Abstract
Background and Aims
The release of hydrogen cyanide (HCN) from injured plant tissue affects multiple ecological interactions. Plant-derived HCN can act as a defence against herbivores and also plays an important role in plant–pathogen interactions. Crucial for activity as a feeding deterrent is the amount of HCN generated per unit time, referred to as cyanogenic capacity (HCNc). Strong intraspecific variation in HCNc has been observed among cyanogenic plants. This variation, in addition to genotypic variability (e.g. in Trifolium repens), can result from modifications in the expression level of the enzymes involved in either cyanogenic precursor formation or HCN release (as seen in Sorghum bicolor and Phaseolus lunatus). Thus, a modification or modulation of HCNc in reaction to the environment can only be achieved from one to the next generation when under genetic control and within days or hours when transcriptional regulations are involved. In the present study, it is shown that in rubber tree (Hevea brasiliensis) HCNc is modulated by post-translational activity regulation of the key enzymes for cyanide release.
Methods
Linamarase (LIN) and hydroxynitrile lyase (HNL) activity was determined by colorimetric assays utilizing dissociation of the substrates p-nitrophenyl-β-d-glucopyranoside and acetone cyanohydrin, respectively.
Key Results
In rubber tree leaves, LIN and HNL show up to ten-fold increased activity in response to tissue damage. This enzyme activation occurs within seconds and results in accelerated HCN formation. It is restricted to the damaged leaf area and depends on the severity of tissue damage.
Conclusions
LIN and HNL activation (in contrast to genetic and transcriptional regulations) allows an immediate, local and damage type-dependent modulation of the cyanogenic response. Accordingly, this post-translational activation plays a decisive role in the defence of H. brasiliensis against herbivores as well as pathogens and may allow more flexible reactions in response to these different antagonists.
Keywords: Cyanogenesis, Hevea brasiliensis, rubber tree, β-glucosidase, linamarase, hydroxynitrile lyase, activation, cyanogenic capacity, plant–herbivore interactions, plant–pathogen interactions
INTRODUCTION
Cyanogenic plants are capable of releasing hydrogen cyanide (HCN) in response to tissue damage. This phenomenon, cyanogenesis, is widespread in the plant kingdom (Møller and Seigler, 1999). More than 3000 cyanogenic plant species have been described (Seigler and Brinker, 1993; Webber and Miller, 2008), including many economically important crops such as cassava (Manihot esculenta), sorghum (Sorghum bicolor), lima bean (Phaseolus lunatus), white clover (Trifolium repens) and the rubber tree Hevea brasiliensis (McMahon et al., 1995). HCN is highly toxic due to inhibition of the mitochondrial respiration pathway (e.g. Solomonson, 1981). Cyanogenesis can therefore function as an efficient defence mechanism, especially against herbivores (Nahrstedt, 1985; Schappert and Shore, 1999; Gleadow and Woodrow, 2002; Ballhorn et al., 2005). On the other hand, HCN can also inhibit active defence reactions in the cyanogenic plant itself (Lieberei et al., 1989, 1996; reviewed by Møller, 2010). Cyanogenic plants have thus repeatedly been found to be susceptible to cyanide-resistant pathogens (Lieberei, 2007; Ballhorn et al., 2010a; Ballhorn, 2011).
HCN is released from cyanogenic precursors, in general from cyanogenic glycosides, consisting of α-hydroxynitriles stabilized by a sugar moiety (Conn, 1981; Hösel, 1981). In rubber tree, the two aliphatic cyanogenic glucosides (CGs) linamarin and lotaustralin are accumulated, with linamarin being the dominating CG (Butler, 1965; Lieberei et al., 1986; Selmar et al., 1988). Linamarin and lotaustralin are synthesized from the protein amino acids valine and isoleucine, respectively (Conn, 1981). This synthesis is catalysed by two types of cytochrome P450 (CYP79D1/D2 and CYP71E) and a UDP-glycosyl transferase as reported for cassava (Hahlbrock and Conn, 1971; Koch et al., 1992; Andersen et al., 2000; Bak et al., 2002). In a first step valine and isoleucine are converted to their respective oximes (catalysed by CYP79D1/D2). The oximes are substrate of CYP71E, which catalyses the formation of nitrile. In a final step the glucose moiety is added by UDP-glycosyl transferase (Siritunga and Sayre, 2004). Nielsen et al. (2008) have shown that in sorghum these enzymes compose a metabolon linked to the endoplasmic reticulum (ER) membrane. In CG degradation, i.e. in HCN release, two enzymes are involved. First, a β-glucosidase, linamarase (LIN) in H. brasiliensis, splits off the sugar moiety (Hösel, 1981). Subsequently, a hydroxynitrile lyase (HNL) catalyses the decomposition of the cyanohydrin to yield HCN and a carbonyl compound. Although acetone cyanohydrin breaks down spontaneously, HNL accelerates HCN formation considerably (Hösel, 1981; Selmar et al., 1989). To avoid the release of HCN from intact tissue, CGs and their degrading enzymes are stored in different cell compartments. In cassava leaves, for instance, the major fractions of LIN and HNL are located in the apoplast whilst linamarin and lotaustralin are stored in the vacuoles (reviewed by McMahon et al., 1995; White et al., 1998). Similar findings have been reported for rubber tree (Gruhnert et al., 1994). In sorghum leaves, additional distribution to different tissues has been observed. Whereas the CG dhurrin is stored primarily in the vacuoles of the epidermal cell layer, dhurrinase (β-glucosidase) and mandelonitrile lyase are located in the chloroplasts and the cytosol of the parenchyma cells, respectively (Kojima et al., 1979; Thayer and Conn, 1981; Wajant et al., 1994).
The amount of HCN released per unit time, i.e. cyanogenic capacity (HCNc; Lieberei, 1988) depends mainly on the activity of LIN and HNL and on the amount of CGs stored in a particular plant tissue. The latter is defined as the cyanogenic potential (HCNp; Loyd and Gray, 1970). HCNc can differ significantly between individuals of the same plant species (Hughes, 1981; Ballhorn et al., 2005) and represents an important parameter for the classification of cyanogenic plants. Depending on HCNc plants are classified as highly (HC) or low (LC) cyanogenic (Ballhorn et al., 2005). Only HC plants release a requisite amount of cyanide at a rate sufficient to repel herbivores (e.g. Ballhorn et al., 2005). Concomitantly, because these plants produce large quantities of HCN, they are also more susceptible to cyanide-resistant pathogens than LC congeners (Lieberei, 2007; Ballhorn et al., 2010a).
HCNc can be regulated at the gene level. Depending on the number of functional alleles encoding LIN as well as the enzymes participating in CG biosynthesis, white clover may be HC, LC or even acyanogenic if no functional alleles are present (Hughes, 1981). The cyanogenic capacity of H. brasiliensis and lima bean also is genotype-dependent (Lieberei, 1988; Ballhorn et al., 2005). In contrast, the CG content of sorghum plants is largely determined by transcriptional activity regulation of the enzymes involved in CG biosynthesis (Busk and Møller, 2002). Whilst developing seedlings up to 7 d old store large amounts of CGs, their HCNp decreases strongly during further development (Halkier and Møller, 1989; Busk and Møller, 2002). Increased β-glucosidase activity in response to herbivore feeding has been observed in P. lunatus leaves 72 h after the onset of attack (Ballhorn et al., 2006). This time frame indicates that β-glucosidase activity, besides genetic control, may also be regulated at the transcript level. White et al. (1998) demonstrated that the absence of HNL activity in cassava root tissue is associated with very low steady-state amounts of HNL transcripts. In rubber tree, decreasing HNL activity has been observed during leaf development (Selmar, 1986). These results suggest strongly that HNL activity, as with β-glucosidase activity, also may be regulated at the transcript level.
Here we show that in H. brasiliensis leaves LIN and HNL activity increases within seconds in response to tissue damage. In contrast to genetic and transcriptional regulation, this post-translational activation of LIN and HNL facilitates an immediate modulation of HCNc in response to attack.
MATERIAL AND METHODS
Plant material
Rubber tree seedlings [Euphorbiaceae: Hevea brasiliensis (Willd. ex. A.Juss) Müll.Arg.] were cultivated under greenhouse conditions at a daytime temperature of 24–28 °C and a night temperature of 22 °C. Relative humidity was maintained at 60–80 %. Plants were illuminated 12 h per day using high-pressure sodium lamps (IP 55 Philips 400 W, Hamburg, Germany). Irrigation was computer-controlled; the amount of water lost to evaporation was replenished automatically.
As with many tropical plants, H. brasiliensis exhibits rhythmic growth (Hallé and Martin, 1968); all leaves of a new shoot develop simultaneously. Four developmental leaf stages can be distinguished (Lieberei, 1984). Developmental stages A, B and C refer to immature leaves showing no lignification, whereas mature D stage leaves are fully lignified (Voß, 2001). Only leaves of developmental stages B, C and D were used in our experiments; stage A leaves were excluded due to their small size (≤3 mm).
Mechanical tissue damage and sampling
Mechanical damage of leaf tissue was achieved using a dissection pin. The number of punctures applied per defined leaf area (0·32 cm2) simulated a low (75 punctures) or a high (150 punctures) degree of tissue damage. Treated leaf areas were excised using a cork borer (diameter = 0·64 cm) 5 min after mechanical damage. Excisions of surrounding, but intact, leaf tissue served as controls. Leaf discs were transferred immediately to Eppendorf tubes containing 100 µL cooled (4 °C) phosphate buffer solution (67 mm phosphate, pH 6·4) as well as polyvinylpoly-pyrrolidone (Sigma Aldrich, Taufkirchen, Germany), then homogenized on ice using a pestle. The pestle was washed with a further 100 µL of phosphate buffer. The homogenates were centrifuged for 20 min at 16 000g (Heraeus Instruments Biofuge fresco, Hanau, Germany). An aliquot of each supernatant (150 µL, defined as protein raw extract) was transferred to a new Eppendorf tube and subsequently used for determination of β-glucosidase (BGLU), LIN and HNL activity. The aliquots were also tested for residual amounts of linamarin and lotaustralin using the Spectroquant Cyanide Test Kit (Merck, Darmstadt, Germany) as described by Ballhorn et al. (2005). No cyanide could be detected in any of the samples (data not shown).
BGLU activity assay
Determination of BGLU activity was carried out according to the method of Hösel and Nahrstedt (1975) using p-nitrophenyl-β-d-glucopyranoside (pNPG; ABCR, Karlsruhe, Germany) as substrate. A 10 mm stock solution of the artificial substrate was prepared in citric acid buffer (50 mm citric acid, 100 mm phosphate, pH 5·6). Activity assays were carried out in 10-mL test tubes. Protein raw extracts (5, 10, 25, 50 or 100 µL) were transferred to test tubes and adjusted to a volume of 1 mL with citric acid buffer. Substrate stock solution (1 mL) was added. Each reaction mixture was incubated for a defined time interval (max. 10 min) at 30 °C in a water bath. The amount of protein raw extract used as well as the incubation time was adapted to the extent of BGLU activity in the respective samples. When samples revealed high enzyme activity in a first measurement the activity assay was repeated with less protein raw extract and if necessary also shorter incubation. Excessive pNPG decomposition, which would have caused substrate shortage and thus underestimation of BGLU activity, was thereby avoided. Samples exhibiting low BGLU activity were tested again using increased amounts of protein raw extract, to obtain sufficient p-nitrophenol formation for precise photometric analyses. This adaptation of the amounts of protein raw extract used was necessary because the activity in the samples differed up to 50-fold (Table 1). The reaction was stopped by transferring aliquots (500 µL) from the reaction mixtures to new test tubes containing cooled (4 °C) sodium carbonate solution (1 mL, 0·5 m). Subsequently, the samples were analysed spectrophotometrically (GE Healthcare Ultrospec 3000, Uppsala, Sweden) at a wavelength of 400 nm. All pNPG-detectable BGLU activity in rubber tree leaves is due to one enzyme, the Hevea LIN (Selmar et al., 1987a). Therefore, all BGLU activity measured with this artificial substrate represents LIN activity.
Table 1.
Tissue damage-dependent activation of linamarase (LIN) and hydroxynitrile lyase (HNL) in rubber tree (H. brasiliensis) leaves
| Activity [μkat (g f.
wt)−1] |
|||||||
|---|---|---|---|---|---|---|---|
| Sample | Intact tissue | s.d. | Damaged tissue | s.d. | AF | Mean AF | |
| LIN | B I | 0·69 | 0·16 | 2·63 | 0·39 | 4 | 8 |
| B II | 0·23 | 0·03 | 3·11 | 0·70 | 14 | ||
| B III | 0·23 | 0·09 | 1·66 | 0·44 | 7 | ||
| C I | 0·69 | 0·13 | 4·65 | 0·57 | 7 | ||
| C II | 0·29 | 0·04 | 3·85 | 0·86 | 14 | ||
| C IV | 1·15 | 0·04 | 11·01 | 1·57 | 10 | ||
| D I | 0·86 | 0·16 | 6·02 | 1·44 | 7 | ||
| D II | 0·57 | 0·09 | 3·65 | 0·65 | 6 | ||
| D V | 1·33 | 0·08 | 8·82 | 0·21 | 7 | ||
| HNL | B VI | 1·84 | 0·13 | 4·33 | 0·82 | 2·4 | 4 |
| B VII | 9·05 | 0·10 | 12·79 | 0·58 | 1·4 | ||
| B VIII | 2·54 | 0·22 | 9·70 | 0·32 | 3·8 | ||
| C IX | 6·03 | 0·78 | 9·11 | 0·64 | 1·5 | ||
| C VI | 1·18 | 0·56 | 3·35 | 0·22 | 2·8 | ||
| C VIII | 2·37 | 0·17 | 6·52 | 0·29 | 2·8 | ||
| D IX | 1·43 | 0·41 | 7·07 | 0·81 | 4·9 | ||
| D IV | 2·99 | 1·42 | 30·43 | 7·71 | 10·2 | ||
| D VIII | 1·33 | 0·08 | 8·82 | 0·21 | 6·6 | ||
Leaf tissue damage was achieved using a dissection pin. Linamarase (LIN; or BGLU) as well as hydroxynitrile lyase (HNL) activity was determined in the soluble protein fraction of samples from damaged and intact leaf areas. The activity of both enzymes increases up to ten-fold in response to tissue damage. Values given are means ± s.d. with n = 3 per treatment (intact and damaged), leaf stage (B, C, D) and seedling (I–IX). AF: activation factor (quotient of mean enzyme activity in samples taken from damaged tissue and mean enzyme activity in samples taken from intact tissue); mean AF: mean of all activation factors; f. wt: fresh weight.
To determine BGLU or LIN activity with linamarin (Sigma Aldrich) as substrate, measurements were carried out in air-tight Thunberg vessels according to Selmar et al. (1987a) to avoid losses of gaseous HCN. Protein raw extract samples (2·5, 5 or 10 µL, depending on the extent of LIN activity) were transferred to the Thunberg vessels and the volume adjusted to 500 µL with citric acid buffer. Subsequently, 500 µL of a 10 mm linamarin stock solution was added. These reaction mixtures were incubated for a defined time interval (max. 10 min, depending on the extent of LIN activity) at 30 °C in a water bath, whereupon the reaction was stopped by adding 1 mm sodium hydroxide solution (0·2 m) from the side bulb of the vessels. In addition to stopping the reaction, sodium hydroxide causes breakdown of acetone cyanohydrin (Cooke, 1978) and leads to the formation of non-volatile sodium cyanide. An aliquot of each reaction mixture (100 µL) was transferred to a 20-mL test tube containing 4·8 mL distilled water and 100 µL hydrochloric acid solution (0·1 m). Cyanide was converted to polymethine dye using a Spectroquant Cyanide Test Kit (Merck). The polymethine concentration and hence amount of cyanide present were determined spectrophotometrically at a wavelength of 585 nm (Ballhorn et al., 2005).
Hydroxynitrile lyase activity assay
Hydroxynitrile lyase activity was determined according to Selmar et al. (1987b) with acetone cyanohydrin as substrate. The assay is based on the detection of HCN. Acetone cyanohydrin is unstable and decomposes, especially at high pH. For this reason, the substrate stock solution was made directly ahead of use. To prepare this solution, freshly distilled acetone cyanohydrin was diluted 1 : 10 in citric acid solution (0·1 m). Aliquots of protein raw extract (10, 20, 50 or 100 µL) were transferred to a test tube. The amount of protein raw extract used was adapted to the extent of HNL activity in the respective samples. When samples revealed high enzyme activity in a first measurement, the activity assay was repeated with less protein raw extract. Samples exhibiting low HNL activity were tested again using increased amounts of protein raw extract. This adaptation of the amounts of protein raw extract used was necessary because the activity in the samples differed up to 25-fold (Table 1). Differences in substrate availability throughout the assay resulting in underestimation of enzyme activity were thus avoided. The volume of each assay was adjusted to 1·98 mL using citric acid buffer (50 mm citric acid, 100 mm phosphate, pH 5·6). Substrate stock solution (20 µL) was added and the reaction mixtures were incubated for 5 min at 30 °C in a water bath. The reaction was stopped by transfer of 50 µL from each reaction mixture to new 20-mL test tubes containing 5 mL distilled water and the first component of the Spectroquant Cyanide Test Kit. Because of the slight acidity of the solution, non-enzymatic acetone cyanohydrin degradation is slow. Due to dissociation, HCN previously formed in the reaction mixture remains in solution as CN− during the incubation step following transfer to the 20-mL test tubes and during conversion to the polymethine dye. In this manner, only HCN released due to HNL activity is quantified. Moreover, no HCN is lost throughout conversion to the polymethine dye (Selmar et al., 1987b). Samples were analysed spectrophotometrically at a wavelength of 585 nm.
To exclude any losses of HCN during incubation of the reaction mixtures, also the total amount of cyanide (HCN and acetone cyanohydrin) was determined as described previously (BGLU activity assay). In parallel, additional trials with the same protein raw extracts were carried out in air-tight Thunberg vessels. No differences in total cyanide were observed between the trials in test tubes and the trials in Thunberg vessels, confirming that no HCN was lost during incubation of the reaction mixtures in test tubes (data not shown).
Quantification of HCN release
To quantify HCN release, protein raw extract from intact and artificially damaged leaf tissue was incubated with a defined amount of linamarin (5 mm). After transfer of protein raw extract (2·5, 5 or 10 µL) to test tubes, the volume was adjusted to 1 mL using citric acid buffer (HNL activity assay), and 1 mL linamarin stock solution (BGLU activity assay) was added. The reaction mixtures were incubated for 10 min at 30 °C in a water bath. To stop the reaction, 20 µL from each reaction mixture were transferred into 20-mL test tubes containing 5 mL distilled water and the first component of the Spectroquant Cyanide Test Kit. The cyanide concentration was determined as described above (BGLU activity assay). The amount of protein raw extract used was adapted to the extent of LIN and HNL activity in the respective samples. This adaptation of the amounts of protein raw extract used was necessary because HCN release in the assays differed up to four-fold (Table 2). Differences in substrate availability throughout the assay resulting in underestimation of HCN release were thus avoided.
Table 2.
Leaf tissue damage-dependent activation of LIN and HNL causes accelerated HCN release in rubber tree
| HCN release [μmol (g f.
wt)−1
min−1] |
|||||
|---|---|---|---|---|---|
| Leaf stage | Intact tissue | s.d. | Damaged tissue | s.d. | Increase |
| B | 49 | 8 | 109 | 15 | 2-fold |
| C | 53 | 3 | 224 | 25 | 4-fold |
| D | 34 | 9 | 216 | 33 | 6-fold |
Protein raw extracts from intact and damaged leaf tissue were incubated with linamarin solution. Hydrogen cyanide (HCN) released during the incubation was determined using the Spectroquant Cyanide Test Kit as described by Ballhorn (2005). HCN release is accelerated up to six-fold in response to tissue damage. Values are means ± s.d. with n = 3 per treatment (intact and damaged) and leaf stage (B, C, D). Increase: increase of HCN release (quotient of mean HCN release measured with samples from damaged tissue and samples from intact tissue); LIN: linamarase; HNL: hydroxynitrile lyase; f. wt: fresh weight.
To exclude any losses of HCN throughout the incubation, additional measurements were carried out as described above for the HNL activity assay. The results confirmed that no HCN was lost during incubation in test tubes (data not shown).
Statistics
Ten different rubber tree seedlings were sampled throughout the experiments. For the determination of enzyme activity as well as HCN liberation kinetics, three individual samples were analysed per treatment (e.g. intact or damaged tissue), leaf stage (B, C, D) and seedling (I–X). Values presented are means of these three determinations ± s.d. The degree of the enzyme activity increase, termed activation factor (AF), is the quotient of mean enzyme activity in samples taken from damaged tissue and mean enzyme activity in samples taken from intact tissue.
RESULTS
LIN and HNL activities in damaged and intact leaf tissue
BGLU activities ranging from 0·23 µkat g−1 f. wt (B stage, seedling II) to 1·33 µkat g −1 f. wt (D, V) were measured with intact leaf tissue (Table 1). In contrast, samples from highly damaged areas (Materials and Methods) of the same leaflets exhibited BGLU activities of 3·1 µkat g−1 f. wt (B, II) and 8·8 µkat g−1 f. wt (D, V). This corresponds to a 14-fold increase in activity for B II and a seven-fold increase for D V, respectively. Similar results were obtained for all other samplings. On average, BGLU activity was eight times higher in damaged than in intact tissues (Table 1). Corresponding incubations with linamarin found that breakdown of this cyanogenic glucoside is accelerated to the same degree in response to tissue damage (data not shown).
Analogous findings resulted from determination of HNL. Intact tissues of B VI and D VIII had HNL activities of 1·8 and 1·3 µkat g −1 f. wt, respectively (Table 1), compared with values of 4·3 and 8·8 µkat g −1 f. wt, respectively, observed for highly damaged leaf areas, corresponding to a 2·4- (B VI) and 6·6-fold (D VIII) increase. Similar results were obtained for all other samplings. On average, HNL activity was four times higher in damaged than in intact tissue (Table 1). This activity increase will subsequently be termed LIN and HNL activation. Its extent will be given as activation factor (AF).
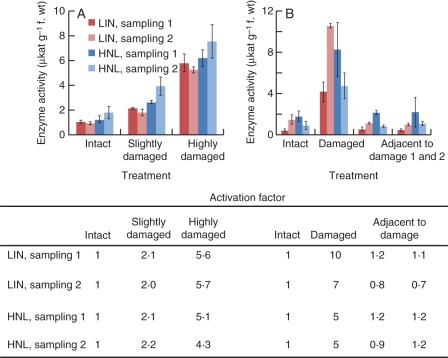
To analyse the impact of the degree or severity of tissue damage on LIN and HNL activation, additional samples with distinct differences in the extent of damage were prepared and examined (Materials and Methods). Whereas a mean AF for LIN of 5–6 and for HNL of 4–5 was noted in highly damaged tissues, the corresponding activation was only two-fold for either enzyme when the leaves had been only slightly damaged (Fig. 1A).
Fig. 1.
(A) Activation of LIN and HNL in differentially damaged rubber tree leaf tissue. (B) Activation of LIN and HNL in the proximity of damaged rubber tree leaf tissue. Leaf tissue damage was achieved using a dissection pin. Linamarase (LIN; or BGLU) as well as hydroxynitrile lyase (HNL) activity was determined in the soluble protein fraction of samples from damaged and intact leaf areas as well as leaf areas adjacent to damaged tissue. With increasing degree of tissue damage also the extent of LIN and HNL activation increased (A). In the proximity of damaged tissue neither LIN nor HNL activation could be observed (B). Values given in the figure are means ± s.d. (error bars) with n = 3 per treatment (intact, slightly damaged, highly damaged, adjacent to damage 1 and 2) and sampling (1 and 2, colour coded). Different rubber tree seedlings were used in each sampling. Samples adjacent to damage were taken on two sides of the treated leaf area (adjacent to damage 1 and 2). Activation factor is the quotient of mean enzyme activity in samples taken from differentially damaged tissue or tissue adjacent to damaged areas and mean enzyme activity in samples taken from intact tissue. Adjacent to damage: samples taken from tissue adjacent to damaged leaf areas.
To determine the spatial extent of the impact of tissue damage on LIN and HNL activation, samples from leaf tissue adjacent to the mechanically damaged areas were tested as described above. Although there was a broad activation of LIN (seven- to ten-fold) and of HNL (five-fold) in the highly damaged areas, no changes in enzyme activity compared with control areas occurred in leaf tissue next to the injuries (Fig. 1B).
Kinetics of LIN and HNL activation
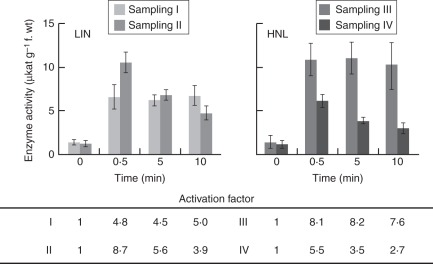
To analyse the velocity of LIN and HNL activation, corresponding samples were taken from leaf tissue 30 s, 5 min and 10 min after tissue damage. In the samples taken after 30 s, LIN activation factors of 4·8 (sampling I) and 8·7 (II) were measured (Fig. 2). In samples taken 5 and 10 min after tissue damage no further LIN activity increase was observed, with AFs of 4·5 (I) and 5·6 (II) in the 5-min samples and 5·0 (I) and 3·9 (II) in the 10-min samples.
Fig. 2.
Kinetics of tissue damage-dependent LIN and HNL activation in rubber tree leaves. Leaf tissue damage was achieved using a dissection pin. Samples from damaged leaf areas were taken 30 s, 5 min and 10 min after tissue damage. Intact tissue served as zero-point sample. Linamarase (LIN; or BGLU) as well as hydroxynitrile lyase (HNL) activity was determined in the soluble protein fraction of the samples. The activation of both enzymes is completed within less than 30 s after tissue damage occurs. Values given in the figure are means ± s.d. with n = 3 per treatment (intact, damaged 30 s, damaged 5 min, damaged 10 min) and sampling or repetition (grey scale code). Different rubber tree seedlings were used in sampling I and II or III and IV. Activation factor is the quotient of mean enzyme activity in samples taken from tissue 0·5, 5 and 10 min after damage and average enzyme activity in samples taken from intact tissue.
Analogous results were obtained for HNL activation. In the samples taken 30 s after tissue damage, AFs of 8·1 (III) and 5·5 (IV) were found (Fig. 2), but, for the 5- and 10-min samples, constant or decreasing activities were observed. AF values of 7·6 (III) and 2·7 (IV) were found for instance regarding the 10-min samplings.
Activation of LIN and HNL is completed within 30 s after tissue damage occurs.
Impact of LIN and HNL activation on HCN liberation kinetics
To verify the impact of LIN and HNL activation on HCNc, protein raw extracts of samples from intact and highly damaged leaf tissues were incubated with linamarin solution and cyanide release was monitored over 10 min. With samples taken from intact stage B leaf tissue, an HCN release rate of 49 µmol (g f. wt)−1 min−1 was observed, but with protein raw extract from damaged tissue, cyanide release rate was 109 µmol (g f. wt)−1 min−1 (Table 2). Thus, HCN formation is twice as fast. Similarly, incubation with protein raw extract from intact stage C leaves caused formation of 53 µmol HCN g−1 (g f. wt)−1 min−1, but samples taken from highly damaged tissue of the same leaf caused release of 224 µmol (g f. wt)−1 min−1, corresponding to a four-fold increase. In the case of intact stage D leaf samples, 34 µmol HCN (g f. wt)−1 min−1 was detected, but protein raw extract from highly damaged tissue accelerated the release six-fold to 216 µmol HCN (g f. wt)−1 min−1.
DISCUSSION
LIN and HNL activation – immediate modulation of the cyanogenic response
Regulation of the amount of CG (HCNp) and BGLU activity at either genetic (e.g. T. repens) or transcriptional (e.g. S. bicolor and P. lunatus) level allows a modification or modulation of HCNc from one to the next generation and within days or hours, respectively (Hughes, 1981; Busk and Møller, 2002). Moreover, both types of regulation, genetic as well as transcriptional, often affect the cyanogenic characteristics (HCNp and HCNc) of the whole plant or plant organs. However, in H. brasiliensis leaves, LIN and HNL activity increases within seconds in response to tissue damage, but the activation response does not spread from the point of injury. LIN and HNL are activated only in leaf areas directly affected by the damage. Moreover, the enhanced activity of either enzyme is related to the severity or type of tissue damage. Extensive damage causes higher activation than minor injuries. In contrast to genetic and transcriptional regulations previously described, activation of LIN and HNL produces immediate, local and damage type-dependent modulation of HCNc.
The rate of activation indicates that the effect is probably induced by post-translational enzyme modification. Protein nitrosylation in animals is known to serve as a rapid mediator of enzyme activation (Murad, 1986). Nitric oxide (NO)-dependent S-nitrosylation has been reported to occur also in plant tissue (Lindermayr et al., 2005). In leaves of tomato (Solanum lycopersicum), NO is synthesized in response to tissue damage (Huang et al., 2004). Whether the observed activation of LIN and HNL in H. brasiliensis leaf tissue is related to NO-induced S-nitrosylation will be a topic for future research.
In addition to synthesis of NO, tissue damage also results in the formation of hydrogen peroxide (H2O2). Besides its function in the signal transduction chain leading to phytoalexin synthesis (reviewed by Buchanan et al., 2002), H2O2 may oxidize cystein residues facilitating enzyme activity modifications similar to the NO-related modulations (Romero-Puertas et al., 2004; Lamotte et al., 2005).
Activation mechanisms – post-translational regulation of BGLU activity in other plants
The BGLU PYK10 from root tissue of Arabidopsis thaliana is stored in so-called ER bodies as an inactive monomer. When PYK10 comes into contact with cytoplasmic PBP1 (PYK Binding Protein 1) upon homogenization, cross-linking of the monomers results in polymerization. The PYK10 (PBP1) polymers are insoluble but are catalytically active (Nagano et al., 2005). A similar mechanism might occur in H. brasiliensis and lead to the activation of LIN as this enzyme tends to form oligomers of variable size (2–26 identical subunits) on native polyacrylamide gels (Selmar et al., 1987a). Nevertheless, incubation of H. brasiliensis leaf homogenates at room temperature did not result in enhanced LIN activity comparable with the activation effect caused by tissue damage (data not shown).
BGLU from leaf tissue of Triticum aestivum forms hexamers and lower order oligomers; only the hexamers are catalytically active (Sue et al., 2006). The same effect might account for the activity of LIN from H. brasiliensis, but to date studying the catalytic activity of specific oligomers has not been conducted mainly because of their unstable state. Once oligomers are separated electrophoretically, each oligomer-type regenerates all oligomer-types (Selmar et al., 1987a). Stabilization of oligomer-types will be necessary for future study of the catalytic activity of these enzymes.
Interestingly, LIN from cassava (also a member of Euphorbiaceae) also forms oligomers (Sermsuvityawong, 1995). The oligomer patterns are highly similar to those reported for rubber tree LIN by Selmar et al. (1987a). Preliminary results on LIN activity in cassava leaf tissue indicate that the enzyme also is activated in response to mechanical tissue damage. Corresponding samples from three individual plants found that the activity increased two- to 30-fold (D. Kadow et al., unpubl. res.). These results point to a relationship between LIN oligomer formation and the LIN activation reported here.
LIN and HNL activation – biochemical and ecological implications
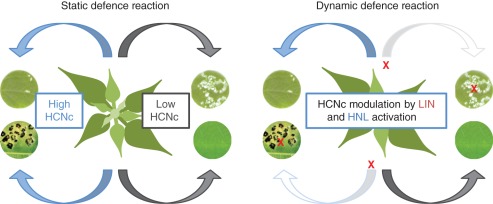
All components needed for cyanogenesis, i.e. CGs and their degrading enzymes, are preformed. While herbivore attack occurs within seconds, modifications of CG level and BGLU activity by mechanisms described so far (i.e. genetic and transcriptional) are not achieved faster than within days or hours. Cyanogenic capacity (HCNc), apart from herbivore impact, thus depends on the preformed components. Accordingly, the defence reaction has to be regarded as static. The immediate up-regulation of HCNc by activation of LIN and HNL makes this static defence reaction a dynamic response and might allow flexible reactions to multiple antagonists such as herbivores and fungal pathogens (Fig 3). In this context, cyanogenesis has been discussed controversially for some time (reviewed by Møller, 2010), because it may act as a resistance- and susceptibility-factor at the same time (Lieberei, 2007; Ballhorn et al., 2010a; Ballhorn, 2011). The interaction of H. brasiliensis with Microcyclus ulei, the fungal agent causing South American Leaf Blight (SALB), demonstrates how problematic the role of cyanogenesis in plant defence can be. Leaves of the rubber tree clone F4542, which is partially resistant to SALB, are weakly cyanogenic, but accumulate up to 3·2 µg scopoletin per gram of leaf dry weight (d. wt) upon inoculation with M. ulei. Scopoletin, a coumarin-derived phytoalexin, inhibits fungal spore germination as well as hyphal growth (Giesemann et al., 1986). By contrast, the strongly cyanogenic clone RRIM600, which is susceptible to SALB, accumulates only 0·5 µg (g d. wt)−1 of scopoletin upon inoculation (Lieberei et al., 1989). However, when HCN – released in response to tissue damage caused by M. ulei – is efficiently removed from the atmosphere surrounding the leaves, RRIM600 leaf tissue accumulates scopoletin to an amount equal to that determined for F4542. Thus, in contrast to plant–herbivore interactions, low cyanogenic capacity is critical for the partial resistance of rubber tree to M. ulei. Similar findings have been reported for lima bean. Genotypes with high HCNc and efficient herbivore repellence are susceptible to fungal attack due to the HCN-mediated inhibition of polyphenol oxidase (PPO), a key enzyme in lima bean defence against fungal pathogens. In contrast, LC genotypes in which PPO is not inhibited show resistance to fungal pathogens, but are susceptible to herbivores (Ballhorn et al., 2010a; Ballhorn, 2011). Cyanogenic plant species may adapt to this by evolving HC as well as LC genotypes as observed for example in white clover and lima bean (Hughes, 1981; Ballhorn, 2011). However, the individual plant can only be herbivore- or pathogen-resistant (static defence reaction). Post-translational regulation of the activity of LIN and HNL may allow plants to combine both properties, high and low HCNc, avoiding ecological costs (dynamic defence reaction). Chewing herbivores that cause major tissue damage may induce strong activation and immediate up-regulation of the HCN release resulting in repellence (Fig. 3). In contrast, no up-regulation may occur in the case of minor damage, e.g. injury caused by initial stages of fungal growth. The small amounts of HCN released possibly would not affect the fungus, but also may not impair alternative plant defence mechanisms, such as phytoalexin biosynthesis (Fig. 3). However, to date no rubber tree clones fully resistant to M. ulei have been described and data on cyanogenic plants resistant to both herbivores and HCN-resistant pathogenic fungi are lacking. Whether LIN and HNL activation provides such a multiple tolerance or resistance will be the focus of future research.
Fig. 3.
Ecological implications of immediate up-regulation of cyanogenic capacity (HCNc). In many cyanogenic plant species, HCNc (Lieberei, 1988) is regulated at genetic and transcriptional level. In these cases, an immediate modulation of HCNc (low HCNc to high HCNc and vice versa) as a result of herbivore or fungal attack is impossible. Therefore, the individual plant can only be herbivore- or pathogenic fungus-resistant (static defence reaction). In contrast, the activation of linamarase (LIN) and hydroxynitrile lyase (HNL) reported here results in immediate up-regulation of HCNc in response to severe, but not to minor, tissue damage (dynamic defence response). This may allow the combination of both properties (low and high HCNc) and might enable resistance to herbivores as well as to fungal pathogens. However, to date no data on cyanogenic plants resistant to both herbivores and HCN-resistant pathogenic fungi have been published. Pictures of leaf discs by: R. Lieberei and V. Noelting, University of Hamburg, Germany; D. J. Ballhorn, Portland State University, USA.
LIN from H. brasiliensis leaf tissue has low substrate specificity. In addition to linamarin this enzyme also decomposes prunasin, dhurrin, coniferin as well as artificial mono-galactosides, mannosides and xylosides (Selmar et al., 1987a). It seems possible that under in vivo conditions substrates other than linamarin may be accepted. For instance, many phytohormones, such as abscisic acid (ABA), are inactivated by glycosylation. In Hordeum vulgare leaves, salt stress causes increased BGLU activity in the apoplast (Dietz et al., 2000) and the subsequently enhanced deglucosylation of ABA glucoside results in reduced stomatal opening. LIN from H. brasiliensis also is located in the apoplasmic space (Selmar, 1986; Gruhnert et al., 1994). If the enzyme is able to deglucosylate ABA glucoside, high levels of activity, indispensable for successful herbivore repellence, would disturb the regulation of stomatal opening, by generating increased amounts of ABA and causing constant stomata closure. Activation of LIN occurring only in response to tissue damage may limit such interferences in intact leaves, but would guarantee at the same time a release of HCN at a rate sufficient to repel herbivores.
In all experiments presented here, tissue damage was achieved mechanically using a dissection pin. Such damage must not necessarily have the same effect as damage caused by herbivores. For example, herbivore attack may result in various types of damage due to differences in feeding styles. Beetles having small mandibles chew the leaf tissue, whilst locust nymphs with relatively large mandibles rather ‘cut’ entire pieces (Ballhorn et al., 2010b). Moreover, insect saliva regularly contains enzymes (e.g. β-glucosidases), inhibitors and elicitors (Mattiaci et al., 1995; Miles, 1999; Zhu-Salzman et al., 2005; Alborn et al., 2007; Harmel et al., 2008; Ballhorn et al., 2010b) potentially affecting the kinetics of HCN release. Increased BGLU activity resulting in accelerated cyanide release has been reported for lima bean 72 h after onset of herbivore attack. In contrast, mechanical damage of the leaves did not result in enhanced enzyme activity (Ballhorn et al., 2006). The authors link this to the presence of elicitors in the insect saliva. However, our results clearly show that in rubber tree leaves LIN and HNL are activated within seconds as a reaction to mechanical tissue damage.
Damage-dependent LIN activation in other cyanogenic plants
As noted above, in lima bean leaves mechanical tissue damage does not result in BGLU activation (Ballhorn et al., 2006). Similar observations have been made in other cyanogenic plants. Preliminary results suggest that neither in flax (Linum usitatissimum) nor in white clover is LIN activation comparable to that described here for rubber tree. In contrast, cassava leaves revealed up to 30-fold increased LIN activity in response to tissue damage (D. Kadow et al., unpubl. res.). Cassava is the agronomically most important of the cyanogenic crops (Siritunga et al., 2004). It is the major source of calories for people living in sub-Saharan Africa (McMahon et al., 1995). Insufficient removal of CGs from the tubers prior to consumption may result in acute or chronic cyanide exposure causing health disorders such as Konzo, a paralytic disorder (acute exposure), or tropical ataxic neuropathy (chronic exposure; Siritunga and Sayre, 2004). Processing of cassava tubers regularly involves enzymatic degradation of linamarin and lotaustralin (Siritunga and Sayre, 2004). If LIN activation were to occur also in the root tissue, this might help to accelerate and thereby optimize the detoxification of cassava tubers. However, Tylleskaer et al. (1992) report that acetone cyanohydrin rather than linamarin is the major source of cyanide in poorly processed cassava.
The cyanogenic status of plants – a complex interaction of multiple factors
Besides genotypic variability and the biotic factors mentioned above, several abiotic factors with influence on plant cyanogenesis have been described. Hughes (1981) reports that leaves of white clover grown at low temperature (19 °C) contain about 50 % more CG than corresponding control plants (grown at 27 °C). Stochmal and Oleszek (1997) observed seasonal changes in HCNp of eight white clover cultivars correlating with mean air temperature. In all cultivars the highest amounts of CG were measured when the air temperature was below 15 °C. With increasing temperature during summer HCNp decreased drastically (Stochmal and Oleszek, 1997). However, when cultivating white clover at a night-time temperature of only 5 °C (15 °C during daytime), Hayden and Parker (2002) found that HCNp decreased in comparison to plants grown at 25/15 °C (day/night). In flax seedlings, CG level increases with increasing temperature (Niedźwiedź-Siegień and Gierasimiuk, 2001). The authors observed highest HCNp in plants grown at 30 °C. Moreover, the light intensity seems to play a key role in the regulation of CG biosynthesis in flax. Shoots of plants exposed to increased light intensities contained up to twice the amount of CG detected in control plants (Trione, 1960; Hughes, 1981; Niedźwiedź-Siegień and Gierasimiuk, 2001). Such modifications of HCNp may be due to altered allocation of nitrogen to CG biosynthesis (e.g. Burns et al., 2002). In contrast, Kongsawadworakul et al. (2009) report that in rubber tree leaves HCNp decreases within hours upon exposure to sunlight. Another important abiotic factor is nitrogen availability. In 5-week-old sorghum plants the application of nitrogen fertilizer caused a pronounced increase in CG content. HCNp of the whole plant was increased by a factor of 7 (Busk and Møller, 2002).
In addition, changes of HCNp have been observed frequently during the course of maturation. While young leaves of lima bean may accumulate huge amounts of CG, HCNp is significantly lower in fully developed leaves (Ballhorn et al., 2005). Similar observations have been made with rubber tree. Immature leaves (stage B and C) have a higher HCNp than stage D (mature) leaves (Kongsawadworakul et al., 2009).
Moreover, CGs not only function as phytoanticipins but also serve the plant as transporters of nitrogen and glucose (reviewed by Møller, 2010). Rubber tree seeds, for instance, store large amounts of linamarin in the endosperm. Upon germination linamarin is converted to the diglucoside linustatin which is then transported to the seedling where HCN is assimilated into asparagine and aspartic acid (Selmar et al., 1988; summarized by Møller, 2010). Similarly, in cassava linamarin synthesized in the leaves is transported to the roots where it is proposed to be the major source of reduced nitrogen for protein biosynthesis (Siritunga and Sayre, 2004). Kongsawadworakul et al. (2009) have suggested that CGs in rubber tree also are a source of nitrogen and glucose in latex production.
The endogenous turnover of CGs may have a key role in the cyanogenic status of the plant as well. In sorghum, a significant turnover of dhurrin even in seedlings has been reported (Adewusi, 1990). Jenrich et al. (2007) suggest a turnover pathway that does not include any cyanohydrin or HCN formation. The authors found that in the course of dhurrin degradation, 4-hydroxyphenylacetonitrile is accumulated and may subsequently be converted to ammonia and 4-hydroxyphenylacetic acid (Jenrich et al., 2007; Møller, 2010).
Taken together, these findings demonstrate that the cyanogenic status of a plant is determined by a complex interaction of multiple factors. The regulatory background of many of the underlying immanent plant processes remains unknown.
Conclusions
Our study shows that the activity of the key enzymes for cyanogenesis – in addition to the genetic and transcriptional control reported previously – can also be regulated at the post-translational level. In rubber tree (H. brasiliensis), LIN and HNL are activated within seconds in response to mechanical tissue damage. In directly affected leaf areas the activity of the two enzymes increases up to ten-fold, depending on the severity or type of tissue damage. This allows an immediate and local modulation of HCNc. Accordingly, LIN and HNL activation may enable more flexible reactions to multiple antagonists such as herbivores and fungal pathogens.
ACKNOWLEDGEMENTS
We thank David Seigler and Daniel J. Ballhorn for critical reading of and valuable comments on earlier versions of this manuscript. We would also like to thank Douglas Steinmacher for fruitful discussions, Marion Kloetzl for maintenance of the rubber tree seedlings and Detlef Boehm as well as Thomas Tumforde for technical assistance. This work was support by the University of Hamburg.
LITERATURE CITED
- Adewusi SRA. Turnover of dhurrin in green sorghum seedlings. Plant Physiology. 1990;94:1219–1224. doi: 10.1104/pp.94.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alborn HT, Hansen TV, Jones TH, et al. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proceedings of the National Academy of Sciences USA. 2007;104:12976–12981. doi: 10.1073/pnas.0705947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen M, Bush P, Svendsen I, Moller B. Cytochromes P450 from cassava catalyzing the first steps in the biosynthesis of the cyanogenic glycosides linamarin and lotaustralin. Journal of Biological Chemistry. 2000;275:1966–1975. doi: 10.1074/jbc.275.3.1966. [DOI] [PubMed] [Google Scholar]
- Bak S, Olsen CE, Halkier BA, Moller BL. Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Physiology. 2002;123:1437–1448. doi: 10.1104/pp.123.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballhorn DJ. Constraints of simultaneous resistance to a fungal pathogen and an insect herbivore in lima bean (Phaseolus lunatus L.) Journal of Chemical Ecology. 2011;37:141–144. doi: 10.1007/s10886-010-9905-0. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Lieberei R, Ganzhorn JU. Plant cyanogenesis of Phaseolus lunatus and its relevance for herbivore–plant interaction: the importance of quantitative data. Journal of Chemical Ecology. 2005;31:1451–1479. doi: 10.1007/s10886-005-5791-2. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Heil M, Lieberei R. Phenotypic plasticity of cyanogenesis in lima bean Phaseolus lunatus – activity and activation of β-glucosidase. Journal of Chemical Ecology. 2006;32:1445–1473. doi: 10.1007/s10886-005-9001-z. [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Pietrowski A, Lieberei R. Direct trade-off between cyanogenesis and resistance to a fungal pathogen in lima bean (Phaseolus lunatus L.) Journal of Ecology. 2010a;98:226–236. [Google Scholar]
- Ballhorn DJ, Kautz S, Lieberei R. Comparing responses of generalist and specialist herbivores to various cyanogenic plant features. Entomologia Experimentalis et Applicata. 2010b;134:245–259. [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists; 2002. [Google Scholar]
- Burns AE, Gleadow RM, Woodrow IE. Light alters the allocation of nitrogen to cyanogenic glycosides in Eucalyptus cladocalyx. Oecologia. 2002;133:288–294. doi: 10.1007/s00442-002-1055-9. [DOI] [PubMed] [Google Scholar]
- Busk PK, Møller BL. Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiology. 2002;129:1222–1231. doi: 10.1104/pp.000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler GW. The distribution of the cyanoglucosides linamarin and lotaustralin in higher plants. Phytochemistry. 1965;4:127–131. [Google Scholar]
- Conn EE. Biosynthesis of cyanogenic glycosides. In: Vennesland B, Conn EE, Knowles CJ, Westby J, Wissing F, editors. Cyanide in biology. London: Academic Press; 1981. pp. 183–196. [Google Scholar]
- Cooke RD. An enzymatic assay for the total cyanide content of cassava (Manihot esculenta Crantz.) Journal of the Science of Food and Agriculture. 1978;29:345. doi: 10.1002/jsfa.2740290408. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Sauter A, Wichert K, Messdaghi D, Hartung W. Extracellular β-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugates in leaves. Journal of Experimental Botany. 2000;51:937–944. [PubMed] [Google Scholar]
- Giesemann A, Biehl B, Lieberei R. Identification of scopoletin as a phytoalexin of the rubber tree Hevea brasiliensis. Journal of Phytopathology. 1986;117:373–376. [Google Scholar]
- Gleadow RM, Woodrow IE. Constraints on effectiveness of cyanogenic glycosides in herbivore defence. Journal of Chemical Ecology. 2002;28:1301–1313. doi: 10.1023/a:1016298100201. [DOI] [PubMed] [Google Scholar]
- Gruhnert C, Biehl B, Selmar D. Compartmentation of cyanogenic glucosides and their degrading enzymes. Plant Physiology. 1994;195:36–42. [Google Scholar]
- Hahlbrock K, Conn EE. Evidence for the formation of linamarin and lotaustralin in flax seedlings by the same glucosyltransferase. Phytochemistry. 1971;10:1019–1023. [Google Scholar]
- Halkier BA, Møller BL. Biosynthesis of the cyanogenic glucoside dhurrin in seedlings of Sorghum bicolor (L.) Moench and partial purification of the enzyme system involved. Plant Physiology. 1989;90:1552–1559. doi: 10.1104/pp.90.4.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallé F, Martin J. Etude de la croissance rhythmique chez ĺHevea (Hevea brasiliensis Muell. Arg. Euphorbiacees – Crotonoides) Adansonia semestrielle. 1968;2:475–503. [Google Scholar]
- Harmel N, Létocart E, Cherqui A, et al. Identification of aphid salivary proteins: a proteomic investigation of Myzus persicae. Insect Molecular Biology. 2008;17:165–174. doi: 10.1111/j.1365-2583.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- Hayden KJ, Parker IM. Plasticity in cyanogenesis of Trifolium repens L.: inducibility, fitness costs and variable expression. Evolutionary Ecology Research. 2002;4:155–168. [Google Scholar]
- Hösel W. The enzymatic hydrolysis of cyanogenic glycosides. In: Vennesland B, Conn EE, Knowles CJ, Westby J, Wissing F, editors. Cyanide in biology. London: Academic Press; 1981. pp. 217–232. [Google Scholar]
- Hösel W, Nahrstedt A. Spezifische Glucosidasen für das Cyanglucosid Triglichinin – Reinigung und Charakterisierung von β-Glucosidasen aus Alocasia macrorrhiza Schott. Hoppe-Seyler's Zeitschrift für physiologische Chemie. 1975;356:1265–1275. [PubMed] [Google Scholar]
- Huang X, Stettmaier K, Michel C, Hutzler P, Mueller MJ, Durner J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta. 2004;218:938–946. doi: 10.1007/s00425-003-1178-1. [DOI] [PubMed] [Google Scholar]
- Hughes MA. The genetic control of plant cyanogenesis. In: Vennesland B, Conn EE, Knowles CJ, Westby J, Wissing F, editors. Cyanide in biology. London: Academic Press; 1981. pp. 495–508. [Google Scholar]
- Jenrich R, Trompetter I, Bak S, Olsen CE, Møller BL, Piotrowski M. Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proceedings of the National Academy of Sciences USA. 2007;104:18848–18853. doi: 10.1073/pnas.0709315104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch B, Nielsen V, Halkier B, Olsen C, Møller B. The biosynthesis of cyanogenic glycosides in seedlings of cassava (Manihot esculenta Crantz) Archives of Biochemistry and Biophysics. 1992;292:141–150. doi: 10.1016/0003-9861(92)90062-2. [DOI] [PubMed] [Google Scholar]
- Kojima M, Poulton JE, Thayer SS, Conn EE. Tissue distribution of dhurrin and enzymes involved in its metabolism in leaves of Sorghum bicolor. Plant Physiology. 1979;67:617–622. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsawadworakul P, Viboonjun U, Romruensukharom P, Chantuma P, Ruderman S, Chrestin H. The leaf, inner bark and latex cyanide potential of Hevea brasiliensis: evidence for involvement of cyanogenic glucosides in rubber yield. Phytochemistry. 2009;70:730–739. doi: 10.1016/j.phytochem.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Barnavon L, Pugin A, Wendehenne D. Nitric oxide in plants: the biosynthesis and cell signaling properties of a fascinating molecule. Planta. 2005;221:1–4. doi: 10.1007/s00425-005-1494-8. [DOI] [PubMed] [Google Scholar]
- Lindermayr C, Saalbach G, Durner J. Proteomic identification of s-nitrosylated proteins in Arabidopsis. Plant Physiology. 2005;137:921–930. doi: 10.1104/pp.104.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberei R. Cyanogenese und Resistenz. Germany: University of Braunschweig; 1984. Habilitation treatise. [Google Scholar]
- Lieberei R. Relationship of cyanogenic capacity (HCN-c) of the rubber tree Hevea brasiliensis to susceptibility to Microcyclus ulei, the agent causing south american leaf blight. Journal of Phytopathology. 1988;122:54–67. [Google Scholar]
- Lieberei R. South American leaf blight of the rubber tree (Hevea spp.): new steps in plant domestication using physiological features and molecular markers. Annals of Botany. 2007;100:1125–1142. doi: 10.1093/aob/mcm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberei R, Selmar D, Biehl B. Metabolization of cyanogenic glucosides in. Hevea brasiliensis. Plant Systematics and Evolution. 1985;150:49–63. [Google Scholar]
- Lieberei R, Nahrstedt A, Selmar D, Gasparotto L. The occurrence of lotaustralin in the genus Hevea and changes of HCN-potential in developing organs of Hevea brasiliensis. Phytochemistry. 1986;25:1573–1578. [Google Scholar]
- Lieberei R, Biehl B, Giesemann A, Junqueira NTV. Cyanogenesis inhibits active defence reactions in plants. Plant Physiology. 1989;90:33–36. doi: 10.1104/pp.90.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberei R, Fock HP, Biehl B. Cyanogenesis inhibits active pathogen defence in plants: inhibition by gaseous HCN of photosynthetic CO2 fixation and respiration in intact leaves. Angewandte Botanik. 1996;70:230–238. [Google Scholar]
- Loyd R, Gray E. Amount and distribution of hydrocyanic acid potential during the life cycle of plants of three Sorghum cultivars. Agronomy Journal. 1970;62:394–397. [Google Scholar]
- Mattiaci L, Dicke M, Posthumus MA. β-glucosidase: an elicitor of herbivore-induced plant odor that attracts hostsearching parasitic wasps. Proceedings of the National Academy of Sciences USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JM, White WLB, Sayre RT. Cyanogenesis in cassava (Manihot esculenta Crantz) Journal of Experimental Botany. 1995;46:731–741. [Google Scholar]
- Miles PW. Aphid saliva. Biological Reviews. 1999;74:41–85. [Google Scholar]
- Møller BL. Functional diversifications of cyanogenic glucosides. Current Opinion in Plant Biology. 2010;13:338–346. doi: 10.1016/j.pbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Møller BL, Seigler DS. Biosynthesis of cyanogenic glycosides, cyanolipids and related compounds. In: Singh BK, editor. Plant amino acids: biochemistry and biotechnology. New York: Marcel Dekker; 1999. pp. 563–609. [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. Journal of Clinical Investigation. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano AJ, Matsushima R, Hara-Nishimura I. Activation of an ER-body-localyzed β-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant and Cell Physiology. 2005;46:1140–1148. doi: 10.1093/pcp/pci126. [DOI] [PubMed] [Google Scholar]
- Nahrstedt A. Cyanogenesis and the role of cyanogenic compounds in insects. Plant Systematics and Evolution. 1985;150:35–47. doi: 10.1002/9780470513712.ch9. [DOI] [PubMed] [Google Scholar]
- Niedźwiedź-Siegień I, Gierasimiuk A. Environmental factors affecting the cyanogenic potential of flax seedlings. Acta Physiologiae Plantarum. 2001;23:383–390. [Google Scholar]
- Nielsen KA, Tattersall DB, Jones PR, Møller BL. Metabolon formation in dhurrin biosynthesis. Phytochemistry. 2008;69:88–98. doi: 10.1016/j.phytochem.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Perazzolli M, Zago ED, Delledonne M. Nitric oxide signaling functions in plant-pathogen interaction. Cellular Microbiology. 2004;6:795–803. doi: 10.1111/j.1462-5822.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Schappert PJ, Shore JS. Cyanogenesis, herbivory and plant defence in Turnera ulmifolia on Jamaica. Ecoscience. 1999;6:511–520. [Google Scholar]
- Seigler DS, Brinker AM. Characterisation of cyanogenic glycosides, cyanolipids, nitroglycosides, organic nitro compounds and nitrile glucosides from plants. In: Waterman PG, Dey PM, Harborne JB, editors. Alkaloids and sulphur compounds 8. Methods in plant biochemistry. London: Academic Press; 1993. pp. 51–131. [Google Scholar]
- Selmar D. Cyanogenese in Hevea: Zwei Wege zur Metabolisierung cyanogener Glycoside. Germany: University of Braunschweig; 1986. [Google Scholar]
- Selmar D, Lieberei R, Biehl B, Voigt J. Hevea linamarase – a nonspecific β-glycosidase. Plant Physiology. 1987a;83:557–563. doi: 10.1104/pp.83.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D, Carvalho FJ, Conn EE. A colorimetric assay for α-hydroxynitrile lyase. Analytical Biochemistry. 1987b;166:208–211. doi: 10.1016/0003-2697(87)90565-3. [DOI] [PubMed] [Google Scholar]
- Selmar D, Lieberei R, Biehl B. Mobilization and utilization of cyanogenic glycosides – the linustatin pathway. Plant Physiology. 1988;86:711–716. doi: 10.1104/pp.86.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D, Lieberei R, Biehl B, Conn EE. α-hydroxynitrile lyase in Hevea brasiliensis and its significance for rapid cyanogenesis. Physiologia Plantarum. 1989;75:97–101. [Google Scholar]
- Sermsuvityawong K, Svasti MRJ, Sawangareetrakul P, Kisamanonta P, Chulavatnatol M. Aggregation of cassava linamarase. Journal of the Science Society of Thailand. 1995;21:283–292. [Google Scholar]
- Siritunga D, Sayre RT. Engineering cyanogen synthesis and turnover in cassava (Manihot esculenta) Plant Molecular Biology. 2004;56:661–669. doi: 10.1007/s11103-004-3415-9. [DOI] [PubMed] [Google Scholar]
- Siritunga D, Arias-Garzon D, White WLB, Sayre RT. Over-expression of hydroxynitrile lyase in transgenic cassava roots accelerates cyanogenesis and food detoxification. Plant Biotechnology Journal. 2004;2:37–43. doi: 10.1046/j.1467-7652.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Solomonson LP. Cyanide as a metabolic inhibitor. In: Vennesland B, Conn EE, Knowles CJ, Westby J, Wissing F, editors. Cyanide in biology. London: Academic Press; 1981. pp. 11–28. [Google Scholar]
- Stochmal A, Oleszek W. Changes of cyanogenic glucosides in white clover (Trifolium repens L.) during the growing season. Journal of Agricultural and Food Chemistry. 1997;45:4333–4336. [Google Scholar]
- Sue M, Yamazaki K, Yajima S, et al. Molecular and structural characterization of hexameric β-d-glucosidases in wheat and rye. Plant Physiology. 2006;141:1237–1247. doi: 10.1104/pp.106.077693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer SS, Conn EE. Subcellular localization of dhurrin β-glucosidase and hydroxynitrile lyase in the mesophyll cells of Sorghum leaf blades. Plant Physiology. 1981;67:617–622. doi: 10.1104/pp.67.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trione EJ. The HCN-content of flax in relation to flax wilt resistance. Phytopathology. 1960;50:482–486. [Google Scholar]
- Tylleskaer T, Banea M, Bikangi N, Cooke RD, Poulter NH, Rosling H. Cassava cyanogens and konzo, an upper motor neuron disease found in Africa. Lancet. 1992;339:208–211. doi: 10.1016/0140-6736(92)90006-o. [DOI] [PubMed] [Google Scholar]
- Voß K. Biologische Bedeutung und Aktivierbarkeit der β-D-Glycosidase in Blättern von Hevea brasiliensis (Willd.) Muell. Arg. (1865) Germany: PhD thesis, University of Hamburg; 2001. [Google Scholar]
- Wajant H, Riedel D, Benz S, Mundry KW. Immunocytological localisation of hydroxynitrile lyase from Sorghum bicolor and Linum usitatissimum. Plant Science. 1994;103:145–154. [Google Scholar]
- Webber BL, Miller RE. Gynocardin from Baileyoxylon lanceolatum and a revision of cyanogenic glycosides in Achariaceae. Biochemical Systematics and Ecology. 2008;36:545–553. [Google Scholar]
- White WLB, Arias-Garzon DI, McMahon JM, Sayre RT. Cyanogenesis in cassava – The role of hydroxynitrile lyase in root cyanide production. Plant Physiology. 1998;116:1219–1225. doi: 10.1104/pp.116.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu-Salzman K, Bi JL, Liu TX. Molecular strategies of plant defence and insect counter-defence. Journal of Insect Science. 2005;12:3–15. [Google Scholar]