Abstract
Experimental evidence suggests that C inhibition and more particularly combined inhibition of C and the TLR co-receptor CD14 may be of therapeutic benefit in sepsis and other inflammatory conditions. A barrier to the testing and further development of many inhibitors is that their activity is species specific. Pig is a relevant species for experimental models of human disease, and this study undertakes a comprehensive comparison of the inhibitory efficacy of the C5 inhibitor Ornithodoros moubata C inhibitor (OmCI) in human and porcine whole blood ex vivo models of Escherichia coli-induced sepsis. The effect of OmCI on complement activity in pigs undergoing E. coli sepsis was also examined.
Porcine and human serum, and whole blood anticoagulated with lepirudin, was incubated with E. coli and the effect of OmCI investigated. The ex vivo results were virtually identical in pig and human. OmCI completely ablated the activity of all three C pathways at 0.64 μM. E. coli-induced C activation and expression of CD11b (wCD11R3 in the pig), was abolished ex vivo at 0.32 μM OmCI. Combining anti-CD14 and OmCI reduced the formation of IL-8 and TNF-α more potently than the single inhibitors. OmCI also efficiently bound E. coli-induced LTB4 in pig and human plasma. In support of our ex vivo findings, in vivo the activity of all C pathways was inhibited at 0.6 mg OmCI/kg pig. In conclusion, OmCI efficiently inhibited pig and human C activation, has accompanying anti-inflammatory effects, and is a promising candidate inhibitor for further in vivo studies of sepsis.
Introduction
C and the TLR family play essential parts in innate immune reactions. Upon pattern recognition these danger sensors identify and eliminate pathogens as well as endogenous danger motifs, thereby protecting the host and maintaining homeostasis (1,2).
The physiological effects of C are numerous and diverse (3). Activation is known to occur via three routes, the classical, the lectin and the alternative pathways, all converging and leading to the cleavage of the central C factor C3. The C3 fragments C3b and iC3b are important opsonins in the defense against bacteria. Activation of the terminal pathway with the cleavage of C5 is biologically highly potent. Release of the anaphylatoxin C5a is known to induce up-regulation of adhesion molecules, stimulate cytokine production, cause paralysis of neutrophils, increase vascular permeability and might lead to disseminated intravascular coagulation among other effects (4). C5b induces assembly of the terminal C5b-9 C complex (TCC), which can lyse certain pathogens and cells when incorporated into their lipid membranes (5).
The TLRs are an important class of pattern recognition receptors (PRR) (6). TLR4 is a PRR for LPS of Gram-negative bacteria, and is dependent on two important co-receptors, MD-2 and CD14 (7,8). TLR signaling induces NF-κB, required for transcription of a wide variety of inflammatory and immune response genes (6).
Uncontrolled systemic activation of these upstream danger sensors may lead to a counterproductive response that endangers self as seen in septic shock (9,10). Ex vivo studies with human whole blood have demonstrated that E. coli-induced inflammation is strongly attenuated by a combined inhibition of C and CD14 (11,12). Consequently we hypothesised that combined inhibition of C and CD14 might prove a useful therapeutic regimen in sepsis and other inflammatory conditions (13). Notably, inhibition of the classical proinflammatory cytokines TNF-α and IL-1β was shown not to have any impact on the E. coli-induced inflammatory reaction (14). We recently showed that inhibition of CD14 attenuated proinflammatory cytokines, granulocyte activation and hypercoagulation in E. coli-induced sepsis in vivo in pigs (15). Furthermore, selective inhibition of C improved organ function in a baboon model of sepsis (16). Combined inhibition of C and CD14 in vivo has yet to be tested.
Ticks produce a plethora of proteins that interfere with host immune and haemostatic defense responses (17). OmCI is a 16.8-kDa saliva protein from the soft tick Ornithodoros moubata (18). This small protein binds directly to C5, prevents cleavage by both the classical and the alternative C5 convertases and thus C5a and TCC is not formed (19). C5 inhibition by OmCI thus has vast anti-inflammatory potential, without affecting beneficial immunoprotective and immunoregulatory functions of upstream C activity (20). OmCI has cross-species C-inhibitory activity that has been demonstrated but not explicitly quantified in human, rat, mouse and guinea pig (18,21). The protein is protective in experimental passive and active autoimmune myasthenia gravis models in rats. OmCI-treated animals, exhibited significantly fewer symptoms and the inflammatory response was substantially attenuated (21,22). Like some of its closest known homologues (23), OmCI has bifunctional properties, capturing the inflammatory mediator leukotriene B4 (LTB4) in an internal binding pocket in addition to binding to C5 (M.A.N., personal observation). LTB4 is a potent chemotactic agent and activator of white cells in particular neutrophils (24). Despite its low molecular weight, the half-life of OmCI is approximately 30 h in rats due to stable binding to C5, making OmCI an interesting agent for use in vivo (21).
The aim of the present study was to compare the efficacy of OmCI in pigs and humans, using functional assays designed for these species. By using a unique whole blood ex vivo model for investigation of the crosstalk of the inflammatory network, C inhibitory efficacy as well as anti-inflammatory properties of OmCI was elucidated. In addition, the C inhibitory effect of OmCI was explored in pilot experiments using an in vivo porcine model of E. coli-induced sepsis.
Material and methods
Reagents and equipment
Sterile PBS pH 7.2 was purchased from Sigma-Aldrich (St. Louis, MO), CryoTubes™ (polypropylene) from Nunc AS (Roskilde, Denmark), Lepirudin (Refludan®) from Pharmion (Hamburg, Germany) and 96 well plates (Costar® 3590) from Corning (New York, NY). Zymosan A (Z-4250) was purchased from Sigma (St. Louis, MO). Human gamma globulin (160 mg/ml, Beriglobin®) was purchased from Behring (Marburg, Germany) and converted to heat-aggregated immunoglobulin G (HAIGG) by heating a 10 mg/ml stock to 63 °C for 15 minutes. Albumin 200 mg/ml was purchased from Octapharma (Hurdal, Norway).
Bacteria
E. coli strain LE392 (ATCC 33572) from the American Type Culture Collection (Manassas, VA) was used in all experiments. E. coli was grown over night in LB-medium (tryptone 1 %, yeast extract 0.5 % and sodium chloride 0.9 %), then centrifuged and washed in PBS. After resuspension in PBS, E. coli was immediately heat-inactivated (1 h, 60 °C) for use in the ex vivo experiments. Growth plates confirmed that all bacteria were killed. Thereafter, the bacteria were washed nine times to remove extra-bacterial LPS and counted by flow cytometry. Live E. coli strain LE392 was used in vivo. Sepsis was induced with an increasing i.v. infusion of E. coli, as previously described (15). Each pig received a total of 1.075 × 108 E. coli/kg during the time course of the experiment, corresponding to 1.1 × 106 bacteria/ml blood.
Inhibitors
Recombinant bacterial OmCI (18), a 16.8 kDa protein, was produced in the laboratory of one of the authers (M.A.N). Before use it was stored in single use aliquots at −70 °C. The mouse anti-human CD14 (clone 18D11) F(ab′)2 and control F(ab′)2 (clone BH1), was purchased from Diatec Monoclonals AS (Oslo, Norway). Mouse anti-porcine CD14 monclonal antibody clone MIL-2, isotype IgG2b, was purchased from Serotec (Oxford, UK). A mouse anti-human IgG2b (clone BH1, prod. no 2070), was purchased from Diatec Monoclonals AS and used as an isotype-matched control. The compstatin analog Ac-I[CV(1MeW)QDWGAHRC]T, which binds to and inhibits cleavage of C3, and the cyclic hexapeptide AcF[OPdChaWR], a C5a receptor antagonist (C5aRa) were produced as previously described (25,26). Eculizumab 10 mg/ml, (Soliris®), a humanized monoclonal IgG2/4k –antibody that binds and inhibits cleavage of C5 was from Alexion Pharmaceuticals (Cheshire, CT), kindly provided by dr. Yngvar Fløysand.
Ex vivo whole blood experiments
The whole blood model has previously been described in detail (27). Briefly, whole blood was collected from 15–60 kg pigs (Sus scrofa domesticus; Landrace; outbread stock) and healthy human donors. Blood was drawn into tubes containing the anticoagulant lepirudin (Refludan®) at a final concentration of 50 μg/ml whole blood. The blood was immediately preincubated with inhibitors, controls or PBS for five minutes at 37 °C. PBS or E. coli, at a final concentration of 105, 106 or 108 E. coli/ml whole blood, was then added and incubated for 2 h at 37 °C. After incubation, the tubes were put on ice and EDTA (final concentration 20 mM) was added to stop the C activation and the tubes were centrifuged for 15 minutes at 1400 × g at 4 °C. The plasma was stored at −70 °C until analysed.
In vivo experiments
Norwegian Landrace pigs (n = 7) of either sex, with a mean weight of 14.4 kg (range 14.0–16.0 kg) were used. Premedication, anesthesia, surgery, hemodynamic monitoring, induction of sepsis and euthanasia were performed as described (15). Pigs received OmCI i.v. as a bolus (22.5, 15, 7.5 and 3.75 mg) and thereafter as a continuous i.v. infusion (0.5 mg/h) for 4 h. The total amounts of OmCI given were 24.5 mg (n = 1), 17 mg (n = 1), 9.5 mg (n = 2) and 5.75 mg (n = 1). Two pigs served as positive controls, receiving saline only (9 mg/ml). Blood samples were drawn and physiological data registered after surgery at baseline (Tbasis), thereafter at 0 (T0), 30, 60, 120, 180, and 240 minutes.
Enzyme Immunoassays
The commercially available enzyme immune assay (EIA) (Wielisa, Wieslab, Lund, Sweden) was used to test functional activity of the classical, lectin and alternative C pathways. The test was designed for human C activity (28), but was later shown to cross-react with pig (29). Human C5a was analysed by a commercial EIA (BD Bioscience, San Jose, CA). Terminal C5b-9 C complex (TCC) was measured in an EIA, previously described in detail (30,31). Briefly, the monoclonal antibody aE11, specific for a C9 neoepitope in TCC, was used as capture antibody and a biotinylated monoclonal anti-C6 (Quidel Corporation, San Diego, CA) was used as detection antibody. Both antibodies cross-react with pig epiotopes; thus the assay can be used to detect porcine as well as human TCC. The porcine cytokines TNF-α, IL-1β, and IL-8 were analysed by Quantikine Porcine Immunoassay kits from R&D Systems (Minneapolis, MN). Human plasma samples were analysed using multiplex technology (Luminex 100; Bio-Rad Labaratories, Hercules, CA) – an immunoassay based on colored beads, each with a unique cytokine detection antibody which permits simultaneous measurement of a wide range of human biomarkers. A 27-plex kit purchased from Bio-Rad Laboratories (Hercules, CA) was used containing the following cytokines: IL-1ra, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, TNF-α, IFN-γ, eotaxin, FGF-basic, G-CSF, GM-CSF, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES and VEGF. The analyses were performed according to the instructions from the manufacturer. Results are presented for the cytokines that exhibited responses to the inhibitors in comparison to the negative controls. LTB4 from pig and human plasma was measured using a competitive enzyme immunoassay from R&D Systems (Minneapolis, MN).
Flow cytometry
For analyses of CD11b (human) or wCD11R3 (pig orthologue) whole blood was preincubated with inhibitor, control or PBS for seven minutes at 37 °C. PBS or E. coli at a final concentration of 108 E. coli/ml whole blood, was added and incubated for 10 minutes before the cells were fixed with 0.5% (v/v) paraformaldehyde for 4 minutes at 37 °C. Human fixed samples were stained with PE conjugated anti human CD11b (catalog no. 333142) or an isotype-matched PE conjugated IgG2a control antibody (catalog no. 349053) (both BD Biosciences Pharmingen, San Jose, CA). Pig fixed samples and EDTA anticoagulated blood from the in vivo experiments were stained with FITC conjugated anti porcine wCD11R3 (catalog no. MCA2309F) or an isotype-matched FITC conjugated IgG1 control antibody (catalog no. MCA928F) (both AbD Serotec, Oxford, UK), and incubated for 15 minutes at room temperature in the dark. The red blood cells were then lysed for 7 minutes thereafter centrifuged and washed before being resuspended in PBS containing 1% albumin. Samples were analysed by FACS (FACScan, Becton Dickinson, Franklin Lakes, NJ). Granulocytes were gated in a FSC/SSC dot plot and CD11b and wCD11R3 expression were given as median fluorescence intensity (MFI).
Statistics
GraphPad Prism version 5 (GraphPad Software, San Diego, CA) was used for the statistical analysis. The data were analysed by one-way ANOVA, followed by Dunnett’s Multiple Comparison Test. A two-tailed p-value below 0.05 was considered statistically significant.
Ethics
Informed written consent was obtained from each blood donor, and the study was approved by the local Ethical Committee. The animals used were treated in adherence to the Norwegian laboratory animal regulations, and the study was approved by the Norwegian Animal Research Authority.
Results
In vitro
The effect of OmCI on the different complement pathways
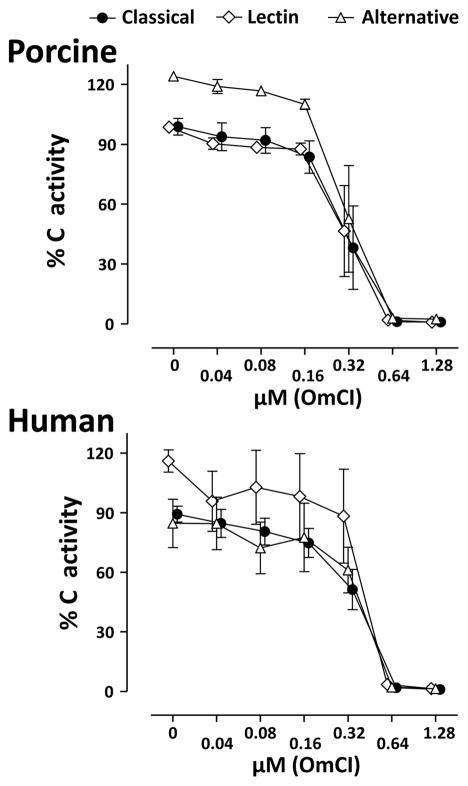
In serum OmCI dose-dependently inhibited all three C activation pathways similarly, and the inhibition was complete at a dose of 0.64 μM OmCI in both pig and human (Figure 1). Concentrations between 1.28 μM and 20 μM were also completely inhibitory (data not shown).
Figure 1. Effect of OmCI on complement functional activity.
The effect of OmCI on the functional activity of the classical, lectin and alternative pathways of C were examined in serum from humans and pigs using the Wielisa assay. The data are expressed as % of a standard defined as 100% activity and are presented as mean ± SEM (n = 3).
The effect of OmCI on the formation of C5a and fluid-phase TCC
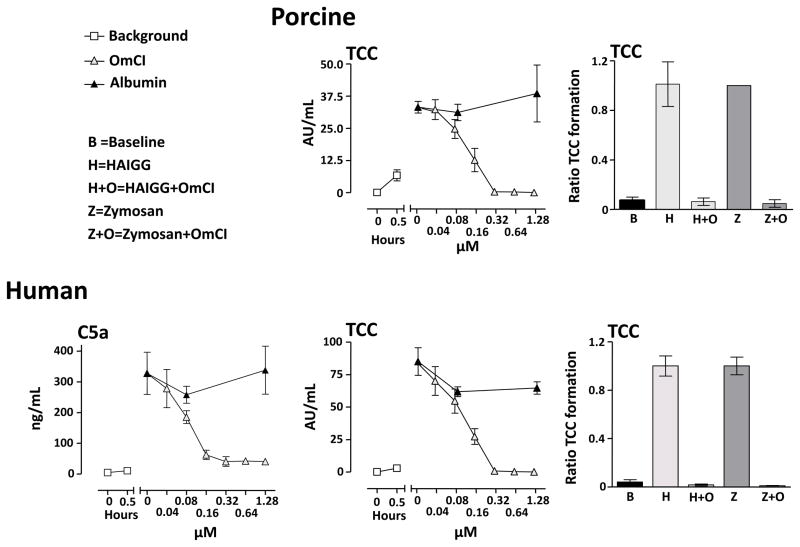
Pig and human whole blood was incubated with 108 E. coli/ml for 30 minutes. OmCI dose-dependently inhibited human C5a formation (Figure 2, left panel). At concentrations of OmCI equal to or higher than 0.32 μM, the formation of human C5a was completely ablated. OmCI also dose-dependently inhibited TCC formation in both pig and human whole blood (Figure 2, middle panels). At concentrations of OmCI equal to or higher than 0.32 μM, the formation of TCC was completely inhibited in both species. When serum was activated with either HAIGG or zymosan, which are potent activators of the classical and lectin/alternative C pathway respectively, TCC formation was completely inhibited by 0.64 μM OmCI in both species (Figure 2, right panels).
Figure 2. Effect of OmCI on complement induced formation of C5a and TCC in vitro.
Left panel: Human whole blood preincubated with OmCI or equimolar amounts of albumin was incubated with 108 E. coli/ml for 30 minutes. Data are expressed as ng/ml and presented as mean ± SEM (n = 3).
Middle panels: Human and pig whole blood preincubated with OmCI or equimolar amounts of albumin was incubated with 108 E. coli/ml for 30 minutes at 37 °C. Data are expressed as AU/ml and presented as mean ± SEM (n = 3 for human and n = 4 for pig).
Right panels: Human and pig serum preincubated with 0.64 μM OmCI or albumin was activated with heat aggregated IgG (HAIGG) or zymosan, both at a final concentration of 1 mg/ml. In all experiments, zymosan induced TCC formation in pig whole blood was higher than the upper standard so these are set to 1.0. Data are expressed as TCC ratio, where 1.0 is defined as the amount of TCC formation induced by HAIGG and zymosan, respectively. The data are presented as mean ± SD (n = 5 for human and n = 3 for pig).
The effect of OmCI on E. coli-induced CD11b/wCD11R3 up-regulation on granulocytes
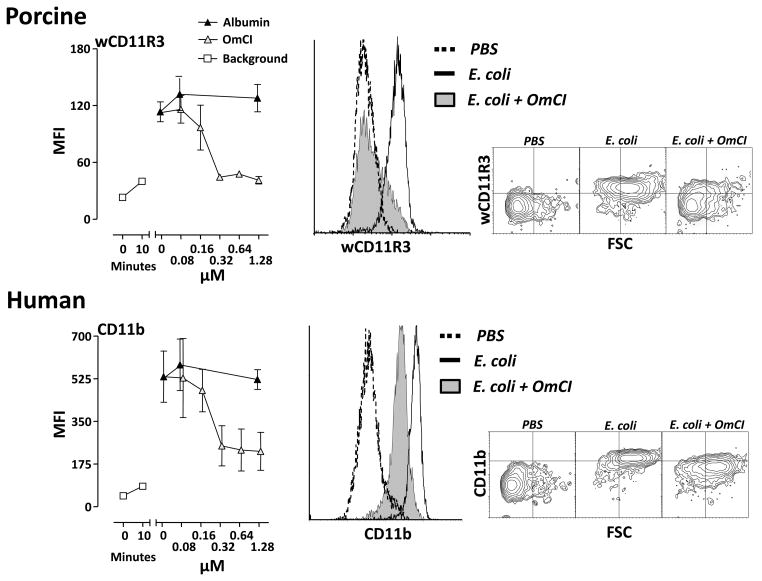
Whole blood was incubated with 108 E. coli/ml for 10 minutes and the up-regulation of CD11b (human) and wCD11R3 (pig orthologue) on granulocytes was examined. OmCI dose-dependently inhibited the expression of the cell surface marker in both species (Figure 3). In human granulocytes the CD11b up-regulation was reduced by more than 60 % and in pigs the wCD11R3 up-regulation was completely abolished at 0.32 μM OmCI.
Figure 3. Effect of OmCI on E. coli-induced expression of CD11b and wCD11R3.
Human and pig whole blood was preincubated with OmCI or equimolar amounts of albumin and then incubated with 108 E. coli/ml for 10 minutes at 37 °C. Expression of human CD11b and pig wCD11R3 were measured using flow cytometry. Median fluorescence intensity (MFI) is expressed as mean ± SD (n = 3 for humans and n = 4 for pigs). To the right, histograms and contour plots of the effect of 1.28 μM OmCI for pig and human.
The effect of OmCI, anti-CD14, and the combination thereof, on E. coli-induced cytokine formation in porcine and human whole blood
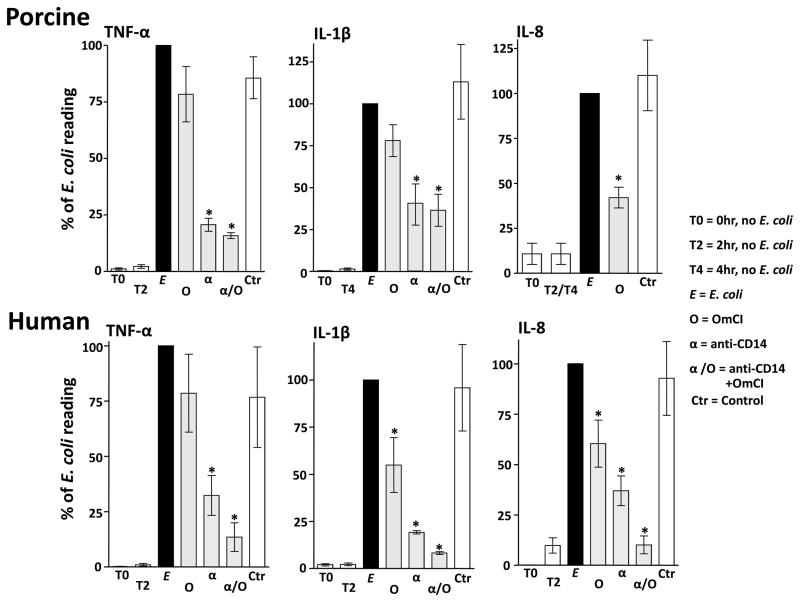
In pig, OmCI significantly reduced the formation of IL-8 compared to the positive control, while TNF-α and IL-1β were non-significantly reduced (Figure 4, upper panels). Inhibition of CD14 significantly reduced the formation of pig TNF-α and IL-1β, whereas combined inhibition by anti-CD14 and OmCI had no additional effect (Figure 4, upper panels). In human, OmCI significantly reduced the formation of IL-1β and IL-8 while TNF-α was non-significantly reduced (Figure 4, lower panels). Inhibition of CD14 significantly reduced the formation of human TNF-α, IL-1β and IL-8 (Figure 4, lower panels). In contrast to pig, combined inhibition in human by anti-CD14 and OmCI appeared to enhance the inhibitory effect observed for cytokines (Figure 4, lower panels). In human, the combined effect of OmCI and anti-CD14 (i.e near complete ablation of TNF-α, IL-1β and IL-8 ) was similar to that of compstatin (C3 inhibitor) or C5 receptor antagonist combined with anti-CD14 (data not shown).
Figure 4. Effect of inhibiting complement, CD14, and a combination thereof, on E. coli-induced cytokine release.
Upper panels: Pig whole blood was preincubated with 0.64 μM OmCI (O), 25 μg/ml anti-CD14 (whole IgG) (α), and the combination of both and then incubated with 106 E. coli/ml for 2 h (TNF-α and IL-8) and/or 105 bacteria/ml for 4 h (IL-1β and IL-8). The effect of anti-CD14 on pig IL-8 could not be measured because of assay intereference. Albumin and an isotype-matched control Ab (IgG2b) were used as controls (Ctr) when analyzing TNF-α and IL-1β, whereas albumin only was used as control when analyzing IL-8. For IL-8, 2 and 4 hour sample data were pooled. Data are normalized to the E. coli group which is defined as 100%. Data are presented as mean ± SEM (n = 3, 4 and 5 for TNF-α, IL-1β and IL-8, respectively).
Lower panels: Human whole blood was preincubated with 0.64 μM OmCI, 10 μg/mL anti-CD14 (F(ab′)2), combinations thereof, and controls for 5 minutes and then incubated for 2 h with 106 E. coli/ml at 37 °C. As control (Ctr) albumin was used in combination with a control F(ab′)2, both in equimolar amounts to OmCI and anti-CD14, respectively. Data are normalized to the E. coli group which is defined as 100% and presented as mean ± SEM (n=3). Statistical comparisons were performed between the effect of OmCI, anti-CD14 and the combinations of both versus no inhibition, *p < 0.05.
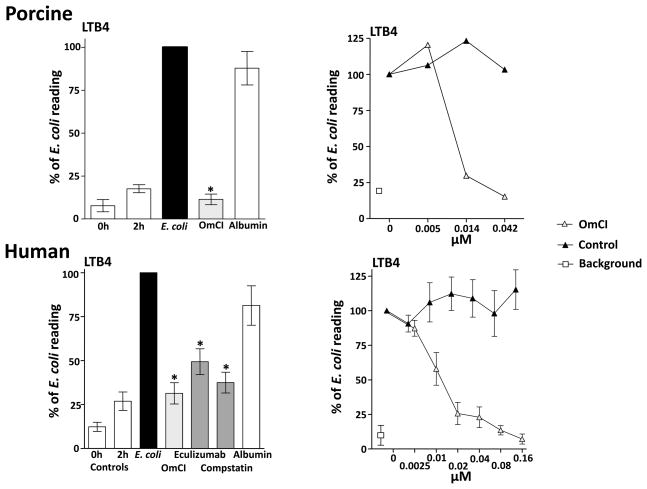
The effect of OmCI on LTB4
Pig blood was preincubated with OmCI and human blood was preincubated with OmCI and the human specific complement inhibitors, eculizumab and compstatin, then activated with 106 E. coli/ml and incubated for 2 h at 37 °C. OmCI, as well as eculizumab and compstatin, significantly reduced the formation of LTB4 (Figure 5, left panels). The data from the specific complement inhibitors indicates that LTB4 formation is C dependent. To demonstrate direct binding of OmCI to LTB4, pig and human whole blood were incubated with E. coli to induce LTB4-enriched plasma, and then equimolar doses of OmCI were added. By sequestering the LTB4 within the β-barrel of the lipocalin structure (23), OmCI dose-dependently decreased the signal in the LTB4 assay by preventing the leukotriene interacting with the capture antibody (Figure 5, right panels). As expected, eculizumab did not have any neutralizing effect on LTB4.
Figure 5. Complement-dependent LTB4 release and binding of LTB4 to OmCI.
Left panels: Pig and human whole blood was preincubated with 0.64 μM OmCI or albumin and human whole blood was also preincubated with the human specific C inhibitors compstatin (25 μM) and eculizumab (0.67 μM), thereafter incubated with 106 E. coli/ml for 120 minutes at 37 °C. Data are presented as mean ± SEM (n = 3 for pig and n = 7 for human). Statistics were performed on the effect of the C inhibitors versus the effect of E. coli only, *p < 0.05.
Right panels: LTB4-enriched plasma was generated by incubating pig and human whole blood with 106 E. coli/ml for 120 minutes at 37 °C. Thereafter OmCI was added to the activated plasma and incubated for 15 minutes at 37 °C. A control peptide or eculizumab were used as controls for pig and human respectively. Samples were then analysed for LTB4. Data are normalized to LTB4 in E. coli activated plasma (defined as 100%). Data are presented as mean ± SEM for human (n = 4), and as mean for pig (n = 2).
In Vivo
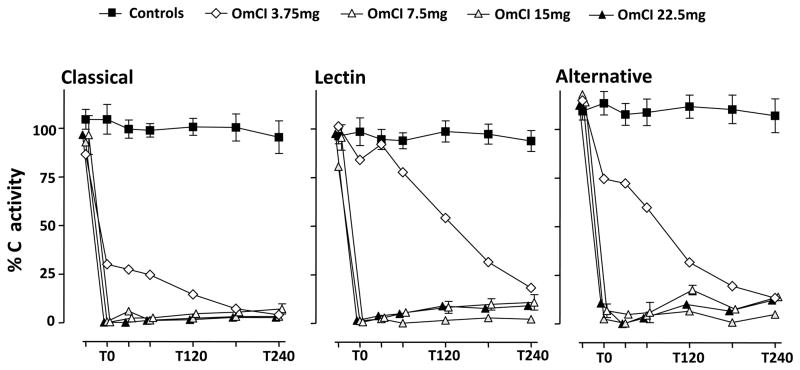
The effect of OmCI on complement activity in an experimental sepsis model in pigs
To investigate the effect of OmCI on functional C activity in vivo, we used our established pig model of Gram-negative sepsis. The effect of different bolus doses of OmCI with uniform continuous infusion of the protein (0.5 mg/h) was assessed in five 15 kg pigs. No pathological side effects were observed when administering OmCI. Two control animals received E. coli only. The three largest bolus doses of OmCI (22.5 mg, 15 mg and 7.5 mg) completely ablated the classical, lectin and alternative pathways immediately after administration and remained effective throughout the experiment (Figure 6). The lowest bolus dose of OmCI (3.75 mg) did not totally ablate C activity at the beginning of the experiment but ablation was almost complete by the end of the experiment.
Figure 6. In vivo effects of OmCI in pigs.
Different amounts of OmCI were tested in a pig model of E. coli-induced sepsis. Five 15 kg pigs received the following boluses of OmCI: 22.5 mg (n = 1), 15 mg (n = 1), 7.5 mg (n = 2) or 3.75 mg (n = 1) prior to the induction of sepsis, and thereafter a continuous infusion of 0.5 mg OmCI/h. Two animals served as positive controls, receiving E. coli and saline (9 mg/ml). Blood samples were drawn at baseline (Tbasis), and then at 0 (T0), 30, 60, 120, 180 and 240 minutes. The effect of OmCI on the functional activity of the three C pathways is shown using the Wielisa assay. The data are expressed in % of a standard defined as 100% activity. Data points are given as single values or as mean with range for 7.5 mg dose and the E. coli positive control.
Discussion
In this study we show for the first time that OmCI is an excellent C inhibitor of humans and is as least as effective in pigs. Additionally, we demonstrated that OmCI attenuated C induced proinflammatory mediators in pig and human.
In the Wielisa analysis OmCI was an equally potent inhibitor of the catalytic activity of the classical/lectin (C4bC3bC2a), and alternative (C3bBb3b) C5 convertases of humans and pigs. These effects were confirmed by dose-dependent inhibition of C5a and TCC formation in whole blood incubated with E. coli. The potency of OmCI as a complement inhibitor was supported by total abrogation of TCC formation in serum activated with HAIGG or zymosan, which are very strong activators of the classical and alternative/lectin pathways, respectively. OmCI is known to bind directly and tightly to the C5 alpha chain in the vicinity of the C5-C345C domain (18,20,32). OmCI binding stabilizes the overall conformation of C5 and inhibits the effect of the C5 convertases without directly blocking the cleavage site on C5 (19). At present, we lack detailed information on the precise locality and binding details of OmCI to C5, so the reason why OmCI is an equally potent inhibitor of both human and pig C5 is unknown.
OmCI significantly decreased the expression of the human cell surface marker CD11b and the pig orthologue wCD11R3, which together with CD18 constitute phagocyte C receptor 3. Since OmCI blocks the formation of C5a (18), this result is consistent with previous work showing that up-regulation of this important inflammatory receptor is mainly dependent on the formation of C5a (33).
E. coli induced cytokine formation was more dependent on CD14 than on C. CD14 is a well known recognition molecule for LPS, co-operating with MD-2 in TLR4 signaling, but CD14 has been implicated in TLR2 and TLR3 signaling (8,34,35) and even acts as co-receptor for TLR7 and TLR9 (36). Thus, CD14 appears to be a promiscuous upstream recognition molecule, reacting with a number of ligands with low affinity, and transferring the ligand to receptors with a higher degree of specificity and affinity (37). The experiments showed that OmCI differentially attenuated E. coli-induced cytokine formation in pig and human, with a significant effect on IL-8 in both species, and on IL-1β in humans. This is consistent with previous findings showing that these cytokines are relatively more dependent on C in Gram-negative-induced inflammation (33). Interestingly, combined inhibition using OmCI and anti-CD14 enhanced the attenuating effect on cytokine formation in human, compared to the individual inhibition by these two upstream recognition molecules of innate immunity. This effect was similar to that of compstatin or C5a receptor antagonist combined with anti-CD14, and fully agrees with previous results (12).
The leukotrienes are important multifunctional mediators of inflammation. LTB4 promotes neutrophil chemotaxis, increases adherence of neutrophils to capillary walls and is a potent inducer of chemokinesis and neutrophil infiltration (38). In the present study we showed that inhibition of C profoundly attenuated the expression of E. coli-induced LTB4. This finding supports existing evidence that LTB4-synthesis is C5a dependent (39). However, because OmCI also captures LTB4 in an internal hydrophobic binding pocket it is difficult to attribute the inhibitory effect of OmCI on the formation on LTB4 that is due to C inhibition or due to direct binding to the leukotriene. OmCIs dual inhibitory activity may provide anti-inflammatory advantages as recently shown in an experimental model of immune complex alveolitis (M.A.N. unpublished). There is evidence that the level of LTB4 significantly increases as an endotoxemic response in pigs (40), and our data indicates that OmCI will inhibit LTB4-induced inflammatory responses.
Data from the experiments on pigs, convincingly demonstrate that OmCI is an efficient inhibitor of C in vivo. The bolus doses that were used, gave estimated blood-concentrations of OmCI (roughly 0.31 – 1.83 μM) corresponding to the ranges that were used in the ex vivo experiments, where 0.64 μM OmCI in undiluted serum completely inhibited C and 0.32 μM OmCI in whole blood ablated TCC formation in response to E.coli. This enables comparison between the ex vivo and the in vivo data. Using the three highest bolus doses of OmCI (roughly 0.61, 1.22 and 1.83 μM) C was completely inhibited in vivo. Whereas, using the smallest bolus dose of OmCI (roughly 0.31 μM in blood) we observed incomplete inhibition of all three C pathways at the beginning of the experiment, but complete inhibition by the end of the experiment. The initial bolus dose was too low to ablate the activity of the standing pool of C5 completely, whereas the continuous infusion gradually blocked more C5. Continuous infusion of OmCI is needed to inhibit newly synthesized C5, since the unexpectedly long half-life of OmCI is dependent on stable binding to C5, thus, surplus OmCI is rapidly cleared from plasma (21).
In conclusion, the present data documents that OmCI is an effective inhibitor of C activation with anti-inflammatory properties in pig and human. The comparable potency of OmCI in the two species and correspondence between the amount of OmCI needed to inhibit C ex vivo and in vivo opens the door to further testing of OmCI in pig models and will facilitate progression towards clinical testing. This is of substantial interest as the list of conditions where C is involved in the disease pathology is extensive and increasing. Given the mortality and morbidity rates associated with sepsis and the existing data on the potential efficacy of combined inhibition of C and CD14 (12,14,33,41) it seems imperative to proceed with animal studies of sepsis combining inhibition of these two important upstream danger sensors of innate immunity.
Footnotes
This work was supported by National Institutes of Health grants AI068730 and GM062134.
Reference List
- 1.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 2.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 4.Ward PA. The harmful role of c5a on innate immunity in sepsis. J Innate Immun. 2010;2:439–445. doi: 10.1159/000317194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 6.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 7.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20:420–425. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 10.Castellheim A, Thorgersen EB, Hellerud BC, Pharo A, Johansen HT, Brosstad F, Gaustad P, Brun H, Fosse E, Tonnessen TI, Nielsen EW, Mollnes TE. New Biomarkers in an Acute Model of Live Escherichia coli-induced Sepsis in Pigs. Scand J Immunol. 2008;2008;68:75–84. doi: 10.1111/j.1365-3083.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- 11.Brekke OL, Christiansen D, Fure H, Fung M, Mollnes TE. The role of complement C3 opsonization, C5a receptor, and CD14 in E. coli-induced up-regulation of granulocyte and monocyte CD11b/CD18 (CR3), phagocytosis, and oxidative burst in human whole blood. J Leukoc Biol. 2007;81:1404–1413. doi: 10.1189/jlb.0806538. [DOI] [PubMed] [Google Scholar]
- 12.Brekke OL, Christiansen D, Fure H, Pharo A, Fung M, Riesenfeld J, Mollnes TE. Combined inhibition of complement and CD14 abolish E. coli-induced cytokine-, chemokine- and growth factor-synthesis in human whole blood. Mol Immunol. 2008;45:3804–3813. doi: 10.1016/j.molimm.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Mollnes TE, Christiansen D, Brekke OL, Espevik T. Hypothesis: combined inhibition of complement and CD14 as treatment regimen to attenuate the inflammatory response. Adv Exp Med Biol. 2008;632:253–263. [PubMed] [Google Scholar]
- 14.Barratt-Due A, Thorgersen EB, Lindstad JK, Pharo A, Brekke OL, Christiansen D, Lambris JD, Mollnes TE. Selective inhibition of TNF-alpha or IL-1 beta does not affect E. coli-induced inflammation in human whole blood. Mol Immunol. 2010;47:1774–1782. doi: 10.1016/j.molimm.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Thorgersen EB, Hellerud BC, Nielsen EW, Barratt-Due A, Fure H, Lindstad JK, Pharo A, Fosse E, Tonnessen TI, Johansen HT, Castellheim A, Mollnes TE. CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs. FASEB J. 2010;24:712–722. doi: 10.1096/fj.09-140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silasi-Mansat R, Zhu H, Popescu NI, Peer G, Sfyroera G, Magotti P, Ivanciu L, Lupu C, Mollnes TE, Taylor FB, Kinasewitz G, Lambris JD, Lupu F. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116:1002–1010. doi: 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunn MA, Sharma A, Paesen GC, Adamson S, Lissina O, Willis AC, Nuttall PA. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol. 2005;174:2084–2091. doi: 10.4049/jimmunol.174.4.2084. [DOI] [PubMed] [Google Scholar]
- 19.Fredslund F, Laursen NS, Roversi P, Jenner L, Oliveira CL, Pedersen JS, Nunn MA, Lea SM, Discipio R, Sottrup-Jensen L, Andersen GR. Structure of and influence of a tick complement inhibitor on human complement component 5. Nat Immunol. 2008;9:753–760. doi: 10.1038/ni.1625. [DOI] [PubMed] [Google Scholar]
- 20.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 21.Hepburn NJ, Williams AS, Nunn MA, Chamberlain-Banoub JC, Hamer J, Morgan BP, Harris CL. In vivo characterization and therapeutic efficacy of a C5-specific inhibitor from the soft tick Ornithodoros moubata. J Biol Chem. 2007;282:8292–8299. doi: 10.1074/jbc.M609858200. [DOI] [PubMed] [Google Scholar]
- 22.Soltys J, Kusner LL, Young A, Richmonds C, Hatala D, Gong B, Shanmugavel V, Kaminski HJ. Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann Neurol. 2009;65:67–75. doi: 10.1002/ana.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mans BJ, Ribeiro JM. Function, mechanism and evolution of the moubatin-clade of soft tick lipocalins. Insect Biochem Mol Biol. 2008;38:841–852. doi: 10.1016/j.ibmb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohnishi H, Miyahara N, Gelfand EW. The role of leukotriene B(4) in allergic diseases. Allergol Int. 2008;57:291–298. doi: 10.2332/allergolint.08-RAI-0019. [DOI] [PubMed] [Google Scholar]
- 25.Katragadda M, Magotti P, Sfyroera G, Lambris JD. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J Med Chem. 2006;49:4616–4622. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- 26.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 27.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 28.Seelen MA, Roos A, Wieslander J, Mollnes TE, Sjoholm AG, Wurzner R, Loos M, Tedesco F, Sim RB, Garred P, Alexopoulos E, Turner MW, Daha MR. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. 2005;296:187–198. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Salvesen B, Mollnes TE. Pathway-specific complement activity in pigs evaluated with a human functional complement assay. Mol Immunol. 2009;46:1620–1625. doi: 10.1016/j.molimm.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Mollnes TE, Lea T, Froland SS, Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985;22:197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 31.Mollnes TE, Redl H, Hogasen K, Bengtsson A, Garred P, Speilberg L, Lea T, Oppermann M, Gotze O, Schlag G. Complement activation in septic baboons detected by neoepitope-specific assays for C3b/iC3b/C3c, C5a and the terminal C5b-9 complement complex (TCC) Clin Exp Immunol. 1993;91:295–300. doi: 10.1111/j.1365-2249.1993.tb05898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roversi P, Lissina O, Johnson S, Ahmat N, Paesen GC, Ploss K, Boland W, Nunn MA, Lea SM. The structure of OMCI, a novel lipocalin inhibitor of the complement system. J Mol Biol. 2007;369:784–793. doi: 10.1016/j.jmb.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lappegard KT, Christiansen D, Pharo A, Thorgersen EB, Hellerud BC, Lindstad J, Nielsen EW, Bergseth G, Fadnes D, Abrahamsen TG, Hoiby EA, Schejbel L, Garred P, Lambris JD, Harboe M, Mollnes TE. Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature. Proc Natl Acad Sci U S A. 2009;106:15861–15866. doi: 10.1073/pnas.0903613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilsen NJ, Deininger S, Nonstad U, Skjeldal F, Husebye H, Rodionov D, von AS, Hartung T, Lien E, Bakke O, Espevik T. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol. 2008;84:280–291. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janot L, Secher T, Torres D, Maillet I, Pfeilschifter J, Quesniaux VF, Landmann R, Ryffel B, Erard F. CD14 works with toll-like receptor 2 to contribute to recognition and control of Listeria monocytogenes infection. J Infect Dis. 2008;198:115–124. doi: 10.1086/588815. [DOI] [PubMed] [Google Scholar]
- 36.Baumann CL, I, Aspalter M, Sharif O, Pichlmair A, Bluml S, Grebien F, Bruckner M, Pasierbek P, Aumayr K, Planyavsky M, Bennett KL, Colinge J, Knapp S, Superti-Furga G. CD14 is a coreceptor of Toll-like receptors 7 and 9. J Exp Med. 2010;207:2689–2701. doi: 10.1084/jem.20101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antal-Szalmas P. Evaluation of CD14 in host defence. Eur J Clin Invest. 2000;30:167–179. doi: 10.1046/j.1365-2362.2000.00610.x. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikai Y. Roles of prostaglandins and leukotrienes in acute inflammation caused by bacterial infection. Curr Opin Infect Dis. 2001;14:257–263. doi: 10.1097/00001432-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Fleming SD, Phillips LM, Lambris JD, Tsokos GC. Complement component C5a mediates hemorrhage-induced intestinal damage. J Surg Res. 2008;150:196–203. doi: 10.1016/j.jss.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson NC, Dobrowsky RT, Fleisher LN. Increased leukotriene B4 in bronchoalveolar lavage fluid and plasma of endotoxemic pigs. Prostaglandins Leukot Essent Fatty Acids. 1988;32:57–62. doi: 10.1016/0952-3278(88)90096-8. [DOI] [PubMed] [Google Scholar]
- 41.Salvesen B, Fung M, Saugstad OD, Mollnes TE. Role of complement and CD14 in meconium-induced cytokine formation. Pediatrics. 2008;121:e496–e505. doi: 10.1542/peds.2007-0878. [DOI] [PubMed] [Google Scholar]