Abstract
Previous studies have demonstrated a female disadvantage in airway diseases, such as asthma and bronchiectasis. The basis for this sex disparity is unknown. We hypothesized that the female sex hormone, progesterone (P4), inhibits functions of the normal airway mucociliary apparatus. P4 receptor (PR) expression was evaluated in human lung and cultured primary human airway epithelial cells isolated from male and female lung transplant donors. PR expression was restricted to the proximal region of the cilia of airway epithelia, and was similar in men and women. Expression of isoform PR-B was more abundant than PR-A in cells from both sexes. Airway epithelial cell exposure to P4 decreased cilia beat frequency (CBF) by 42.3% (±7.2). Inhibition of CBF was prevented by coadministration of P4 with the active form of estrogen, 17β-estradiol, or the PR antagonist, mifepristone. P4 inhibition was time and dose dependent, with a significant decrease by 8 hours and maximal effect at 24 hours, accompanied by translocation of PR from the cilia to the nucleus. Inhibition of cilia beat was also prevented by treatment of cells with actinomycin D, suggesting that CBF inhibition is a transcriptionally mediated event. Together, these findings indicate that sex hormones influence the function of a key component of the mucociliary apparatus. These mechanisms may contribute to the sex disparity present in airway diseases and provide therapeutic targets for the treatment of these debilitating airway diseases.
Keywords: cilia, progesterone receptor, cilia beat frequency, sex disparity, lung disease
Clinical Relevance
Women have an increased incidence and greater severity of airway diseases than men. However, mechanisms to explain this sex disparity remain unknown. Here, we demonstrate that expression of the progesterone (P4) receptor in cilia of airway epithelium is regulated by sex hormones, and that P4 inhibits cilia beat frequency. This effect may impact mucociliary clearance and thus contribute to the female disadvantage in airway disease, suggesting hormone receptors as a therapeutic target.
Multiple epidemiologic studies have established that women have more severe airway disease than men (1, 2). Women with asthma and chronic obstructive lung disease have more frequent and severe exacerbations than men, and a lower disease-specific quality of life (1, 3). Bronchiectasis affects women more commonly, and is more aggressive than in men (2, 4). For example, in cystic fibrosis (CF), a disease in which the impact of bronchiectasis has been extensively studied, women have worse outcomes and a decreased life expectancy compared with men, despite a similar cause of disease. This has been attributed specifically to a greater severity of lung disease, and is present even after adjusting for potentially confounding morphometric, nutritional, compliance, and microbial factors (4, 5). In addition, in models of Pseudomonas aeruginosa infection, one of the most common pathogens found in bronchiectasis, female mice are more affected than males, again for unclear reasons (6). Defects in mucociliary clearance are a central component to the development of bronchiectasis. We, therefore, hypothesize that female sex hormones regulate the mucociliary apparatus.
The major female sex hormones, estrogen and progesterone (P4), vary in levels during ovulatory cycles, pregnancy, menopause, and with exogenous delivery. Although their roles in the uterus, ovary, oviduct, and breast have been well studied, expression and function in other tissues are less well described (7–10). The classic mechanism of steroid hormone action involves binding to specific intracellular receptors that translocate to the nucleus and regulate cell-specific transcription. However, it is now broadly accepted that steroid hormones also function via nongenomic mechanisms (11–15).
The two major subtypes of estrogen receptors (ERs), ER-α and ER-β, each have a high affinity for 17β-estradiol (E2), the predominant form of estrogen (7, 16). Both receptors are expressed in normal lung. ER-β is more abundant and expressed in bronchial epithelial cells (16). Experimental studies show that E2 regulates CF transmembrane conductance regulator (CFTR) function, potentially affecting the course of bronchiectasis. E2 may be beneficial in CF lung disease by preventing the degradation and promoting trafficking of the mutant ΔF508 CFTR (17). Furthermore, E2 inhibits IL-8 release from CF bronchial epithelial cells (18). However, other work shows that E2 decreases the height of the airway epithelial surface liquid layer by inhibiting a non-CFTR, calcium-associated chloride channel (19). These findings are intriguing, but their clinical impact remains unknown (5, 18).
Although early work in this field has focused on estrogens, the role of P4 in the airway has garnered less attention. P4 acts via two main receptors, P4 receptor (PR)-A and PR-B, transcribed from a single gene. PR-B differs from PR-A by a 164 amino acid extension at the aminoterminus (8). These receptors induce both overlapping and distinct responses that are promoter and cell context dependent. For example, PR-A is necessary and sufficient for female fertility, whereas PR-B is required for mammary gland alveologenesis during pregnancy (8, 9). In endometrial tissue, PR-A and PR-B dually serve an anti-inflammatory role (20). Expression of PR in the lungs has been reported, but cell-specific localization and function has not been characterized (12, 13, 16, 21).
Our prior studies in ciliated epithelial cells led us to investigate potentially shared features of airway and fallopian tube epithelium, especially given the common ciliated surface (22, 23). Evidence supports critical roles for sex hormone regulation of cilia function in the fallopian tubes (15, 24–26). Fallopian tube cilia are lost after ovariectomy in baboons and rabbits, and in postmenopausal women (24). Notably, studies report that P4, but not E2, regulates cilia beat frequency (CBF) in the fallopian tubes, presumably to optimize conditions for fertilization (12, 15, 25, 26). The goal of our study was, therefore, to determine the impact of E2 and P4 on cilia function in the airways to improve the understanding of sex disparities in airway diseases and as a foundation for the development of novel therapies.
Materials and Methods
Cell Culture and Lung Tissues
Cell lines T-47D (human breast cancer; ATCC, Manasas, VA), and SW480 (human colon adenocarcinoma; provided by Brian Dieckgraefe, Washington University, St. Louis, MO) were cultured in RPMI-1640 medium supplemented with 0.2 U/ml insulin and 10% FBS. Primary human tracheal epithelial cells (hTECs) from healthy donors for lung transplantation were cultured on membranes (Transwell; Corning, Corning, NY) and differentiated using air–liquid interface (ALI) conditions (27, 28). Lung tissues were from healthy donors (provided by Michael Holtzman and John Battaile, Washington University). The Institutional Review Committee at Washington University approved all studies.
Cell Treatments
P4, E2, and mifepristone were solubilized in DMSO. The final DMSO concentration in medium was 0.2%. Actinomycin D was solubilized in water (2.5 μg/ml). All reagents were from Sigma-Aldrich (St. Louis, MO).
Immunofluorescent Staining and Microscopy
See online supplement for details. Briefly, hTECs on membranes were fixed with 4% paraformaldehyde and processed on membranes as previously described (27), or embedded in paraffin and processed for sectioning. hTECs and human lung deparaffinized sections were treated with Antigen Unmasking Solution, pH 6.0 (Vector Laboratories, Burlingame, CA). Primary antibody, mouse anti-acetylated α-tubulin (Sigma-Aldrich), rabbit anti-PR (sc-539; Santa Cruz Biotechnology, Santa Cruz, CA), and mouse anti-Foxj1 (29) were detected using secondary antibodies with Alexa Fluor dyes (Invitrogen, Carlsbad, CA). PR was detected in paraffin-embedded tissues using a tyramide amplification modified from the manufacturer's protocol (see the online supplement). Immunofluorescent photomicrographs were obtained using a Retiga 2000R camera and QCapture Pro software (both from Q Imaging, Surrey, BC, Canada). Images were globally adjusted for contrast and brightness and composed using Photoshop and Illustrator software (Adobe Systems, San Jose, CA).
Quantitative PCR
RNA was isolated using an Illustra RNAspin kit (GE Healthcare, Buckinghamshire, UK). RNA was reverse transcribed using a cDNA Reverse Transcription Kit, then amplified using the TaqMan Fast Universal PCR Master Mix (both from Applied Biosystems, Carlsbad, CA) with PR primers 5′-TGGAAGAAATGACTGCATCG-3′ and 5′-TAGGGCTTGGCTTTCATTTG-3′ and probe (HS00172183_mL; Applied Biosystems), as used by others to detect both PR-A and PR-B (30). PR expression was normalized to glyceraldehyde 3-phosphate dehydrogenase expression.
Protein Blot Analysis
See the online supplement for details. Briefly, cells lysates were separated by PAGE and transferred to polyvinylidene fluoride membranes (29). PR was detected using the anti-PR antibody (Santa Cruz Biotechnology). Densitometry of scanned immunoblot image was performed using Image J software (National Institutes of Health, Bethesda, MD) (31).
CBF Assay
See online supplement for details. Briefly, well differentiated hTECs (ALI culture over 21 d) were imaged at 37°C on membranes using an inverted microscope with a 20× phase contrast objective (Eclipse Ti-U; Nikon, Melville, NY). Cilia beat was captured using a high-speed video camera and analyzed using the Sisson-Ammons Video Analysis system (Ammons Engineering, Mount Morris, MI) (32). At least five fields per membrane were captured for whole-field analysis.
Statistical Analysis
For statistical analysis of RT-PCR, densitometry, and CBF data, an unpaired t test was used to compare two samples. ANOVA was used to compare greater than two samples, with the Bonferroni correction used for post hoc analysis.
Results
PR Is Expressed in Cilia of Airway Epithelial Cells
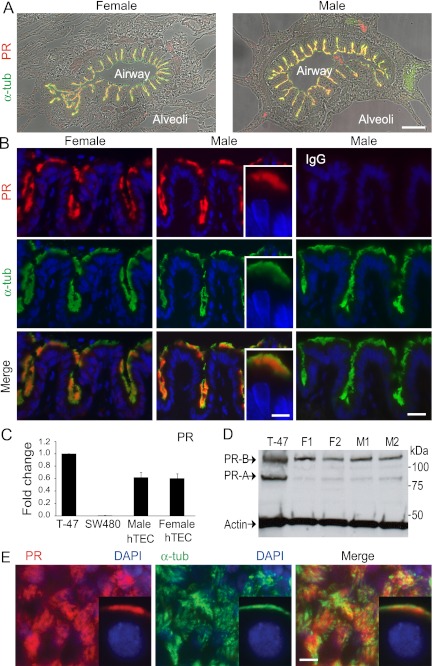
To localize PR expression in lung, and specifically in airway epithelium, we used tracheal tissue obtained from healthy male and female lung transplantation donors. PR was clearly present in airways of samples from both sexes and colocalized with cilia marker protein, acetylated α-tubulin (Figures 1A and 1B). Within the cilia, immunostaining was restricted to the proximal region (Figure 1B, insets). Evaluation of at least three different donor samples from each sex, consistently revealed this pattern of expression. This pattern of PR expression in airway epithelia mirrors that seen in the ciliated epithelial cells of the oviduct (12).
Figure 1.
Progesterone (P4) receptor (PR) is expressed in cilia of airway epithelial cells. (A) Lung tissue from lung transplant donors of indicated sex immunostained for PR (red) and cilia marker, acetylated α-tubulin (α-tub; green), overlayed with differential interference contrast microscopy images show airway expression of PR in cilia. (B) Representative high-power images from (A) are shown. Rabbit IgG was substituted for anti-PR antibody in samples shown in the far right panel. Image detail shows PR expression in the proximal cilia (insets, middle panels). Images are representative of at least three samples from each sex. (C) Expression of PR in RNA isolated from human tracheal epithelial cell (hTEC) preparations relative to that in T-47D and SW480 cell lines. Shown are the mean (±SD) of PR in hTEC preparations derived from three different male and female donors, normalized to glyceraldehyde 3-phosphate dehydrogenase. (D) Protein blot analysis for PR-A and -B isoforms in hTECs from four different donors. (E) PR expression in cilia of fully differentiated hTEC preparation (male, air–liquid interface [ALI] Day 70) immunostained as in (A), viewed en face. Inset shows PR expression in hTECs sectioned from cells on membrane. Scale bars, 100 μm (A), 30 μm (B), 10 μm (E).
We next assayed PR expression in airway epithelium using RT-PCR, immunoblotting, and immunostaining in single-donor hTEC cultures as an enriched ciliated epithelial cell source (n ≥ 3 independent donor preparations from each sex). Quantitative PCR assay of PR expression in RNA from normal male and female hTECs showed that total PR was of similar abundance (Figure 1C) (33). Protein blot analysis demonstrated that PR-B is more abundant than PR-A in both male and female hTEC preparations (Figure 1D). There was a trend toward increased expression of both isoforms in cells derived from female donors, but this was not found to be significant (see Figure E1 in the online supplement). To study PR expression further, we immunostained primary culture hTECs differentiated at ALI. Identical to expression in lung sections, PR was colocalized with cilia marker acetylated α-tubulin within the proximal region of the cilia (Figure 1E). Importantly, all analyses showed similar PR expression in samples from male and female donors.
PR Localization Is Differentiation and Ligand Dependent in Airway Epithelial Cells
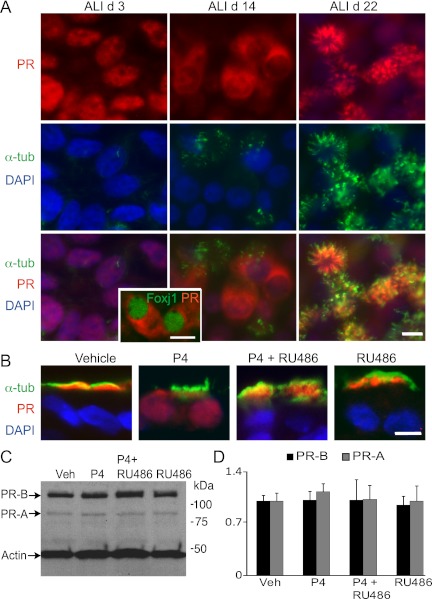
Given that hormone receptors are classically known to be nuclear receptors, we were interested in determining if the state of differentiation and presence of ligand impacted the cellular localization of PR in airway epithelial cells (34). Using hTEC culture as a model of ciliated cell differentiation, we found that undifferentiated airway epithelium (ALI Day 3) expressed PR in the nucleus of cells before motile cilia formation (Figure 2A). As ciliogenesis progressed, PR moved out of the nucleus into the cytoplasm (e.g., ALI Day 14), and then into the cilia (e.g., ALI Day 22). We confirmed PR localization to be in the cytoplasm at the earliest stages of ciliogenesis by demonstrating cytoplasmic PR staining when the ciliogenesis-related transcriptional factor, Foxj1, localized to the nucleus at a time when motile cilia were yet to be generated (Figure 2A). The shift in nuclear to cilia expression was observed in hTEC preparations obtained from donors of both sexes, and PR remained in the nucleus of some nonciliated cells.
Figure 2.
PR localization is differentiation and ligand dependent in airway epithelial cells. (A) Differentiation-dependent PR expression of hTEC preparations immunostained for PR (red) and acetylated α-tubulin (green) shown en face. Nuclei are stained with DAPI (blue). ALI Day 3 shows PR in the nuclei of all cells. As differentiation proceeds, PR moves to the cytoplasm in some cells (ALI Day 14). Inset shows hTECs immunostained for PR (red) and Foxj1 (green) during differentiation, demonstrating cytoplasmic PR during early ciliogenesis (ALI Day 11). In differentiated hTECs, PR is expressed in the cilia (ALI Day 22). Top right panel shows PR expression in cilia, and top left panel shows PR expression in nuclei of nonciliated cells, within a plane of focus below the level of the cilia. (B) P4-induced nuclear localization of PR in fully differentiated hTEC preparations. hTECs treated with P4, mifepristone (Mife), or P4 plus mifepristone, and immunostained as in (A). (C) Protein blot analysis of hTECs treated as in (B) show no change in the expression of PR isoforms. (D) Densitometry analysis of studies in (C) expressed as the mean (±SD) of fold change of PR-A and -B normalized to actin and relative to vehicle-treated samples from three independent experiments. Scale bars, 10 μm in (A and B).
We next examined PR trafficking under ligand-stimulated conditions. We evaluated PR localization in well differentiated hTEC preparations treated with P4 or mifepristone, a synthetic steroid compound that serves as a competitive PR antagonist and does not bind to the ERs (35). Although PR was expressed in the cilia of nonstimulated, mature hTECs, P4 treatment (24 h) resulted in PR trafficking to the nucleus and cytoplasm, an effect blocked by the simultaneous addition of mifepristone (Figure 2B). Similar effects were observed in cell preparations from both men and women. Protein blot analysis showed no significant change in PR expression during ligand stimulation or antagonism (Figures 2C and 2D). These observations provide further support for the hypothesis that one component of receptor engagement is ciliated cell specific.
P4 Decreased CBF in Airway Epithelial Cells
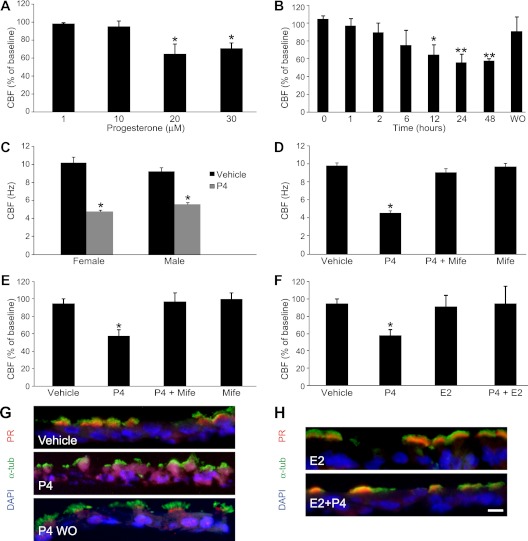
Prior studies in oviduct epithelium have shown that P4 inhibited CBF, whereas E2 has little impact on CBF (25, 26). We thus sought to determine if P4 also inhibits CBF in airway epithelium under similar conditions. The mean (±SD) baseline CBF in hTECs, before any treatment, was 9.8 (±0.9) Hz in 28 independent preparations (female, 10.4 ± 0.9 Hz, 12 independent preparations; male, 9.3 ± 0.2 Hz, 16 independent preparations; P = 0.081). CBF preparations after application of P4 resulted in a dose-dependent decrease in CBF compared with vehicle-treated samples, with a maximal nadir at 20 μM when measured at 24 hours (Figure 3A). The CBF inhibition by P4 was time dependent, with the nadir occurring after 24 hours of exposure, and was reversible 24 hours after the P4-containing medium was replaced with fresh complete medium (washout [WO]; Figure 3B). The effect in hTEC preparations from male and female donors was not significantly different: the CBF after 20-μM P4 treatment for 24 hours was 4.75 (±0.38) in cells from females and 5.51 (±0.45) in male donors (P = 0.22), but both were significantly decreased compared with vehicle treatment (Figure 3C). Additional CBF measurements obtained 5, 15, and 30 minutes after P4 exposure showed no significant change from baseline (Figure E2). Treatment with mifepristone blocked the P4 inhibition of CBF, suggesting that the inhibition is PR dependent (Figures 3D and 3E). We were also interested in the effect of E2 on CBF and P4-mediated inhibition. E2 alone did not affect CBF. However, E2 prevented the P4-mediated inhibition of CBF when added concurrently, revealing a balance in sex hormone function (Figure 3F).
Figure 3.
P4 decreased cilia beat frequency (CBF) in hTEC preparations. Baseline CBF was measured at time 0 before treatments. (A) Dose–response of P4 on change in CBF measured after 24 hours of P4 treatment. *P < 0.001 relative to baseline. (B) Time-dependent effect of P4 on CBF relative to pretreatment baseline with recovery after 24-hour washout (WO). *P < 0.001 relative to 0–6 hours and **P < 0.001 relative to 0–12 hours. (C) CBF measured 24 hours after P4 (20 μM) or vehicle of hTECs from female and male donors. *P < 0.001 relative to vehicle conditions. Baseline CBF of male and female samples was not different (P = 0.08). (D and E) Effect of blockade of P4 by Mife after 24 hours. *P < 0.001 relative to all other conditions on absolute and relative CBF. (F) 17β-estradiol (E2) prevents P4 inhibition of CBF after 24 hours. *P < 0.001 relative to all other conditions. Data shown are the mean (±SD). CBF measured at 37°C from 5 to 10 fields of each sample from 3 to 10 male and female donors. (G) Representative images of PR localization after vehicle, P4 treatment, and WO, demonstrating PR trafficking. (H) E2 inhibits P4-mediated PR trafficking. Cells were immunostained as in Figure 2B. Scale bars, 10 μm in (G and H).
Having observed the loss of P4-dependent inhibition of CBF after WO (Figure 3B) and concurrent E2 treatment (Figure 3F), we next evaluated the associated changes in PR localization within the cell. Consistent with the loss of inhibition of CBF observed 24 hours after WO of P4, there was decreased nuclear expression and return of PR to the cilia (Figure 3G). After WO, some PR expression remained present within the cytoplasm and nucleus. As expected, E2 did not change PR localization, and P4 did not induce PR trafficking in the presence of E2 (Figure 3H). These observations further support an interplay of the two sex hormones and a role for P4-mediated activation of PR in the regulation of cilia function.
P4-Dependent Inhibition of CBF Is Abrogated by Actinomycin
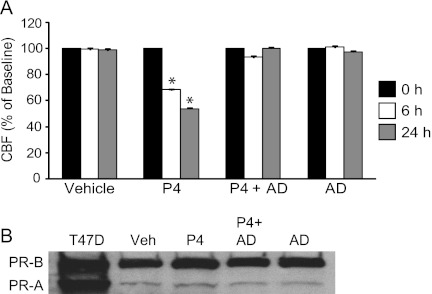
Temporal features of P4-mediated inhibition of CBF in mouse and human fallopian tube suggest that both nongenomic and genomic mechanisms are at play (36). However, in airway epithelia, the relatively slow onset of inhibition of CBF and delayed reversal of the P4 effect (24 h) supports a genomic response. We further investigated this by pretreating cells for 24 hours with transcription inhibitor, actinomycin D. Actinomycin D pretreatment, followed by the addition of P4 to actinomycin D for 24 hours, resulted in the failure of P4 to inhibit CBF in hTECs (Figure 4A). This effect occurred without a decrease in PR-A or -B expression under treatment conditions, as determined by protein blot (Figure 4B) and densitometry analysis (Figure E3). These results suggest that P4-induced inhibition of CBF is through a transcriptional (genomic) program.
Figure 4.
P4-dependent inhibition of CBF is abrogated by actinomycin D (AD). (A) CBF of hTEC preparations treated with vehicle, P4, P4 plus AD, or AD alone at indicated times. *P < 0.001 relative to time 0. (B) Representative protein blot analysis of PR expression after 24 hours, as in Figure 2. Cells were exposed to AD for 24 hours before adding P4, and were then treated for indicated additional times.
Discussion
It has long been noted that women with asthma, bronchiectasis, and related airway diseases are prone to a more aggressive course than men (1–5, 21, 37), although the mechanism behind this observation remains elusive. Sex hormones, such as estrogen and P4, are natural candidates to explain this disparity. In the human lung, we show, for the first time, localization of PR to motile cilia of airway epithelial cells and an inhibitory effect of P4 on CBF that could be blocked by concurrent E2. After P4 ligand incubation, PR trafficked between the cilia and nucleus, but was blocked by concurrent E2, indicating that the relative balance of sex hormones could alter epithelial cell function. The potent effect of P4 alone to slow CBF demonstrates the importance that shifts in hormones may have on the lung. Given the critical role of cilia in mucociliary clearance, our observations contribute to work in this nascent area of investigation, which we believe will now require concerted in vitro and in vivo studies to elucidate the biologic roles of the sex hormones in airway disease.
The PR expression that we observed in the cilia of human airway epithelial cells mirrors that of oviduct epithelium (12) and in the mouse airway (10, 12). PR expression in the airway was dependent on both cell differentiation state and hormone environment, similar to responses in the oviduct (12). In undifferentiated airway epithelial cells, PR localized to the nucleus; as differentiation progressed, PR trafficked to the cilia, suggesting a highly regulated process in ciliated cells. However, PR remained in the nucleus of a subpopulation of nonciliated cells, as in the oviduct, possibly to engage in a different program. Trafficking from the cilia back to the nucleus was also ligand dependent, which may explain the clinical differences in the severity of airway diseases in men and women. We found that PR could be stimulated and trafficked in cells from both sexes, indicating that the hormonal environment results in phenotypic differences. In addition, we cannot exclude that engagement of PR-A or -B isoforms may be different between sexes, or that other endogenous steroids may alter expression. In contrast to our finding that PR-B was dominant in hTECs, Shao and colleagues (13) reported dominant PR-A expression in the mouse lung. This difference might be related to species or strain, tissue examined, or the stage of development (9, 13).
A significant limitation in this work is that the dose of P4 used in our experiments is higher than the typical circulating blood levels in women. We did not perform detailed experiments to quantify the amount of active P4 in the preparation we used or receptor–ligand binding affinity assays in our system. However, the dose was similar to those used in prior studies in oviduct of CBF (25, 26), and the P4 concentration in lung epithelium or lining fluid is unknown. How the levels of P4 in the lung surface fluid may affect CBF in vivo remains undefined. Thus, given that little information is known about the concentration of sex hormones in the lungs, in vivo studies are necessary to confirm the impact of P4 on cilia function in the airway epithelium.
The degree of P4-mediated inhibition of CBF was similar in the oviduct and airway epithelia. In the oviduct, CBF was depressed by approximately 50% in the presence of P4 concentrations similar to those that we used (15, 25, 26). In contrast to a rapid, nongenomic mechanism described in oviduct epithelium, where specific interactions of sperm and ovum are required (36, 38), we found that the inhibition in the airway epithelium and reversal was slow (24 h), suggesting that P4 transcriptionally reprograms the cell to regulate CBF. This was supported by the ability of actinomycin D to completely block the effect of P4 without diminishing PR expression, although further studies will be required to confirm transcriptional mechanisms. As noted, another class of P4 membrane receptors, with structures unrelated to classic PR, are also expressed in oviduct cilia. These may be responsible for rapid responses (14), but we did not observe a significant acute change in CBF (0–30 min) after the immediate addition of P4 to our hTEC preparations (Figure E2).
Factors that control PR-mediated CBF in oviduct and lung are unknown; however, one potential regulator of CBF is the cilia-based protein known as transient receptor potential cation channel subfamily V member V4, which binds and is regulated by PR (39). Transient receptor potential cation channel subfamily V member V4 is a nonselective calcium channel that increases intracellular calcium and CBF in response to shear stress and increased viscosity (39). Several other intracellular signals have been demonstrated to mediate changes of CBF in response to stimuli, including β-adrenergic agonists, nitric oxide, and calcium (40–42). P4 has been shown to inhibit nitric oxide synthases in a variety of cell types, and inhibition of these proteins is known to inhibit CBF (40, 43, 44). It is also noteworthy that the expression of PR is restricted to the proximal cilia, a region that corresponds with the transition zone, which, among other functions, controls entry and assembly of proteins that move in and out of the cilia, suggesting other mechanisms for CBF regulation (45).
Physiologic roles for sex hormone receptors are evolving, and the function of PR in the lung is unclear. In the oviduct, CBF regulation of the speed at which the oocyte moves to the uterus is an event without parallel in the lung. We could only speculate that the effect of P4 on slowing CBF could work in concert with P4-induced changes in ventilation (46), or with the effects of estrogen. Regardless, our findings point to a balance between the major female sex hormones, E2 and P4, which impact CBF. This may be the case in the oviduct, where ERβ is also expressed in epithelial cell cilia. Although the function of this ER in the oviduct relative to CBF is not defined, it has recently been shown that, in the lung, ERβ mediates a rapid, nongenomic effect to speed CBF (47, 48). In our studies, we did not find that E2 alone directly affects CBF when assayed at 24 hours; however, E2 blocked P4-mediated CBF inhibition at that time, emphasizing the importance of hormone balance. During normal ovulatory cycles, both E2 and P4 are increased, whereas, during pregnancy, P2 serum levels are high and E2 low. Women with asthma are known to be prone to exacerbations during pregnancy, but the protean physiologic changes of pregnancy make it difficult to directly implicate P4 alone. Thus, P4 may also function as a rheostat in the presence of other hormones that increase CBF, such as endogenous catacholamines or sex hormones, as levels change with pregnancy, normal ovulatory cycles, and postmenopausal states.
The P4-associated fall in CBF that we saw was significant, although CBF is only one tool used to assess mucociliary clearance. The inhibitory effect of P4 on CBF does not necessarily translate directly to a decrement in mucociliary clearance in vivo; however, the 40% fall in CBF is noteworthy relative to previous studies. Freshly isolated epithelia from patients with chronic obstructive pulmonary disease and pneumonia showed a 27% decrease in CBF relative to healthy control subjects, cigarette smoke condensate decreased forskolin-stimulated CBF by 21% in human sinonasal epithelia, and alcohol increased CBF by roughly 40% in isolated, demembranated cilia from bovine trachea (42, 49, 50). In those reports, the effect of sex was not studied, and we are not aware of studies that have specifically assessed sex differences in mucociliary clearance.
In summary, this study indicates an important role for PR regulation in cilia function. Observations here lead to questions of the role for PR in airway epithelial cell differentiation, injury, and repair responses, and how this hormone impacts airway diseases. Given the critical role of cilia function in airway clearance, we suggest that our findings support a role of P4 in the variation in airway diseases seen between males and females. Interestingly, we are not aware of any published reports indicating a sex disparity in patients with primary ciliary dyskinesia, possibly due to the immotile state of their cilia being unable to respond to differences in hormone environment, whereas a clear female disadvantage has been described in other airway diseases, such as CF and asthma (1–5). Studies to date have focused primarily on potential advantages of increased CBF as a therapeutic intervention, rather than blocking inhibition. Further work will be required to determine whether hormonal fluctuations in people impact lung function or respiratory symptoms, and whether hormone receptor manipulation may serve as a potential therapy for patients with airway diseases.
Supplementary Material
Acknowledgments
The authors thank Jian Xu and Cassandra Mikols for assistance with airway epithelial cell culture, Sean Gunsten for assistance with RT-PCR, Brian Dieckgraefe for cells, Michael Holtzman, and John Battaile for providing human lung samples, and Yael Alevy and Michael Holtzman for providing glyceraldehyde 3-phosphate dehydrogenase primers.
Footnotes
This work was supported by National Institutes of Health (NIH) grant KL2 RR024994/UL1RR024992 (R.J.), American Thoracic Society career development award (R.J.), NIH R01 HL56244 (S.L.B.), and the Children's Discovery Institute at St. Louis Children's Hospital and Washington University (S.L.B.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0107OC on October 27, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.The ENFUMOSA Cross-Sectional European Multicentre Study of the Clinical Phenotype of Chronic Severe Asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J 2003;22:470–477 [DOI] [PubMed] [Google Scholar]
- 2.Tam A, Don M, Wadsworth S, Dorscheid D, Man SFM, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health 2011;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celli B, Vestbo J, Jenkins CR, Jones PW, Ferguson GT, Calverley PM, Yates JC, Anderson JA, Willits LR, Wise RA. Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease: the TORCH experience. Am J Respir Crit Care Med 2011;183:317–322 [DOI] [PubMed] [Google Scholar]
- 4.Morrissey BM, Harper RW. Bronchiectasis: sex and gender considerations. Clin Chest Med 2004;25:361–372 [DOI] [PubMed] [Google Scholar]
- 5.Zeitlin PL. Cystic fibrosis and estrogens: a perfect storm. J Clin Invest 2008;118:3841–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guilbault C, Stotland P, Lachance C, Tam M, Keller A, Thompson-Snipes L, Cowley E, Hamilton TA, Eidelman DH, Stevenson MM, et al. Influence of gender and interleukin-10 deficiency on the inflammatory response during lung infection with Pseudomonas aeruginosa in mice. Immunology 2002;107:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morani A, Warner M, Gustafsson JA. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J Intern Med 2008;264:128–142 [DOI] [PubMed] [Google Scholar]
- 8.Conneely OM, Jericevic BM. Progesterone regulation of reproductive function through functionally distinct progesterone receptor isoforms. Rev Endocr Metab Disord 2002;3:201–209 [DOI] [PubMed] [Google Scholar]
- 9.Aupperlee MD, Smith KT, Kariagina A, Haslam SZ. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology 2005;146:3577–3588 [DOI] [PubMed] [Google Scholar]
- 10.Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction 2004;128:139–146 [DOI] [PubMed] [Google Scholar]
- 11.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol Rev 2000;52:513–556 [PubMed] [Google Scholar]
- 12.Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol 2006;191:525–535 [DOI] [PubMed] [Google Scholar]
- 13.Shao R, Egecioglu E, Weijdegard B, Ljungstrom K, Ling C, Fernandez-Rodriguez J, Billig H. Developmental and hormonal regulation of progesterone receptor A-form expression in female mouse lung in vivo: interaction with glucocorticoid receptors. J Endocrinol 2006;190:857–870 [DOI] [PubMed] [Google Scholar]
- 14.Nutu M, Weijdegard B, Thomas P, Thurin-Kjellberg A, Billig H, Larsson DG. Distribution and hormonal regulation of membrane progesterone receptors beta and gamma in ciliated epithelial cells of mouse and human fallopian tubes. Reprod Biol Endocrinol 2009;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessel T, Schuchter U, Walt H. Ciliary motility in bovine oviducts for sensing rapid non-genomic reactions upon exposure to progesterone. Horm Metab Res 2004;36:136–141 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz AG, Prysak GM, Murphy V, Lonardo F, Pass H, Schwartz J, Brooks S. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res 2005;11:7280–7287 [DOI] [PubMed] [Google Scholar]
- 17.Fanelli T, Cardone RA, Favia M, Guerra L, Zaccolo M, Monterisi S, De Santis T, Riccardi SM, Reshkin SJ, Casavola V. Beta-oestradiol rescues DeltaF508CFTR functional expression in human cystic fibrosis airway CFBE41o- cells through the up-regulation of NHERF1. Biol Cell 2008;100:399–412 [DOI] [PubMed] [Google Scholar]
- 18.Chotirmall SH, Greene CM, Oglesby IK, Thomas W, O'Neill SJ, Harvey BJ, McElvaney NG. 17β-estradiol inhibits IL-8 in cystic fibrosis by up-regulating secretory leucoprotease inhibitor. Am J Respir Crit Care Med 2010;182:62–72 [DOI] [PubMed] [Google Scholar]
- 19.Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, Fricks I, Young SL, Tarran R. 17beta-estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest 2008;118:4025–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 2008;149:1190–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cagle PT, Mody DR, Schwartz MR. Estrogen and progesterone receptors in bronchogenic carcinoma. Cancer Res 1990;50:6632–6635 [PubMed] [Google Scholar]
- 22.Okada A, Ohta Y, Brody SL, Iguchi T. Epithelial c-jun and c-fos are temporally and spatially regulated by estradiol during neonatal rat oviduct differentiation. J Endocrinol 2004;182:219–227 [DOI] [PubMed] [Google Scholar]
- 23.Okada A, Ohta Y, Brody SL, Watanabe H, Krust A, Chambon P, Iguchi T. Role of foxj1 and estrogen receptor alpha in ciliated epithelial cell differentiation of the neonatal oviduct. J Mol Endocrinol 2004;32:615–625 [DOI] [PubMed] [Google Scholar]
- 24.Comer MT, Leese HJ, Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod 1998;13:3114–3120 [DOI] [PubMed] [Google Scholar]
- 25.Mahmood T, Saridogan E, Smutna S, Habib AM, Djahanbakhch O. The effect of ovarian steroids on epithelial ciliary beat frequency in the human Fallopian tube. Hum Reprod 1998;13:2991–2994 [DOI] [PubMed] [Google Scholar]
- 26.Paltieli Y, Eibschitz I, Ziskind G, Ohel G, Silbermann M, Weichselbaum A. High progesterone levels and ciliary dysfunction—a possible cause of ectopic pregnancy. J Assist Reprod Genet 2000;17:103–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002;283:L1315–L1321 [DOI] [PubMed] [Google Scholar]
- 28.Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, You Y, Brody SL. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol 2010;43:731–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J, You Y, Huang T, Brody SL. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci 2007;120:1868–1876 [DOI] [PubMed] [Google Scholar]
- 30.Ishibashi H, Suzuki T, Suzuki S, Moriya T, Kaneko C, Takizawa T, Sunamori M, Handa M, Kondo T, Sasano H. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab 2003;88:2309–2317 [DOI] [PubMed] [Google Scholar]
- 31.Abràmoff MM, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics International 2004;11:36–42 [Google Scholar]
- 32.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 2003;211:103–111 [DOI] [PubMed] [Google Scholar]
- 33.Savouret JF, Fridlanski F, Atger M, Misrahi M, Berger R, Milgrom E. Origin of the high constitutive level of progesterone receptor in T47-D breast cancer cells. Mol Cell Endocrinol 1991;75:157–162 [DOI] [PubMed] [Google Scholar]
- 34.DeFranco DB. Regulation of steroid receptor subcellular trafficking. Cell Biochem Biophys 1999;30:1–24 [DOI] [PubMed] [Google Scholar]
- 35.Heikinheimo O, Kekkonen R, Lahteenmaki P. The pharmacokinetics of mifepristone in humans reveal insights into differential mechanisms of antiprogestin action. Contraception 2003;68:421–426 [DOI] [PubMed] [Google Scholar]
- 36.Bylander A, Nutu M, Wellander R, Goksor M, Billig H, Larsson DG. Rapid effects of progesterone on ciliary beat frequency in the mouse fallopian tube. Reprod Biol Endocrinol 2010;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieberman D, Kopernik G, Porath A, Lazer S, Heimer D. Sub-clinical worsening of bronchial asthma during estrogen replacement therapy in asthmatic post-menopausal women. Maturitas 1995;21:153–157 [DOI] [PubMed] [Google Scholar]
- 38.Publicover S, Barratt C. Reproductive biology: progesterone's gateway into sperm. Nature 2011;471:313–314 [DOI] [PubMed] [Google Scholar]
- 39.Jung C, Fandos C, Lorenzo IM, Plata C, Fernandes J, Gene GG, Vazquez E, Valverde MA. The progesterone receptor regulates the expression of TRPV4 channel. Pflugers Arch 2009;459:105–113 [DOI] [PubMed] [Google Scholar]
- 40.Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun 1993;191:83–88 [DOI] [PubMed] [Google Scholar]
- 41.Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol 2002;538:633–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sisson JH, Pavlik JA, Wyatt TA. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide–dependent kinase mechanism. Alcohol Clin Exp Res 2009;33:610–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salzman AL, Linn SC, Szabo C. Progesterone inhibits inducible nitric oxide synthase mRNA expression in human intestinal epithelial cells. Int J Mol Med 2000;6:209–216 [DOI] [PubMed] [Google Scholar]
- 44.Zicari A, Centonze C, Realacci M, Buchetti B, Pietropolli A, Ticconi C. Estradiol 17-beta and progesterone modulate inducible nitric oxide synthase and high mobility group box 1 expression in human endometrium. Reprod Sci 2008;15:559–566 [DOI] [PubMed] [Google Scholar]
- 45.Benzing T, Schermer B. Transition zone proteins and cilia dynamics. Nat Genet 2011;43:723–724 [DOI] [PubMed] [Google Scholar]
- 46.Das TK. Effects of the menstrual cycle on timing and depth of breathing at rest. Indian J Physiol Pharmacol 1998;42:498–502 [PubMed] [Google Scholar]
- 47.Shao R, Weijdegard B, Fernandez-Rodriguez J, Egecioglu E, Zhu C, Andersson N, Thurin-Kjellberg A, Bergh C, Billig H. Ciliated epithelial–specific and regional-specific expression and regulation of the estrogen receptor-beta2 in the fallopian tubes of immature rats: a possible mechanism for estrogen-mediated transport process in vivo. Am J Physiol Endocrinol Metab 2007;293:E147–E158 [DOI] [PubMed] [Google Scholar]
- 48.Jia S, Zhang X, He DZ, Segal M, Berro A, Gerson TG, Wang Z, Casale TB. Expression and function of a novel variant of estrogen receptor-α36 in mouse airway. Am J Respir Cell Mol Biol 2011;45:1084–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen NA, Zhang S, Sharp DB, Tamashiro E, Chen B, Sorscher EJ, Woodworth BA. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope 2009;119:2269–2274 [DOI] [PubMed] [Google Scholar]
- 50.Piatti G, Ambrosetti U, Santus P, Allegra L. Effects of salmeterol on cilia and mucus in COPD and pneumonia patients. Pharmacol Res 2005;51:165–168 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.