Abstract
Influenza A virus (IAV) is a worldwide public health problem causing 500,000 deaths each year. Palmitoyl-oleoyl-phosphatidylglycerol (POPG) is a minor component of pulmonary surfactant, which has recently been reported to exert potent regulatory functions upon the innate immune system. In this article, we demonstrate that POPG acts as a strong antiviral agent against IAV. POPG markedly attenuated IL-8 production and cell death induced by IAV in cultured human bronchial epithelial cells. The lipid also suppressed viral attachment to the plasma membrane and subsequent replication in Madin-Darby canine kidney cells. Two virus strains, H1N1-PR8-IAV and H3N2-IAV, bind to POPG with high affinity, but exhibit only low-affinity interactions with the structurally related lipid, palmitoyl-oleoyl-phosphatidylcholine. Intranasal inoculation of H1N1-PR8-IAV in mice, in the presence of POPG, markedly suppressed the development of inflammatory cell infiltrates, the induction of IFN-γ recovered in bronchoalveolar lavage, and viral titers recovered from the lungs after 5 days of infection. These findings identify supplementary POPG as a potentially important new approach for treatment of IAV infections.
Keywords: antiviral, innate immunity, pulmonary surfactant
Clinical Relevance
This research identifies the pulmonary surfactant lipid, phosphatidylglycerol, as a potential new agent for the prevention of influenza A virus infection. The lipid may also be useful for treating established virus infections.
Influenza A virus (IAV) is one of the most common viruses causing global health problems and life-threatening infections, resulting in an estimated 500,000 deaths each year (1). In the United States, 5–20% of the population is infected annually, producing 200,000 hospitalizations and 36,000 deaths (1–3). Patients with chronic pulmonary disease (e.g., chronic obstructive pulmonary disease, asthma) are more susceptible to IAV infection, and typically develop more severe symptoms requiring hospitalization or intensive care unit admissions (4–6). In 2009–2010, the pandemic influenza A outbreak was caused by a novel IAV of swine origin and the H1N1 subtype. This pandemic spread rapidly, and is illustrative of the problems of emergence of new strains (2, 7). Vaccination is the standard strategy for prevention of influenza, but this effect varies, and depends upon successful matching of the vaccine antigen with the epidemic, or pandemic virus, and population compliance with vaccination programs (1). Vaccine shortages for rapidly spreading pandemic viruses can also limit population coverage and further intensify disease outbreaks and persistence.
Two classes of drugs currently available for treatments of influenza in nonimmune individuals are the ion channel inhibitors (e.g., amantadine, remantadine) and the neuraminidase (NA) inhibitors (NAIs) (e.g., oseltamivir, zanamivir, peramivir) (1). The near-complete loss of efficacy of the ion channel inhibitors has led to heavy reliance upon NAIs (8), which are currently standard drugs of choice for both prophylaxis and the early treatment of IAV infection. The frequency of NAI-resistant IAV is approximately 1% in adults and 4–8% in children (9). This resistance usually develops from prior application of or prophylaxis treatment with NAIs. However, the Centers for Disease Control and Prevention recently reported that seasonal oseltamivir-resistant IAV has appeared independently of oseltamivir use. The osteltamvir-resistant IAV is a more frequent and serious problem in children (10). The growing frequency of NAI-resistant IAV strains (9–11) highlights the importance of developing new agents for the treatment of influenza infection with novel mechanisms of action.
Pulmonary surfactant is a lipid and protein complex that regulates biophysical properties of the alveoli and innate immune responses in the lung (12). It is well recognized that the hydrophilic surfactant proteins (SPs), SP-A and SP-D, play multiple roles in regulating host defense. SP-A and SP-D bind to a variety of bacteria, fungi, and viruses with high affinity, and regulate the innate immune responses to these pathogens in the lung (12, 13). SPs are minor components of the surfactant complex accounting for approximately 10% of the material. The major constituents of pulmonary surfactant are phospholipids, with phosphatidylcholines (PCs) as the dominant molecular class. Dipalmitoyl-PC is the most abundant lipid molecular species in surfactant, and is the lipid most responsible for the reduction of surface tension at the air–tissue interface, within the alveolar compartment (13). Phosphatidylglycerol (PG) is also present in surfactant, and comprises approximately 10-mole % of the lipids. In humans, palmitoyl-oleoyl-PG (POPG) is the most abundant molecular species present within the PG class (14). The concentration of phospholipids in the extracellular pulmonary surfactant present in the alveolar hypophase is estimated to be approximately 35 mg/ml (15). These extraordinarily high extracellular phospholipid levels are not found in any other organ system. In addition, no other organ has such high levels of PG, although trace levels of PG are found in numerous organs where this lipid primarily functions at the subcellular level as a precursor to mitochondrial cardiolipin. The functions of such high levels of extracellular PG within the lung have been unclear, but recent studies now provide evidence that this lipid plays an important role in regulating innate immunity and viral infection (16–21). We recently reported that POPG suppresses LPS-induced inflammatory responses in vivo and in vitro through direct interactions with CD14 and MD2 (16). Previous studies have also reported that PG antagonizes ligand recognition by LPS-binding protein and CD14, and reduces LPS-induced inflammatory responses (17–19). In addition to regulating cellular responses to LPS, CD14 has been implicated in the innate immune response to respiratory syncytial virus (RSV) (22). This latter connection prompted recent examination of the effects of POPG upon RSV-induced inflammation and infection (20). These studies produced the unanticipated finding that POPG blocks RSV infection in vitro and in vivo by disrupting viral attachment to epithelial cell surfaces. An additional unanticipated finding was that supplemental POPG, administered intranasally, markedly attenuated RSV infection in vivo in mice (20). This unexpected antiviral activity of surfactant lipid led us to examine the effect of POPG as an IAV antagonist. The goals of this study were to determine if POPG could: (1) suppress the inflammatory response and cell death induced by IAV infection in epithelial cells in vitro; (2) inhibit viral attachment and subsequent replication in epithelial cells; (3) directly interact with IAV; and (4) attenuate IAV infection in vivo. Our findings demonstrate that supplemental POPG is a potent antiviral agent against IAV. These findings strongly suggest that POPG and related compounds play an important role in pulmonary innate immunity, and could be developed and used as a novel therapy against IAV infection.
Materials and Methods
Viruses, Tissue Culture, Infection, and Surfactant Lipid Treatments
IAVs, Philippines 82/H3N2 and H1N1/PR8, were prepared as previously described (23–25). Madin-Darby canine kidney (MDCK) cells and those from a human bronchial epithelial cell line (Beas2B) were obtained from ATCC (Manassas, VA). Phospholipids were obtained from Avanti (Alabaster, AL), and unilamellar liposomes were prepared as previously reported (16, 20). To examine the effects of phospholipids on H3N2-IAV infection, cells were pretreated for 1 hour with POPG or palmitoyl-oleoyl-PC (POPC) liposomes (20).
Viral Protein Expression
MDCK cells were grown in 24-well plates and pretreated for 1 hour with phospholipids before virus addition. Viruses were added to cells in the presence or absence of phospholipids, and the total well lysates were subjected to immunoblotting after 36 hours using Goat polyclonal anti-IAV antibody (Millipore, Billerica, MA) and β-actin (Cell Signaling Technology, Danvers, MA). Quantification of M1 protein (MP) and NA protein expression was performed using NIH Image J1.34 software (National Institutes of Health, Bethesda, MD).
Hemagglutinin Messenger RNA Analysis by Quantitative RT-PCR
MDCK cells were grown in 24-well plates, and H3N2-IAV adsorption was performed using a multiplicity of infection (MOI) of 0.5–1.0 for 2 hours at 37°C. Immediately after the adsorption, and at 24 hours, total well contents were processed for RNA extraction using a Qiagen RN-easy kit (Qiagen, Germantown, MD). Hemagglutinin (HA) messenger RNA (mRNA) expression was quantified using a quantitative RT-PCR kit (Invitrogen, Camarillo, CA).
Binding of IAVs to Phospholipids and MDCK Cells
To examine the direct interactions between IAV and phospholipids, solid-phase binding assays were performed (20). Phospholipid-coated wells were incubated for 2 hours at 37°C with varying concentrations of viruses. Viral attachment was detected with goat anti-IAV antibody added with 3% BSA at 37°C. The bound viruses were quantified by absorbance at 450 or 490 nm.
For cellular binding studies, MDCK cells were grown in 24-well plates and IAV was adsorbed to the monolayers for 2 hours at 19°C, either in the absence or presence of phospholipids. At 19°C, endocytosis by MDCK cells is minimal, and this temperature allows viral binding to reach equilibrium within 2 hours. The cell monolayers were processed at 0°C for subsequent analysis by quantitative immunoblotting.
In Vivo Suppression of Influenza A Infection
Female BALB/c mice (6 wk old) were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were anesthetized with 0.25 g/kg avertin introduced intraperitoneally (20). Anesthetized mice were inoculated intranasally with a total volume of 50 μl of PBS in groups consisting of sham infection, IAV infection (80 plaque-forming units [pfu]/mouse), IAV infection plus POPG, and POPG alone. POPG liposomes were prepared in PBS (16, 20), and mice were inoculated with 3 mg of the lipid premixed with the virus. On specific days, mice were killed by intraperitoneal injection of 0.25 ml of Nembutal (10 mg/ml). Bronchoalveolar lavage fluid was used for differential cell quantification and IFN-γ analysis (20). Homogenates of the left lungs were used for IAV plaque assays (26). The right lungs were processed for lung histopathology score (20, 27). Animal studies followed all prescribed guidelines, and were approved by the Institutional Animal Care and Use Committee.
Statistical Analysis
All results are shown as means (±SE). ANOVA was used to determine the level of significant difference among all groups. Differences among groups were considered significant at P less than 0.05.
Results
POPG Attenuates H3N2-IAV–Induced Cytokine Production in Human Bronchial Epithelial Cells
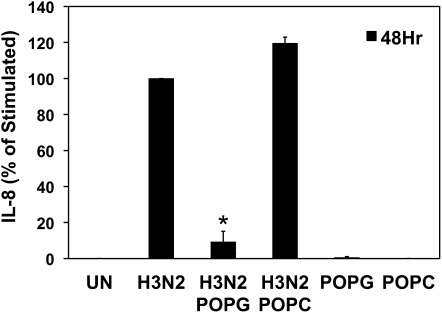
We first examined the effects of POPG upon IL-8 production induced by H3N2-IAV in the Beas2B cell line. IL-8 is a typical early alarm cytokine released by tissues to recruit neutrophils to sites of injury and infection. Cells were pretreated with POPG, in the form of small unilamellar vesicles, for 1 hour and challenged with H3N2-IAV at an MOI of 2 per cell. As shown in Figure 1, H3N2-IAV induced a 6,000-fold increase of IL-8 compared with uninfected cells. POPG (200 μg/ml) treatment inhibited H3N2-IAV–induced IL-8 production by 91%. A control lipid, POPC, did not alter the virally induced IL-8 production. POPG and POPC contain identical hydrophobic domains, but differ in their hydrophilic domains, which contain phosphoglycerol and phosphocholine, respectively. Treatment of Beas2B cells with either POPG or POPC in the absence of virus had no effect upon basal IL-8 production. From these experiments we conclude that POPG acts as a potent inhibitor of the inflammatory response elicited by H3N2-IAV in cultured human epithelial cells. These results also indicate that the polar portion of POPG plays a major role in dictating the specificity of the lipid as an antagonist of H3N2-IAV induction of IL-8 production.
Figure 1.
Palmitoyl-oleoyl-phosphatidylglycerol (POPG) attenuates H3N2–influenza A virus (IAV)–induced IL-8 production by bronchial epithelial cells. IL-8 production by cells from a human bronchial epithelial cell line (Beas2B) was determined by ELISA after a 48-hour H3N2-IAV challenge in either the absence or presence of 200 μg/ml of POPG or palmitoyl-oleoyl-phosphatidylcholine (POPC). The cells were either sham treated (UN) or challenged with virus at a multiplicity of infection (MOI) of 2. Values shown are means (±SE) for three independent experiments; *P < 0.05. UN, uninfected.
The concentration of POPG used in these experiments was less than 10% of the PG levels found in pulmonary surfactant, suggesting that in vivo resident PG pools may provide significant protection from the virus. Previous studies have shown that the actions of POPG are not broadly pleiotropic for inhibition of IL-8 production, because the lipid does not suppress the expression of the cytokines induced by the Toll-like receptor (TLR) 5 agonist, flagellin (20). Additional control experiments demonstrated that POPG does not alter cellular protein synthesis or growth. These findings clearly demonstrate that POPG can significantly suppress H3N2-IAV–induced inflammatory cytokine production.
POPG Prevents Cell Death and Suppresses IAV Protein Expression
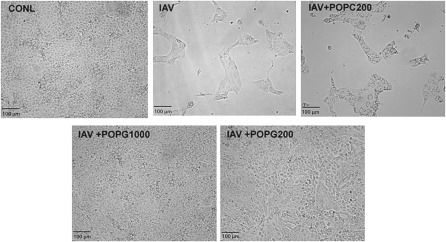
We next investigated the action of POPG upon the cytopathic effects of the virus against MDCK cells, which are routinely used for plaque assays and in vitro propagation of many strains of IAV. As shown in Figure 2, untreated MDCK cells form monolayers with a typical cobblestone appearance. Infection of the cells with H3N2-IAV, at an MOI of 1, destroys the cell monolayer after 36 hours. In contrast, treatment of the cells with virus in the presence of 1 mg/ml POPG completely protects the cells from the cytopathic effects of IAV. At 200 μg/ml, POPG also provides significant protection of the monolayer from the lytic effects of the virus, although a few cytopathic foci are evident. Treatment of the cultures with virus in the presence of 200 μg/ml POPC fails to prevent cell death by IAV infection. From these data, we conclude that POPG acts early in the infectious cycle to protect cells from IAV.
Figure 2.
POPG prevents the cytopathic effects of H3N2 upon cultured Madin-Darby canine kidney (MDCK) cells. Monolayers of MDCK cells were either uninfected (CONL) or treated with H3N2-IAV at an MOI of 1 (IAV), in either the absence or presence of POPG (200 μg/ml or 1,000 μg/ml) or POPC (200 μg/ml), as indicated. After 36 hours, the cultures were examined by light microscopy.
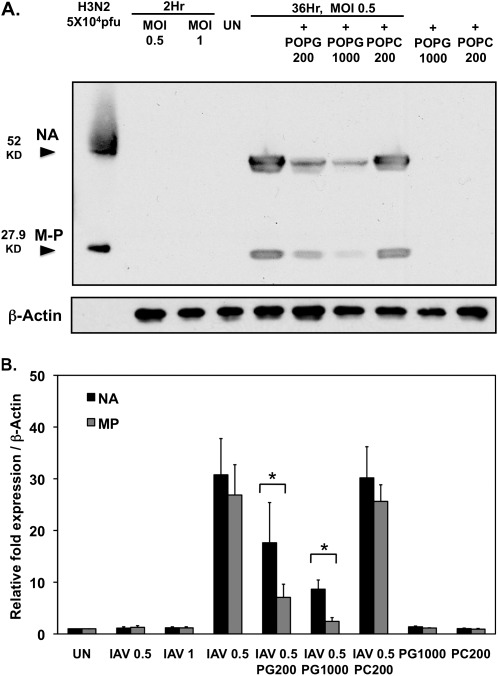
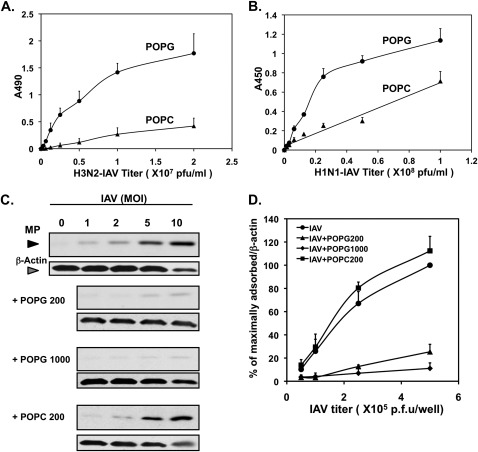
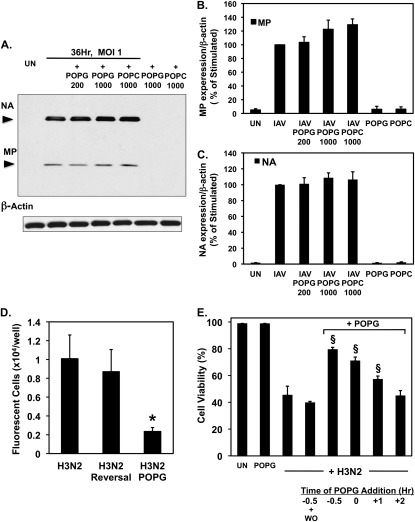
To estimate phospholipid antagonism of virus propagation in MDCK cells, we examined the effects of POPG upon the expression of MP and NA, which were measured after infection of the cells with H3N2-IAV for 36 hours. As shown in Figure 3A, at 2 hours after infection, using viral MOIs of either 0.5 or 1.0, neither MP nor NA protein were detectable; however, after 36-hour infection, MP and NA protein expression clearly increased, and was readily measurable. POPG treatment attenuated both MP and NA protein expression in a dose-dependent manner, with 1 mg/ml being significantly more effective than 200 μg/ml (Figures 3A and 3B). At 1 mg/ml, POPG inhibited NA expression by 80% and MP expression by 75%. In contrast to POPG, POPC was completely ineffective. In addition to the H3N2 strain, we also performed experiments with the mouse-adapted H1N1-PR8-IAV strain, because it is routinely used to perform in vivo studies with mice. As shown in Figure E1 in the online supplement, infection of MDCK cells with H1N1-PR8-IAV at an MOI of 0.5, for 36 hours in the presence of 1 mg/ml POPG or POPC, produced results similar to those found for H3N2-IAV. The treatment with POPG at 1 mg/ml attenuated MP expression by 70%, whereas POPC at 1 mg/ml did not alter MP expression. From these results, we conclude that POPG can suppress IAV protein expression from both H3N2-IAV and H1N1-PR8 strains, and that the effect is dependent upon phospholipid structure.
Figure 3.
POPG suppresses neuraminidase (NA) and M1 protein expression elicited by H3N2-IAV infection of MDCK cells. (A) Monolayers of MDCK cells were either uninfected (UN) or infected with H3N2-IAV at MOIs of 0.5 or 1 for 2 hours in either the absence or presence of POPG (200 μg/ml or 1,000 μg/ml) or POPC (200 μg/ml). After 36 hours, the wells were harvested and analyzed for the expression of NA and M1 (M-P) by SDS-PAGE and immunoblotting. Control experiments also included incubation with POPG (1,000 μg/ml) and POPC (200 μg/ml) in the absence of viral infection. (B) Quantification of NA and M1 protein (MP) expression from three independent experiments. Values shown are means (±SE). *P < 0.05, upon comparison of virally infected cells without phosphatidylglycerol (PG) addition to those with PG addition.
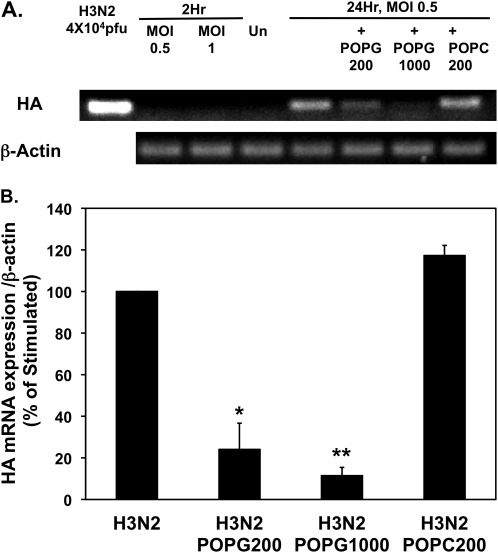
We also examined the mRNA expression for the HA gene using quantitative RT-PCR. The results from these experiments are shown in Figure 4. After 2 hours of viral adsorption, RNA for HA was not detectable at MOIs of either 0.5 or 1.0. After 24 hours, a robust RT-PCR signal was obtained from cells infected at an MOI of 0.5 (Figure 4A). POPG treatment significantly attenuated the HA-mRNA signal, but POPC did not alter the HA-mRNA signal. Quantitative analysis of the RT-PCR data in Figure 4B shows that the inclusion of 200 μg/ml POPG during infection suppressed HA gene expression by 75%, and 1 mg/ml POPG suppressed the expression by 88%. In contrast to the findings with POPG, the treatment with POPC was ineffective. These data demonstrate that POPG suppresses IAV HA-mRNA expression in MDCK cells. Collectively, the experiments examining cytopathology, protein expression, and mRNA expression demonstrate that POPG disrupts the IAV infection process at an early stage, and consequently prevents viral replication and cell death. These findings suggested that POPG might directly interact with the virus and interfere with cell binding, and additional experiments were conducted to test this idea.
Figure 4.
POPG inhibits hemagglutinin (HA) messenger RNA (mRNA) expression in H3N2-IAV–infected MDCK cells. (A) Monolayers of MDCK cells were either uninfected (UN) or infected at MOIs of 0.5, or 1 for 2 hours in either the absence or presence of POPG (200 μg/ml or 1,000 μg/ml) or POPC (200 μg/ml). After 24 hours, the wells were harvested and processed for RNA extraction and subjected to quantitative RT-PCR and gel electrophoresis. (B) The results from three independent experiments are shown. Values are means (±SE). *P < 0.02; **P < 0.001.
POPG Binds to IAV with High Affinity
To investigate the mechanism of the antiviral effect of POPG, we examined the binding interaction between IAV and the lipid. Figure 5A shows the direct binding of H3N2-IAV to a POPG solid phase adsorbed to an ELISA plate. This binding interaction is virus concentration–dependent, high affinity, and saturable. In contrast to the virus binding to POPG, the binding to POPC has the characteristic of a low-affinity, nonspecific interaction. Figure 5B shows the results of similar experiments using H1N1-PR8-IAV with POPG, and also demonstrates high-affinity, concentration-dependent, and saturable binding. Compared with POPG, the lipid POPC is a weak-binding ligand for H1N1-PR8-IAV, and the interaction is nonsaturable and nonspecific.
Figure 5.
POPG binds IAV with high affinity and inhibits cell surface binding of H3N2-IAV. (A) Aliquots of 1.25 nmol phospholipid (POPG or POPC) were adsorbed onto microtiter wells, and the indicated concentrations of H3N2-IAV were added and incubated at 37°C for 2 hours. The viral binding was detected by ELISA and quantified by A490. (B) Solid-phase lipids were prepared as in (A), and the binding of H1N1-PR8-IAV was performed at 37°C for 2 hours. The viral binding was detected by ELISA and quantified by A450. (C) Monolayers of MDCK cells, at 19°C, were challenged with H3N2-IAV at MOIs of 0–10, as indicated, in either the absence or presence of POPG (200 μg/ml or 1,000 μg/ml) or POPC (200 μg/ml). The cultures were harvested and processed for SDS-PAGE and immunoblotting. (D) Quantification of three immunoblotting experiments performed as described for (C). Values shown in (A), (B), and (D) are means (±SE) for three independent experiments. p.f.u., plaque-forming units.
Additional experiments examined whether POPG could interrupt binding of H3N2-IAV to cell surfaces. In these studies, H3N2-IAV was adsorbed to MDCK cells at varying multiplicities of infection for 2 hours at 19°C (to block endocytosis) in either the absence or presence of POPG. After viral adsorption, cell monolayers were washed with PBS to remove unbound viruses, and processed to detect attached viruses by immunoblotting for MP. The results presented in Figure 5C demonstrate that MP detection increased with increasing H3N2-IAV titer. The attachment of the virus to the cell surface was high affinity and saturable. The recovery of MP was inhibited 75% by 200 μg/ml POPG, and 93% by 1 mg/ml POPG, at viral MOIs as high as 10 per cell (Figure 5D). POPC failed to block the binding between MDCK cells and IAV. These data provide clear evidence that POPG binds directly to IAV and disrupts viral adsorption to epithelial cell surfaces, thereby suppressing infection (Figures 2–4) and the inflammatory response (Figure 1).
POPG Antagonism of H3N2-IAV Infection Is Reversible and Dependent upon the Timing of Lipid Addition
The data presented in Figure 5 provide compelling evidence that POPG directly binds to IAV, and this interaction inhibits cell surface attachment of the virus. However, these findings do not provide any information about the consequences of POPG binding to IAV upon the integrity of the virus. To examine this issue in more detail and assess whether the lipid has direct virucidal activity, we conducted two types of experiments. In the first line of experimentation, H3N2-IAV (MOI of 1) was preadsorbed to MDCK cells in culture for 4 hours at 8°C (a temperature that inhibits all viral endocytosis), and then the adsorbed virus was exposed to POPG at either 200 or 1,000 μg/ml for an additional 4 hours at 8°C. As a control phospholipid, POPC was added to separate MDCK monolayers also harboring preadsorbed virus. After the incubation with lipid, the cultures were washed and warmed to 37°C to allow the viral infection to proceed. We reasoned that if the POPG acted as a virucidal agent to compromise the integrity of the virus, the lipid treatment should reduce the subsequent progress of the infection. As shown in Figures 6A–6C, treatment of cell surface–associated virus with POPG failed to disrupt cell infection, as monitored by the production of the viral proteins, NA and MP, at 36 hours after the infection.
Figure 6.
The POPG effect upon IAV infection is reversible and dependent upon the timing of lipid and virus addition. (A) Monolayers of MDCK cells were infected with IAV at an MOI of 1 at 8°C for 4 hours, and then treated with POPG (200 μg/ml or 1,000 μg/ml) or POPC (1,000 μg/ml) at 8°C for 4 hours. The cultures were shifted to 37°C and the infections were allowed to proceed for 36 hours. The contents of each tissue culture well were recovered and processed for immunoblotting with polyclonal goat anti-IAV and rabbit anti–β-actin antibodies. Immunoblots from a representative experiment are shown. (B and C) Quantification of MP and NA expression, normalized to β-actin expression, from three experiments is summarized. (D) Aliquots of H3N2-IAV (108 pfu/ml) were incubated for 1 hour at 37°C in either the absence or presence of 1,000 μg/ml POPG. Subsequently, the viral aliquots were diluted 103-fold in either the absence (Reversal) or presence of POPG (POPG) and used to infect monolayers of MDCK cells at an MOI of 0.05. At 7 hours after the initiation of infection, the cultures were washed with cold PBS and the monolayers were fixed with paraformaldehyde, permeabilized, and stained for the presence of viral antigens with polyclonal goat anti-IAV antibody and rabbit anti-goat Alexa 548 antibody. Fluorescent foci were scored using a Zeiss 200-M microscope (Zeiss, Oberkochen, Germany) and Slidebook software (Leeds Precision Instruments, Minneapolis, MN) at 10× magnification. (E) Cells were either uninfected (UN), treated with 1,000 μg/ml POPG alone (POPG), or challenged with virus (+ H3N2) in either the absence or presence of POPG, as indicated. POPG was added either 0.5 or 0 hours before (designated by minus sign), or 1 or 2 hours after (designated by plus sign) viral addition. In one group of 0.5-hour lipid treatment before viral addition, the POPG was washed out (WO) of the culture well before viral infection. In all other groups the POPG level was maintained until cell harvest. In each case, viral adsorption was conducted for 1 hour at 37°C, followed by removal of unbound virus. Culture wells were harvested and the cells present in both the supernatant and the adherent monolayer were counted and stained for viability using 0.02% trypan blue. Viral infection at an MOI of 0.05 occurred at 0 hours. Values shown in bar graphs are means (±SE) for three independent experiments. *P < 0.05; §P < 0.001.
As a second approach to examining whether POPG was directly acting as a virucidal agent, we tested the reversibility of the interaction of POPG with IAV. In these experiments, H3N2-IAV (108 pfu/ml) was incubated with 1 mg/ml POPG at 37°C for 1 hour. After the incubation, the virus and lipid were diluted 103-fold in either the absence, or presence of 1 mg/ml POPG, and then used to infect monolayers of MDCK cells. As a control for these manipulations, identical aliquots of H3N2-IAV were incubated at 37°C for 1 hour in the complete absence of phospholipid. The infectivity of the IAV in these experiments was examined by quantifying fluorescent foci of viruses formed on the MDCK monolayers 6 hours after infection, detected by antibody. The results presented in Figure 6D show that H3N2-IAV alone produced 1.01 (±0.25) × 104 fluorescent foci per well, and virus transiently exposed to 1 mg/ml lipid and then diluted 103-fold produced 0.87 (±0.23) × 104 foci per well, whereas virus exposed to a constant level of POPG produced 0.23 (±0.04) × 104 foci per well. These results demonstrate that the effects of POPG upon H3N2-IAV are reversible. Together, the data in Figures 6A–6D provide strong evidence that POPG is not directly virucidal.
An additional conclusion from the data in Figures 6A–6C is that POPG must act before viral attachment to the cell surface, because virus already bound to cells is resistant to the antagonistic effects of the lipid. To examine further this latter conclusion, we applied a viral challenge to MDCK monolayers that were exposed to POPG for various periods before and after the addition of virus. To quantify the effects of viral infections in these experiments, we measured cell viability at 36 hours after adding viruses to the cultures. The results of these experiments are presented in Figure 6E. The viability of uninfected cells (98.7 [±0.24]%), and cells treated with POPG alone (98.6 [±0.3]%), was equivalent. The addition of H3N2-IAV at an MOI of 0.5 reduced cell viability to 45.2 (±6.8)%. Pretreatment of MDCK cells with POPG, followed by washout of the lipid before addition of virus, resulted in 44.7 (±3.9)% viability, whereas omission of the lipid washout resulted in 79.3 (±1.7)% viability. Simultaneous addition of lipid and virus provided significant protection to the monolayer, and produced 70.8 (±3.0)% viability. The addition of lipid 1 hour after infection showed marginal protection, and yielded 57.1 (±2.5)% viability. When the lipid was added at 2 hours after infection, there was no significant protection of the cultures from the extent of cell death produced by virus alone. Collectively, these data show that: (1) preincubation of cells with POPG followed by washout, before viral challenge, does not block infection; (2) the antiviral effects of POPG are nearly the same whether cells are preincubated with lipid or simultaneously incubated with lipid at the time of viral challenge; and (3) the protective effects of the lipid diminish rapidly with time after viral challenge. All of these conclusions are consistent with the lipid acting before viral binding to the cell surface. These in vitro properties of POPG suggested that the lipid might function as an effective antiviral agent in vivo, and further experiments were conducted to examine in vivo efficacy.
Intranasal Administration of POPG Suppresses IAV Infection in Mice
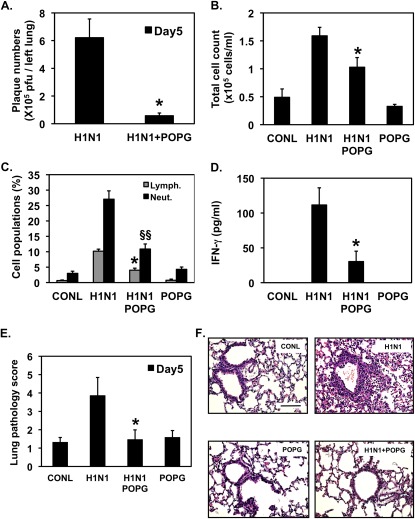
We examined the potency of POPG as an anti-IAV agent using a mouse model of viral infection. Female BALB/c mice (6 wk old) were inoculated intranasally, with the mouse-adapted influenza strain, H1N1-PR8-IAV (80 pfu/mouse), in either the absence or presence of 3 mg POPG. At 5 days after infection, the animals were killed, and the lungs were lavaged, harvested, and analyzed for the effects of viral infection (26). The results presented in Figure 7A demonstrate that the POPG treatment clearly suppressed viral propagation in the lung by a factor of 10 (IAV infection = 6.21 ± 0.6 × 105 pfu; IAV + POPG = 0.6 ± 0.2 × 105 pfu). No plaques were obtained from uninfected animals, animals challenged with ultraviolet light–inactivated IAV, or animals treated with POPG alone. Lavage from control mice produced a total cell number of 5.3 (±1.6) × 104 cells/ml, and H1N1-IAV increased the total cells recovered in lavage to 15.3 (±1.5) × 104 cells/ml (Figure 7B). The POPG treatment reduced the total cell number in lavage, induced by virus, by 50%. The data presented in Figure 7C demonstrate that POPG significantly suppressed the proportional increase in lymphocytic and neutrophilic cellular infiltrates in lavage by 60%. IFN-γ production was undetectable in lavage from control animals, and was 111.5 (±24.7) pg/ml after virus infection. The POPG treatment suppressed the virally induced IFN-γ response by 81% (Figure 7D).
Figure 7.
POPG inhibits H1N1-PR8-IAV infection and inflammation in vivo and suppresses the histopathology elicited by the virus. BALB/c mice were infected with 80 pfu H1N1-PR8 (H1N1) by intranasal inoculation in either the absence or presence of 3 mg of POPG, as indicated. Sham (CONL) and lipid-only treatments (POPG) were also performed. After 5 days of infection, the animals were killed. (A) Amount of virus present in the left lung was quantified using plaque assays. *P < 0.01. (B) Lavage fluid collected from animals was used to quantify total cells recovered from the bronchoalveolar compartment. *P < 0.02. (C) Cytospin preparations were used to quantify the percentage of lymphocytes (Lymph.) and neutrophils (Neut.) present in the lavage fluid. *P < 0.01, §§P < 0.001. (D) The production of IFN-γ was measured in cell-free lavage fluid by ELISA. *P < 0.01. Each group contains four to six animals per individual experiment. (E) Paraffin sections (4 μm) were stained with hematoxylin and eosin, analyzed by light microscopy, and assigned a histopathology score. *P < 0.05. (F) Representative micrographs from the experiment. Values shown in A–E are means (±SE) for three independent experiments. Scale bar, 200 μm.
Lung tissue from experimental animals was examined and assigned a histopathology score (20), and the data are shown in Figure 7E. H1N1-IAV–infected animals had a threefold higher histopathology score than sham-infected control animals, and animals receiving virus plus POPG were not significantly different from sham-infected animals. Representative micrographs are shown in Figure 7E and reveal that H1N1-IAV infection elicited a significant influx of inflammatory cells in alveolar and peribronchial areas and pneumonia. POPG treatment markedly attenuated these virus-induced inflammatory changes, and the lipid treatment alone did not cause significant histological changes. From the data shown in Figures 6 and 7, we conclude that POPG suppresses H1N1-PR8-IAV infection and viral replication in vivo, and markedly reduces the inflammatory responses to the virus. These findings strongly suggest that supplementary POPG could be an important and novel approach for prevention and treatment of IAV infections.
Discussion
In this article, we provide strong evidence that supplementary POPG, the major molecular species of PG present in pulmonary surfactant, potently suppresses the infection of epithelial cells by IAV in vitro and in vivo. By interfering with the initial infective process, the lipid also disrupts the release of inflammatory cytokines, such as IL-8, by the epithelium. The in vitro doses of POPG capable of disrupting IAV infection are similar to in vitro doses of the lipid effective against RSV (20). In contrast, the in vivo doses of POPG required to attenuate IAV infection (3 mg/mouse) are much higher than those effective against RSV (150 μg/mouse). The reasons for these differences are not yet completely understood. One important difference between RSV and mouse-adapted IAV is the efficiency of the infection. Based on our experimental data, only 80 pfu of IAV are required to produce a robust in vivo infection, yielding 2 × 103 pfu/left lung on Day 1, 3 × 105 pfu/left lung on Day 3, and 6 × 105 pfu/left lung on Day 5 after infection, whereas 107 RSV are required to produce an in vivo yield of 5 × 103 pfu/left lung after 5 days (20). The infectivity, thermal stability, and replication kinetics of IAV make this virus less susceptible to POPG than RSV. However, the 3-mg dose of POPG used in mice in these experiments did not produce any deleterious effects in either animal behavior or tissue histopathology.
We are currently examining the delivery and turnover of POPG administered to mice. The turnover rate of POPG will be an important determinant of the window of efficacy of the exogenously applied lipid. The turnover of POPG is expected to be very high in mice, because of their respiratory rate (250–300 breaths/min). By comparison, the respiratory rate in human newborns is 25–30 breaths/minute and the estimated half-life of PG is 30 hours (28). Thus, the anticipated antiviral effects of exogenous POPG in humans may be achievable with reasonable dosing, and can reasonably be expected to be relatively long lived.
The role of PG in pulmonary surfactant has long been enigmatic. Our recent work and earlier studies (16, 18–21) provide strong evidence that PG plays an important role in suppressing TLR-mediated inflammatory processes. We propose that the inhibitory actions of PG against TLRs function to set a high threshold for the engagement of inflammatory cascades in the lung. Fundamentally, this threshold prevents inflammation by casual environmental stimuli, such as ambient levels of microparticulate LPS, but enables engagement of inflammatory processes once the threshold is exceeded by sufficient quantities of TLR agonists. During established infections by bacteria and viruses, the quantity of TLR agonists produced is expected to exceed the inhibitory threshold that results from POPG, thereby allowing a robust inflammatory response to proceed.
In addition to inhibiting TLR activation, emerging research, including the present study, now demonstrates that PG can interfere with viral infections involving the respiratory tract. Our work demonstrates the action of POPG against RSV and IAV. Studies by Perino and colleagues (29) demonstrate that dipalmitoyl-PG also disrupts vaccinia virus infection in vitro and in vivo. Interestingly, the major route of infection for vaccinia virus is respiratory. The principal mechanism of antiviral action of POPG against RSV and IAV is by inhibition of viral attachment to epithelial cell surfaces, and dipalmitoyl-PG antagonizes vaccinia virus in the same manner. In this article, we provide direct evidence that POPG binds to IAV with high affinity (Figures 5A and 5B), and that this binding reaction disrupts the adsorption of viral particles to cell surfaces (Figures 5C and 5D). In mice, the net effects of POPG treatment are to reduce the viral burden by a factor of 10, suppress the influx of inflammatory cells by a factor of 2, reduce the neutrophilic and lymphocytic populations by a factor of 4, and limit IFN-γ production by 80%. We interpret the reduction of IFN-γ levels as a measure of success in reducing the viral burden in vivo. The POPG treatment also reduced the lung histopathology to the level of uninfected animals. Collectively, these findings demonstrate that POPG is an important lead compound for developing new classes of antiviral agents.
The antiviral actions of POPG are likely to be complementary to those of the SPs, SP-A and SP-D, which also bind IAV and markedly attenuate the host inflammatory response. In mouse models of IAV infection, the absence of SP-A is associated with reduced viral clearance and elevated inflammatory responses (relative to wild-type strains), both of which are rectified by providing supplementary SP-A at the time of infection (30). Likewise, genetic ablation of SP-D results in increased viral loads and higher inflammatory cytokine responses compared with wild-type strains; and these reactions are alleviated by adding SP-D at the time of infection (31). In general, the action of SP-D against IAV appears more robust than that of SP-A. The binding of SP-D to IAV is critically dependent upon N-linked glycosylation of asparagine 165 of the viral HA1 protein, whereas the binding of SP-A to IAV is dependent upon the presence of sialic acid in the N-linked oligosaccharide in the C-terminal domain of the SP-A protein. The potential therapeutic application of POPG, or related lipids, has some advantages over the use of SPs, insofar as POPG: (1) is of low molecular weight; (2) likely to be nonimmunogenic; (3) can be chemically synthesized in large amounts; and (4) is chemically stable. The recently discovered activity of different molecular species of PG against multiple viruses (RSV, IAV, and vaccinia) with structurally different surface proteins suggests that single-point mutations to viral surface proteins (e.g., asparagine 165 of HA1) may be insufficient to enable viral evasion of the inhibitory effects of the phospholipid.
Although the antiviral properties of PG were not anticipated, it is clear that in vitro administration of this lipid, which is part of the intrinsic pulmonary surfactant system, can disrupt the infective processes of RSV and IAV, which are serious, problematic pathogens worldwide. Most importantly, our data demonstrate that supplemental POPG introduced via the airways can significantly attenuate IAV infection in mice. This property of POPG suggests that the lipid has significant potential for preventing viral infections in at-risk human populations, and perhaps treating viral infections after they have become established.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants HL094629, HL 073907, and CBDE-2009.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0194OC on November 3, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Glezen WP. Clinical practice: prevention and treatment of seasonal influenza. N Engl J Med 2008;359:2579–2585 [DOI] [PubMed] [Google Scholar]
- 2.Gooskens J, Jonges M, Claas E, Meijer A, van den Broek P, Kroes A. Morbidity and mortality associated with nosocomial transmission of oseltamivir-resistant influenza A (H1N1) virus. JAMA 2009;301:1066. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet 2003;362:1733–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan V, Diette GB, Rand CS, Bilderback AL, Merriman B, Hansel NN, Krishnan JA. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med 2006;174:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy 2009;39:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009;361:1935–1944 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Seasonal flu. Washington, D.C.: World Health Organization; 2009 [Google Scholar]
- 8.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med 2005;353:1363–1373 [DOI] [PubMed] [Google Scholar]
- 9.Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA 2009;301:1066–1069 [DOI] [PubMed] [Google Scholar]
- 10.Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, Hayden FG, Sugaya N, Kawaoka Y. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 2004;364:759–765 [DOI] [PubMed] [Google Scholar]
- 11.Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, St George K, Epperson S, Brammer L, Klimov AI, et al. Infections with oseltamivir-resistant influenza a(H1N1) virus in the United States. JAMA 2009;301:1034–1041 [DOI] [PubMed] [Google Scholar]
- 12.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 2005;5:58–68 [DOI] [PubMed] [Google Scholar]
- 13.Numata M, Voelker DR. Asthma and infections. : Martin RJ, Sutherland ER, Lung biology in health and disease. New York: Informa Healthcare; 2010. pp. 45–165 [Google Scholar]
- 14.Wright SM, Hockey PM, Enhorning G, Strong P, Reid KB, Holgate ST, Djukanovic R, Postle AD. Altered airway surfactant phospholipid composition and reduced lung function in asthma. J Appl Physiol 2000;89:1283–1292 [DOI] [PubMed] [Google Scholar]
- 15.Lewis JF, Jobe AH. Surfactant and the adult respiratory distress syndrome. Am Rev Respir Dis 1993;147:218–233 [DOI] [PubMed] [Google Scholar]
- 16.Kuronuma K, Mitsuzawa H, Takeda K, Nishitani C, Chan ED, Kuroki Y, Nakamura M, Voelker DR. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J Biol Chem 2009;284:25488–25500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang PY, Kitchens RL, Munford RS. Phosphatidylinositides bind to plasma membrane CD14 and can prevent monocyte activation by bacterial lipopolysaccharide. J Biol Chem 1998;273:24309–24313 [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto M, Asai Y, Ogawa T. Treponemal phospholipids inhibit innate immune responses induced by pathogen-associated molecular patterns. J Biol Chem 2003;278:44205–44213 [DOI] [PubMed] [Google Scholar]
- 19.Mueller M, Brandenburg K, Dedrick R, Schromm AB, Seydel U. Phospholipids inhibit lipopolysaccharide (LPS)-induced cell activation: a role for LPS-binding protein. J Immunol 2005;172:1091–1096 [DOI] [PubMed] [Google Scholar]
- 20.Numata M, Chu HW, Dakhama A, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus–induced inflammation and infection. Proc Natl Acad Sci USA 2010;107:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandasamy P, Zarini S, Chan ED, Leslie CC, Murphy RC, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits Mycoplasma pneumoniae–stimulated eicosanoid production from human and mouse macrophages. J Biol Chem 2011;286:7841–7853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 2000;1:398–401 [DOI] [PubMed] [Google Scholar]
- 23.Daly K, Nguyen P, Woodland DL, Blackman MA. Immunodominance of major histocompatibility complex class I–restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J Virol 1995;69:7416–7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis 2009;9:493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartshorn KL, White MR, Tecle T, Holmskov U, Crouch EC. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J Immunol 2006;176:6962–6972 [DOI] [PubMed] [Google Scholar]
- 26.Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Suzuki H, Nishimura H, Mitamura K, Sugaya N, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 2004;431:703–707 [DOI] [PubMed] [Google Scholar]
- 27.Cimolai N, Taylor GP, Mah D, Morrison BJ. Definition and application of a histopathological scoring scheme for an animal model of acute mycoplasma pneumoniae pulmonary infection. Microbiol Immunol 1992;36:465–478 [DOI] [PubMed] [Google Scholar]
- 28.Hallman M, Merritt TA, Bry K. The fate of exogenous surfactant in neonates with respiratory distress syndrome. Clin Pharmacokinet 1994;26:215–232 [DOI] [PubMed] [Google Scholar]
- 29.Perino J, Crouzier D, Spehner D, Debouzy JC, Garin D, Crance JM, Favier AL. Lung surfactant DPPG phospholipid inhibits vaccinia virus infection. Antiviral Res 2011;89:89–97 [DOI] [PubMed] [Google Scholar]
- 30.LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol 2002;282:L563–L572 [DOI] [PubMed] [Google Scholar]
- 31.Hawgood S, Brown C, Edmondson J, Stumbaugh A, Allen L, Goerke J, Clark H, Poulain F. Pulmonary collectins modulate strain-specific influenza A virus infection and host responses. J Virol 2004;78:8565–8572 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.