Abstract
Background and Aims
The smoke-derived chemical karrikinolide (KAR1) shows potential as a tool to synchronize the germination of seeds for weed management and restoration. To assess its feasibility we need to understand why seeds from different populations of a species exhibit distinct responses to KAR1. Environmental conditions during seed development, known as the parental environment, influence seed dormancy so we predicted that parental environment would also drive the KAR1-responses of seeds. Specifically, we hypothesized that (a) a common environment will unify the KAR1-responses of different populations, (b) a single population grown under different environmental conditions will exhibit different KAR1-responses, and (c) drought stress, as a particular feature of the parental environment, will make seeds less dormant and more responsive to KAR1.
Methods
Seeds of the weed Brassica tournefortii were collected from four locations in Western Australia and were sown in common gardens at two field sites, to test whether their KAR1-responses could be unified by a common environment. To test the effects of drought on KAR1-response, plants were grown in a glasshouse and subjected to water stress. For each trial, the germination responses of the next generation of seeds were assessed.
Key Results
The KAR1-responses of seeds differed among populations, but this variation was reduced when seeds developed in a common environment. The KAR1-responses of each population changed when seeds developed in different environments. Different parental environments affected germination responses of the populations differently, showing that parental environment interacts with genetics to determine KAR1-responses. Seeds from droughted plants were 5 % more responsive to KAR1 and 5 % less dormant than seeds from well-watered plants, but KAR1-responses and dormancy state were not intrinsically linked in all experiments.
Conclusions
The parental environment in which seeds develop is one of the key drivers of the KAR1-responses of seeds.
Keywords: Brassica tournefortii, butenolide, drought stress, germination, karrikinolide, maternal environment, parental environment, phenotypic plasticity, physiological dormancy, seed development, seed dormancy, weed seed bank
INTRODUCTION
The management of many weed species is complicated by seed dormancy, an inbuilt mechanism preventing seeds from germinating in environmental conditions that are suitable for germination (Vleeshouwers et al., 1995; Benech-Arnold et al., 2000; Baskin and Baskin, 2004). Many agronomic weeds have physiologically dormant seeds that remain alive in the soil seed bank and cycle in and out of dormancy, resulting in germination being distributed over time in sporadic flushes (Benech-Arnold et al., 2000). Constantly applying weed control measures, every time new weeds emerge, is inefficient and costly for management in the long-term (Benech-Arnold et al., 2000; Foley, 2001; Stevens et al., 2007). If the germination of weeds could be synchronized, and their soil seed bank depleted, weed control would be more effective, more sustainable and cheaper (Dyer, 1995; Adkins and Peters, 2001; Stevens et al., 2007).
A germination stimulant showing potential to be used as a tool to synchronize the germination of weed seeds is karrikinolide (KAR1, 3-methyl-2H-furo[2,3-c]pyran-2-one; Daws et al., 2007; Stevens et al., 2007). Karrikinolide, a butenolide identified from smoke, stimulates germination in a wide range of smoke-responsive species (Flematti et al., 2004; Merritt et al., 2006, 2007; Commander et al., 2008, 2009; Chiwocha et al., 2009), as well as species for which an ecological role for fire or smoke responses are lacking (Daws et al., 2007, 2008; Chiwocha et al., 2009), including some crop (Light et al., 2009) and weed species (Daws et al., 2007; Stevens et al., 2007; Long et al., 2011a). Since species from a wide range of families have been found to respond to KAR1 (Chiwocha et al., 2009), we might expect that a broad range of weed species would also respond to KAR1. However, not all species do respond to KAR1, and even for those that do, the magnitude of response is not always the same (Stevens et al., 2007; Long et al., 2011b). If KAR1 is to be used effectively, we need to understand why seeds vary in their response, and how this response may be affected by conditions before and after seeds are dispersed.
Post-dispersal factors that influence the KAR1-response of seeds are relatively well studied (Merritt et al., 2006; Commander et al., 2009; Long et al., 2011a) but less is known about what factors determine the inherent KAR1-response of a seed (Long et al., 2011a). After seed dispersal, whether or not a seed germinates in response to KAR1 can depend on germination conditions such as light exposure (Merritt et al., 2006; Nelson et al., 2009; Long et al., 2011a), temperature (Merritt et al., 2006; Commander et al., 2009; Long et al., 2011a), hydration state (Long et al., 2010) and dormancy state (Nelson et al., 2010; Long et al., 2011a). Yet when seeds from populations of the same species, collected from different locations or seasons are tested under the same conditions, we see that they can respond differently to KAR1 and that this seems to be linked to the dormancy state of the seeds (Stevens et al., 2007; Nelson et al., 2010; Long et al., 2011a). Such differences in germination under the same conditions indicate that the populations either differ genetically, or that the dormancy state and KAR1-response of their seeds are influenced by the different environments in which they developed (Stevens et al., 2007; Long et al., 2011a).
The environmental conditions experienced by plants during seed development, known as the parental (or maternal) environment, can influence seed dormancy in many species, and thus seed dormancy is a plastic trait (Roach and Wulff, 1987; Donohue, 2009). When a seed is freshly mature, the primary dormancy it displays developed while the seed was maturing on the parent plant (Finch-Savage and Leubner-Metzger, 2006; Donohue, 2009). During seed development, maternal processes supply the seed with nutrients, hormones, proteins and transcripts, which will influence the seed's metabolism and gene expression (Donohue, 2009). These processes can be regulated by environmental factors such as photoperiod, temperature, water availability, vegetative canopy development and nutrient supply (Fenner, 1991; Gutterman, 2000; Donohue, 2009). The effect of a parental environment factor may depend on the degree of stress that a particular plant perceives. In particular, the effects of parental photoperiod and temperature on dormancy show trends that are consistent among a range of species, with seeds that develop in a warmer environment or with shorter days often being less dormant (Fenner, 1991; Gutterman, 2000; Donohue, 2009). The effect of parental water supply is not as clear (Gutterman, 2000); the majority of studies show that seeds that develop with a lower water supply are less dormant (e.g. Arnold et al., 1992; Meyer and Allen, 1999; Luzuriaga et al., 2006). However, other studies have shown no effect (Swain et al., 2006; Hoyle et al., 2008a, b) or even an increase in dormancy (Sharif-Zadeh and Murdoch, 2000). Understanding more about the effect of parental water supply on seed germination would be especially useful when attempting to synchronize seedling emergence from seed banks in rain-fed agricultural cropping zones of the world.
To determine the robustness of KAR1 as a weed management tool, we need to understand the plasticity of KAR1-responses, i.e. to understand how the germination response of seeds to KAR1 changes with parental environment. Given that we see variation in the KAR1-responses of seeds of different populations of the same dormant species, and that seed dormancy can be affected by parental environment, we reasoned that the parental environment in which seeds develop will drive differences in the KAR1-responses of seeds. We hypothesized that (a) a common environment will unify the KAR1-responses of different populations, (b) a single population grown under different environmental conditions will exhibit different KAR1-responses and (c) drought stress, as a particular feature of the parental environment, will make seeds less dormant and more responsive to KAR1.
To test these hypotheses, we used the KAR1-responsive, self-pollinated weed species Brassica tournefortii as a model. We identified four populations that exhibited distinct responses to KAR1, then grew plants from these populations in common gardens. One population was additionally subjected to controlled drought conditions, and the harvested seeds from all experiments were tested for their germination responses.
MATERIALS AND METHODS
Original seed collections
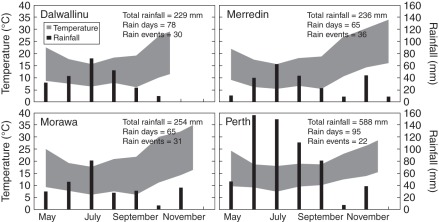
Seeds of Brassica tournefortii were collected at maturity from four sites across the Western Australian wheatbelt in 2009: Dalwallinu (30 °16′49·0''S, 116 °39′28·9″E) in October, Morawa (29 °14′29·9″S, 115 °48′8·4″E) and Perth (31 °58′15·1″S, 115 °49′39·7″E) in November and Merredin (31 °30′1·3″S, 118 °12′55·3″E) in December 2009 (Fig. 1). These seed collections were chosen to include different original germination profiles. After collection and prior to germination testing, filled viable seeds were separated from the chaff and small unviable seeds by passing samples through a gravity seed separator (‘Zig Zag’ Selecta, Machinefabriek BV, Enkhuizen, The Netherlands). Seeds were dried at 15 % relative humidity (RH) and 15 °C for up to 1 month after being collected, then stored in air-tight bags at −20 °C.
Fig. 1.
Climate data for four sites from which Brassica tournefortii seeds were collected in 2009, from the estimated start of germination events (May) until the month of seed collection for Dalwallinu (October), Merredin (December), Morawa (November) and Perth (November). The shaded area indicates the monthly temperature range. The black columns are the total monthly rainfall. ‘Total rainfall’ = cumulative rainfall; ‘Rain days’ = number of days on which rainfall was recorded; ‘Rain events’ = number of days or consecutive days of rainfall, bounded by ≥1 d with zero rainfall. All data from Australian Government Bureau of Meteorology (2010).
Germination testing
The germination response of each seed collection was tested within 3 weeks of harvest with a full-factorial experimental design of eight temperature regimes, in 12-h alternating light (30 µm m−2 s−1) or constant darkness, with or without KAR1 (Flematti et al., 2005). Three replicates of 50 seeds were placed on 10 g L−1 agar, with or without 1 µm KAR1, in Petri dishes. To exclude light, seeds were placed on Petri dishes containing agar media in a dark room and wrapped in aluminium foil. The seeds were incubated at constant temperatures of 10, 15, 20, 25, 30, 35 °C and 12-hourly alternating temperatures of 20/10 °C and 35/20 °C for 3 weeks. Seeds were counted as germinated if the radicle had visibly protruded at least 1 mm.
Common-garden experiments
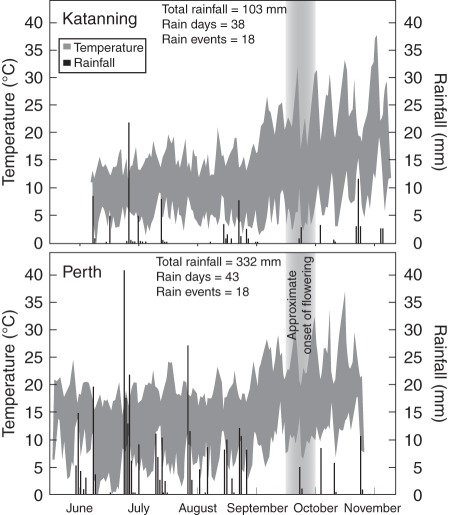
To test the effects of parental environment on KAR1-response and seed dormancy, the four original seed collections were sown in common gardens at two field sites: Perth (31 °56′55″S, 115 °47′34″E) on 3 June 2010 and Katanning (33 °40′59·0″S, 117 °36′43·6″E) on 23 June 2010. Both sites had sandy loam soil types but differed in rainfall and temperature conditions (Fig. 2). At each site, four 2 × 1 m rectangular plots of each seed collection were arranged in a completely randomized design. For each plot, 200 seeds were primed with 1 µm KAR1 solution for 12 h at 20 °C to overcome dormancy, then re-dried, mixed with 50 g of clean white sand and sprinkled in four even rows, 0·5–1 cm below the soil surface of the plot. The soil at the Perth site was very low in nutrients, so Thrive Soluble All Purpose (Yates) fertilizer was applied at a rate of 8 g/4·5 L per plot three times before harvest. The plots were thinned to a minimum of 25 evenly spaced plants per plot 12 weeks after sowing. Plots were harvested 22 weeks after sowing. Plants in each plot were counted, cut at the base and placed into a bag to collect the seeds, pooling the seeds of the 25 plants in each plot. To verify that plants from the different seed collections had grown equally, the heights of three plants per plot were measured, one at each end of the plot and a plant from the centre. The plant height, along with the number of plants, seed mass and seed number are shown in Table 1. The bags were gently crushed to remove the seeds from their siliquae and filled seeds were separated from other plant material as above.
Fig. 2.
Climate data for common-garden sites, from sowing of seeds to seed collection for Katanning (23 June to 26 November) and Perth (3 June to 12 November) 2010. The shaded area indicates the daily temperature range. The black columns are the total daily rainfall. ‘Total rainfall’ = cumulative rainfall; ‘Rain days’ = number of days on which rainfall was recorded; ‘Rain events’ = number of days or consecutive days of rainfall, bounded by ≥1 d with zero rainfall. All data from Australian Government Bureau of Meteorology (2011).
Table 1.
Mean number of plants per plot, plant height, seed mass and seed number (± s.e.) of Brassica tournefortii plants at harvest when grown from four different seed collections, in four plots each, in two common gardens
| Site | Seed collection | No. of plants** | Plant height (cm) | Mass of 1000 seeds (g)*** | No. of seeds per plant |
|---|---|---|---|---|---|
| Katanning | Dalwallinu | 30 ± 3 | 45 ± 3 | 1·26 ± 0·012 | 500 ± 110 |
| Merredin | 38 ± 3 | 46 ± 2 | 1·25 ± 0·016 | 300 ± 30 | |
| Morawa | 33 ± 7 | 44 ± 3 | 1·21 ± 0·014 | 400 ± 60 | |
| Perth | 33 ± 4 | 43 ± 3 | 1·27 ± 0·018 | 200 ± 90 | |
| Mean | 33 ± 2 | 45 ± 1 | 1·25 ± 0·012 | 400 ± 50 | |
| Perth | Dalwallinu | 26 ± 2 | 41 ± 3 | 1·36 ± 0·024 | 600 ± 120 |
| Merredin | 25 ± 2 | 42 ± 2 | 1·46 ± 0·025 | 300 ± 20 | |
| Morawa | 26 ± 0 | 44 ± 2 | 1·35 ± 0·014 | 400 ± 90 | |
| Perth | 27 ± 2 | 40 ± 2 | 1·41 ± 0·016 | 300 ± 60 | |
| Mean | 26 ± 1 | 42 ± 1 | 1·40 ± 0·019 | 400 ± 40 |
Significant differences between site means at P ≤ 0·01 and 0·001 are indicated by ** and ***, respectively.
Seeds from the common-garden experiments were stored at 15 % RH and 15 °C for 2 weeks after harvest then the germination response of each population was tested with a full-factorial experimental design (KAR1 × temperature × light × population), as for the previous experiment. Fifty seeds from each plot (four plots per original seed collection) were plated for each treatment, and germination was scored after 2 weeks, as we found that maximum germination of B. tournefortii seeds occurred within 1 week under these conditions (data not shown). The seed water content of each harvested sample was determined on a dry weight basis (Katanning collections averaged 6·7 ± 0·1 %; Perth collections averaged 7·4 ± 0·1 %), and assumed to have no impact on KAR1-response (Long et al., 2010).
Glasshouse experiment testing the effect of parental drought
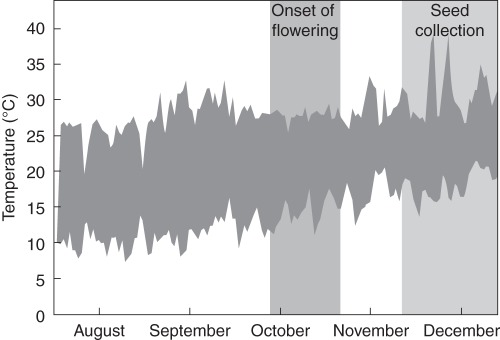
To test the effect of parental drought on KAR1-response and seed dormancy, B. tournefortii plants from the Merredin population were grown in a glasshouse at Kings Park and Botanic Garden in Perth (Fig. 3), and were exposed to either drought or well-watered conditions. Plants were grown from seeds that were sown on 4 August 2010 and arranged in a randomized complete block design with three blocks, with a minimum of ten replicates per treatment per block. To overcome dormancy prior to sowing, seeds were placed in 1 g L−1 agar containing 1 µm KAR1 and incubated overnight in constant darkness at 20 °C. The seed/agar mixture was pipetted into 13·5 × 13·5 × 15 cm square free-draining 2-L pots filled with Richgro red potting mix (Perth, Western Australia), approx. 1 cm below the surface at a density of about ten seeds per pot.
Fig. 3.
Daily temperature range in Perth during the drought experiment, from sowing of seeds to seed collection, 3 August to 31 December 2010. Data from Australian Government Bureau of Meteorology (2011).
Seedlings were evenly thinned to only one plant per pot at the two-leaf stage. At the onset of flowering, defined as when the first yellow petal was visible on each plant, each pot was randomly allocated to either the drought or watered treatment. Pots were individually watered to weight, with watered pots being kept at a soil water potential greater than −0·01 MPa, and droughted pots being kept at a soil water potential between −0·1 and −0·5 MPa.
To determine the final pot weight for the two treatments, a soil water potential curve for the potting mix was calculated using the pressure plate method (Richard and Fireman, 1943) and the core bulk density: θv = 0·20422 + 0·0025234/Ψsoil, where θv is soil volumetric water content and Ψsoil is soil water potential (MPa). After determining the amount of dry soil in individual pots, the volumes of water equating to −0·01 MPa (watered treatment), −0·1 MPa and −0·5 MPa (upper and lower limits for drought stress treatment) were calculated.
Seeds were harvested from each plant separately when the whole plant turned straw-coloured, 17–21 weeks after sowing. The number of plants, time of seed maturation, seed mass and seed number for droughted and well-watered plants are shown in Table 2. The seeds were cleaned as before.
Table 2.
Mean number of plants per block, time from the onset of flowering to seed collection, seed mass and seed number (± s.e.) of Brassica tournefortii plants which were either treated with drought (−0·1< Ψsoil > −0·5 MPa) or were well-watered (ψsoil > −0·01 MPa)
| Treatment | No. of plants | Weeks from flowering to collection | Mass of 1000 seeds (g)** | No. of seeds per plant*** |
|---|---|---|---|---|
| Drought | 17 ± 1 | 8 ± 0·2 | 1·21 ± 0·019 | 1200 ± 110 |
| Well-watered | 13 ± 3 | 8 ± 0·2 | 1·13 ± 0·023 | 2200 ± 200 |
Significant differences at P ≤ 0·01 and 0·001 are indicated by ** and ***, respectively.
The germination response of seeds from droughted and watered plants was tested with a full-factorial experimental design (KAR1 × temperature × light × drought treatment), as in the previous experiments, after the seeds had been stored at 15 % RH and 15 °C for 3·5 weeks after the last plant was collected. Three technical replicates of 50 seeds per treatment per glasshouse block were plated and the number of germinated seeds was counted after 2 weeks. The water content of seeds prior to germination testing was determined to be 8·2 ± 0·4 % for seeds from droughted plants and 8·4 ± 0·5 % for seeds from well-watered plants, therefore imparting no influence on KAR1-response (Long et al., 2010).
Statistical analysis
To determine if the germination profiles of the populations differed from one another when grown at the original collection sites and in the common-garden experiments, and to compare the germination profiles of seeds from the glasshouse drought experiment, binomial data were analysed using a generalized linear model with a logit link-function and stepwise-addition of factors to simplify the model (GenStat 10th edition; McCullagh and Nelder, 1989). The factors important to the models were determined according to their P-value using a χ2 test. Factors relating to replication (‘glasshouse block’ and ‘technical replicate’) were tested for their significance as individual factors, but their interactions with other factors were not modelled. Two-sample binomial tests were used to compare individual treatments; data for replicates and blocks were pooled when these factors were not significant. Germination profiles of the different populations were considered distinct if pooling their data did not afford a better model by decreasing the residual deviance.
RESULTS
Responses of original collections
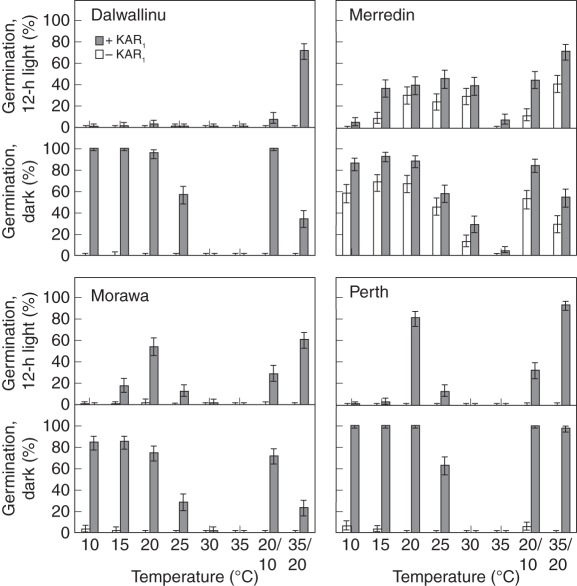
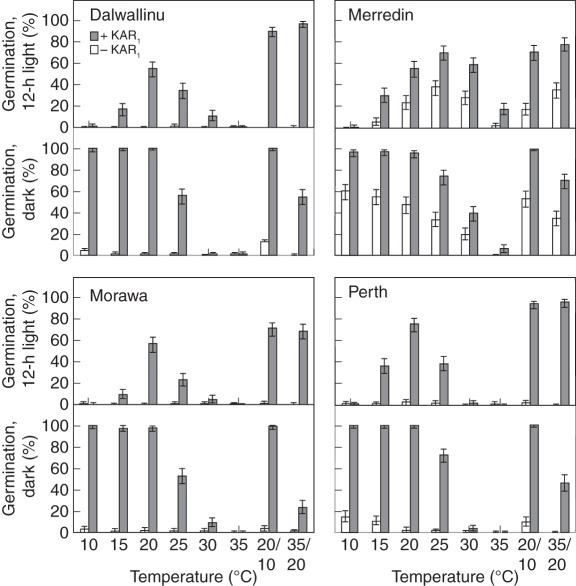
Seeds collected from four different sites were tested with and without KAR1 to see how different populations of B. tournefortii varied in their germination responses. All the populations had different profiles of KAR1-response (P < 0·001; Fig. 4). In general, germination was best in darkness at temperatures of ≤20 °C. The Merredin population germinated under the widest range of temperatures in both alternating-light and darkness, and was the only population to germinate at the highest constant temperatures of 30 °C and 35 °C. Under alternating-light conditions, the Dalwallinu population only germinated at 35/20 °C, while the Morawa and Perth populations also germinated to levels of ≥50 % at 20 °C. In response to alternating temperatures, all populations germinated best at the higher regime of 35/20 °C under alternating-light conditions, yet in darkness more seeds germinated at 20/10 °C, indicating that light and temperature interacted to influence KAR1-responses (P < 0·001). On water media (without KAR1), all the populations also had different germination profiles (P < 0·001), but only one of the four populations, Merredin, germinated to >1 % in total (maximum germination of 30 %), indicating that the Merredin population was less dormant than the others (Fig. 4).
Fig. 4.
Germination response of four different collections of Brassica tournefortii seeds following collection from original source sites in 2009. Seeds were tested in 12-h alternating light or constant darkness, with or without 1 µm KAR1, under eight temperature regimes. Germination was scored 3 weeks after sowing. Bars indicate mean ± 95 % confidence interval for binomial estimates; n = 150 seeds per treatment.
Responses of different populations grown in a common environment
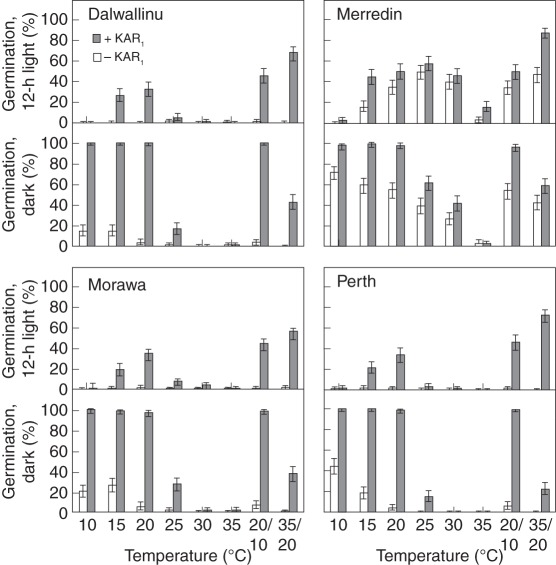
Seeds of the same four populations that were used in the initial germination testing were grown in two separate common gardens and the resultant seeds were tested to see if a common environment would unify their KAR1-response. All the populations still had different KAR1-response profiles when harvested from both of the trials (P < 0·001; Figs 5 and 6). However, the deviance in the model explained by ‘population’ and its interactions decreased from 18 % for the original collection sites to 10 % and 11 % for Katanning and Perth, respectively, indicating that the KAR1-responses of the populations became more uniform when grown in common gardens. For both common-garden experiments, seeds of all four populations also exhibited different germination profiles without KAR1 (P < 0·001). The deviance in the model explained by population and its interactions decreased from 70 % for the original collection sites to 62 % and 56 % for Katanning and Perth, respectively, indicating that the germination responses without KAR1 also became more uniform when grown in common gardens.
Fig. 5.
Germination response of Brassica tournefortii seeds collected from plants grown in a common garden at Katanning, sown from four different original collection sources. Seeds were tested in 12-h alternating light or constant darkness, with or without 1 µm KAR1, under eight temperature regimes. Germination was scored 2 weeks after sowing. Bars indicate mean ± 95 % confidence interval for binomial estimates; n = 200 seeds per treatment.
Fig. 6.
Germination response of Brassica tournefortii seeds collected from plants grown in a common garden at Perth, sown from four different original collection sources. Seeds were tested in 12-h alternating light or constant darkness, with or without 1 µm KAR1, under eight temperature regimes. Germination was scored 2 weeks after sowing. Bars indicate mean ± 95 % confidence interval for binomial estimates; n = 200 seeds per treatment.
Responses of individual populations when grown in different environments
Germination responses of seeds from individual populations that developed at different sites were compared to see whether a single population grown in different environments would exhibit different KAR1-responses. Each population exhibited different germination profiles, both with and without KAR1, depending on the site at which the seeds developed (P < 0·001). The Merredin population was the least plastic in its KAR1-response, with the variation between the germination profiles for seeds collected from different environments being half of that of the Dalwallinu and Perth populations (‘site’ explained 5 % of the deviance in the model for Merredin, 7 % for Morawa and 10 % for the Dalwallinu and Perth populations). The Merredin population was also least plastic in its germination responses without KAR1, with site explaining 6 %, 13 %, 23 % and 30 % of deviance in the models for the Merredin, Perth, Dalwallinu and Morawa populations, respectively.
Overall, all populations germinated to higher percentages with KAR1 when collected from the Katanning trial compared with those collected from the original locations; for Dalwallinu an extra 16 % of seeds germinated, for Merredin and Morawa an extra 11 %, and for Perth an extra 6 % (Dalwallinu 1641/3201 versus 858/2400; Merredin 1923/3198 versus 1180/2400; Morawa 1421/3190 versus 816/2400; Perth 1734/3213 versus 1125/2350; P < 0·001). Without KAR1 the Dalwallinu, Morawa and Perth populations germinated to higher overall percentages – to 2 %, 1 % and 3 %, respectively (Dalwallinu 51/3200 versus 2/2400; P < 0·001; Morawa 33/3200 versus 14/2400, P = 0·035; Perth 89/3200 versus 21/2400, P < 0·001), while the Merredin population germinated to 28 %, the same percentage as originally (911/3199 versus 718/2400, P = 0·241). All populations also germinated to higher percentages with KAR1 overall when collected from the Katanning trial than from the Perth trial – Dalwallinu another 11 %, Merredin another 3 %, Morawa another 5 % and Perth another 16 % (Dalwallinu 1641/3201 versus 1281/3200; Merredin 1923/3198 versus 1817/3200; Morawa 1421/3190 versus 1260/3200; Perth 1734/3213 versus 1227/3200; P < 0·001). Interestingly, the trend was reversed without KAR1, with more seeds from the Perth trial germinating without KAR1 than from the Katanning trial; Dalwallinu another 1 %, Merredin another 7 %, Morawa another 3 % and Perth another 2 % (Dalwallinu 84/3200 versus 51/3200 and Merredin 1147/3200 versus 911/3199, P < 0·001; Morawa 132/3200 versus 33/3200, P = 0·002; Perth 151/3200 versus 89/3200, P < 0·001, respectively). The environmental conditions differed at the Perth and Katanning common gardens, with the Katanning site being drier and warmer throughout the growing period (Fig. 2).
Glasshouse experiment testing the effect of parental drought
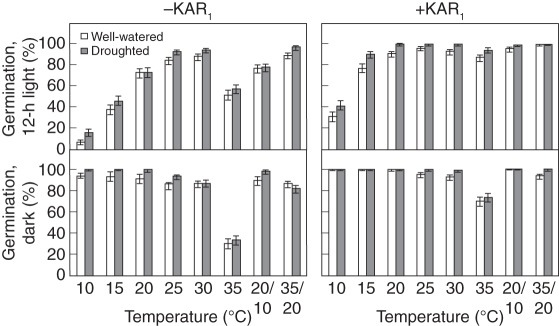
To test whether drought would enhance the KAR1-response of B. tournefortii seeds, we tested the germination response of seeds harvested from plants of the Merredin population were grown under either drought (−0.1 < Ψsoil > −0·5 MPa) or well-watered conditions (Ψsoil > −0·01 MPa) in a glasshouse. Overall, seeds collected from droughted plants germinated 5 % more in response to KAR1 than seeds collected from well-watered plants (6695/7173 versus 6381/7198, P < 0·001; Fig. 7). Seeds collected from droughted plants were also 5 % less dormant than seeds collected from well-watered plants, according to the germination of seeds without KAR1 (5581/7199 versus 5201/7175, P < 0·001; Fig. 7). Compared with the germination responses of seeds from the original collection and common-garden trials, seeds of the Merredin population collected from the drought experiment germinated to high percentages even in the light and without KAR1. Germination was >70 % in all of the 32 combinations of conditions in which the seeds were tested, except at 10 °C and 15 °C in alternating-light without KAR1, at 10 °C in alternating-light with KAR1, and at 35 °C without KAR1 in both alternating-light and darkness.
Fig. 7.
Germination response of Brassica tournefortii seeds collected from plants grown under droughted (−0·1 < Ψsoil > −0·5 MPa) or well-watered (Ψsoil > −0·01 MPa) conditions, germinated in 12-h alternating light or constant darkness, with or without 1 µm KAR1, under eight temperature regimes. Germination was scored 2 weeks after sowing. Bars indicate mean ± 95 % confidence interval for binomial estimates; n = 450 seeds per treatment.
DISCUSSION
The parental environment in which B. tournefortii seeds developed had a strong influence on the subsequent KAR1-responses of the seeds in this study. Nevertheless, parental environment was not the only driver of differences in KAR1-responses and, as such, our first hypothesis, that a common environment will unify the KAR1-responses of different populations, was only partially supported. Different populations retained distinct profiles of KAR1-response even when grown under common environments. However, the KAR1-responses of seeds became more uniform for three of the four populations – Dalwallinu, Morawa and Perth – when the seeds developed in common environments at two geographically distinct sites (Figs 5 and 6 versus Fig. 4). However, the Merredin population, which initially germinated with KAR1 across a wider range of temperature and light conditions than the other three populations (Fig. 4), maintained a distinctive response even when grown together with the other three populations (Figs 5 and 6). Our second hypothesis that a single population grown under different environmental conditions will exhibit different KAR1-responses was supported as, within a population, the germination profiles of seeds from different parental environments were always different. Our third hypothesis that drought would make seeds more responsive to KAR1 was supported, as 5 % more seeds collected from drought-stressed plants germinated compared with seeds from well-watered plants (Fig. 7). Taken together, our results indicate that parental environment is a key driver of seed responses to KAR1, but also suggest that genetics strongly influences KAR1-responses.
Parental environment and genetics appear to interact to determine KAR1-responses, just as they do seed dormancy (Roach and Wulff, 1987; Donohue, 2009). If the KAR1-response of B. tournefortii was only affected by parental environment, we would expect the germination profiles of different populations to be identical to one another when seeds developed in common gardens. Equally, if KAR1-responses depended solely on genetics, then we would expect the germination responses of each population to remain the same even when seeds developed in different environments. Yet in this study the various parental environments affected the subsequent germination capacity of seeds differently, depending on the population. Characteristics of the environments in our study that could have influenced the dormancy state and inherent capacity of seed populations to respond to KAR1 include temperature, water supply, nutrient availability and photoperiod (Fenner, 1991; Gutterman, 2000; Donohue, 2009). Plants can differ in their sensitivity to environmental stressors (Meyer and Allen, 1999; Andrade et al., 2009), and temperature and photoperiod, in particular, are well-known to impart epigenetic effects on plant development (Boyko and Kovalchuk, 2011). Genetic and physiological aspects that underpin seed germination responses and which may have been influenced by the various parental environments in our study include (a) genetic differences in dormancy-related genes (Gao et al., 2003; Bentsink et al., 2006; Kaga et al., 2008), (b) different regulation of these dormancy-related genes (Gao et al., 2003; Barrero et al., 2010) and (c) differing concentrations of, or sensitivities to, hormones that are involved in dormancy and germination, such as abscisic acid and gibberellins (Finkelstein et al., 2008; Long et al., 2010). In addition to these dormancy-related factors, the differing KAR1-responses could also be accounted for by (a) genetic differences in KAR1 responsive genes, (b) different regulation of these KAR1-responsive genes or (c) differing sensitivities to KAR1 (Long et al., 2010). Preliminary investigations have identified several putative KAR1-related genes in Arabidopsis thaliana (Nelson et al., 2011; Waters et al., 2012) but we do not know if these genes operate similarly to determine KAR1-responses in wild species. Just which of the many genetic, physiological and environmental factors contribute to the observed variation in KAR1-response and seed dormancy state is yet to be determined. In any case, the parental environment is not the only factor influencing the germination response of B. tournefortii seeds to KAR1, and it can interact with other genetic and hormonal factors.
One specific feature of the parental environment that could be expected to affect seed dormancy or KAR1-response, especially in an agricultural context, is water supply. We found that drought stress imposed during the reproductive growth phase made seeds 5 % more responsive to KAR1 and 5 % less dormant in our glasshouse trial (Fig. 7). Our results are supported by others that have shown parental drought to decrease dormancy in the weed species Sorghum halepense (Arnold et al., 1992) and Lolium rigidum (Steadman et al., 2004). Contrasting with these findings, parental water supply during the reproductive growth phase had no effect on the seed dormancy of the native Australian daisy Actinobole uliginosum (Hoyle et al., 2008a) or perennial Goodenia fascicularis (Hoyle et al., 2008b). Such discrepancies among the reports of the impact of drought on seed dormancy may be due to the intensity of the imposed drought stress (Steadman et al., 2004) or its timing, as the impacts of environmental stresses such as drought on seed characteristics can vary depending on whether they occur during vegetative growth, pre- or post-zygotic reproductive growth, or throughout a plant's life span (Kochanek et al., 2011). Indeed, given the high variability in germination percentages that can be seen for different populations of B. tournefortii (Fig. 4), the 5 % difference in germination that we observed in our study, although statistically significant, indicates that parental water supply during the reproductive phase of plant development did not have a large effect on KAR1-response or seed dormancy. In our study, the fact that Merredin seeds collected from plants grown in the glasshouse germinated more than those from all other parental environments in this study (Fig. 7 versus Figs 4, 5 and 6), suggests that other environmental characteristics, most likely temperature and photoperiod (Donohue, 2009), have a greater effect on KAR1-response and seed dormancy than parental water supply. Thus, although parental drought had a slight effect on B. tournefortii germination, factors other than water supply during the reproductive phase are likely to have a greater effect on dormancy, and KAR1-response.
A subtle yet interesting finding from our experiments is that the interaction of KAR1 with seed dormancy state may not be as clear as implied in earlier studies (Long et al., 2011a). If the KAR1-response was dependent solely on dormancy state, we would expect to be able to draw the same conclusions from germination profiles with and without KAR1. However, we found that more seeds germinated with KAR1 when harvested from the Katanning common-garden trial than from the Perth trial, whilst the opposite trend was true for germination without KAR1, with seeds from the Perth trial being more germinable (Figs 5 and 6). Given these results, the germination response to KAR1 may not necessarily be directly linked to seed dormancy.
Our study highlights the importance of considering parental environment factors when managing seed banks, and controlling the parental environment of seeds used in KAR1 experiments. Other parental environment factors known to affect seed dormancy, such as temperature and photoperiod, should be tested for their effects on the KAR1-response of seeds. To see if parental water supply can impart a greater effect on KAR1-response or seed dormancy, or whether it generally only affects KAR1-response by a small percentage it would be useful to test the effects of drought on a more dormant population than the one we used, with different watering regimes and drought levels applied at different developmental stages. Resolving the relationship between KAR1-response and seed dormancy state is important for understanding the underlying mechanisms of both traits, and this can be achieved by investigating genetic and physiological factors contributing to variation in KAR1-responses; sensitivities to stresses, hormones and KAR1, the concentrations of hormones, genetic variation among populations, as well as variation in gene regulation all need to be explored. When studying germination responses to KAR1, as well as seed dormancy, the environment in which the seeds developed should be taken into account. This may mean taking care with how the parent plants are grown, especially during the reproductive phase, or noting the characteristics of field environments where seeds are collected. Our study also shows the importance of testing multiple populations of the same species and the risks of drawing conclusions about the KAR1-response of a species from studies using single populations. A parental environment effect on KAR1-response means that environmental characteristics of previous seasons need to be measured and, where possible, future characteristics modelled when planning strategies for managing seed banks. In short, variation in the parental environment will need to be considered and better understood if KAR1 is to be used as a tool to stimulate the germination of seed banks.
ACKNOWLEDGEMENTS
We thank Michael Renton (UWA School of Plant Biology) for his statistical advice, Gary Cass and Elizabeth Halladin (UWA School of Earth and Environment) for assisting with deriving the soil moisture curve, Dave Symons (Kings Park and Botanic Garden) for help with field work, the Kings Park Writing Group, Steven Smith (UWA ARC Centre of Excellence in Plant Energy Biology) and anonymous reviewers for their helpful comments on earlier versions of this manuscript. Alan Harrod and Vince Lambert (Department of Agriculture and Food Western Australia) and Michael Blair (UWA Faculty of Natural and Agricultural Sciences) provided access to field sites and staff and students at Kings Park and Botanic Garden and UWA helped with glasshouse and field work. This work was supported by an Australian Research Council Linkage Grant (LP 0776951) and a Rural Industries Research and Development Corporation National Weeds and Productivity Research Program Grant (PRJ 006918).
LITERATURE CITED
- Adkins SW, Peters NCB. Smoke derived from burnt vegetation stimulates germination of arable weeds. Seed Science Research. 2001;11:213–222. [Google Scholar]
- Andrade A, Vigliocco A, Alemano S, Alvarez D, Abdala G. Differential accumulation of abscisic acid and its catabolites in drought-sensitive and drought-tolerant sunflower seeds. Seed Science Research. 2009;19:201–211. [Google Scholar]
- Arnold RLB, Fenner M, Edwards PJ. Changes in dormancy level in Sorghum halepense seeds induced by water stress during seed development. Functional Ecology. 1992;6:596–605. [Google Scholar]
- Australian Government Bureau of Meteorology. Climate Data Online. 2010 http://www.bom.gov.au/climate/data/ . 19 May 2010. [Google Scholar]
- Australian Government Bureau of Meteorology. Climate Data Online. 2011 http://www.bom.gov.au/climate/data/ . 30 June 2011. [Google Scholar]
- Barrero JM, Millar AA, Griffiths J, et al. Gene expression profiling identifies two regulatory genes controlling dormancy and ABA sensitivity in Arabidopsis seeds. The Plant Journal. 2010;61:611–622. doi: 10.1111/j.1365-313X.2009.04088.x. [DOI] [PubMed] [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Benech-Arnold RL, Sanchez RA, Forcella F, Kruk BC, Ghersa CM. Environmental control of dormancy in weed seed banks in soil. Field Crops Research. 2000;67:105–122. [Google Scholar]
- Bentsink L, Jowett J, Hanhart CJ, Koornneef M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 2006;103:17042–17047. doi: 10.1073/pnas.0607877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Kovalchuk I. Genome instability and epigenetic modification – heritable responses to environmental stress? Current Opinion in Plant Biology. 2011;14:260–266. doi: 10.1016/j.pbi.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Chiwocha SDS, Dixon KW, Flematti GR, et al. Karrikins: a new family of plant growth regulators in smoke. Plant Science. 2009;177:252–256. [Google Scholar]
- Commander LE, Merritt DJ, Rokich DP, Flematti GR, Dixon KW. Seed germination of Solanum spp. (Solanaceae) for use in rehabilitation and commercial industries. Australian Journal of Botany. 2008;56:333–341. [Google Scholar]
- Commander LE, Merritt DJ, Rokich DP, Dixon KW. Seed biology of Australian arid zone species: germination of 18 species used for rehabilitation. Journal of Arid Environments. 2009;73:617–625. [Google Scholar]
- Daws MI, Davies J, Pritchard HW, Brown NAC, Van Staden J. Butenolide from plant-derived smoke enhances germination and seedling growth of arable weed species. Plant Growth Regulation. 2007;51:73–82. [Google Scholar]
- Daws MI, Pritchard HW, Van Staden J. Butenolide from plant-derived smoke functions as a strigolactone analogue: evidence from parasitic weed seed germination. South African Journal of Botany. 2008;74:116–120. [Google Scholar]
- Donohue K. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society B – Biological Sciences. 2009;364:1059–1074. doi: 10.1098/rstb.2008.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer WE. Exploiting weed seed dormancy and germination requirements through agronomic practices. Weed Science. 1995;43:498–503. [Google Scholar]
- Fenner M. The effects of the parent environment on seed germinability. Seed Science Research. 1991;1:75–84. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annual Review of Plant Biology. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. A compound from smoke that promotes seed germination. Science. 2004;305:977. doi: 10.1126/science.1099944. [DOI] [PubMed] [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. Synthesis of the seed germination stimulant 3-methyl-2H-furo[2,3-c]pyran-2-one. Tetrahedron Letters. 2005;46:5719–5721. [Google Scholar]
- Foley ME. Seed dormancy: an update on terminology, physiological genetics, and quantitative trait loci regulating germinability. Weed Science. 2001;49:305–317. [Google Scholar]
- Gao W, Clancy JA, Han F, Prada D, Kleinhofs A, Ullrich SE. Molecular dissection of a dormancy QTL region near the chromosome 7 (5H) L telomere in barley. Theoretical and Applied Genetics. 2003;107:552–559. doi: 10.1007/s00122-003-1281-5. [DOI] [PubMed] [Google Scholar]
- Gutterman Y. Maternal effects on seeds during development. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. Cambridge: CABI Publishing; 2000. pp. 59–84. [Google Scholar]
- Hoyle GL, Daws MI, Steadman KJ, Adkins SW. Pre- and post-harvest influences on physiological dormancy alleviation of an Australian Asteraceae species: Actinobole uliginosum (A. Gray) H. Eichler. Seed Science Research. 2008a;18:191–199. [Google Scholar]
- Hoyle GL, Steadman KJ, Daws MI, Adkins SW. Pre- and post-harvest influences on seed dormancy status of an Australian Goodeniaceae species, Goodenia fascicularis. Annals of Botany. 2008b;102:93–101. doi: 10.1093/aob/mcn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga A, Isemura T, Tomooka N, Vaughan DA. The genetics of domestication of the azuki bean (Vigna angulatis) Genetics. 2008;178:1013–1036. doi: 10.1534/genetics.107.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek J, Steadman KJ, Probert RJ, Adkins SW. Parental effects modulate seed longevity: exploring parental and offspring phenotypes to elucidate pre-zygotic environmental influences. New Phytologist. 2011;191:223–233. doi: 10.1111/j.1469-8137.2011.03681.x. [DOI] [PubMed] [Google Scholar]
- Light ME, Daws MI, Van Staden J. Smoke-derived butenolide: towards understanding its biological effects. South African Journal of Botany. 2009;75:1–7. [Google Scholar]
- Long RL, Williams K, Griffiths EM, et al. Prior hydration of Brassica tournefortii seeds reduces the stimulatory effect of karrikinolide on germination and increases seed sensitivity to abscisic acid. Annals of Botany. 2010;105:1063–1070. doi: 10.1093/aob/mcq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RL, Stevens JC, Griffiths EM, et al. Seeds of Brassicaceae weeds have an inherent or inducible response to the germination-stimulant karrikinolide. Annals of Botany. 2011a;108:933–944. doi: 10.1093/aob/mcr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RL, Stevens JC, Griffiths EM, Adamek M, Powles SB, Merritt DJ. Detecting karrikinolide responses in seeds of the Poaceae. Australian Journal of Botany. 2011b;59:609–619. [Google Scholar]
- Luzuriaga AL, Escudero A, Perez-Garcia F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae) Weed Research. 2006;46:163–174. [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- Merritt D, Kristiansen M, Flematti G, et al. Effects of a butenolide present in smoke on light-mediated germination of Australian Asteraceae. Seed Science Research. 2006;16:29–35. [Google Scholar]
- Merritt DJ, Turner SR, Clarke S, Dixon KW. Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany. 2007;55:336–344. [Google Scholar]
- Meyer SE, Allen PS. Ecological genetics of seed germination regulation in Bromus tectorum L. II. Reaction norms in response to a water stress gradient imposed during seed maturation. Oecologia. 1999;120:35–43. doi: 10.1007/s004420050830. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Riseborough JA, Flematti GR, et al. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiology. 2009;149:863–873. doi: 10.1104/pp.108.131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DC, Flematti GR, Riseborough JA, Ghisalberti EL, Dixon KW, Smith SM. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA. 2010;107:7095–7100. doi: 10.1073/pnas.0911635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DC, Scaffidi A, Dun EA, et al. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA. 2011;108:8897–8902. doi: 10.1073/pnas.1100987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard LA, Fireman M. Pressure-plate apparatus for measuring moisture sorption and transmission by soils. Soil Science. 1943;56:395–404. [Google Scholar]
- Roach DA, Wulff RD. Maternal effects in plants. Annual Review of Ecology and Systematics. 1987;18:209–235. [Google Scholar]
- Sharif-Zadeh F, Murdoch AJ. The effects of different maturation conditions on seed dormancy and germination of Cenchrus ciliaris. Seed Science Research. 2000;10:447–457. [Google Scholar]
- Steadman KJ, Ellery AJ, Chapman R, Moore A, Turner NC. Maturation temperature and rainfall influence seed dormancy characteristics of annual ryegrass (Lolium rigidum) Australian Journal of Agricultural Research. 2004;55:1047–1057. [Google Scholar]
- Stevens JC, Merritt DJ, Flematti GR, Ghisalberti EL, Dixon KW. Seed germination of agricultural weeds is promoted by the butenolide 3-methyl-2H-furo 2,3-c pyran-2-one under laboratory and field conditions. Plant and Soil. 2007;298:113–124. [Google Scholar]
- Swain AJ, Hughes ZS, Cook SK, Moss SR. Quantifying the dormancy of Alopecurus myosuroides seeds produced by plants exposed to different soil moisture and temperature regimes. Weed Research. 2006;46:470–479. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology. 1995;83:1031–1037. [Google Scholar]
- Waters MT, Nelson DC, Scaffidi A, Flematti GR, Sun YK, Dixon KW, Smith SM. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development. 2012;139:1285–1295. doi: 10.1242/dev.074567. [DOI] [PubMed] [Google Scholar]