The Asterothamnus group occurs primarily in central Asia and is characterized by a woody stem base, caespitose habit, sessile–glandular and tomentose stems and leaves, few or solitary heads and strongly coiling rays (Nesom, 1994b). Most of these features may be convergent characters resulting from adaptive modification under harsh environmental conditions (drought or cold), however. Our samples were limited to Asterothamnus, Rhinactinidia and Arctogeron (Appendix) because Kemulariella and Psychrogeton materials were unavailable.
Asterothamnus
This was segregated from Aster in 1950 by Novopokrovskiy, and its generic status has been accepted by several authors (Tamamschyan, 1959; Ling et al., 1985; Zhang and Bremer; 1993; Bremer, 1994; Nesom, 1994b; Czerepanov, 1995; Nesom and Robinson, 2007). The genus comprises seven species endemic to deserts and desert steppes in central Asia (Ling et al., 1985; Zhao, 1996). Asterothamnus has distinctive characters: it is a strongly branching sub-shrub with a woody rhizome, linear or narrowly elliptic leaves, densely or thinly tomentose stems and leaves and solitary or few heads in a loose corymb, reflecting adaptation to drought. In our phylogenetic trees (Figs 1B and 2) Asterothamnus belongs to the Aster clade (clade 8) and should be treated as a member of Aster. The two species sampled form a well-supported sub-clade (PP = 1·00 and BS = 100) that is nested in clade 7 with A. sikuensis, A. poliothamnus, A. falcifolius, Arctogeron and eastern Asian Doellingeria in both phylogenetic analyses (Figs 1B and 2). Asterothamnus is obviously different in morphology from the other members of clade 7 and should be regarded as a section of Aster.
Arctogeron gramineum
This is the only species of Arctogeron and is distributed in north-eastern China, Mongolia and eastern Russia. It occurs on dry mountain slopes or stony slopes and displays characters linked to drought adaptation such as low-growing and mat-forming habit and linear-subulate leaves. The species was originally described in 1753 by Linnaeus as a member of Erigeron and then established as a separate genus in 1836 by de Candolle and transferred to Aster in 1907 by Komarov (reviewed by Ling et al., 1985). Like Asterothamnus, Arctogeron belongs to the Aster clade (clade 8; Figs 1B and 2) and should be treated as a member of Aster. It is well nested in clade 7 (Figs 1B and 2) and should be treated as a monotypic section of Aster.
Rhinactinidia
This is a genus of four species native to central Asia and Siberia (Ling et al., 1985; Czerepanov, 1995). It was established as a genus in 1831 by Lessing and was later included in Aster s.l. (Ling et al., 1985). Its generic status is currently generally accepted (Tamamschyan, 1959; Ling et al., 1985; Zhang and Bremer, 1993; Bremer, 1994; Nesom, 1994b; Czerepanov, 1995; Nesom and Robinson, 2007). Nesom (1994b) suggested that Asterothamnus and Krylovia (= Rhinactinidia) are closely related in terms of similarities such as keeled phyllaries, a coiling–reflexing disc corolla, and two-veined achenes with glandular surfaces. Rhinactinidia is considered different from Aster in its diagnostic characters and zygomorphic disc corollas (Ling et al., 1985), but these features can also be found in Aster s.s. Our study shows (Figs 1B and 2) that Rhinactinidia is well nested within the Aster clade, belongs to the A. amellus clade (clade 3) and has no close relationship with Asterothamnus. Two samples of Rhinactinidia form a well-supported clade (PP = 1·00 and BS = 100 in Figs 1B and 2), and Rhinactinidia should be treated as a series of section Aster.
Status of the ‘Galatella group’
According to Nesom (1994a, b), the Galatella group of Asterinae s.s. includes three genera, Galatella (approx. 30 species), Crinitina (13 species) and Tripolium (a monotypic genus). These genera have been treated as three sections of Aster (Galatella, Linosyris and Tripolium, respectively) by some botanists but as segregate genera in other studies (reviewed by Ling et al., 1985; Nesom, 1994b). Furthermore, Nesom was indecisive about whether Galatella and Crinitina might belong in Solidagininae (Nesom, 1991) or whether they are more closely related to typical Aster (Nesom, 1994b). Based on ITS data, Fiz et al. (2002) and Brouillet et al. (2009b) found that Galatella and Crinitina form a well-supported clade, and a few studies have shown that Galatella or Crinitina are weakly related to Bellidinae rather than to Aster (Noyes and Rieseberg, 1999; Fiz et al., 2002; Karaman, 2006). Our phylogenetic analyses (Figs 1 and 2) show that Galatella, Crinitina and Tripolium constitute a well-supported clade (clade 22: PP = 1·00 and BS = 92 in Fig. 1A; PP = 1·00 and BS = 95 in Fig. 2). Furthermore, in the combined analysis (Fig. 2), Crinitina linosyris, Crinitina villosa and Galatella dahurica form a well-supported clade (clade 21: PP = 1·00, BS = 97), which would support the merger of Crinitina into Galatella. Whether Tripolium deserves generic status or whether the three genera should be merged into a single genus remains to be determined. If the latter is reasonable, the oldest name would have to be used for the genus, i.e. Galatella. In our analyses the Galatella–Crinitina–Tripolium clade (clade 22; Figs 1A and 2) is closely related to neither the Aster clade nor Solidago decurrens (a representative of sub-tribe Solidagininae). Similarities between the Galatella group and Aster in leaves, disc style branches, achenes and heads (Nesom, 1994b) are superficial and have developed in parallel, and the Galatella group should be separated from Aster. The trees show a moderate to weak relationship (clade 23: PP = 0·89 in Fig. 1A; PP = 0·91 and BS = 57 in Fig. 2) between the three genera of the Galatella group and Bellis, which is consistent with the conclusions of Fiz et al. (2002). The systematic position of the Galatella group remains unresolved.
Redelimitation of Aster
According to our data, all existing generic delimitations of Aster are problematic. The EA Aster as delimited by some botanists (e.g. Ling et al., 1985; Nesom, 1994b; Nesom and Robinson, 2007) is paraphyletic because it excludes some of the descendants of the most recent common ancestor. Therefore, monophyletic Aster should include such genera as Sheareria, Rhynchospermum, Kalimeris (excluding K. longipetiolata), Heteropappus, Miyamayomena, Rhinactinidia, Turczaninowia, Asterothamnus, Arctogeron and eastern Asian Doellingeria. Conversely, EA Aster as delimited by other botanists (e.g. Merxmüller et al., 1976; Ito and Soejima, 1995) is polyphyletic because it includes morphologically similar but distantly related taxa. Callistephus, Galatella, Crinitina and Tripolium should be excluded from Aster. The Aster clade (clade 8) is strongly supported in both the ITS tree (PP = 0·99; Fig. 1B) and the combined tree (PP = 1·00, BS = 98; Fig. 2), so the Aster clade is the recircumscribed genus Aster. Molecular data (Figs 1 and 2) revealed, however, that many Chinese Aster spp. should be excluded from Aster, although their status as Aster species, except for series Albescentes, has not been doubted. Of 41 sampled species of Aster s.s. (Ling et al., 1985; Chen, 1988; Ito and Soejima, 1995; Li and Liu, 2002), 17 should be removed from the genus.
Series Hersileoides (Aster section Orthomeris, sensu Ling et al., 1985) is endemic to western China and consists of two restricted species, A. hersileoides and A. nitidus (Ling et al., 1985; Yin et al., 2010). They are characterized by a shrubby habit, solitary capitula at the apex of branches, membranous receptacular bracts and a short outer pappus. A karyotypic study of these species (Yin et al., 2010) showed that they are diploid and have shorter chromosomes and higher asymmetry of karyotype than that with A. ageratoides. Our study demonstrates that the series is a well-supported monophyletic group (clade 9: PP = 0·99 and BS = 98 in Fig. 1A; PP = 1·00 and BS = 100 in Fig. 2). Although the systematic position of the series has never been questioned, the ITS phylogenetic tree (Fig. 1A) shows that clade 9, series Hersileoides, is not closely related to clade 8, the Aster clade, and in the combined tree (Fig. 2) the sister relationship between clades 8 and 9 is only weakly supported (BS = 54), even though the Bayesian PP is high (0·99). Therefore, the series should be removed from Aster, and it might be reasonable to elevate the series to a generic level in sub-tribe Asterinae.
Aster albescens var. albescens, A. argyropholis and A. lavanduliifolius are representative of series Albescentes. Western China is the centre of diversity of this series, with six of the seven species being endemic to the region (the exception being A. albescens which is distributed from western China to the southern Himalayas; Ling et al., 1985; Chen, 1988). Ling et al. (1985) established the series and placed it within Aster section Orthomeris. The series differs from others in the section with its shrubby habit, pinnate primary lateral leaf veins, relatively small heads, small rays and four- to six-veined, sub-cylindric achenes. Our studies (Figs 1A and 2) demonstrate that series Albescentes is a well-supported monophyletic taxon (clade 12: PP = 1·00 and BS = 98 in Fig. 1A; PP = 1·00 and BS = 100 in Fig. 2) and should be removed from Aster. Nesom (1994b) suggested that series Albescentes is closely related to the NA group, in which it would be positioned near NA Doellingeria. The present results provide no evidence to support this relationship, however. On the contrary, series Albescentes occurs in a polytomy (clade 18: PP = 1·00 in Fig. 2) with Myriactis and other segregates of Aster s.s., implying that series Albescentes may belong to the Australasian lineages rather than to the NA clade (clade 20; Fig. 2). In the ITS analysis the series occurs at an unresolved position within a polytomy (Fig. 1A) in Astereae. Its systematics requires further investigation; however, series Albescentes should undoubtedly be removed from Aster and be considered for generic rank.
According to Ling et al. (1985), A. auriculatus and A. panduratus belong to section Aster series Auriculati, and Aster pycnophyllus belongs to section Orthomeris series Sikkimenses. In the trees (Figs 1A and 2), the three species are well nested in a clade with Myriactis (clade 11: PP = 1·00 and BS = 97 in Fig.1A; PP = 1·00 and BS = 100 Fig. 2) and distantly related to Aster, suggesting that they should be removed from Aster. Although Myriactis is quite different from these three species with its two- to multiple-seriate ray florets, male disc florets and glandular collar, they do not form a sub-clade sister to Myriactis. Therefore, the relationships among the three species and Myriactis require further study.
Of the 15 sampled species of Aster section Alpigenia (Appendix), seven fall in the Aster clade, and the other eight fall outside it (Figs 1A and 2). Of these eight species, six (A. asteroides, A. brachytrichus, A. diplostephioides, A. flaccidus, A. setchuenensis and A. yunnanensis) form a well-supported clade (clade 10: PP = 0·99 in Fig. 1A; PP = 1·00 and BS = 98 in Fig. 2), implying that these species might become a new genus. The systematic position of this group is unresolved, however. In the ITS tree (Fig. 1A) clade 10 falls within a big polytomy, and in the combined tree (Fig. 2) it belongs to clade 18, a polytomy, with Myriactis. Aster senecioides, the sole member of a monotypic series of section Alpigenia, forms a strongly supported clade (clade 15: PP = 1·00 and BS = 93 in Fig. 1; PP = 1·00 and BS = 100 in Fig. 2) with A. fuscescens, also the sole member of a monotypic series of section Aster (Ling et al., 1985). These two species are at an unresolved position within the big polytomy in the ITS tree (Fig. 1A) and belong to a polytomy (clade 18; Fig. 2) in the combined tree. Clade 15 might be treated as a separate genus. Similarly, in the ITS tree (Fig. 1A), A. batangensis (clade 16) occupies an unresolved position within the big polytomy of EA Astereae, and, in the combined tree (Fig. 2), clade 16 belongs to clade 18. Our phylogenetic trees (Figs. 1A and 2) show that A. batangensis seems to deserve the status of a monotypic genus. Thus, A. series Hersileoides, A. series Albescentes, a six-species group including A. asteroides, a group composed of A. senecioides and A. fuscescens, and A. batangensis should be elevated to generic level, and, together with the Aster clade, placed with the Australasian lineages.
Nesom (2000) stated that Aster, even in its more restricted morphological definition, still encompasses a great deal of variation, and the description remains correspondingly general. Herein, Aster is expanded to include some segregates of Aster s.l. and other genera, making Aster more complex in some morphological features. For example, treating Sheareria as a section of Aster adds to Aster some new characters such as bract-like leaves, assimilating branches, small heads with only 5–8 florets, and functionally staminate disc flowers. Arctogeron brings to Aster such new features as caespitose herbs, narrow grass-like leaves with a scabrous ciliate margin and densely silvery pubescent cypselas. The high morphological diversity implies that Aster has undergone an evolutionary radiation since it originated. Aster displays a broad morphological variability in pappi (e.g. pappi are one- to four-seriate or absent, short or long, persistent or caducous) that, as mentioned above, has been used as a diagnostic character in delimiting some genera. Pappi are absent in clades 2 and 4 and in the M. piccolii–M. simplex clade of clade 3 (Figs 1B and 2), which implies that the disappearance of a pappus has evolved independently at least three times in Aster. Kalimeris, A. smithianus, A. dolichopodus and A. souliei share reduced pappi but occur in different sub-clades (Figs 1B and 2), suggesting convergent evolution toward pappus shortening. Dimorphic pappi (different lengths of pappi between ray and disc florets) are a diagnostic feature of Heteropappus, but dimorphic pappi are also found in A. homochlamydeus (W.-P. Li, unpubl. res.), which is another example of convergent evolution of pappi. Furthermore, no evolutionary relationships occur among dimorphic pappi, short pappi and absent pappi, i.e. no evolutionary series from dimorphic pappi to epappi exists. According to Ling et al. (1985), series Turbinati is characterized by four- to seven-seriate phyllaries, whereas our phylogenetic trees (Figs 1 and 2) show that three species (A. turbinatus, A. baccharoides and A. sampsonii), A. jishouensis (series Turbinati), with multiseriate phyllaries, and R. verticillatum, with two- to three-seriate phyllaries, form a clade, and this clade is not closely related to another species with multiseriate phyllaries, A. sampsonii. Therefore, the multiseriate phyllaries feature has arisen more than once in Aster. Noticeably, six species in one sub-clade of clade 7 (Figs 1B and 2) share a more or less shrubby habit, and clade 9 (sister to clade 8 in Fig. 2) is also characterized by a shrubby habit, which might mean that shrubby habit may represent a symplesiomorphy in clades 8 and 9. Whether EA Aster, with predominantly herbaceous perennials, originated from a woody ancestor is worth considering. Nonetheless, in clade 6 (Figs. 1B and 2), the shrubby habit of A. baccharoides and A. smithianus seems to be a convergence because these species occur within clades 1 and 3, respectively (Figs 1B and 2), and are not closely related to each other, and each of them is the only shrub in its clade. The morphology of Aster is so complex that further tracing of important morphological characters in the phylogenetic trees is necessary to reveal their phylogenetic significance.
Infrageneric classification of Aster
After extensive changes in the generic delimitation of Aster, its infrageneric systematics should be reconstructed. Three infrageneric taxonomic schemes of EA Aster s.s. have been described. First, Ling et al. (1985) divided Chinese Aster s.s. into three sections [Aster, Orthomeris (a name based on an NA type in genus Oclemena) and Alpigenia] and 27 series. Next, Ito and Soejima (1995) recognized five sections of Japanese Aster: Tripolium (a monotypic section), Pseudocalimeris (largely equal to the genus Heteropappus), Teretiachaenium (including the taxa of Miyamayomena and eastern Asian Doellingeria), Asteromoea (similar to Kalimeris) and Aster (largely equal to Aster s.s.). Finally, Nesom (1994b) divided Aster into four sections and taxa incertae sedis. The former includes sections Aster, Alpigeni (including sub-sections Homochaeta, Heterochaeta and Senecioides), Ageratoides and Calimeridei, and the latter is a six-species group. The current study supports none of these taxonomic systems, however.
We suggest clade 8 (Figs 1B and 2) as the genus Aster and clades 6 and 7 (Figs 1 and 2) as two subgenera of Aster. In clade 6, each of four sub-clades (clades 1–4; Figs 1B and 2) could be treated as a section. As mentioned above, M. angustifolius (clade 4; Figs 1B and 2) and S. nana (clade 2; Figs 1B and 2) may be treated as monotypic sections. Clade 1 is well supported in all analyses (PP = 1·00 and BS = 99 in Fig. 1B; PP = 1·00 and PP = 100 in Fig. 2) and could be regarded as section Ageratoides, typified by A. ageratoides (Nesom, 1994b). Section Ageratoides includes all taxa of section Pseudocalimeris and section Asteromoea and some members of section Teretiachaenium and section Aster (sensu Ito and Soejima, 1995); it corresponds more or less to sections Ageratoides of Nesom (1994b) and Orthomeris of Ling et al. (1985). Clade 3 is well supported only by BI (PP = 0·96 in Fig. 1B; PP = 1·00 in Fig. 2) but not by MP analysis. It might be treated as section Aster, typified by A. amellus. Although all previous schemes have recognized section Aster, their circumscriptions differ from ours. Some members (e.g. A. dolichopodus, A. mangshanensis, A. smithianus and A. vestitus) of section Aster of Ling et al. (1985) are nested in clade 1 (section Ageratoides) rather than in clade 3 (section Aster), and some members of section Orthomeris (e.g. A. sampsonii, A. turbinatus, A. baccharoides and A. jishouensis; Ling et al., 1985; Li and Liu, 2002) are nested in clade 3 rather than in clade 1. In fact, Nesom (1994b) agreed with Ling et al. (1985) in the circumscription of section Aster. As mentioned above, in Flora of Japan (Ito and Soejima, 1995) section Aster has a much wider definition than ours. Some species of section Alpigenia in the classifications of Ling et al. (1985) and Nesom (1994b) belong to clade 1 (section Ageratoides) or clade 3 (section Aster), and the others occur outside of the Aster clade, suggesting that section Alpigenia should be abandoned.
Clade 7, the other sub-clade of clade 8, is moderately to well supported (PP = 0·99 and BS = 79 in Fig. 1B; PP = 1·00 and BS = 92 in Fig. 2) and could be treated as the other subgenus of Aster. The subgenus consists of three segregates (eastern Asian Doellingeria, Asterothamnus and Arctogeron) of Aster s.l. and three species (A. falcifolius, A. poliothamnus and A. sikuensis) of Aster s.s. As discussed above, eastern Asian Doellingeria, Asterothamnus and Arctogeron should be treated as three different sections. According to Ling et al. (1985), A. falcifolius is the only member of series Falcifolii of section Orthomeris, and A. poliothamnus and A. sikuensis belong to series Vestiti of section Aster. These three species have more or less woody stems that are similar to those of the other taxa of clade 7, Asterothamnus and Arctogeron. Aster falcifolius is characterized by solitary flowers and bracteole leaves that become denser until grading into phyllaries. It should be raised to the sectional level. Aster poliothamnus and A. sikuensis share some features, such as four- to five-seriate phyllaries and the absence of rhizomes, and form a strongly to weakly supported clade (PP = 1·00, BS = 71; Fig. 2). These two species may deserve the status of a section. As a result, the subgenus (clade 7; Figs 1B and 2) would comprise five sections.
According to Ling et al. (1985), the recircumscribed Aster has seven series with two or more species included in our analyses. None of these is monophyletic, however. All three species of series Vestiti (A. vestitus, A. poliothamnus and A. sikuensis; Ling et al., 1985) were sampled and occur in clades 6 and 7 (see Figs 1B and 2), and they should be placed in different subgenera. Aster alpinus, A. handelii, A. heterolepis and A. oreophilus are assigned to series Alpigenia (Ling et al., 1985) but occur in four clades of section Ageratoides (clade 1; Figs 1B and 2) and section Aster (clade 3; Figs 1B and 2). Although A. fanjingshanicus, A. tongolensis and A. souliei of series Tongolenses (Ling et al., 1985) belong to clade 1, they are not closely related to one another (Figs 1B and 2). All of the series (sensu Ling et al., 1985) of Aster must be re-evaluated.
More than half the species of Aster (Tamamschyan, 1959; Grieson, 1975; Merxmüller and Schreiber, 1976; Ling et al., 1985; Czerepanov, 1995; Ito and Soejima, 1995) are not included in our study; therefore, a more extensive taxon sampling of molecular sequence data is necessary for a full phylogenetic reconstruction of Aster. Because more than half of the sampled species of section Alpigenia (sensu Ling et al., 1985) should be excluded from Aster, it is particularly important to collect molecular data for all the species. Because the combined analysis shows better resolution than that of the ITS phylogeny in Aster s.l., the combined data for the Australasian lineages are needed to resolve the origin and systematic position of Aster and its segregates.
ACKNOWLEDGEMENTS
We thank the referees for their useful comments, suggestions and questions; Professor De-Yuan Hong, Liang-Bi Chen and Qiner Yang for their generous concern and help; the management departments of many of China's National Nature Reserves; the faculty of the natural sciences departments at Shumen University; Professors Gong-Xi Chen and Dun-Yan Tan for field assistance; the curators of the PE, WUK, SZ, CDBI, HNNU, KUN, IBSC, IBK, NAS, HGAS, CCNU, HIB, HIMC and FUS herbaria for access to specimens; Professor Dong-Ping Li for kindly offering research facilities for our use; Professor Xiang Hu, Professor Ping Zhang, Ming Tang and Feng-Ming Qian for technical assistance; and Professor Chengqi Ao for providing the leaves of M. angustifolius. This study was financed by the National Natural Science Foundation of China (grant nos 30470131 and 39899400) and by the Scientific Research Fund of Hunan Provincial Education Department (grant no. 08A046).
Table 1.
Taxa sampled, phylogenetic lineages, vouchers and GenBank accessions.
| Present taxonomy* | Phylogenetic lineages and infrageneric classification of Aster† | Numbers, locations and altitudes of vouchers‡ | GenBank accession number§ |
||
|---|---|---|---|---|---|
| ITS | ETS | trnL-F | |||
| Unplaced taxa | |||||
| Doellingeria umbellata | NA | – | AF046966 | NA | |
| Eurybia sibirica | NA | – | AY772421 | AY772435 | GU480699 |
| Nannoglottis delavayi | BL | – | AY017167 | NA | |
| Sub-tribe Homochrominae | |||||
| Felicia filifolia | BL | – | FJ457937 | NA | |
| Sub-tribe Hinterhuberinae | |||||
| Celmisia mackaui | NZ | – | AF422115 | NA | |
| Chiliotrichum diffusum | PSA | – | AF046945 | DQ479128 | AF452501 |
| Madagaster madagascariensis | BL | – | DQ479031 | NA | |
| Mairia hirsuta | BL | – | FJ457929 | NA | |
| Olearia astroloba | AL | – | AF497646 | NA | |
| Olearia ballii | AL | – | AF497662 | NA | |
| Olearia calcarea | AL | – | AF497663 | NA | |
| Olearia ciliata | AL | – | AF497667 | NA | |
| Olearia cordata | AL | – | AF497668 | NA | |
| Olearia covenyi | NZ | – | AF497711 | NA | |
| Olearia rudis | AL | – | AF497677 | NA | |
| Olearia tomentosa | AL | – | AF497650 | NA | |
| Oritrophium hieracioides | PSA | – | DQ479116 | NA | |
| Pleurophyllum hookeri | NZ | – | HQ439864 | NA | |
| Printzia polifolia | BL | – | FJ457927 | NA | |
| Pteronia camphorata var. camphorata | BL | – | DQ479118 | NA | |
| Remya kauaiensis | AL | AF497684 | NA | ||
| Sub-tribe Brachyscominae | |||||
| Brachyscome rigidula | AL | – | DQ478994 | NA | |
| Calotis hispidula | AL | – | AB196597 | NA | |
| Sub-tribe Bellidinae | |||||
| Bellis perennis | BE | LWP1003008; Changsha, cultivated | JN315918 | JN315942 | JN315894 |
| Sub-tribe Grangeinae | |||||
| Grangea maderaspatana | GR | LWP0802034; Zhaoqing City, 200 m | JN315920 | JN315944 | JN315896 |
| Dichrocephala auriculata | GR | LWP0708234; Dali City, 2300 m | JN315919 | JN315943 | JN315895 |
| Sub-tribe Lagenophorinae | |||||
| Myriactis nepalensis | AL | LWP0509002; Kunming City, 2300 m | JN315921 | JN315945 | JN315897 |
| Myriactis wightii | AL | LWP0509010; Kunming City, 2200 m | JN315922 | JN315946 | JN315898 |
| Rhynchospermum verticillatum | AL | LWP0607065; Mt. Emei, 1200 m | JN543706 | JN543707 | JN543708 |
| Sheareria nana | AL | LWP0701001; Changsha City, 30m | JN543703 | JN543704 | JN543705 |
| Sub-tribe Baccharidinae | |||||
| Baccharis neglecta | SA | – | U97604 | NA | |
| Sub-tribe Podocominae | |||||
| Camptacra gracilis | AL | – | AF247069 | NA | |
| Kippistia suaedifolia | AL | – | AF497660 | NA | |
| Minuria integerrima | AL | – | AF046957 | NA | |
| Minuria macrorhiza | AL | – | AF247076 | ||
| Tetramolopium humile var. humile | AL | – | DQ479040 | NA | |
| Sub-tribe Asterinae | |||||
| Arctogeron gramineum | SEA | LWP0606014; Wulanhaote City, 300 m | JN315928 | JN315952 | JN315904 |
| Aster | Eurasian Aster s.s. | ||||
| Aster amellus | Section Aster series Amelli | LWP0408002; Shumen, Bulgaria, 400 m | JN543742 | JN543743 | JN543744 |
| Aster maackii | Section Aster series Macrocephali | LWP0609043; Yichun City, 200 m | JN543745 | JN543746 | JN543747 |
| Aster tataricus | Section Aster series Macrocephali | LWP0108018; Xinglong County, 400 m | JN543748 | JN543749 | JN543750 |
| Aster fuscescens | Section Aster series Fuscescentes | YGS1007021; Gongshan County, 2500 m | JN543751 | JN543752 | JN543753 |
| Aster auriculatus | Section Aster series Auriculati | LWP0509059; Yongsheng County, 2200 m | JN543754 | JN543755 | JN543756 |
| Aster panduratus | Section Aster series Auriculati | LWP1012067; Guiyang City, 1100 m | JN543757 | JN543758 | JN543759 |
| Aster mangshanensis | Section Aster series Auriculati | LWP0511034; Mt. Mang, 1670 m | JN543760 | JN543761 | JN543762 |
| Aster poliothamnus | Section Aster series Vestiti | LWP0506001; Zhang County, 500 m | JN543763 | JN543764 | JN543765 |
| Aster sikuensis | Section Aster series Vestiti | LWP0510025; Lueyang County, 300 m | JN543766 | JN543767 | JN543768 |
| Aster vestitus | Section Aster series Vestiti | LWP0509023; Lijiang City, 2610 m | JN543769 | JN543770 | JN543771 |
| Aster taliangshanensis | Section Aster series Taliangshanensis | LWP0607056; Xichang City, 2800 m | JN543772 | JN543773 | JN543774 |
| Aster dolichopodus | Section Aster series Smithiani | LWP0409060; Maerkang City, 2500 m | JN543775 | JN543776 | JN543777 |
| Aster smithianus | Section Aster series Smithiani | LWP0508034; Maerkang City, 2600 m | JN543778 | JN543779 | JN543780 |
| Aster ageratoides var. lasiocladus | Section Orthomeris series Ageratoides | LWP0112018; Changsha City, 110 m | JN543781 | JN543782 | JN543783 |
| Aster homochlamydeus | Section Orthomeris series Ageratoides | LWP0508004; Li County, 2600 m | JN543784 | JN543785 | JN543786 |
| Aster hersileoides | Section Orthomeris series Hersileoides | LWP0807002; Li County, 2100 m | JN543787 | JN543788 | JN543789 |
| Aster nitidus | Section Orthomeris series Hersileoides | LWP0505007; Nanchuan County, 660 m | JN543790 | JN543791 | JN543792 |
| Aster albescens var. albescens | Section Orthomeris series Albescentes | LWP0508123; Baoxing County, 2010 m | JN543862 | JN543863 | JN543864 |
| Aster argyropholis | Section Orthomeris series Albescentes | LWP0409045; Maerkang City, 2500 m | JN543793 | JN543794 | JN543795 |
| Aster lavanduliifolius | Section Orthomeris series Albescentes | LWP0708053; Yajiang county, 2720 m | JN543796 | JN543797 | JN543798 |
| Aster pycnophyllus | Section Orthomeris series Sikkimenses | LWP0509091; Dali City, 2800 m | JN543799 | JN543800 | JN543801 |
| Aster falcifolius | Section Orthomeris series Falcifolii | LWP0410050; Mt. Huping, 400 m | JN543802 | JN543803 | JN543804 |
| Aster baccharoides | Section Orthomeris series Turbinati | LWP0802001; Zhuhai City, 100 m | JN543805 | JN543806 | JN543807 |
| Aster jishouensis | Section Orthomeris series Turbinati | LWP1012015; Jishou City, 600 m | JN543808 | JN543809 | JN543810 |
| Aster sampsonii | Section Orthomeris series Turbinati | LWP0511060; Mt. Mang, 1100 m | JN543811 | JN543812 | JN543813 |
| Aster turbinatus | Section Orthomeris series Turbinati | LWP0110029; Fenghua City, 60 m | JN543814 | JN543815 | JN543816 |
| Aster alpinus | Section Alpinenia series Alpini | LWP0607020; Wulumuqi City, 2320 m | JN543817 | JN543818 | JN543819 |
| Aster handelii | Section Alpinenia series Alpini | LWP0708174; Zhongdian County, 3400 m | JN543820 | JN543821 | JN543822 |
| Aster heterolepis | Section Alpinenia series Alpini | LWP0507004; Jiuzhai County, 2600 m | JN543823 | JN543824 | JN543825 |
| Aster oreophilus | Section Alpinenia series Alpini | LWP0509016; Lijiang City, 3000 m | JN543826 | JN543827 | JN543828 |
| Aster fanjingshanicus | Section Alpinenia series Tongolensis | LWP0606082; Mt. Fangjing, 2300 m | JN543829 | JN543830 | JN543831 |
| Aster tongolensis | Section Alpinenia series Tongolensis | LWP0708147; Xiangcheng County, 3300 m | JN543832 | JN543833 | JN543834 |
| Aster souliei | Section Alpinenia series Tongolensis | LWP0708084; Litang County, 4000 m | JN543835 | JN543836 | JN543837 |
| Aster brachytrichus | Section Alpinenia series Latibracteati | LWP0607075; Xichang City, 2800 m | JN543838 | JN543839 | JN543840 |
| Aster asteroides | Section Alpinenia series Asteroides | LWP0708112; Daocheng County, 2780 m | JN543841 | JN543842 | JN543843 |
| Aster flaccidus | Section Alpinenia series Asteroides | LWP0607026; Wulumuqi City, 3700 m | JN543844 | JN543845 | JN543846 |
| Aster diplostephioides | Section Alpinenia series Diplostephioides | LWP0507020; Jiuzhai County, 2600 m | JN543847 | JN543848 | JN543849 |
| Aster setchuenensis | Section Alpinenia series Diplostephioides | LWP0508007; Maerkang City, 2800 m | JN543850 | JN543851 | JN543852 |
| Aster yunnanensis | Section Alpinenia series Diplostephioides | LWP0508089; Kangding City, 3500 m | JN543853 | JN543854 | JN543855 |
| Aster senecioides | Section Alpinenia series Senecioides | LWP0708215; Lijiang City, 2800 m | JN543856 | JN543857 | JN543858 |
| Aster batangensis | Section Alpinenia series Batangenses | LWP0606039; Lijiang City, 2700 m | JN543859 | JN543860 | JN543861 |
| Asterothamnus centrali-asiaticus | SEA | LWP0607045; Yinchuan City, 1630 m | JN315930 | JN315954 | JN315906 |
| Asterothamnus fruticosus | SEA | LWP0607005; Wulumuqi City, 950 m | JN315929 | JN315953 | JN315905 |
| Callistephus chinensis | SEA | LWP0108021; Anshan City, 340 m | JN315931 | JN315955 | JN315907 |
| Crinitina linosyris | SEA | LWP0408001; Shumen, Bulgaria, 400 m | JN315932 | JN315956 | JN315908 |
| Crinitina villosa | SEA | LWP0408009; Shumen, Bulgaria, 400 m | JN315933 | JN315957 | JN315909 |
| Doellingeria scaber | SEA | LWP0108025; Anshan City, 350 m | JN315934 | JN315958 | JN315910 |
| Galatella dahurica | SEA | LWP0609047; Mt. A'er, Nei Mongol, 400 m | JN315935 | JN315959 | JN315911 |
| Heteropappus altaicus var. millefolius | SEA | LWP0506010; Zhang County, 600 m | JN543709 | JN543710 | JN543711 |
| Heteropappus crenatifoliu | SEA | LWP0409037; Maerkang City, 3200 m | JN543712 | JN543713 | JN543714 |
| Kalimeris indica | SEA | LWP0806017; Changsha City, 80 m | JN543715 | JN543716 | JN543717 |
| Kalimeris incisa | SEA | LWP0609107; Tonghua County, 560 m | JN543721 | JN543722 | JN543723 |
| Kalimeris integrifolia | SEA | LWP0609077; Mudanjiang City, 360 m | JN543718 | JN543719 | JN543720 |
| Kalimeris longipetiolata | SEA | LWP0508104; Baoxing County, 2600 m | JN315936 | JN315960 | JN315912 |
| Miyamayomena angustifolius | SEA | DBY9206; Yongjia County. 200 m | JN543736 | JN543737 | JN543738 |
| Miyamayomena piccolii | SEA | LWP0510055; Mei County. 300 m | JN543730 | JN543731 | JN543732 |
| Miyamayomena simplex | SEA | LWP0508083; Kangding City, 2800 m | JN543733 | JN543734 | JN543735 |
| Rhinactinidia eremophila | SEA | LWP0607036; Wulumuqi City, 2620 m | JN543727 | JN543728 | JN543729 |
| Rhinactinidia limoniifolia | SEA | LWP0607012; Wulumuqi City, 1800 m | JN543724 | JN543725 | JN543726 |
| Tripolium vulgare | SEA | LWP0311001; Varna, Bulgaria, 1 m | JN315937 | JN315961 | JN315913 |
| Turczaninowia fastigiata | SEA | LWP0609030; Daqin City, 150 m | JN543739 | JN543740 | JN543741 |
| Sub-tribe Solidaginae | |||||
| Solidago decurrens | NA | LWP0510116; Lichuan County, 1050 m | JN204176 | JN204177 | JN204178 |
| Sub-tribe Pentachaetinae | |||||
| Pentachaeta aurea | NA | – | AF046972 | NA | |
| Sub-tribe Boltoniinae | |||||
| Boltonia asteroides | NA | – | AF477632 | NA | |
| Sub-tribe Machaerantherinae | |||||
| Machaeranthera tanacetifolia | NA | – | AF477661 | NA | |
| Sub-tribe Symphyotrichinae | |||||
| Symphyotrichum novi-belgii | NA | LWP0606002; Beijing, cultivated. | JN315926 | JN315950 | JN315902 |
| Symphyotrichum subulatum | NA | LWP1010007; Changsha City, 40 m | JN315927 | JN315951 | JN315903 |
| Sub-tribe Astranthiinae | |||||
| Astranthium integrifolium | NA | – | AF046984 | NA | |
| Sub-tribe Chrysopsidinae | |||||
| Chrysopsis mariana | NA | – | GQ892729 | NA | |
| Sub-tribe Conyzinae | |||||
| Conyza japonica | AIS | LWP0606032; Lijiang City, 2500 m | JN315938 | JN315962 | JN315914 |
| Conyza sumatrensis | NA | LWP1009002; Changsha City, 35 m | JN315923 | JN315947 | JN315899 |
| Erigeron annus | NA | LWP1010009; Changsha City, 40 m | JN315924 | JN315948 | JN315900 |
| Erigeron breviscapus | NA | LWP0606055; Lijiang City, 2500 m | JN315925 | JN315949 | JN315901 |
| Tribe Anthemideae | |||||
| Chrysanthemum coronarium | OG | LWP1004010; Changsha, cultivated. | JN315939 | JN315963 | JN315915 |
| Dendranthema indicum | OG | LWP1012002; Changsha City, 80 m | JN315940 | JN315964 | JN315916 |
| Tribe Calenduleae | |||||
| Calendula officinalis | OG | LWP1004006; Changsha, cultivated. | JN315941 | JN315965 | JN315917 |
* Generic circumscriptions and nomenclature of Astereae follow Nesom and Robinson (2007) except Turczaninowia which follows Ling et al. (1985) and Crinitina Soják is substituted for Crinitaria Cass. The name Aster setchuenensis follows the International Plant Names Index (IPNI).
† Phylogenetic lineages: follows Brouillet et al. (2009b); infrageneric classification of Aster follows Ling et al. (1985). AIS, Astereae incertae sedis; AL, Australasian lineages; BE, Bellidinae; BL, early-branching lineages; ETS, external transcribed spacer; GR, Grangeinae; ITS, internal transcribed spacer; NA, North American lineage; NZ, New Zealand clade; OG, outgroup; PSA, palaeo South American clade; SA, South American lineages; SEA, segregates of Eurasian Aster s.l.
‡ Information is omitted for the accessions that were obtained from GenBank. Four species were collected from Bulgaria and the others from China.
§ One or two sequence (ETS, trnL-F) data unavailable.
LITERATURE CITED
- Andrus N, Tye A, Nesom G, et al. Phylogenetics of Darwiniothamnus (Asteraceae: Astereae) – molecular evidence for multiple origins in the endemic flora of the Galápagos Islands. Journal of Biogeography. 2009;36:1055–1069. [Google Scholar]
- Baldwin BG, Markos S. Phylogenetic utility of the external transcribed spacer (ETS) of 18S–26S nrDNA: congruence of ETS and ITS trees of Calycadenia (Compositae) Molecular Phylogenetics and Evolution. 1998;10:449–463. doi: 10.1006/mpev.1998.0545. [DOI] [PubMed] [Google Scholar]
- Bentham G. Flora Hongkongensis. London: Lovell Reeve; 1861. [Google Scholar]
- Bentham G. Compositae. In: Bentham G, Hooker JD, editors. Genera Plantarum. London: Lovell Reeve; 1873. pp. 163–533. [Google Scholar]
- Bremer K. Asteraceae – cladistics and classification. Portland, OR: Timber Press; 1994. [Google Scholar]
- Brouillet L, Allen GA, Semple JC, Ito M. ITS phylogeny of North American asters (Asteraceae: Astereae) 2001 Botany 2001 [ASPT/BSA/IOPB joint meeting] Albuquerque, New Mexico 12–16 August 2001. Abstract. http://bsa2001.scientific-conference.net/section12/abstracts/150.shtml. (accessed 23 November 2011) [Google Scholar]
- Brouillet L, Anderberg AA, Nesom GL, Lowrey TK, Urbatsch LE. Welwitschiella is a member of the African subtribe Grangeinae (Asteraceae Astereae): a new phylogenetic position based on ndhF and ITS sequence data. Kew Bulletin. 2009a;64:645–660. [Google Scholar]
- Brouillet L, Lowrey TK, Urbatsch L, et al. Astereae. In: Funk VA, Susanna A, Stuessy T, Bayer R, editors. Systematics, evolution and biogeography of the Compositae. Vienna: IAPT; 2009b. pp. 449–490. [Google Scholar]
- Chen YL. Sheareria. In: Ling R, Chen YL, Shi Z, et al., editors. Flora Reipublicae Popularis Sinicae. Beijing: Science Press; 1979. pp. 318–323. [Google Scholar]
- Chen YL. Systematic notes on the genus Miyamayomena Kitam. (Compositae) Bulletin of Botanical Research. 1986;6:37–46. [Google Scholar]
- Chen YL. Two new species of Aster L. from China. Bulletin of Botanical Research. 1988;8:11–16. [Google Scholar]
- Chen YL. New plants from the Hengduan Mountains. Acta Phytotaxonomica Sinica. 1990;28:483–490. [Google Scholar]
- Cross EW, Quinn CJ, Wagstaf SJ. Molecular evidence for the polyphyly of Olearia (Astereae: Asteraceae) Plant Systematics and Evolution. 2002;235:99–120. [Google Scholar]
- Czerepanov SK. Vascular plants of Russia and adjacent states (the former USSR) Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Dorn R. Asters retreat to Eurasia. Castilleja. 2003;22:3. [Google Scholar]
- Doyle JJ, Doyle JD. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, Botanical Society of America. 1987;19:11–15. [Google Scholar]
- Farris JS, Kallersjo M, Kluge AG, Bult C. Testing significance of congruence. Cladistics. 1994;10:315–319. [Google Scholar]
- Fiz O, Valcarcel V, Vargas P. Phylogenetic position of Mediterranean Astereae and character evolution of daisies (Bellis, Asteraceae) inferred from nrDNA ITS sequences. Molecular Phylogenetics and Evolution. 2002;25:157–171. doi: 10.1016/s1055-7903(02)00228-2. [DOI] [PubMed] [Google Scholar]
- Fu JQ. The new plants of the Compositae from north-western China. Bulletin of Botanical Research. 1983;3:110–128. [Google Scholar]
- Gao TG, Wang W, Bayer RJ, Li DZ. Systematic position of the enigmatic genus Sheareria (Asteraceae) – evidence from molecular, morphological and cytological data. Taxon. 2009;58:769–780. [Google Scholar]
- Garcia S, Panero JL, Siroky J, Kovarik A. Repeated reunions and splits feature the highly dynamic evolution of 5S and 35S ribosomal RNA genes (rDNA) in the Asteraceae family. BMC Plant Biology. 2010;10:176. doi: 10.1186/1471-2229-10-176. http://dx.doi.org/10.1186/1471-2229-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson JJC. A revision of the asters of the Himalayan area. Notes from the Royal Botanic Garden. Edinburgh. 1964;26:67–163. [Google Scholar]
- Grieson AJC. Aster L. In: Davis PH, editor. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press; 1975. pp. 118–121. [Google Scholar]
- Gu HY, Zhao XL, Qu LJ, Wen LX, Chen ZL. Preliminary studies in the phylogeny of Kalimeris yomena subsp. yomena and two other taxa using RFLP analysis. Cathaya. 1994;6:27–34. [Google Scholar]
- Gu HY, Hoch PC. Systematics of Kalimeris (Asteraceae: Astereae) Annals of the Missouri Botanical Garden. 1997;84:762–814. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hoffmann O. Compositae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. Leipzig: Engelmann; 1890. pp. 87–391. 4(5) [Google Scholar]
- Hu SY. The Compositae of China. V. Quarterly Journal of the Taiwan Museum. 1967;20:1–77. [Google Scholar]
- Huang H. Plant diversity and conservation in China: planning a strategic bioresource for a sustainable future. Botanical Journal of the Linnean Society. 2011;166:282–300. doi: 10.1111/j.1095-8339.2011.01157.x. [DOI] [PubMed] [Google Scholar]
- Huziwara Y. Heterokalimeris maruyamae Kitamura. A new intergeneric hybrid of Kalimeris and Heteropappus. Japanese Journal of Genetics. 1950;25:25–26. [Google Scholar]
- Ito M, Soejima A. In: Aster. Flora of Japan Vol. IIIb, Angiospermae, Dicotyledoneae, Sympetalae (b) Iwatsuki K, Yamazaki T, Boufford DE, Ohba H, editors. Tokyo: Kodansha; 1995. pp. 59–73. [Google Scholar]
- Ito M, Soejima A, Nishino T. Phylogeny and speciation of Asian. Aster. Korean Journal of Plant Taxonomy. 1994;24:133–143. [Google Scholar]
- Ito M, Soejima A, Hasebe M, Watanabe K. A chloroplast-DNA phylogeny of Kalimeris and Aster, with reference to generic circumscription. Journal of Plant Research. 1995;108:93–96. [Google Scholar]
- Ito M, Soejima A, Watanabe K. Phylogenetic relationships of Japanese Aster (Asteraceae, Astereae) sensu lato based on chloroplast-DNA restriction site mutations. Journal of Plant Research. 1998;111:217–223. [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends in Biochemical Sciences. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Karaman-Castro V, Urbatsch LE. Phylogeny of Hinterhubera group and related genera (Hinterhuberinae: Astereae) based on the nrDNA ITS and ETS sequences. Systematic Botany. 2009;34:805–817. [Google Scholar]
- Karaman V. Phylogeny of Hinterhubera, Novenia and related genera based on the nuclear ribosomal (nr) DNA sequence data (Asteraceae: Astereae) Louisiana State University: Baton Rouge; 2006. [Google Scholar]
- Kitamura S. Compositae Japonicae. Pars Prima. Memoirs of the College of Science, Kyoto Imperial University. Series B. Biology. 1937;8:299–399. [Google Scholar]
- Kitamura S. Change in the generic name Gymnaster. Acta Phytotaxonomy and Geobotany. 1982;33:409. [Google Scholar]
- Lessing CF. Synopsis Generum Compositarum. Berlin: Duncker and Humblot; 1832. p. 189. [Google Scholar]
- Li WP. Natural hybridization between Aster ageratoides var. scaberulus and Kalimeris indica (Asteraceae): evidences from morphology, karyotype and ITS sequences. Botanical Studies. 2006;47:191–197. [Google Scholar]
- Li WP, Liu SX. Aster jishouensis (Asteraceae), a new species from Hunan, China. Acta Phytotaxonomica Sinica. 2002;40:455–457. [Google Scholar]
- Li WP, Zhang ZG. Aster shennongjiaensis (Asteraceae), a new species from Central China. Botanical Bulletin of Academia Sinica. 2004;45:95–99. [Google Scholar]
- Li WP, Zhang P, Yin GS. New evidence for the tribal placement of Sheareria within Astereae (Compositae) Journal of Systematics and Evolution. 2008;46:608–613. [Google Scholar]
- Lin YR. Astereae. In: Wu TL, Hu Q, Chen Z, editors. Flora of Guangdong. Guangzhou: Guangdong Science and Technology Press; 2007. pp. 114–193. [Google Scholar]
- Ling R, Chen YL, Shi Z. Astereae. In: Ling R, Chen YL, Shi Z, editors. Flora Reipublicae Popularis Sinicae. Beijing: Science Press; 1985. pp. 70–353. [Google Scholar]
- Liu JQ, Gao TG, Chen ZD, Lu AM. Molecular phylogeny and biogeography of the Qinghai–Tibet Plateau endemic Nannoglottis (Asteraceae) Molecular Phylogenetics and Evolution. 2002;23:307–325. doi: 10.1016/s1055-7903(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Lowrey TK, Quinn CJ, Taylor RK, Chan R, Kimball RT, De Nardi JC. Molecular and morphological reassessment of relationships within the Vittadinia group of Astereae (Asteraceae) American Journal of Botany. 2001;88:1279–1289. [PubMed] [Google Scholar]
- Lowell EU, Roberts RP, Karaman V. Phylogenetic evaluation of Xylothamia, Gundlachia, and related genera (Asteraceae, Astereae) based on ETS and ITS nrDNA sequence data. American Journal of Botany. 2003;90:634–649. doi: 10.3732/ajb.90.4.634. [DOI] [PubMed] [Google Scholar]
- Markos S, Baldwin BG. Higher-level relationships and major lineages of Lessingia (Compositae, Astereae) based on nuclear rDNA internal and external transcribed spacer (ITS and ETS) sequences. Systematic Botany. 2001;26:168–183. [Google Scholar]
- Merxmüller H, Schreiber A, Yeo PF. Aster L. In: Tutin TG, Burges NA, Chater AO, et al., editors. Flora Europaea, Vol. 4: Plantaginaceae to Compositae (and Rubiaceae) London: Cambridge University Press; 1976. pp. 112–116. [Google Scholar]
- Nesom GL. Morphological definition of the Gutierrezia group (Asteraceae: Astereae) Phytologia. 1991;71:252–262. [Google Scholar]
- Nesom GL. Taxonomy of Doellingeria (Asteraceae: Astereae) Phytologia. 1993;75:452–462. [Google Scholar]
- Nesom GL. Subtribal classification of the Astereae (Asteraceae) Phytologia. 1994a;76:193–274. [Google Scholar]
- Nesom GL. Review of the taxonomy of Aster sensu lato (Asteraceae: Astereae), emphasizing the New World species. Phytologia. 1994b;77:141–297. [Google Scholar]
- Nesom GL. Generic conspectus of the tribe Astereae (Asteraceae) in North America, Central America, the Antilles, and Hawaii. Sida, Botanical Miscellany. 2000;20:1–100. [Google Scholar]
- Nesom GL, Robinson H. Tribe Astereae Cass. In: Kadereit JW, Jeffrey C, editors. The families and genera of vascular plants. Flowering plants: Eudicots: Asterales. Berlin: Springer; 2007. pp. 284–342. [Google Scholar]
- Noyes RD, Rieseberg LH. ITS sequence data support a single origin for North American Astereae (Asteraceae) and reflect deep geographic divisions in Aster s.l. American Journal of Botany. 1999;86:398–412. [PubMed] [Google Scholar]
- Nylander JAA. 2004. MrModeltest 2·2. Distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden. http://www.abc.se/~nylander/ . [Google Scholar]
- Panero JL, Funk VA. The value of sampling anomalous taxa in phylogenetic studies: major clades of the Asteraceae revealed. Molecular Phylogenetics and Evolution. 2008;47:757–782. doi: 10.1016/j.ympev.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Roberts RP. Phylogeny of Ericameria, Chrysothamnus and related genera (Asteraceae: Astereae) based on nuclear ribosomal DNA sequence data. PhD Thesis. Louisiana State University: Baton Rouge; 2002. [Google Scholar]
- Roberts RP, Urbatsch LE. Molecular phylogeny of Chrysothamnus and related genera (Asteraceae, Astereae) based on nuclear ribosomal 3'ETS and ITS nrDNA sequence data. Systematic Botany. 2004;29:199–215. [Google Scholar]
- Robinson H. A revision of the tribal and subtribal limits of the Heliantheae (Asteraceae) Smithsonian Contributions to Botany. 1981;51:1–102. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kokubugata G, Moller M. Molecular evidence for a natural hybrid origin of Doellingeria × sekimotoi (Asteraceae) using ITS and matK sequences. International Journal of Plant Science. 2007;168:469–476. [Google Scholar]
- Selliah S, Brouillet L. Molecular phylogeny of the North American eurybioid asters (Asteraceae, Astereae) based on the nuclear ribosomal internal and external transcribed spacers. Canadian Journal of Botany. 2008;86:901–915. [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4·0b10. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- Tamamschyan SG. Astereae. In: Komarov VL, editor. Flora URSS. Moscow: Leningrad; 1959. pp. 24–290. [Google Scholar]
- Tara M. Cytogenetic studies on natural intergeneric hybridization on Aster alliances. I. Aster ageratoides subsp. ovatus (2n = 36) × Kalimeris incisa (2n = 72) Botanical Magazine (Tokyo) 1972;85:219–240. [Google Scholar]
- Tara M. Cytogenetic studies on natural intergeneric hybridization on Aster alliances. II. Heteropappus hispidus (2n = 36) × Kalimeris incisa (2n = 72) Journal of Science of the Hiroshima University, Series B, Division 2. 1973;14:107–140. [Google Scholar]
- Tara M. Relationships between genomic constitutions and pappus lengths in the natural intergeneric F1 hybrid of Aster ageratoides subsp. ovatus × Kalimeris incisa and its descendants. Bulletin of the School of Education, Okayama University. 1989;82:139–166. [Google Scholar]
- Urbatsch LE, Roberts RP. Molecular phylogeny of Ericameria (Asteraceae, Astereae) based on nuclear ribosomal 3' ETS and ITS sequence data. Taxon. 2003;52:209–228. [Google Scholar]
- Urbatsch LE, Roberts RP, Karaman V. Phylogenetic evaluation of Xylothamia, Gundlachia, and related genera (Asteraceae, Astereae) based on ETS and ITS nrDNA sequence data. American Journal of Botany. 2003;90:634–649. doi: 10.3732/ajb.90.4.634. [DOI] [PubMed] [Google Scholar]
- Vaezi J, Brouillet L. Phylogenetic relationships among diploid species of Symphyotrichum (Asteraceae: Astereae) based on two nuclear markers, ITS and GAPDH. Molecular Phylogenetics and Evolution. 2009;51:540–553. doi: 10.1016/j.ympev.2009.03.003. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and application. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Xiang C, Semple JD. Molecular systematic study of Aster sensu lato and related genera (Asteraceae: Astereae) based on chloroplast DNA restriction site analyses and mainly North American taxa. In: Hind DJN, Beentje HJ, editors. Compositae: systematics, Vol. 1. Proceedings of the International Compositae Conference, Kew, 1994. Kew: Royal Botanic Gardens; 1996. pp. 393–423. [Google Scholar]
- Yin GS, Li WP, Chen SM, Liu SX. A karyotypic study on Aster series Hersileoides Ling (Asteraceae) Journal of Wuhan Botanical Research. 2010;28:406–409. [Google Scholar]
- Zhang XP, Bremer K. A cladistic analysis of the tribe Astereae (Asteraceae) with notes on their evolution and subtribal classification. Plant Systematics and Evolution. 1993;184:259–283. [Google Scholar]
- Zhao YZ. A study on floristic analysis of Asterothamnus. Acta Scientiarum Naturalium Universitatis NeiMonggol. 1996;27:659–661. [Google Scholar]
- Zhu ZY, Min BQ. A new species of Aster from Sichuan. Bulletin of Botanical Research. 1990;10:61–63. [Google Scholar]
- Zhuang X. Astereae. In: Wu CY, editor. Flora Yunnanica. Beijing: Science Press; 2004. pp. 46–131. [Google Scholar]