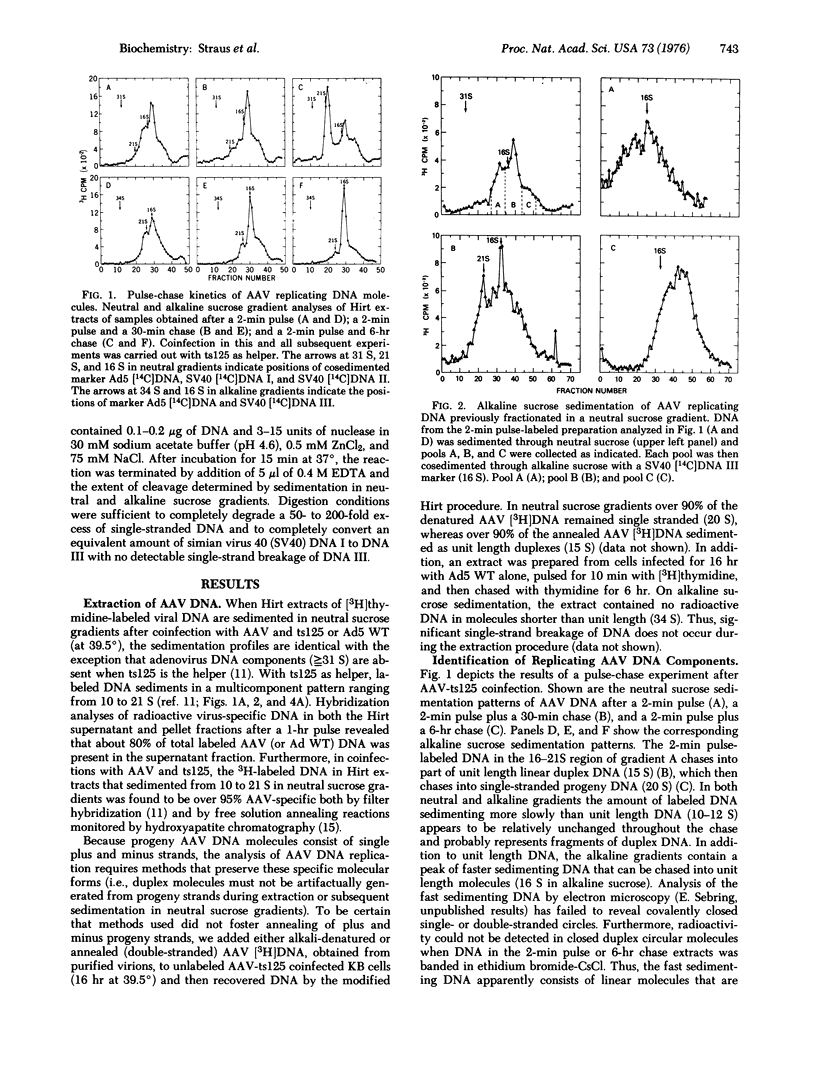
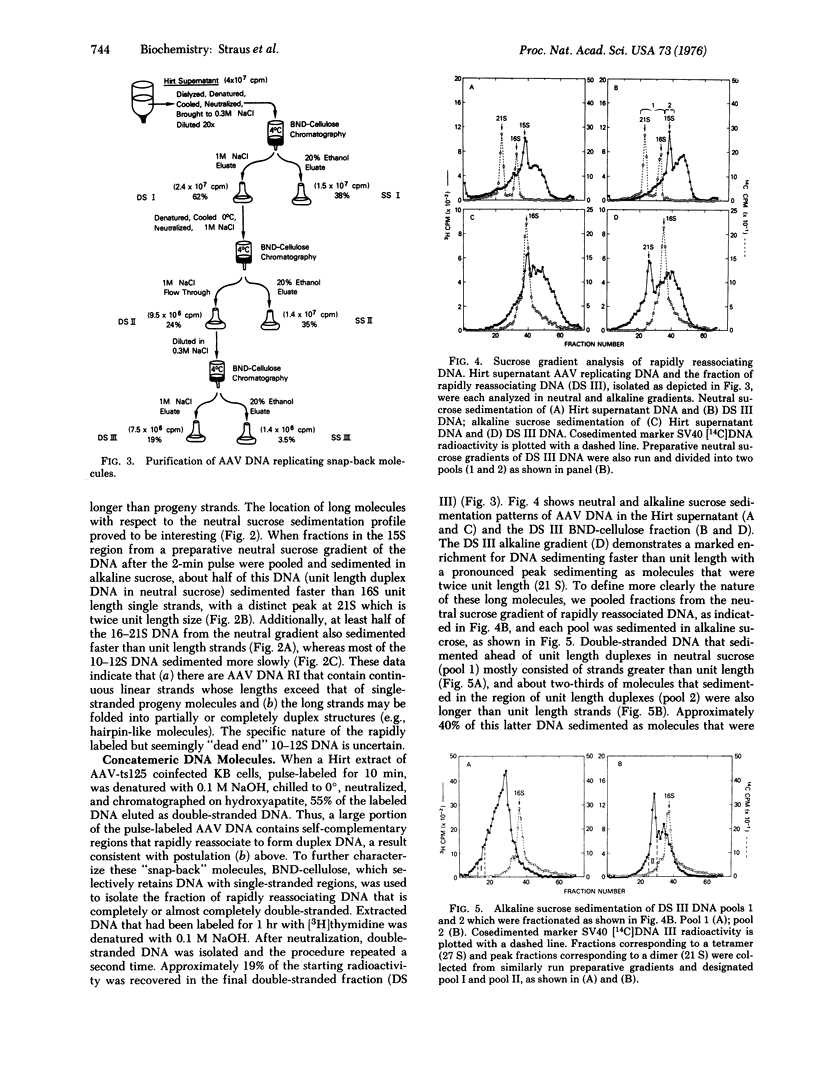
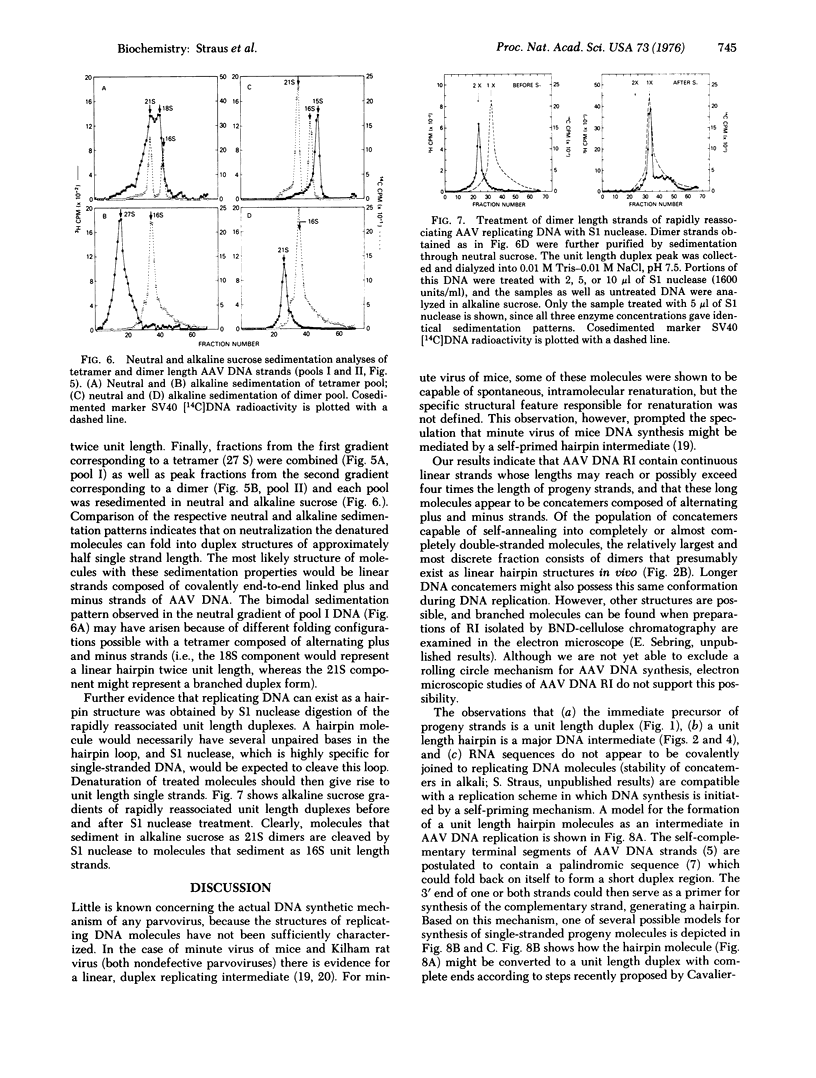
Abstract
Replicating DNA molecules of adenovirus-associated virus (AAV) were selectively extracted from KB cells coinfected at 39.5 detrees with a DNA minus, temperature-sensitive mutant of adenovirus 5 (ts125) as helper. Under these conditions AAV DNA replication proceeds normally, but there is little, if any, adenovirus DNA synthesis. An analysis of the replicating molecules in sucrose density gradients reveals that there are AAV DNA intermediates which consist of covalently linked plus and minus DNA strands. Under denaturing conditions, these concatemers are linear single strands whose lengths can reach at least four times the size of the AAV genome. The most abundant concatemeric species is a dimer which presumably exists in vivo as a unit length hairpin. Unit length linear duplexes appear to be immediate precursors of plus and minus progeny strands. These findings are compatible with a self-priming mechanism for the synthesis of AAV DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Kelly T. J., Jr Letter: Visualization of the inverted terminal repetition in adeno-associated virus DNA. J Mol Biol. 1974 Jan 15;82(2):267–271. doi: 10.1016/0022-2836(74)90344-1. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G. Specific cleavage of adenovirus-associated virus DNA by restriction endonuclease R-EcoRI--characterization of cleavage products. Virology. 1975 Feb;63(2):523–538. doi: 10.1016/0042-6822(75)90325-6. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Koczot F. J., Garrison J., Rose J. A., Dolin R. Separate helper functions provided by adenovirus for adenovirus-associated virus multiplication. Nat New Biol. 1973 Jul 18;244(133):71–73. doi: 10.1038/newbio244071a0. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. Arrangement of sequences in the inverted terminal repetition of adenovirus 18 DNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3039–3043. doi: 10.1073/pnas.72.8.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerry H. W., Kelly T. J., Jr, Berns K. I. Arrangement of nucleotide sequences in adeno-associated virus DNA. J Mol Biol. 1973 Sep 15;79(2):207–225. doi: 10.1016/0022-2836(73)90001-6. [DOI] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle G., Patch C., Khoury G., Rose J. Isolation and partial characterization of single-stranded adenoviral DNA produced during synthesis of adenovirus type 2 DNA. J Virol. 1975 Oct;16(4):775–782. doi: 10.1128/jvi.16.4.775-782.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VII. Helper requirement for viral deoxyribonucleic acid and ribonucleic acid synthesis. J Virol. 1972 Jul;10(1):1–8. doi: 10.1128/jvi.10.1.1-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. In vivo conversion of the single-stranded DNA of the kilham rat virus to a double-stranded form. J Virol. 1973 Feb;11(2):299–305. doi: 10.1128/jvi.11.2.299-305.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedat J. W., Kelly R. B., Sinsheimer R. L. Fractionation of nucleic acid on benzoylated-naphthoylated DEAE cellulose. J Mol Biol. 1967 Jun 28;26(3):537–540. doi: 10.1016/0022-2836(67)90321-x. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Ginsberg H. S., Rose J. A. DNA-minus temperature-sensitive mutants of adenovirus type 5 help adenovirus-associated virus replication. J Virol. 1975 Jan;17(1):140–148. doi: 10.1128/jvi.17.1.140-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C., Ellens D. J., Jansz H. S. Linear intermediates in the replication of adenovirus DNA. Nat New Biol. 1972 Sep 13;239(89):47–49. [PubMed] [Google Scholar]
- Tattersall P., Crawford L. V., Shatkin A. J. Replication of the parvovirus MVM. II. Isolation and characterization of intermediates in the replication of the viral deoxyribonucleic acid. J Virol. 1973 Dec;12(6):1446–1456. doi: 10.1128/jvi.12.6.1446-1456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoren M. M., Sebring E. D., Salzman N. P. Specific initiation site for simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):462–468. doi: 10.1128/jvi.10.3.462-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]



