Abstract
Although inhaled bronchodilators are commonly used in the treatment of airway disease to dilate airway smooth muscle, little is known regarding the mechanisms that regulate albuterol movement across the epithelium to reach its target, the airway smooth muscle. Because the rate of onset depends on the transepithelial transport of albuterol, to determine the mechanisms that regulate the transepithelial movement of albuterol is essential. Human bronchial epithelial cells, fully redifferentiated in culture at the air–liquid interface, were used to study the cellular uptake and total transepithelial flux of 3H-albuterol from the apical to the basolateral surfaces. 3H-mannitol and transepithelial electrical resistance were used to quantify changes in paracellular permeability. The majority of albuterol flux across the epithelium occurred via the paracellular route. The cellular uptake of albuterol was found to be saturable, whereas transepithelial flux was not. Cellular uptake could be inhibited by the amino acids lysine and histidine, with no effect on net transepithelial flux. Transepithelial flux was altered by maneuvers that collapsed or disrupted intercellular junctions. Acidification, usually seen in exacerbations of airway disease, decreased albuterol flux. In addition, albuterol increased its own paracellular permeability. The ability of albuterol to modulate paracellular permeability was blocked by the β2-adrenergic receptor–selective antagonist ICI 118551. Albuterol mainly crosses the epithelium via the paracellular pathway, but has the ability to modulate its own permeability through changes in the leakiness of tight junctions, which is modulated through the signaling of the β2-adrenergic receptor.
Keywords: albuterol, transepithelial flux, β2-adrenergic receptor signaling, airway epithelial permeability modulation, tight junctions
Clinical Relevance
The application of albuterol as rescue medication in the treatment of airway disease exacerbations is staggering, and transepithelial transport could exert a significant impact on its rate of onset and clinical outcomes. Here we demonstrate for the first time, to the best of our knowledge, that the rapid onset of albuterol is attributable to its ability to enhance its own paracellular transport by binding to receptors on airway epithelial cells. The implications of this discovery in relation to clinical outcomes in airway disease or in smokers with asthma are discussed. The ability of albuterol to enhance epithelial permeability could also revolutionize the systemic delivery of drugs via the pulmonary route, when coadministered with inhaled β2-agonists.
Albuterol is a β-adrenergic agonist commonly used in the treatment of airway disease, including asthma and chronic obstructive pulmonary disease. Albuterol binds to β2-adrenergic receptors on bronchial smooth muscle cells. This binding results in the activation of adenylyl cyclases, which in turn results in the cyclic adenosine monophosphate (cAMP)–mediated activation of protein kinase A, and thereby smooth muscle relaxation (1). The bioavailability of inhaled drugs is determined by transepithelial flux across the airway epithelium and by tissue retention. Transepithelial flux could depend on either of two parallel pathways (or a combination of both), namely a transcellular pathway and a paracellular pathway, also known as the shunt pathway. Although previous studies looked at the transport of bronchodilators, including albuterol, in bronchial cell lines or other epithelia (2, 3), little is known about the predominant route of transepithelial albuterol flux.
The transcellular pathway could be mediated by simple diffusion across the apical and basolateral membranes (unlikely, given that albuterol is charged at neutral and slightly acidic pH), or by ion channels or transporters that mediate active transport or facilitate diffusion. A number of studies, including ours, that relied on the ability of albuterol to compete with known substrates of transporters suggested a potential role of organic cation transporters for cellular uptake (4, 5), but no complete transporter system has been identified.
The paracellular pathway or shunt pathway provides a route for passive transepithelial flux, with solute molecules moving in either direction driven solely by their concentration gradient. However, even the paracellular pathway can be regulated by tight junctions and by changes in the volume of lateral intercellular spaces (6–8) or changes in the protein composition of the junction. Permeability across tight junctions is size-selective and charge-selective, and is regulated by the number of tight junction strands and the charge on claudins (9, 10). The paracellular space of airway epithelia is cation-selective (11, 12) and this cation selectivity is regulated by the amino acids in the extracellular loop of claudins (13). Transport studies with more than 50 different nitrogenous cations demonstrated that proton-rich solutes tend to be more permeant for two reasons: stronger binding energies to proton acceptor sites in the paracellular space, and a smaller effective size in a proton acceptor environment (14).
Because albuterol is a hydrophilic molecule that carries a net positive charge over a broad pH range, we reasoned that that the paracellular pathway could account for at least part of albuterol flux by permeation through the hydrated cation channels of the tight junctions and lateral intercellular spaces. The transcellular pathway, on the other hand, could not only have a bearing on overall flux, but could also determine tissue retention and duration of action, thus affecting the bioavailability of albuterol.
In this study, we demonstrate that albuterol transport across the airway epithelium is nonsaturable, and the majority of total flux is paracellular. Cellular uptake, on the other hand, is saturable and contributes a minor component to the net flux. Cellular uptake at the air–liquid interface model is insensitive to competitors or inhibitors of organic cation transporters (OCTs), but can be inhibited by the cationic amino acids lysine and histidine. We also demonstrate that albuterol decreases the transepithelial electrical resistance (TEER) of normal human bronchial epithelial cell (NHBE) monolayers, and increases the epithelial permeability of mannitol and itself.
Materials and Methods
Unless otherwise stated, all materials were obtained from Sigma Chemical Co. (St. Louis, MO). 3H-albuterol (10 Ci/mmol) was purchased as a racemic mixture from Moravek, Inc. (Brea, CA). The purity of 3H albuterol was confirmed according to HPLC. 3H (11.7 Ci/mmol) and 14C (56.5 mCi/mmol) mannitol were purchased from Perkin Elmer Life Sciences (Boston, MA).
Cell Culture
Air–liquid interface (ALI) cultures of human airway epithelial cells were prepared as previously described (15).
Transport and Uptake Studies
Transport and uptake studies were performed as described in the online supplement.
Effect of extracellular pH on transport.
The methodology regarding the effects of pH is described in the online supplement.
Sodium dependence of transport.
The methodology regarding sodium dependence is described in the online supplement.
Tight junction perturbation.
The methodology of tight junction perturbation experiments is described in the online supplement.
To study the effects of albuterol on paracellular permeability, NHBE cultures were pretreated with transport buffer alone or transport buffer containing 10 μM cold albuterol for 1 hour. Then the apical solution was replaced with 0.4 ml of transport buffer containing 500 nM 3H-mannitol. The appearance of mannitol in the basolateral compartment was evaluated as a function of time.
Ussing chamber experiments.
Lobar bronchial tissue was dissected, rinsed with the appropriate bathing solution, and mounted in Ussing chambers (EasyMount Chamber; Physiologic Instruments, San Diego, CA). The apical and basolateral bath solution (Krebs-Henseleit) consisted of 118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM NaH2PO4 ⋅ H2O, 1.2 mM CaCl2 ⋅ 2H2O, and 5.5 mM glucose, pH 7.35, when gassed with 95% O2/5% CO2. Both apical and basolateral solutions were maintained at 37°C by heated water jackets and continuously bubbled with a 95% O2/5% CO2 mixture. To monitor the short-circuit current, the transepithelial membrane potential was clamped at 0 mV with a two-channel voltage clamp (model VCC MC2; Physiologic Instruments), using Ag/AgCl electrodes in agar bridges. Signals were digitized and recorded with DAQplot software (VVI Software, College Station, PA) via a LabJack A/D converter (LabJack Corp., Lakewood, CO). The input resistance of each filter was measured by the application of 1-mV bipolar pulses of 2-second duration. The initial resistance of the tissue was measured similarly. Albuterol (10 μM) was added apically. Fifteen minutes after the addition of albuterol, the resistance of tissue was measured every 90 seconds by the application of 1-mV bipolar pulses of 2-second duration, up to the end of the experiment at 30 minutes.
Confocal microscopy.
The methodology for confocal microscopy experiments is described in the online supplement.
Data analysis
Data are expressed as the means ± SE for four cultures from at least two lungs. Statistical significance was evaluated using unpaired t tests for two groups, or ANOVA followed by the Tukey-Kramer honestly significant difference test for multiple comparisons, as appropriate. P < 0.05 was considered significant.
Apparent permeability (Papp) was determined using the equation:
where dQ/dT is the flux determined from the amount transported (Q) over time (T) during the experiment, A is the surface area of the filter, and C0 is the initial concentration on the donor side.
Results
Kinetic Studies
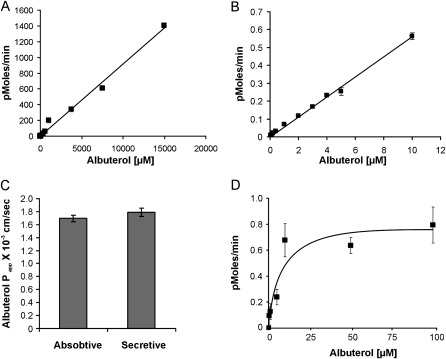
Albuterol flux was studied over an extended concentration range (25 nM to 15 mM). Flux was not saturable over the concentration range studied, implying that flux was mainly driven by a concentration gradient (Figures 1A and 1B). Across the NHBE monolayer, transport showed no net flux, that is, the Papp from the apical to basolateral (absorptive) direction was nearly equal to the Papp from the basolateral to apical (secretory) direction (Figure 1C). To determine whether this seemingly diffusive pathway occurred through cells, cellular uptake was studied over a concentration range of 250 nM to 100 μM. In contrast to overall transport, cellular uptake was saturable, with a Km of 5.6 μM (Figure 1D). Therefore, the data suggest a disconnect between cellular uptake kinetics, which were saturable, and overall flux kinetics, which were not saturable. This finding is best explained in terms of albuterol crossing the airway epithelium, mostly by paracellular diffusion, whereas the transcellular compartment contributes only a small amount to this flux.
Figure 1.
Transepithelial flux studies. (A) Albuterol demonstrates nonsaturable transport kinetics. A broad albuterol concentration range of 0.25–15,000 μM was studied. Saturation was not observed throughout the concentrations tested. Error bars are attached to symbols. (B) No saturable overall transepithelial flux is evident at lower albuterol concentrations of 25 nM to 10 μM. Error bars are attached to symbols. (C) Permeability of albuterol across normal human bronchial epithelial (NHBE) air–liquid cultures. NHBE cells were grown on Transwell filter inserts, and secretory (basolateral to apical) and absorptive (apical to basolateral) flux was determined. NHBE cells demonstrate a symmetric albuterol flux. (D) Albuterol demonstrates saturable uptake kinetics into cells. A concentration range of 0.25–100 μM was tested. A sigmoidal Michaelis-Menton curve was observed, with an apparent Km of 5.6 μM. All data represent the mean ± SE for n = 4 experiments from two different lungs. *P < 0.05.
Cellular Uptake
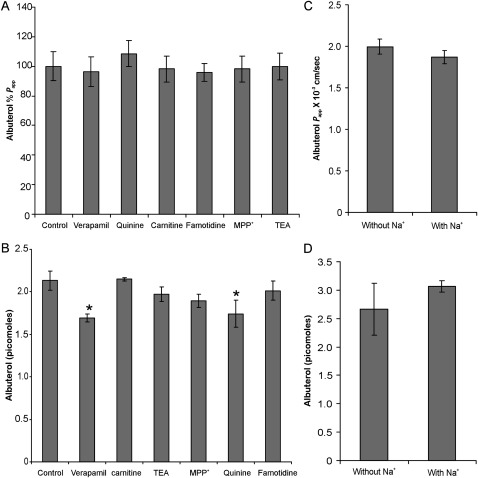
Cellular uptake may play a role in determining tissue retention and the duration of action of any drug. Uptake followed by a prolonged release could increase the duration of action of a drug. Earlier studies by our laboratory and others suggested a potential role of organic cation transporters (the SLC22 family) in the epithelial uptake of albuterol (4, 5). The endogenous substrates for these include monoamine neurotransmitters, choline, L-carnitine, aketoglutarate, cAMP, cGMP, prostaglandins, and urate. Apart from these endogenous substrates, SLC22 family members also transport structurally similar cations. In our laboratory, we demonstrated that albuterol can inhibit the uptake of carnitine, 1-methyl-4-phenylpyridinium (MPP), and ASP+, which are substrates of OCTs (5). To determine whether OCTs play a role in the cellular uptake of albuterol, transport and cellular uptake were studied in the presence and absence of substrates or inhibitors of OCTs. OCT substrates and inhibitors did not demonstrate an inhibition of net transepithelial albuterol flux (Figure 2A). Only quinine and verapamil demonstrated a small, but statistically significant, inhibition of cellular uptake (Figure 2B). Famotidine, an inhibitor of OCTs 1, 2, and 3 (16), did not affect transepithelial flux or the cellular uptake of albuterol. Both transepithelial flux and the cellular uptake of albuterol were also found to be sodium-independent (Figures 2C and 2D), suggesting that OCTN2 transport is not involved.
Figure 2.
Effects of organic cation transporter (OCT) inhibitors and sodium depletion on cellular uptake and overall transepithelial flux of albuterol. (A) Effect of OCT inhibitors (all at 1 mM) on albuterol permeability. Both competitive and noncompetitive inhibitors of OCT exert no significant effect on albuterol permeability (P > 0.05, according to ANOVA). (B) Only quinine and verapamil demonstrate a small but statistically significant effect on the cellular uptake of albuterol. (C and D) Sodium depletion demonstrates no statistically significant effect on albuterol permeability (C) or cellular uptake (D), suggesting that both transepithelial flux and the uptake of albuterol are sodium-independent. All data represent the mean ± SE for n = 4 experiments from two different lungs. *P < 0.05. MPP, 1-methyl-4-phenylpyridinium; TEA, tetraethyl ammonium.
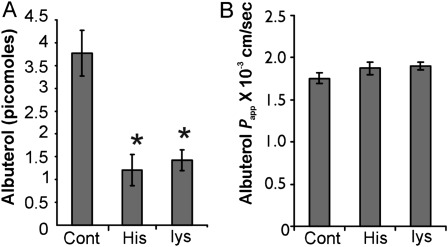
In addition to OCTs, the amino-acid transporters are the only other known systems capable of transporting hydrophilic cationic molecules (reviewed in Ref. 17). These transport systems have broad specificity, are highly redundant, and hence could play a potential role in the transport of hydrophilic cationic drugs. Heterodimeric amino-acid transporters were previously implicated in drug transport (18). Therefore, the transport of albuterol was studied in the presence and absence of 10 mM lysine or histidine. As shown in Figure 3, the cellular accumulation of albuterol was inhibited by both lysine and histidine (Figure 3A). On the other hand, the net apical-to-basolateral flux was not affected by either amino acid (Figure 3B).
Figure 3.
Effect of cationic amino acids on cellular uptake and permeability of albuterol. (A) Two amino acids, lysine (lys) and histidine (His), known to be transported by cationic amino-acid transporters, were assessed for their ability to inhibit cellular albuterol uptake. Histidine and lysine demonstrated a potent inhibition of cellular uptake, suggesting that amino-acid transporters may be involved in the cellular uptake of albuterol. (B) Neither histidine nor lysine showed any significant inhibition of transepithelial albuterol flux (P > 0.05). All data represent the mean ± SE for n = 4 experiments from two different lungs. *P < 0.05. Papp, apparent permeability.
Paracellular Albuterol Flux
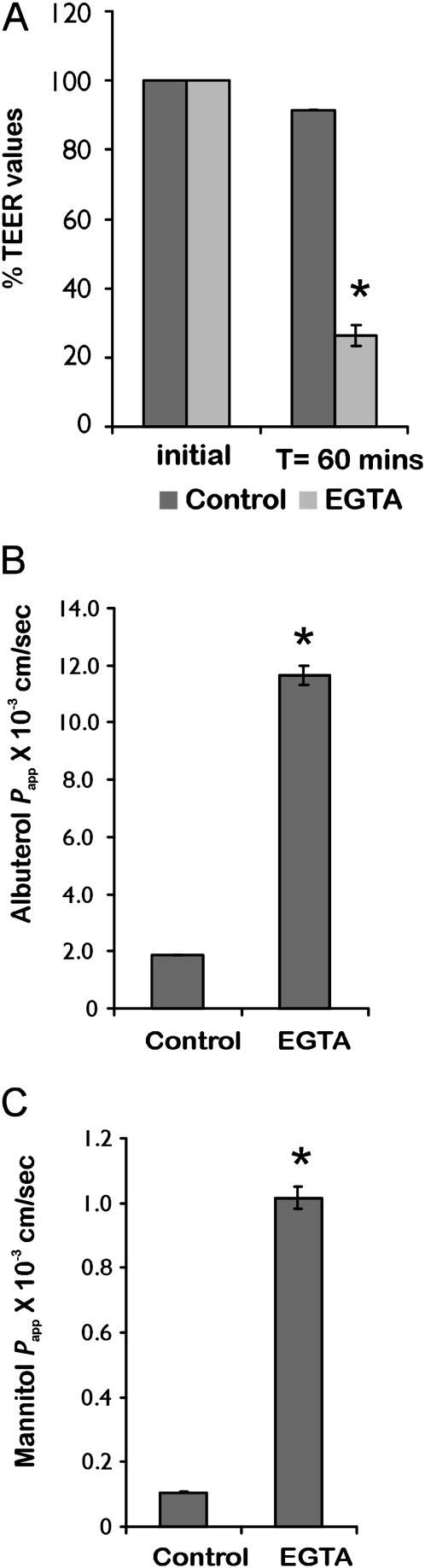
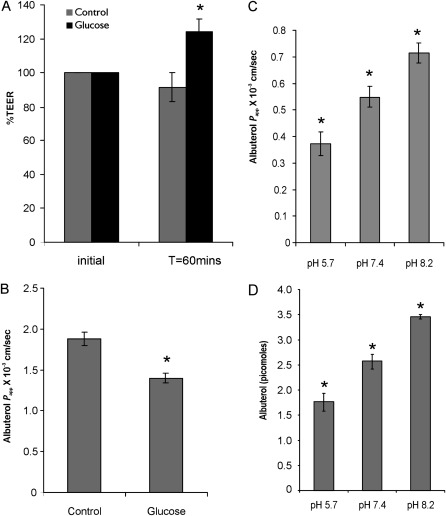
Given these results, the paracellular pathway was examined with respect to net albuterol flux. The paracellular pathway was modulated by calcium chelation to loosen tight junctions (19), and by luminal hypertonicity to collapse lateral spaces and decrease paracellular permeability (20, 21). ALI cultures were preincubated with 6 mM ethylene glycol tetraacetic acid (EGTA) for 1 hour. This resulted in a rapid decrease in TEER values for the NHBE monolayer (Figure 4A). A 6-fold increase in transepithelial albuterol flux was observed, along with a similar increase in mannitol flux, monitored in the same experiment (Figures 4B and 4C). In the second approach, NHBE cells were subjected to luminal hypertonicity, because changes in resistance associated with osmotic gradients have been attributed to the changing geometry of lateral spaces (22) or to increasing numbers and depth of tight junction strands (21). NHBE cells were preincubated with 100 mM glucose to determine its effect on TEER and transepithelial albuterol flux. Luminally applied glucose increased TEER, with a corresponding decrease in albuterol flux (Figures 5A and 5B).
Figure 4.
Effect of tight junction perturbation by ethylene glycol tetraacetic acid (EGTA). (A) As expected, EGTA treatment demonstrated a significant decrease in transepithelial electrical resistance (TEER). (B) Tight junction disruption demonstrated a 6-fold increase in albuterol permeability. (C) A similar increase in permeability was observed for the paracellular marker mannitol. A correlation between tight junction perturbation and a concomitant increase in both albuterol and mannitol transport implies that the paracellular pathway exerts a significant effect on transepithelial albuterol flux. All data represent the mean ± SE for n = 4 experiments from two different lungs. *P < 0.05. T, time.
Figure 5.
Effect of luminal hypertonicity and pH. (A) Luminal hypertonicity after the application of 100 mM glucose increased TEER by 27%, in line with earlier observations of the effects of luminal hypertonicity on other epithelia (20, 21). (B) A concomitant 26% decrease in albuterol permeability was observed in cells pretreated with luminal hypertonicity. (C) Effect of pH on overall albuterol transport in NHBE cells. The transport of albuterol was studied at pH 5.7, 7.4, and 8.2. A decrease in pH is known to change the proton acceptor sites in tight junctions and decrease the paracellular permeability of cations. As expected, a decrease in pH also decreases albuterol transport. (D) Effect of pH on albuterol uptake into cells. A lower pH also decreased the cellular uptake of albuterol. All data represent the mean ± SE for n = 4 experiments from two different lungs. *P < 0.05.
Transport across the paracellular pathway is pH-dependent, because a change in pH can alter the negative charges, resulting in a change in flux of charged molecules across the paracellular pathway (23). Because albuterol is cationic, the paracellular flux of albuterol should increase with increasing pH. As seen in Figure 5C, a decrease in pH decreases albuterol flux and vice versa, whereas an increase in pH enhances albuterol flux. In addition, the cellular uptake of albuterol was found to be pH-dependent. We observed a decrease in the intracellular accumulation of albuterol with decreasing pH (Figure 5D).
Albuterol Enhances Its Own Transepithelial Flux by Increasing Paracellular Permeability
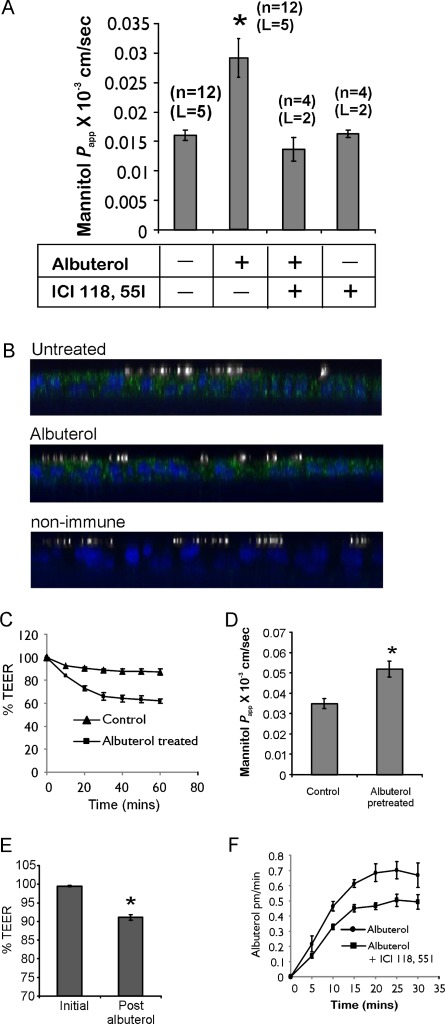
Tight junctions composed of claudins and the lateral intercellular spaces modulate paracellular permeability. Claudins interact with each other in a “kissing” interaction, resulting in the formation of pores (24). Moreover, this interaction is dynamic, in that it opens and reforms (25). Changes in intracellular cAMP and calcium, as well as the membrane potential, were shown to modulate tight junction permeability (26–28). Airway epithelia contain a high density of β2-receptors (29, 30). The activation of β2-receptors results in the generation of cAMP, the activation of PKA, and the phosphorylation of proteins, including those regulating calcium homeostasis (31). Thus, we determined the effect of apically applied albuterol on paracellular permeability. NHBE cells were pretreated with albuterol in the presence or absence of a β2-receptor–specific antagonist, ICI 118551 (100 nM). Apical-to-basolateral mannitol flux was measured at designated intervals. As depicted in Figure 6A, albuterol-pretreated cells showed an increase in the flux of the paracellular marker mannitol, suggesting that albuterol can modulate the paracellular pathway. The ability to modulate the paracellular pathway was completely blocked by ICI 118551. When added alone, ICI 118551 did not demonstrate any effect on epithelial permeability.
Figure 6.
Albuterol increases tight junction permeability. (A) The pretreatment of NHBE cells with albuterol causes a significant increase in permeability of the paracellular marker mannitol. The albuterol-mediated increase in epithelial permeability could be completely blocked by ICI 118551. The numbers involved in each experiment are indicated (n, number of experiments; L, number of lungs). (B) NHBE cells express β2-adrenergic receptor (β2-AR). NHBE cells were double-stained for β2-AR (green) and acetylated tubulin (white), as described in Materials and Methods. NHBE cells demonstrated both apical and basolateral expression of β2-AR, with a higher level of expression apically. No redistribution of β2-AR occurred after albuterol treatment. (C) Effect of albuterol on TEER. Cystic fibrosis (CF) cells were pretreated to rule out the contribution of transcellular currents, as described in Results. Cells were pretreated with albuterol, and TEER was measured every 10 minutes. Albuterol caused a rapid and sustained decrease in TEER. Data represent the mean ± SE for n = 4 experiments from two different lungs. (D) The permeability of the paracellular marker mannitol after measurements of TEER demonstrated a concomitant increase in mannitol permeability for albuterol-pretreated CF cells. Data represent the mean ± SE for n = 4 experiments from two different lungs. (E) Effect of albuterol on bronchial tissue. Bipolar pulses of 2 mV at 90-second intervals were used to determine TEER. For each lung, the highest initial resistance was considered 100%, and the rest of the measurements were normalized accordingly. Albuterol was added to the mucosal side of the bronchial tissue, and the change in TEER as a function of paracellular permeability was determined. Data represent the mean ± SE for bronchi from four different lungs. (F) Albuterol increases its own permeability by binding to the β2-receptor. Cells were treated with albuterol alone (10 μM) or albuterol and ICI 118551 (100 nM). Blocking the β2-receptor decreased albuterol transport by interfering with the ability of albuterol to increase epithelial permeability. Data represent the mean ± SE for n = 6 experiments from three different lungs. *P < 0.05.
To determine whether NHBE cells grown at the ALI express β2-adrenergic receptor (β2-AR) and its subcellular localization changes in the presence of albuterol, NHBE cells were treated apically with 10 μM albuterol or vehicle, and double-stained with antibodies to β2-AR or nonimmune IgG and acetylated tubulin (to label cilia). In polarized NHBE cells, β2-AR was significantly expressed at or near the apical cell surface (Figure 6B). Staining was also observed at the lateral and basal surfaces, similar to the staining observed by Naren and colleagues (32). We did not observe a significant cellular redistribution of β2-AR upon treatment with albuterol (Figure 6B).
To investigate this pathway further, we sought to eliminate the contribution of ion fluxes through the transcellular pathway. As described previously (12), cystic fibrosis (CF) epithelia that lack CF transmembrane conductance regulator anion channels with added inhibitors are suitable for this purpose. We confirmed that albuterol demonstrated identical flux kinetics in CF and NHBE cells (data not shown).
CF cells cultured at the ALI were pretreated with amiloride (10 μM) to inhibit epithelial Na+ channels, 4,4′-dinitrostilbene-2,2′-disulphonic acid (100 μM) to inhibit other chloride channels, and BaCl2 (5 mM) to inhibit potassium channels that are known to interact with and that are activated by β2-receptors (33). These inhibitors were present for the remainder of the experiment. Where indicated, 10 μM of albuterol were added apically, and TEER values were measured every 10 minutes. The donor solution was replaced with transport buffer containing 3H-mannitol. The transport of mannitol was determined as a function of time. As shown in Figure 6C, albuterol caused a modest but reproducible and sustained decrease in TEER. The maximum decrease was observed within the first 20 minutes. To determine whether the TEER decrease translated into an increase in permeability, mannitol was applied apically to control and albuterol-pretreated cells. Albuterol-pretreated cells showed an increased rate of mannitol flux (Figure 6D), suggesting that the decrease in TEER was attributable to increased epithelial permeability. We also determined whether albuterol could exert a similar effect on intact bronchial tissue. Lobar bronchi were dissected and mounted in Ussing chambers in Krebs-Henseleit buffer and pretreated with amiloride (10 μM), and the initial TEER was determined by an application of 1-mV bipolar pulses of 2-second duration. Pulses were discontinued, and albuterol (10 μM) was added apically. Fifteen minutes after the addition of albuterol, changes in TEER were determined. As shown in Figure 6E, albuterol treatment demonstrated a significant change in tissue resistance, which could only be attributable to an increase in paracellular permeability, in light of our other data. The bronchial epithelium contributed significantly to the overall resistance of the bronchial tissue, because tissue in which the epithelium was removed demonstrated a 62% lower resistance than tissue with intact epithelium, and this resistance did not change after the addition of albuterol (data not shown).
To determine whether the ability of albuterol to increase permeability can enhance its own transport, NHBE cells were treated with 10 μM 3H-albuterol in the presence or absence of ICI 118551 (100 nM), and albuterol flux was determined. If albuterol enhances its own transport by signaling through β2 receptors, then ICI 118551 should decrease albuterol's permeability. As shown in Figure 6F, ICI 118551 demonstrated a decreased initial rate of transport that was significantly different within 10 minutes after the addition of albuterol. The transport rate reached its maximum within 25 minutes, and was consistently higher than the rate observed in the presence of ICI 118551, suggesting that albuterol can increase its own transepithelial permeability.
Discussion
We demonstrate for the first time, to the best of our knowledge, that albuterol can facilitate its own transport via the paracellular pathway by increasing epithelial permeability. In the absence of a specific transporter, cationic hydrophilic drugs are preferentially transported paracellularly, because the paracellular spaces of most epithelia are cation-selective. The transport is rapid, and the only rate-limiting conditions are the concentration, size, and charge on the particle (3). In the case of albuterol, the onset of action as a bronchodilator is rapid, but lasts for a relatively short time (4 hours). Because the paracellular pathway of the airway epithelium is cation-selective, a significant proportion of the transport of albuterol, which is a cationic hydrophilic molecule, could occur via the paracellular pathway. In this report, we tried to estimate the contributions of two parallel and competing pathways (i.e., the transcellular and paracellular pathways), and found the paracellular route to be the major contributor to albuterol flux across the epithelium.
We observed a clear disconnect between cellular uptake data and the transepithelial flux of albuterol. Although transepithelial flux is nonsaturable, cellular uptake showed saturation kinetics. Similarly, the inhibition of cellular uptake by the amino acids lysine and histidine did not translate into a significant inhibition of overall transport, suggesting that the transport of albuterol is predominantly paracellular. Moreover, the modulation of tight junctions, either by calcium depletion or luminal hypertonicity, translated into a concomitant change in albuterol's transepithelial flux.
Although the transcellular pathway does not play a major role in albuterol transport, it could exert an impact on the duration of action. Interestingly, the cellular uptake of albuterol was inhibited by the amino acids lysine and histidine, pointing to a potential role of amino-acid transporters in the uptake of albuterol. Cationic amino-acid transporters could be good candidates for albuterol uptake, because they transport positively charged amino acids and demonstrate a high level of redundancy (17). Four distinct mechanisms, designated as systems y+, y+L, b0,+, and B0,+, account for cationic amino-acid transport in mammalian tissues (17, 34). The systems y+, b0,+, and y+L are sodium-independent, whereas B0,+ is sodium-dependent (17, 34). Our data indicate that transport and uptake are sodium-independent, suggesting the potential involvement of systems y+, y+L, or b0,+. The experiments required to identify the transporter involved in cellular albuterol uptake are beyond the scope of this study.
In our previous studies on drug uptake into primary airway epithelia, we observed a significant inhibition of OCT substrate uptake by albuterol, suggesting that albuterol competes with these substrates for transport (5). However, none of these substrates could compete with albuterol for cellular uptake, suggesting that the earlier inhibition that we observed may have been attributable to a noncompetitive inhibition of OCTs by albuterol. ICI 118551 did not inhibit cellular uptake, suggesting that β2-AR binding and receptor internalization via the endosomal pathway do not contribute significantly to albuterol uptake (data not shown).
Notably, the airway epithelia express high concentrations of β2-AR (30) Figure 6B), and hence these drugs initiate signaling cascades in epithelial cells even before they contact receptors on smooth muscle. These signaling events in the context of the pharmacology and dynamics of inhaled drugs are important to understand. We demonstrate that albuterol's ability to increase paracellular permeability could be blocked by the β2-selective antagonist ICI 118551, suggesting that signaling via β2-receptors is involved. Salmeterol, a long-acting β2-agonist, also increased epithelial permeability to an extent comparable to that of albuterol (data not shown). We did not see a significant redistribution of β2-AR on NHBE cells after albuterol treatment. Although agonist binding is known to be responsible for the desensitization and internalization of β2-AR, the process is dynamic and results in the recycling of the receptor back to the cell membrane. Earlier reports demonstrated that the β-adrenergic agonist terbutaline inhibited the microvasculature leakage induced by histamine in guinea pig lungs (35). We do not consider that work to contradict our results, because those measurements involved endothelial leakage and did not assess contributions of the airway epithelium in this process (personal communication from Dr. C.G. Persson).
The use of albuterol as rescue medication in exacerbations of airway disease is staggering. Although the clinical action of albuterol on airway smooth muscle is well explored, the role of transepithelial transport in determining clinical outcomes during acute exacerbations has received scant attention. A delayed onset of action when albuterol is used as a rescue medication could have serious repercussions, including lung hyperinflation and increased work of breathing. Although albuterol transport is paracellular, this transport could be affected in several disease states. In exacerbations of airway disease (36), the airway acidifies, as measured by low pH in exhaled breath condensate. Inflammation causes persistent intercellular and extracellular acidification, possibly through the cytokine-mediated increased activity of a dual nicotinamide adenine dinucleotide phosphate-reduced oxidase that produces intracellular H+ (37). Consistent with the reported preference for cations in paracellular transport (23), the transepithelial transit of albuterol was found to decrease with decreasing pH. This could potentially decrease transepithelial albuterol flux and delay the rate of onset. In such chronically inflamed airways, any interference with the ability of albuterol to modulate epithelial permeability and facilitate its own transport would reduce the bronchodilatory effects of medications. In patients who smoke and have asthma, this pathway could be further compromised, because cigarette smoke could also interfere with albuterol transport by altering the expression pattern of claudins and affecting tight-junction permeability (38), or by interfering with β2-receptor signaling, either through down-regulating receptor density (39) or via proinflammatory cytokines such as transforming growth factor–β1 (40, 41), which can induce β2-receptor desensitization (42). Indeed, admission rates to hospitals for asthma and hospital-based care are increased in smokers (43, 44).
The administration of therapeutics via aerosol to the pulmonary epithelium for systemic delivery represents a significant opportunity for many classes of drugs, and for both small molecules and macromolecules (45). The ability of albuterol and other β2-agonists to increase paracellular permeability could also find applications in enhancing the bioavailability of other systemic drugs delivered via the pulmonary route.
Supplementary Material
Acknowledgments
The authors acknowledge Lisa Novak for help with confocal imaging experiments. Part of this work was accepted as an abstract for a poster presentation (Poster J84) at the American Thoracic Society 2011 International Conference.
Footnotes
This study was supported by National Institutes of Health grants HL-060644 and HL-089399 (M.S.) and HL-066125 (G.E.C.), and by American Lung Association Biomedical Research Grant RG-196042-N (H.J.U.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0220OC on December 8, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Anderson GP. Current issues with beta2-adrenoceptor agonists: pharmacology and molecular and cellular mechanisms. Clin Rev Allergy Immunol 2006;31:119–130 [DOI] [PubMed] [Google Scholar]
- 2.Forbes B, Shah A, Martin GP, Lansley AB. The human bronchial epithelial cell line 16HBE14O− as a model system of the airways for studying drug transport. Int J Pharm 2003;257:161–167 [DOI] [PubMed] [Google Scholar]
- 3.Knipp GT, Ho NF, Barsuhn CL, Borchardt RT. Paracellular diffusion in CACO-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J Pharm Sci 1997;86:1105–1110 [DOI] [PubMed] [Google Scholar]
- 4.Ehrhardt C, Kneuer C, Bies C, Lehr CM, Kim KJ, Bakowsky U. Salbutamol is actively absorbed across human bronchial epithelial cell layers. Pulm Pharmacol Ther 2005;18:165–170 [DOI] [PubMed] [Google Scholar]
- 5.Horvath G, Schmid N, Fragoso MA, Schmid A, Conner GE, Salathe M, Wanner A. Epithelial organic cation transporters ensure pH-dependent drug absorption in the airway. Am J Respir Cell Mol Biol 2007;36:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu AS. Claudins and epithelial paracellular transport: the end of the beginning. Curr Opin Nephrol Hypertens 2003;12:503–509 [DOI] [PubMed] [Google Scholar]
- 7.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963;17:375–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 2006;68:403–429 [DOI] [PubMed] [Google Scholar]
- 9.Claude P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol 1978;39:219–232 [DOI] [PubMed] [Google Scholar]
- 10.Claude P, Goodenough DA. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol 1973;58:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 2003;285:L1166–L1178 [DOI] [PubMed] [Google Scholar]
- 12.Flynn AN, Itani OA, Moninger TO, Welsh MJ. Acute regulation of tight junction ion selectivity in human airway epithelia. Proc Natl Acad Sci USA 2009;106:3591–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 2002;283:C142–C147 [DOI] [PubMed] [Google Scholar]
- 14.Moreno JH, Diamond JM. Nitrogenous cations as probes of permeation channels. J Membr Biol 1975;21:197–259 [DOI] [PubMed] [Google Scholar]
- 15.Nlend MC, Bookman RJ, Conner GE, Salathe M. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol 2002;27:436–445 [DOI] [PubMed] [Google Scholar]
- 16.Bourdet DL, Pritchard JB, Thakker DR. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (HOCT1; SLC22A1), HOCT2 (SLC22A2), and HOCT3 (SLC22A3). J Pharmacol Exp Ther 2005;315:1288–1297 [DOI] [PubMed] [Google Scholar]
- 17.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 2008;88:249–286 [DOI] [PubMed] [Google Scholar]
- 18.Kageyama T, Nakamura M, Matsuo A, Yamasaki Y, Takakura Y, Hashida M, Kanai Y, Naito M, Tsuruo T, Minato N, et al. The 4F2HC/LAT1 complex transports L-dopa across the blood-brain barrier. Brain Res 2000;879:115–121 [DOI] [PubMed] [Google Scholar]
- 19.Rothen-Rutishauser B, Riesen FK, Braun A, Gunthert M, Wunderli-Allenspach H. Dynamics of tight and adherens junctions under EGTA treatment. J Membr Biol 2002;188:151–162 [DOI] [PubMed] [Google Scholar]
- 20.Bindslev N, Tormey JM, Wright EM. The effects of electrical and osmotic gradients on lateral intercellular spaces and membrane conductance in a low resistance epithelium. J Membr Biol 1974;19:357–380 [DOI] [PubMed] [Google Scholar]
- 21.Madara JL. Increases in guinea pig small intestinal transepithelial resistance induced by osmotic loads are accompanied by rapid alterations in absorptive-cell tight-junction structure. J Cell Biol 1983;97:125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright EM, Smulders A, Tormey J. The role of lateral intercellular spaces and solute polarization effects in the passive flow of water across the rabbit gall bladder. J Membr Biol 1972;7:198–219 [DOI] [PubMed] [Google Scholar]
- 23.Powell DW. Barrier function of epithelia. Am J Physiol 1981;241:G275–G288 [DOI] [PubMed] [Google Scholar]
- 24.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–338 [DOI] [PubMed] [Google Scholar]
- 25.Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci USA 2003;100:3971–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Palomo A, Meza I, Beaty G, Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol 1980;87:736–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krizbai IA, Deli MA. Signalling pathways regulating the tight junction permeability in the blood–brain barrier. Cell Mol Biol (Noisy-le-grand) 2003;49:23–31 [PubMed] [Google Scholar]
- 28.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 2008;1778:729–756 [DOI] [PubMed] [Google Scholar]
- 29.Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis 1985;132:541–547 [DOI] [PubMed] [Google Scholar]
- 30.Davis PB, Silski CL, Kercsmar CM, Infeld M. Beta-adrenergic receptors on human tracheal epithelial cells in primary culture. Am J Physiol 1990;258:C71–C76 [DOI] [PubMed] [Google Scholar]
- 31.Hirota S, Helli PB, Catalli A, Chew A, Janssen LJ. Airway smooth muscle excitation–contraction coupling and airway hyperresponsiveness. Can J Physiol Pharmacol 2005;83:725–732 [DOI] [PubMed] [Google Scholar]
- 32.Naren AP, Cobb B, Li C, Roy K, Nelson D, Heda GD, Liao J, Kirk KL, Sorscher EJ, Hanrahan J, et al. A macromolecular complex of beta 2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA 2003;100:342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanrachakul B, Pipkin FB, Khan RN. Contribution of coupling between human myometrial beta2-adrenoreceptor and the Bk(Ca) channel to uterine quiescence. Am J Physiol Cell Physiol 2004;287:C1747–C1752 [DOI] [PubMed] [Google Scholar]
- 34.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. Cats and hats: the SLC7 family of amino acid transporters. Pflugers Arch 2004;447:532–542 [DOI] [PubMed] [Google Scholar]
- 35.Persson CG, Erjefalt I. Terbutaline and adrenaline inhibit leakage of fluid and protein in guinea-pig lung. Eur J Pharmacol 1979;55:199–201 [DOI] [PubMed] [Google Scholar]
- 36.Manzanares D, Monzon ME, Savani RC, Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via Rhamm and Ron. Am J Respir Cell Mol Biol 2007;37:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fragoso MA, Fernandez V, Forteza R, Randell SH, Salathe M, Conner GE. Transcellular thiocyanate transport by human airway epithelia. J Physiol 2004;561:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merikallio H, Kaarteenaho R, Paakko P, Lehtonen S, Hirvikoski P, Makitaro R, Harju T, Soini Y. Impact of smoking on the expression of claudins in lung carcinoma. Eur J Cancer 2011;47:620–630 [DOI] [PubMed] [Google Scholar]
- 39.Laustiola KE, Lassila R, Kaprio J, Koskenvuo M. Decreased beta-adrenergic receptor density and catecholamine response in male cigarette smokers: a study of monozygotic twin pairs discordant for smoking. Circulation 1988;78:1234–1240 [DOI] [PubMed] [Google Scholar]
- 40.Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys 2005;43:167–188 [DOI] [PubMed] [Google Scholar]
- 41.Wang RD, Wright JL, Churg A. Transforming growth factor–beta1 drives airway remodeling in cigarette smoke–exposed tracheal explants. Am J Respir Cell Mol Biol 2005;33:387–393 [DOI] [PubMed] [Google Scholar]
- 42.Mak JC, Rousell J, Haddad EB, Barnes PJ. Transforming growth factor–beta1 inhibits beta2-adrenoceptor gene transcription. Naunyn Schmiedebergs Arch Pharmacol 2000;362:520–525 [DOI] [PubMed] [Google Scholar]
- 43.Prescott E, Lange P, Vestbo J. Effect of gender on hospital admissions for asthma and prevalence of self-reported asthma: a prospective study based on a sample of the general population: Copenhagen City Heart Study Group. Thorax 1997;52:287–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sippel JM, Pedula KL, Vollmer WM, Buist AS, Osborne ML. Associations of smoking with hospital-based care and quality of life in patients with obstructive airway disease. Chest 1999;115:691–696 [DOI] [PubMed] [Google Scholar]
- 45.Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc 2004;1:338–344 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.