Abstract
Pim kinases are a family of serine/threonine kinases whose activity can be induced by cytokines involved in allergy and asthma. These kinases play a role in cell survival and proliferation, but have not been examined, to the best of our knowledge, in the development of allergic disease. This study sought to determine the role of Pim1 kinase in the development of allergic airway responses. Mice were sensitized and challenged with antigen (primary challenge), or were sensitized, challenged, and rechallenged with allergen in a secondary model. To assess the role of Pim1 kinase, a small molecule inhibitor was administered orally after sensitization and during the challenge phase. Airway responsiveness to inhaled methacholine, airway and lung inflammation, cell composition, and cytokine concentrations were assessed. Lung Pim1 kinase concentrations were increased after ovalbumin sensitization and challenge. In the primary allergen challenge model, treatment with the Pim1 kinase inhibitor after sensitization and during airway challenges prevented the development of airway hyperresponsiveness, eosinophilic airway inflammation, and goblet cell metaplasia, and increased Th2 cytokine concentrations in bronchoalveolar fluid in a dose-dependent manner. These effects were also demonstrated after a secondary allergen challenge, where lung allergic disease was established before treatment. After treatment with the inhibitor, a significant reduction was evident in the number of CD4+ and CD8+ T cells and concentrations of cytokines in the airways. The inhibition of Pim1 kinase was effective in preventing the development of airway hyperresponsiveness, airway inflammation, and cytokine production in allergen-sensitized and allergen-challenged mice. These data identify the important role of Pim1 kinase in the full development of allergen-induced airway responses.
Keywords: airway hyperresponsiveness, inflammation, Pim1 kinase, T cells
Asthma is a complex inflammatory disorder, characterized by persistent airway inflammation and airway hyperresponsiveness (AHR) as a result of cellular and molecular responses induced by allergen exposure, infectious pathogens, or chemical agents (1, 2). Several clinical and experimental investigations showed that T cells, and especially Th2-type cells, play a pivotal role in the development of AHR and eosinophilic inflammation through the secretion of a variety of cytokines, including IL-4, IL-5, and IL-13 (3, 4). These cytokines bind to the extracellular Janus kinase (JAK) receptors and subsequently induce the phosphorylation and activation of signal transducers and activators of transcription (STATs), which translocate into the nucleus, where they bind to DNA and affect basic cell functions, including cell growth, differentiation, and death (5).
Pim kinases represent a family of three serine/threonine kinases that control cell survival, proliferation, differentiation, and apoptosis (6–8). Unlike other serine/threonine kinases, these are regulated via JAK/STAT activation–driven transcription of the Pim gene, rather than by membrane recruitment and phosphorylation (8). The overexpression of Pim kinase has been demonstrated in various human lymphomas, leukemias, and prostatic cancers (9). The role of Pim-induced oncogenic transformation was extensively studied in hematopoietic tumors (10–13). Despite numerous studies on the role of Pim kinase in the development of tumor cells, studies exploring the role of these kinases in immune cells have been limited. Pim1 kinase was expressed in human eosinophils, and played a major role in the IL-5–induced survival of eosinophils (14, 15). Furthermore, Pim1 expression was increased in eosinophils from bronchoalveolar lavage (BAL) fluid, compared with blood from patients with asthma after an allergen provocation (16). In a recent study, Pim1 kinase was shown to promote cell survival in T cells (17).
CD4+ T cells play a central role in the development of allergic inflammation (18). CD4+ T cells, especially Th2 cells producing IL-4, IL-5, and IL-13, were identified in the BAL fluid and airway tissue in patients with asthma (4). The transfer of Th2 cells, followed by airway allergen challenge in mice, was sufficient to induce airway eosinophilia and AHR (19, 20). Recent studies demonstrated increased numbers of CD8+ T cells in the lung tissue of patients with asthma (21). These studies suggest that not only CD4+ T cells but also CD8+ T cells may be essential in the development of AHR and allergic inflammation (22–25). Subsets of CD8+ T cells that produce IL-4, IL-5, and IL-13, but not IFN-γ, labeled as Tc2 cells, are known to increase AHR and airway inflammation (26–28).
In this study, we determined the role of Pim1 kinase in the development of allergen-induced AHR and airway inflammation in vivo, using a Pim1 kinase inhibitor. The inhibitor was examined in two distinct models of allergic airway inflammation. First, the development of allergen-induced airway responses was examined in mice that were systemically sensitized to ovalbumin (OVA), followed by 3 consecutive days of OVA challenge via the airways (primary allergen challenge). Second, effects on established airway disease were examined in mice that were sensitized and challenged with OVA, and when their allergic airway inflammation had resolved, they were exposed to a single provocative airway challenge with allergen (secondary allergen challenge). This condition may more closely resemble allergic asthma, where subjects are already sensitized and repeatedly exposed to allergen. The results demonstrated that the administration of Pim1 kinase inhibitor prevented the development of AHR, airway inflammation, and BAL cytokine production in mice sensitized and challenged to allergen. The administration of Pim1 kinase inhibitor also attenuated the consequences of secondary challenge in previously sensitized and challenged mice. These suppressive effects were manifested in both CD4+ and CD8+ T cells, and may help identify a novel role for Pim1 kinase in the development of allergen-induced AHR and airway inflammation.
Materials and Methods
Animals
Female BALB/c mice, 8–12 weeks of age and free of pathogens, were purchased from Harlan Laboratory (Indianapolis, IN). The animals were maintained on an OVA-free diet. Experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of National Jewish Health.
Sensitization and Challenge with Allergen
Our experimental protocol for the sensitization and primary and secondary challenges to allergen was described previously (29). Briefly, in the primary allergen challenge protocol, mice were sensitized by an intraperitoneal injection of 20 μg OVA (Fisher Scientific, Pittsburgh, PA), emulsified in 2.0 mg of alum (AlumImuject; Pierce, Rockford, IL) on Days 1 and 14, followed by aerosolized OVA challenge (1% in saline for 20 minutes) on Days 28, 29, and 30. Control mice were sensitized with PBS followed by OVA challenge in the same way. In the secondary allergen challenge protocol, mice were sensitized with 10 μg OVA with alum on Days 1 and 7, followed by 0.2% OVA challenge on Days 14–16 (primary allergen challenge). Fourteen days after the final primary allergen challenge, mice were challenged again with 1% OVA for 20 minutes (secondary allergen challenge). A group of mice were sensitized with PBS, followed by primary and secondary challenges with OVA. In all groups, assays were performed 48 hours after the final allergen challenge.
Pim Kinase Inhibitor Treatment
To determine the role of Pim1 kinase in the development of allergen-induced AHR and airway inflammation, we used the Pim1 kinase inhibitor, AR00460770 (Array Pharma, Boulder, CO). To characterize AR00460770 in vitro, the cellular half-maximal inhibitory concentration (IC50) and kinase selectivity assays were determined. The cellular IC50 of AR00460770 was analyzed by the Ser112 phosphorylation of transiently transfected Bcl-2–associated death promoter (BAD) in HEK-293 cell lines, engineered to express human Pim1 and Pim2 (Millipore, Billerica, MA) and rat Pim3 (Array BioPharma, Boulder, CO) in conjunction with a DNA vector construct directing the expression of the Pim kinase substrate glutathione S-transferase (GST)-BAD (pEBG-mBAD). Cells were treated with serial dilutions of AR00460770 for 1.5 hours, and then labeled with an antibody specific for phospho-BAD (Ser112) and an antibody against GST (Cell Signaling Technology, Danvers, MA) as a normalization control. Immunoreactivity was detected using infrared (IR) fluorophore–conjugated secondary antibodies, and quantified on an imager (Aerius; Li-Cor, Lincoln, NE). The kinase selectivity of AR00460770 was evaluated using the Kinase Profiler service (Millipore) (30–32). The properties and specificity of the inhibitor are described in Tables 1 and 2.
TABLE 1.
CELLULAR HALF-MAXIMAL INHIBITORY CONCENTRATIONS FOR PIM INHIBITION WERE DETERMINED FOR AR00460770 (IC50) BY ASSESSING SER112 PHOSPHORYLATION OF TRANSIENTLY TRANSFECTED BAD IN HEK CELL LINES ENGINEERED TO EXPRESS PIM1, PIM2, OR PIM3
| Pim1 | Pim2 | Pim3 | |
| AR00460770 | 93 | 9,200 | 340 |
Definition of abbreviations: SER, serine.
Cellular half-maximal inhibitory concentration values are reported in nanomole.
TABLE 2.
CHARACTERIZATION OF PIM INHIBITOR AR00460770
| Kinase | Abbreviation | Enzyme IC50 (nm) |
| Proto-oncogene serine/threonine-protein kinase | Pim1 | 0.300 |
| Pim2 | 71 | |
| Pim3 | 4 | |
| Proline–alanine–rich sterile (STE)20–related kinase | PASK | 62 |
| Tyrosine kinase, non–receptor 2 | TNK2 | 3,000 |
| Ca2+/calmodulin-dependent protein kinase II–γ | CAMK2γ | 6,000 |
| FMS-like tyrosine kinase receptor–3 | Flt3 | >10,000 |
| Platelet-derived growth factor receptors | PDGFRs | >10,000 |
| MAP/microtubule affinity–regulating kinase 1 | MARK1 | >1000 |
| Ca2+/calmodulin–dependent protein kinase II–β | CAMK2β | >10,000 |
| 5′ AMP-activated protein kinase | AMPK | >100,000 |
| Ribosomal S6 kinase | RSK | >10,000 |
Definition of abbreviations: IC50, half-maximal inhibitory concentration; MAP, mitogen-activated protein.
AR00460770 was tested at 10 μM against 230 kinases in enzymatic assays (Millipore Kinase Profiler). It was determined to be selective for the three Pim isoforms.
Western Blot Analysis
Lung tissue was homogenized, and lysates were cleared of debris and resuspended in an equal volume of 2 × Laemmli buffer. Lysates were loaded onto a 4–10% gradient reducing gel, subjected to electrophoresis, and transferred to nitrocellulose membranes. The membranes were blotted with goat anti-Pim1 (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-GAPDH (R&D Systems, Minneapolis, MN), anti-goat IgG (Invitrogen, Carlsbad, CA), and anti-rabbit IgG (Rockland, Gilbertson, PA). Images were captured and quantitatively analyzed using an Odyssey infrared imager (Li-Cor).
Assessment of Airway Function
Airway responsiveness was assessed as previously described, by measuring changes in pulmonary resistance (RL) in response to increasing doses of inhaled methacholine (MCh; Sigma-Aldrich, St. Louis, MO) in anesthetized and ventilated mice (33). The values of peak airway responses to inhaled MCh were recorded.
Bronchoalveolar Lavage and Lung Histology
Lungs were lavaged with 1 ml of Hanks’ balanced salt solution through the trachea immediately after the assessment of AHR. Numbers of total leukocytes were counted with a hemocytometer, and cell differentiation was performed on cytospin slides prepared with Wright-Giemsa stain. The numbers of inflammatory and mucus-containing cells were quantitated as previously described (34).
Measurement of Cytokines
Cytokine concentrations in BAL fluid and cell culture supernatants were measured by ELISA, as previously described (34).
Isolation of Lung Mononuclear Cells and Flow Cytometry
Lung mononuclear cells (MNCs) were isolated as described previously using collagenase digestion, and their cellular composition was identified as described elsewhere (35).
CD4+ and CD8+ T Cell Purification and Cell Proliferation Assay
The purification of CD4+ and CD8+ T cells was conducted as previously described (24). The purity of CD4+ and CD8+ T-cell populations exceeded 95%, as assessed by flow cytometry.
In cell proliferation assays, an anti-mouse CD3e monoclonal antibody (mAb) (5 μg/ml; R&D Systems) was immobilized on 96-well flat-bottom plates overnight at 4°C. Purified CD4+ and CD8+ T cells were incubated the with inhibitor or PBS as vehicle (2 × 105 cells/well), and anti-CD28 mAb (5 μg/ml; R&D Systems) was added to the anti-CD3–precoated plates and incubated at 37°C for 24 hours. After 24 hours, 1 μCi tritium-labeled thymidine per well (Perkin-Elmer, Boston, MA) was added to 96-well plates for 6 hours and harvested with distilled water, followed by counting in a microplate scintillation and luminescence counter (Packard, Meriden, CT). The cell viability of CD4+ and CD8+ T cells was assessed 24 hours after incubation with 10 μM of inhibitor by a vital stain with trypan blue, and determined using an automated cell counter (Countess; Invitrogen).
Statistical Analysis
Results were expressed as means ± SEM. The t test was used to determine differences between the two groups. For comparisons between multiple groups, the Tukey-Kramer test was used. Nonparametric analyses, using the Mann-Whitney U test or Kruskal-Wallis test, were also applied to confirm that statistical differences remained significant, even if the underlying distribution was uncertain. Differences were regarded as statistically significant when P < 0.05.
Results
In Vitro Characterization of AR00460770
The cellular IC50 and kinase selectivity of AR00460770 were determined and, as shown in Tables 1 and 2, exhibited strong inhibition specific to Pim1 kinase.
Lung Pim1 Kinase Concentrations Are Increased after Sensitization and Challenge with Allergen
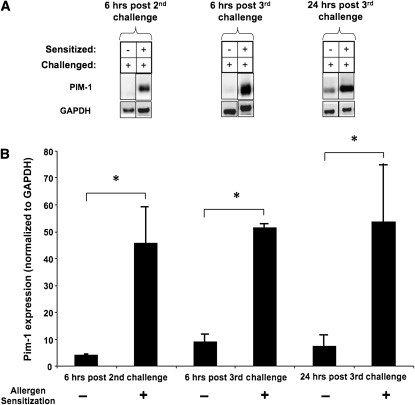
To determine the importance of Pim1 kinase after allergen challenge, we evaluated protein expression levels of the kinase in lung tissue after the OVA challenge of sensitized mice. Pim1 expression levels in OVA-sensitized mice were markedly increased after OVA challenge compared with levels in nonsensitized, challenged-only mice. This up-regulation was detected in OVA-sensitized mice 6 hours after their second OVA challenge, and remained high up to 24 hours after the third OVA challenge (Figure 1).
Figure 1.
Expression levels of Pim1 kinase in lungs after sensitization and challenge with ovalbumin (OVA). Pim1 kinase concentrations were determined by Western blot analyses in lungs of mice that were sensitized and challenged with OVA, or that received sham sensitization and OVA challenge. Expression levels were examined at three time points: 6 hours after the second OVA challenge, 6 hours after the third OVA challenge, and 24 hours after third OVA challenge. Experiments were repeated at least three times. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control (A), and the average optical densitometry was expressed by standardizing to GAPDH (B). *P < 0.05 compared with the sham-sensitized group.
Pim1 Kinase Inhibitor Treatment Prevents Development of AHR and Airway Inflammation after Primary Allergen Challenge
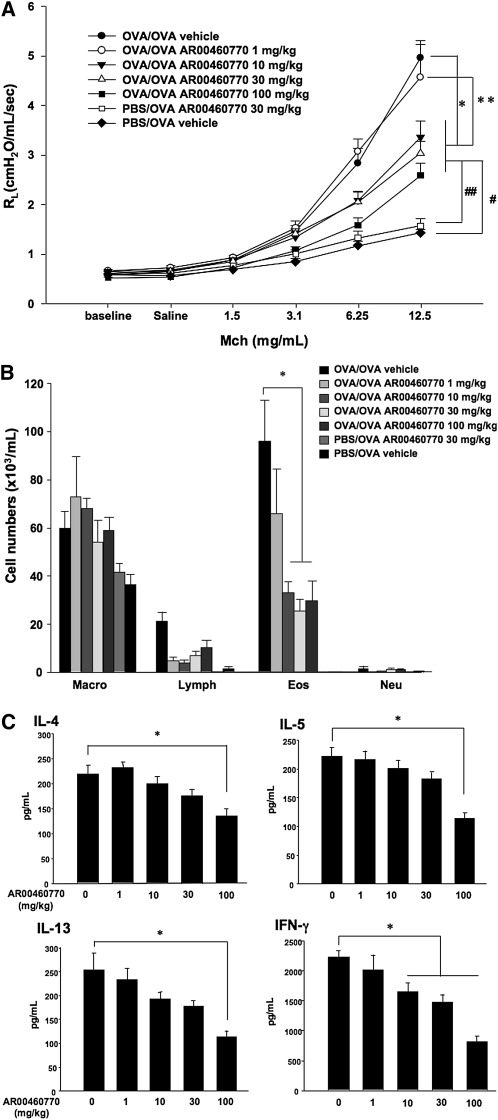
To determine the effects of Pim1 kinase inhibitor treatment on allergen-induced airway inflammation and AHR, mice were treated with the inhibitor or vehicle during the OVA challenge phase in the primary allergen challenge model. As shown in Figures 2A and 2B, vehicle-treated mice developed greater airway responses to MCh and eosinophil numbers in BAL fluid after sensitization and challenge with OVA, compared with sham-sensitized, OVA-challenged mice. Mice treated with the Pim1 inhibitor at doses of 10, 30, or 100 mg/kg developed significantly lower airway responsiveness to inhaled MCh and lower BAL eosinophil numbers, compared with the vehicle-treated group. Sham-sensitized but OVA-challenged mice were treated with 100 mg/kg of the inhibitor to assess its potential effects on smooth muscle contraction. Treatment with the inhibitor in this way did not alter the development of increasing RL with increasing concentrations of inhaled MCh.
Figure 2.
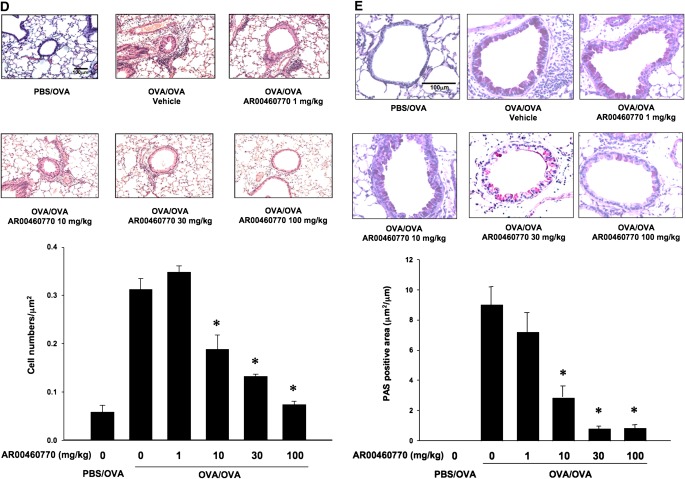
Effect of Pim1 kinase inhibition on airway responses after primary allergen challenge. The effects of the Pim1 kinase inhibitor were determined in the primary allergen challenge model. (A) Changes in pulmonary resistance (RL) in response to increasing doses of methacholine (MCh). (B) Cell composition in bronchoalveolar lavage (BAL) fluid. Macro, macrophages; Lympho, lymphocytes; Eos, eosinophils; Neu, neutrophils. (C) BAL fluid cytokine concentrations. (D) Lung tissue histology after staining with hematoxylin and eosin (H&E). (E) Lung tissue histology after staining with periodic acid–Schiff (PAS). Quantitative analyses of inflammatory and PAS+ cells in lung tissue were performed as described in Materials and Methods. Mice were sham-sensitized, followed by OVA challenge (PBS/OVA), or sensitized and challenged with OVA (OVA/OVA). The Pim1 inhibitor, AR00460770, was administered at doses of 1, 10, 30, or 100 mg/kg. #P < 0.05, compared with PBS/OVA vehicle. **P < 0.05, compared with OVA/OVA AR00460770 at 1 mg/kg. ##P < 0.05, compared with PBS/OVA AR00460770 at 30 mg/kg.
As shown in Figure 2C, the inhibitor treatment of sensitized and challenged mice reduced the concentrations of IL-4, IL-5, IL-13, and IFN-γ in BAL fluid in a dose-dependent manner. Significant changes were evident primarily at the highest administered dose of the inhibitor (100 mg/kg).
Histopathological analyses of lung-tissue sections revealed that the numbers of inflammatory cells, including eosinophils in peribronchial and perivascular areas, were increased in mice after OVA sensitization and challenge, compared with sham-sensitized and challenged mice (Figure 2D). Similarly, the numbers of periodic acid–Schiff–positive (PAS+) mucus-containing goblet cells were increased in the sensitized and challenged mice (Figure 2E). The administration of inhibitor significantly decreased the numbers of inflammatory cells and PAS+ mucus-containing goblet cells in lung tissue in a dose-dependent manner (Figure 2E).
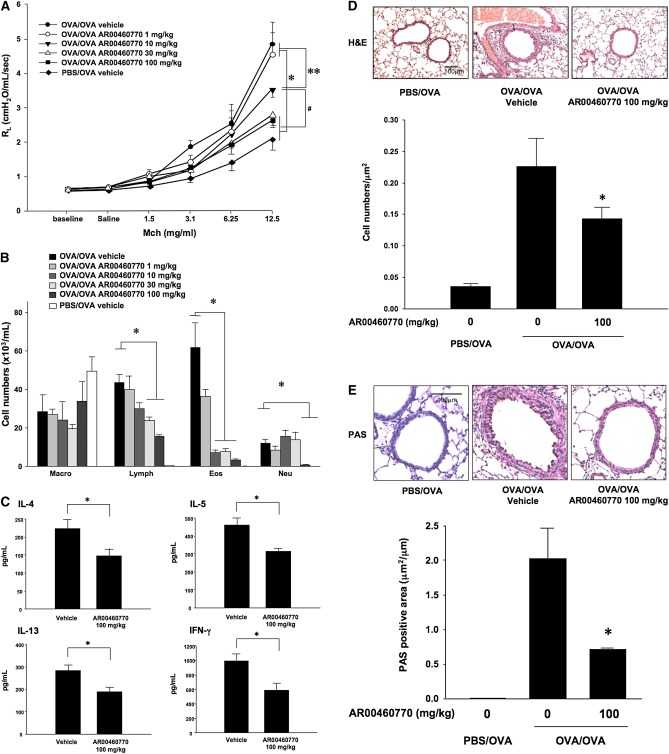
Inhibition of Pim1 Kinase Attenuates the Development of AHR and Airway Inflammation in the Secondary Allergen Challenge Model
The airway responses in the primary allergen challenge model reflect the first immune responses in the lungs, where adaptive immunity is initiated in response to airborne allergen exposure. For the most part, patients with asthma have already developed allergic airway inflammation and airway dysfunction before the initiation of treatment. Immune responses to allergen and tissue remodeling of the airways are generally already established. The secondary allergen challenge model is an approach to examine the response to allergen provocation where allergen-induced airway inflammation is previously established. To determine the effects of Pim1 kinase inhibition in the secondary allergen challenge model, we measured AHR, cell composition, and cytokine concentrations in BAL fluid, 48 hours after a single provocative allergen challenge. As in the primary allergen challenge model, vehicle-treated mice developed significantly higher airway responsiveness to MCh and eosinophils in BAL fluid after OVA sensitization and secondary allergen challenge. Similar to the results observed in the primary allergen challenge model, treatment with the Pim1 kinase inhibitor (at 10, 30, and 100 mg/kg) significantly decreased levels of airway responsiveness and the number of eosinophils in BAL fluid in a dose-dependent manner, compared with vehicle-treated groups (Figures 3A and 3B). Moreover, a significant reduction in numbers of lymphocytes and neutrophils was evident at the higher doses of inhibitor. Assays of BAL cytokine concentrations demonstrated that IL-4, IL-5, IL-13, and IFN-γ were decreased in Pim1 kinase inhibitor (100 mg/kg)–treated mice that had been sensitized and challenged with OVA (Figure 3C). Histopathological analyses revealed that Pim1 kinase inhibition decreased numbers of inflammatory cell in the lungs and goblet cell metaplasia along the airways (Figure 3D).
Figure 3.
Effects of Pim1 kinase inhibition on airway responses in the secondary allergen challenge model. The effects of Pim1 kinase inhibition were determined in the secondary allergen challenge model. (A) Changes in RL in response to increased doses of MCh. (B) Cell composition in BAL fluid. (C) BAL fluid cytokine concentrations. (D) Lung tissue histology after staining with H&E. (E) Lung tissue histology after staining with PAS. Quantitative analyses of inflammatory and goblet cells were performed as described in Materials and Methods. Mice were sham-sensitized, followed by OVA challenge (PBS/OVA), or sensitized and challenged with OVA (OVA/OVA). The Pim1 inhibitor was administered at doses of 1, 10, 30, or 100 mg/kg. Control groups received vehicle (n = 8). *P < 0.05, compared with OVA/OVA vehicle or OVA/OVA AR00460770 at 1 mg/kg. #P < 0.05, compared with OVA/OVA AR00460770 10 mg/kg. **P < 0.05, compared with OVA/OVA vehicle or OVA/OVA AR00460770 at 1 mg/kg.
Decrease of CD4+ and CD8+ T Cells in the Lungs of Sensitized and Challenged Mice after Treatment with the Pim1 Kinase Inhibitor
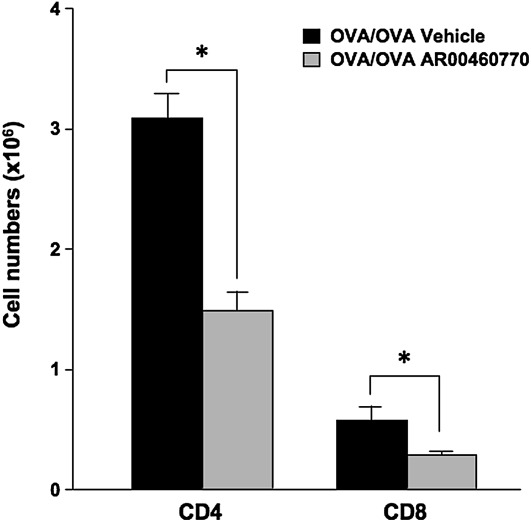
Because both CD4+ and CD8+ T cells are potent effector cells in the development of allergic inflammation, their numbers were examined after treatment with inhibitor in sensitized and challenged mice. Lungs from OVA-sensitized and OVA-challenged mice that received either inhibitor or vehicle were excised, and lung MNCs were purified. Numbers of CD4+ and CD8+ T cells were determined by flow cytometry. As shown in Figure 4, the overall number of CD4+ T cells was significantly lower in the inhibitor-treated mice (1.48 ± 0.26 × 106 cells/lung, versus 3.09 ± 0.35 × 106 cells/lung in vehicle-treated mice). CD8+ T cells were also significantly decreased after Pim1 kinase inhibition, from 0.57 ± 0.21 × 106 cells/lung to 0.29 ± 0.06 × 106 cells/lung. These results demonstrate that Pim1 kinase inhibition in vivo reduces the numbers of CD4+ and CD8+ T cells that accumulate in the lungs of sensitized and challenged mice.
Figure 4.
The effects of Pim kinase inhibition on numbers of CD4+ and CD8+ T cells. In OVA-sensitized and OVA-challenged mice, the numbers of CD4+ T cells (CD4) and CD8+ T cells (CD8) in the lungs of mice treated with the Pim1 kinase inhibitor (OVA/OVA AR00460770) or vehicle (OVA/OVA vehicle) were determined. Mononuclear cells isolated from lungs were stained with anti-CD3, anti-CD4, and anti-CD8 for flow cytometry analysis, as described in Materials and Methods. The data shown are representative of three independent experiments. *P < 0.05, compared with vehicle.
Reduction of CD4+ and CD8+ T-Cell Proliferation and Cytokine Production In Vitro after Pim1 Kinase Inhibitor Treatment
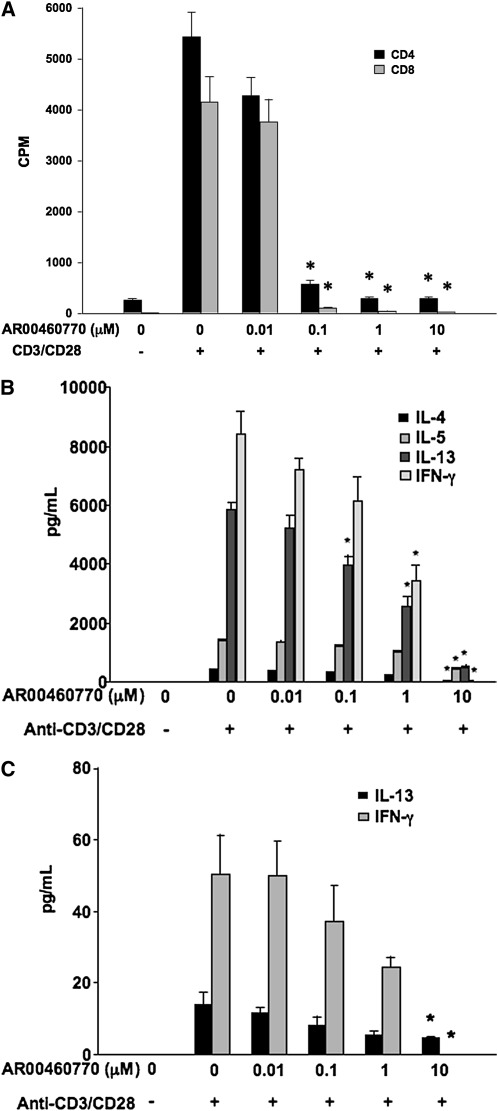
To examine the proliferative capacity of T cells after the inhibition of Pim1 kinase, CD4 and CD8 T cells were isolated and purified from spleens and incubated with a combination of anti-CD3 and anti-CD28 for 24 hours. The cell viabilities of CD4+ or CD8+ T cells were determined in the presence of 10 μM of the inhibitor. After 24 hours, inhibitor treatment did not show significant effects on cell viabilities compared with vehicle control samples (from 90.0–90.3% in CD4+ T cells, and from 80.2–82.8% in CD8+ T cells, respectively). In a dose-dependent manner, the Pim1 kinase inhibitor reduced the CD4+ and CD8+ T-cell proliferation triggered by the combination of anti-CD3 and anti-CD28. In stimulated cell cultures, increased concentrations of IL-4, IL-5, IL-13, and IFN-γ were detected. Treatment with the inhibitor decreased the concentrations of all of these cytokines in a dose-dependent fashion (Figure 5).
Figure 5.
Effects of Pim1 kinase inhibition on cell proliferative responses and cytokine production from CD4+ and CD8+ T cells. Purified spleen CD4+ and CD8+ T cells were preincubated with the Pim1 kinase inhibitor, followed by anti-CD3 and anti-CD28 stimulation. (A) Cell proliferation assays were performed 24 hours after anti-CD3/anti-CD28 stimulation, and calculated from the uptake of tritium-labeled thymidine (n = 8). CPM, counts per minute. (B) Quantitation of cytokine concentrations in supernates from anti-CD3/anti-CD28–stimulated CD4+ cells. (C) Quantitation of cytokine concentrations in supernates from anti-CD3/anti-CD28–stimulated CD8+ T cells. CPM, counts per minute. *P < 0.05, compared with vehicle-treated cells.
Discussion
In these initial studies using an experimental model of asthma, we demonstrated that concentrations of Pim1 kinase were elevated in the lungs of OVA-sensitized and OVA-challenged mice, compared with concentrations in the lungs of nonsensitized, challenged-only mice. Pim kinases are among the few to be up-regulated during cell activation through increased transcription (8). There are three subtypes of Pim kinases. Pim1 and Pim2 are primarily restricted to hematopoietic cells, and Pim3 is expressed in brain, kidney, and mammary tissue (36). To date, only a single study demonstrated that the expression of Pim1 was induced in human immune cells, and that was in eosinophils from either whole blood or BAL fluid after stimulation with IL-5 (16). Concentrations of Pim1 kinase in BAL eosinophils, compared with blood eosinophils, were further increased after segmental allergen challenge in patients with asthma (16). Linking the increases in Pim1 kinase concentrations in sensitized and challenged mice to function, we demonstrated that sensitized and challenged mice treated with a Pim1 kinase inhibitor developed significantly lower levels of AHR, cytokines, eosinophilic airway inflammation, lung inflammatory cell accumulation, and goblet cell metaplasia. The effects of the kinase inhibitor in vivo on the development of AHR, BAL eosinophilia, airway inflammation, and goblet cell metaplasia were seen at doses (10–30 mg/kg) that, based on the cellular activity of AR460770 (Table 1), were specific to Pim1 kinase inhibition. The suppressive effects associated with Pim1 kinase inhibition were observed in both the primary and secondary allergen challenge protocols. The primary challenge model reflects the initial immune responses of the airways to airborne allergen exposure, and best defines the development of lung allergic responses. In contrast, the secondary allergen challenge model detects airway secondary immune responses in lungs on a background of previously established allergic airway inflammation. In both models, the reduction of eosinophils in the BAL appeared at lower doses of inhibitor, and these doses did not alter BAL cytokine production levels. Together with the data on human eosinophils (16), our findings suggest that the inhibition of Pim1 kinase directly affects eosinophil function. The comparable suppressive effects of Pim1 kinase inhibition after both primary and secondary allergen challenge imply that the activation of this kinase plays a critical role in both the initiation and recall responses induced by allergen challenges of sensitized mice.
Pim1 kinase controls cellular survival, and the inhibition of this kinase can cause cell death. The antibody targeting of Pim kinase synergistically enhanced the cytotoxicity of anticancer agents, and the inhibition of Pim1 kinase triggered the death of leukemic cells (37, 38). To address the possibility that the suppressive effects of Pim1 kinase inhibition on allergen-induced airway responses were attributable to its toxic effects on immune cells, an evaluation of cell viability was performed. At concentrations up to 10 μM, the inhibitor had no effect on cell number or viability, suggesting its effects on airway responses were not attributable to drug-mediated cellular toxicity. These findings are similar to those described in Pim-deficient mice, in which the rate of apoptosis of T cells was not different than in wild-type mice (36). Furthermore, treatment with Pim1 kinase inhibitor in sham-sensitized but OVA-challenged mice did not alter airway responsiveness to MCh, indicating that the inhibitor less likely exhibited toxic effects on lung resident cells, including airway smooth muscle. Microarray data indicate that Pim1 is expressed in airway epithelial cells, indicating the potential for additional suppressive effects of the inhibitor on airway epithelial cell function, as well as eosinophil and T-cell function in the development of AHR and allergic airway inflammation (39). Although the specificity of the inhibitor was demonstrable in vitro, the effects of the inhibitor on other cells cannot be completely ruled out at this time.
The pathogenesis of asthma is complex, and the role of Th2 CD4+ T cells secreting proallergic cytokines such as IL-4, IL-5, and IL-13 has been emphasized (1, 3, 19). The role of IFN-γ may be more complex. In several studies, IFN-γ was shown to be a potent suppressor of allergen-induced AHR, lung pathology, and Th2 cytokine production (40–42). In other studies, IFN-γ was shown to be essential in the development of AHR (43), and the administration of IFN-γ was shown to be ineffective in preventing AHR or airway eosinophilia (40). In patients with asthma, elevations of IFN-γ were shown to correlate with asthma severity (44) and bronchial hyperresponsiveness (45). Given the distribution of Pim1 kinase, several points of action likely explain the attenuation of AHR, BAL cytokine concentrations, and lung inflammation in primary or secondary allergen challenge models. Effector CD4+ and CD8+ T cells play a role in the pathogenesis of asthma through cell proliferation to specific allergens, followed by prolonged survival (25, 45). T cells are also required for the development or initiation of airway eosinophilia and goblet cell metaplasia through the release of specific cytokines (46, 47). The inhibition of Pim1 kinase altered the activities of these effector cells in the airways, because both the numbers of CD4+ and CD8+ T cells in the lungs and BAL cytokine concentrations were decreased in inhibitor-treated, sensitized, and challenged mice. Further, according to in vitro experiments, inhibitor treatment demonstrated suppressive effects on the proliferation of CD4+ and CD8+ T cells induced in response to stimulation with anti-CD3 and anti-CD28. In parallel, concentrations of cytokines, IL-4, IL-5, IL-13, and IFN-γ, in stimulated cell culture supernates were also reduced in a dose-dependent manner. Of interest here, Pim-deficient T cells were shown to display normal proliferative responses to high concentrations of anti-CD3 and IL-2, but Pim was required for normal proliferation at lower levels of the stimuli (36). Together, the results suggest that under experimental conditions in vivo and in vitro, the inhibition of Pim1 kinase limits responses through interference with the expansion and activities of critical effector cells, CD4+ and CD8+ T cells in the airways, and possibly eosinophils, as reported elsewhere (18–25, 48, 49).
Although survival kinases, such as the Pim kinases, are known to regulate common substrates such as BAD or 4EBP1 (50), the downstream activities of individual Pim kinases may be different. Targeting Pim1 kinase may account for effects on allergic lung responses in other ways. The suppressor of cytokine signaling-1, c-myc, Pim-associated protein-1, protein tyrosine phosphatase U2S, and heterochromatin protein 1 are all potential downstream targets of Pim1 kinase (51–56). Recently, the nuclear factor of activated T cells (NFAT) was reported to be a potential downstream substrate of Pim1 kinase (57). The regulation of NFAT activity was shown to be important for the normal selection of thymocytes and may play a role in the functional development of T cells. The down-regulation of NFAT may play a role in the suppression of both CD4+ and CD8+ T-cell proliferation and T-cell cytokine production as a downstream substrate of the kinase.
Asthma is a chronic airway inflammatory disease, as demonstrated by the infiltration and proliferation of inflammatory cells in the airways (48, 59). As a result, interference with inflammatory cell accumulation in the airways and cell expansion represent potential strategies in the treatment of inflammatory diseases such as asthma. Despite the successful introduction of anti-inflammatory drugs and immunomodulators in the treatment of autoimmune diseases, few have produced similar benefits in asthma (60, 61). This finding may suggest that the inflammatory pathways in asthma differ from those in other diseases, and novel strategies are required. To date, compelling evidence for the involvement of Pim1 kinase in the development and progression of several different diseases has made it a potential pharmaceutical target. Limited numbers of Pim kinase inhibitors have been available for study. One recent study using a Pim1 kinase inhibitor demonstrated therapeutic effects in chronic lymphocytic leukemia (37). The data reported here demonstrate for the first time, to the best of our knowledge, that targeting Pim1 kinase effectively reduces the development of the full spectrum of allergen-induced lung inflammatory responses, at least in part through limiting the expansion and activities of effector CD4+ and CD8+ T cells. As such, the inhibition of Pim1 kinase represents a novel therapeutic target in the treatment of asthma.
Supplementary Material
Acknowledgments
The assistance of Ms. Diana Nabighian in the preparation of the manuscript is gratefully acknowledged.
Footnotes
This study was supported by National Institutes of Health grants HL-36577 and AI-77609, and by Array Biopharma.
The contents of this work are solely the responsibility of the authors, and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0190OC on November 10, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med 2001;344:350–362 [DOI] [PubMed] [Google Scholar]
- 2.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol 2002;3:715–720 [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261 [DOI] [PubMed] [Google Scholar]
- 4.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304 [DOI] [PubMed] [Google Scholar]
- 5.Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science 2002;296:1653–1655 [DOI] [PubMed] [Google Scholar]
- 6.Bachmann M, Moroy T. The serine/threonine kinase Pim-1. Int J Biochem Cell Biol 2005;37:726–730 [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Bhattacharya N, Weaver M, Petersen K, Meyer M, Gapter L, Magnuson NS. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J Vet Sci 2001;2:167–179 [PubMed] [Google Scholar]
- 8.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest 2005;115:2618–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer. 2011;11:23–34 [DOI] [PubMed] [Google Scholar]
- 10.Amson R, Sigaux F, Przedborski S, Flandrin G, Givol D, Telerman A. The human protooncogene product p33Pim is expressed during fetal hematopoiesis and in diverse leukemias. Proc. Natl. Acad. Sci. USA 1989;86:8857–8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdman A, Fang X, Pang ST, Ekman P, Egevad L. Pim-1 expression in prostatic intraepithelial neoplasia and human prostate cancer. Prostate 2004;60:367–371 [DOI] [PubMed] [Google Scholar]
- 12.Cibull TL, Jones TD, Li L, Eble JN, Ann Baldridge L, Malott SR, Luo Y, Cheng L. Overexpression of Pim-1 during progression of prostatic adenocarcinoma. J Clin Pathol 2006;59:285–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieborowska-Skorska M, Hoser G, Kossev P, Wasik MA, Skorski T. Complementary functions of the antiapoptotic protein A1 and serine/threonine kinase Pim-1 in the BCR/ABL-mediated leukemogenesis. Blood 2002;99:4531–4539 [DOI] [PubMed] [Google Scholar]
- 14.Temple R, Allen E, Fordham J, Phipps S, Schneider HC, Lindauer K, Hayes I, Lockey J, Pollock K, Jupp R. Microarray analysis of eosinophils reveals a number of candidate survival and apoptosis genes. Am J Respir Cell Mol Biol 2001;25:425–433 [DOI] [PubMed] [Google Scholar]
- 15.Andina N, Didichenko S, Schmidt-Mende J, Dahinden CA, Simon HU. Proviral integration site for Moloney murine leukemia virus 1, but not phosphatidylinositol-3 kinase, is essential in the antiapoptotic signaling cascade initiated by IL-5 in eosinophils. J Allergy Clin Immunol 2009;123:603–611 [DOI] [PubMed] [Google Scholar]
- 16.Stout BA, Bates ME, Liu LY, Farrington NN, Bertics PJ. IL-5 and granulocyte–macrophage colony–stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J Immunol 2004;173:6409–6417 [DOI] [PubMed] [Google Scholar]
- 17.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med 2005;201:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busse WW, Coffman RL, Gelfand EW, Kay AB, Rosenwasser LJ. Mechanisms of persistent airway inflammation in asthma. A role for T cells and T-cell products. Am J Respir Crit Care Med 1995;152:388–393 [DOI] [PubMed] [Google Scholar]
- 19.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med 1997;186:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell–regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol 1998;161:1501–1509 [PubMed] [Google Scholar]
- 21.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, Assoufi B, Collins JV, Durham S, Kay AB. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis 1990;142:1407–1413 [DOI] [PubMed] [Google Scholar]
- 22.Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter TA, Larsen GL, Gelfand EW. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a marine model of airway sensitization. J Exp Med 1996;183:1719–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isogai S, Taha R, Tamaoka M, Yoshizawa Y, Hamid Q, Martin JG. CD8+ alphabeta T cells can mediate late airway responses and airway eosinophilia in rats. J Allergy Clin Immunol 2004;114:1345–1352 [DOI] [PubMed] [Google Scholar]
- 24.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, Miyahara S, Balhorn A, Dakhama A, Gelfand EW. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol 2004;172:2549–2558 [DOI] [PubMed] [Google Scholar]
- 25.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. 2004 Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med 10:865–869 [DOI] [PubMed] [Google Scholar]
- 26.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)–4 and IL-12 in promoting Type 2 versus Type 1 cytokine profiles. J Exp Med 1994;180:1715–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seder RA, Boulay JL, Finkelman F, Barbier S, Ben-Sasson SZ, Le Gros G, Paul WE. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol 1992;148:1652–1656 [PubMed] [Google Scholar]
- 28.Coyle AJ, Erard F, Bertrand C, Walti S, Pircher H, Le Gros G. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med 1995;181:1229–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Miyahara N, Kodama T, Taube C, Balhorn A, Dakhama A, Kitamura K, Hirano A, Tanimoto M, Gelfand EW. S-carboxymethylcysteine normalises airway responsiveness in sensitized and challenged mice. Eur Respir J 2005;26:577–585 [DOI] [PubMed] [Google Scholar]
- 30.López-Ramos M, Prudent R, Moucadel V, Sautel CF, Barette C, Lafanechère L, Mouawad L, Grierson D, Schmidt F, Florent JC, et al. New potent dual inhibitors of CK2 and Pim kinases: discovery and structural insights. FASEB J 2010;24:3171–3185 [DOI] [PubMed] [Google Scholar]
- 31.Yan Bin, Zemskova M, Holder S, Chin V, Kraft A, Koskinen PJ, Lilly M. The Pim-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem 2003;278:45358–45367 [DOI] [PubMed] [Google Scholar]
- 32.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev 2003;17:1841–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda K, Shiraishi Y, Matsubara S, Miyahara N, Matsuda H, Okamoto M, Joetham A, Gelfand EW. Effects of combination therapy with montelukast and carbocysteine in allergen-induced airway hyperresponsiveness and airway inflammation. Br J Pharmacol 2010;160:1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med 2001;163:721–730 [DOI] [PubMed] [Google Scholar]
- 35.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, Gelfand EW. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest 1996;97:1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikkers H, Nawijn M, Allen J, Brouwers C, Verhoeven E, Jonkers J, Berns A. Mice deficient for all Pim kinases display reduced body size and impaired responses to hematopoietic growth factors. Mol Cell Biol 2004;24:6104–6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen LS, Redkar S, Bearss D, Wierda WG, Gandhi V. Pim kinase inhibitor, SGI-1776, induces apoptosis in chronic lymphocytic leukemia cells. Blood 2009;114:4150–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu XF, Li J, Vandervalk S, Wang Z, Magnuson NS, Xing PX. Pim-1–specific mAb suppresses human and mouse tumor growth by decreasing Pim-1 levels, reducing Akt phosphorylation, and activating apoptosis. J Clin Invest 2009;119:362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kicic A, Hallstrand TS, Sutanto EN, Stevens PT, Kobor MS, Taplin C, Paré PD, Beyer RP, Stick SM, Knight DA. Decreased fibronectin production significantly contributes to dysregulated repair of asthmatic epithelium. Am J Respir Crit Care Med. 2010;181:889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lack G, Renz H, Saloga J, Bradley KL, Loader JE, Leung DYM, Larsen GL, Gelfand EW. Nebulized but not parenteral IFN-γ decreases IgE production and normalizes airway function in a murine model of allergen sensitization. J Immunol 1994;152:25446–25454 [PubMed] [Google Scholar]
- 41.Flaishon L, Topilaki I, Shoseyov D, Hershkoviz R, Fireman E, Levo Y, Marmor S, Shachar I. Anti-inflammatory properties of low levels of IFN-γ. J Immunol 2002;168:3707–3711 [DOI] [PubMed] [Google Scholar]
- 42.Yoshida M, Leigh R, Matsumoto K, Wattie J, Ellis R, O'Byrne PM, Inman MD. Effect of interferon-γ on allergic airway responses in interferon-gamma–deficient mice. Amer J Resp Crit Care Med 2002;166:451–456 [DOI] [PubMed] [Google Scholar]
- 43.Hessel EM, Van Oosterhout AJ, Van Ark I, Van Esch B, Hofman G, Van Loveren H, Savelkoul HF, Nijkamp FP. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Amer J Resp Cell Molec Biol 1997;16:325–334 [DOI] [PubMed] [Google Scholar]
- 44.ten Hacken NHT, Oosterhoff Y, Kauffman HF, Guevarra L, Satoh T, Tollerud DJ, Postma DS. Elevated serum interferon-γ in atopic asthma correlates with increased airways responsiveness and circadian peak expiratory flow variation. Eur Resp J 1998;11:312–316 [DOI] [PubMed] [Google Scholar]
- 45.Heaton T, Rowe J, Turner S, Aalberse RC, de Klerk N, Suriyaarachchi D, Serralha M, Holt BJ, Hollams E, Yerkovich S, et al. An immunoepidemiological approach to asthma: identification of in-vitro T cell response patterns associated with different wheezing phenotypes in children. Lancet 2005;365:142–149 [DOI] [PubMed] [Google Scholar]
- 46.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med 1988;167:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laoukili J, Perret E, Willems T, Minty A, Parthoens E, Houcine O, Coste A, Jorissen M, Marano F, Caput D, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest 2001;108:1817–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohn L, Tepper JS, Bottomly K. IL-4–independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J Immunol 1998;161:3813–3816 [PubMed] [Google Scholar]
- 49.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol 2007;119:1303–1310 [DOI] [PubMed] [Google Scholar]
- 50.Blanco-Aparicio C, Collazo AM, Oyarzabal J, Leal JF, Albaran MI, Lima FR, Pequeno B, Ajenjo N, Becerra M, Alfonso P, et al. Pim 1 kinase inhibitor ETP-45299 suppresses cellular proliferation and synergizes with PI3K inhibition. Cancer Lett 2011;300:145–153, 2011 [DOI] [PubMed] [Google Scholar]
- 51.van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T, Berns A. Predisposition to lymphomagenesis in Pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus–induced tumors. Cell 1989;56:673–682 [DOI] [PubMed] [Google Scholar]
- 52.Yan B, Zemskova M, Holder S, Chin V, Kraft A, Koskinen PJ, Lilly M. The Pim-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem 2003;278:45358–45367 [DOI] [PubMed] [Google Scholar]
- 53.Chen XP, Losman JA, Cowan S, Donahue E, Fay S, Vuong BQ, Nawijn MC, Capece D, Cohan VL, Rothman P. Pim serine/threonine kinases regulate the stability of SOCS-1 protein. Proc. Natl. Acad. Sci. USA 2002:99:2175–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maita H, Harada Y, Nagakubo D, Kitaura H, Ikeda M, Tamai K, Takahashi K, Ariga H, Iguchi-Ariga SM. PAP-1, a novel target protein of phosphorylation by Pim-1 kinase. Eur J Biochem 2000;267:5168–5178 [DOI] [PubMed] [Google Scholar]
- 55.Koike N, Maita H, Taira T, Ariga H, Iguchi-Ariga SM. Identification of heterochromatin protein 1 (HP1) as a phosphorylation target by Pim-1 kinase and the effect of phosphorylation on the transcriptional repression function of HP1(1). FEBS Lett 2000;467:17–21 [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Bhattacharya N, Meyer MK, Seimiya H, Tsuruo T, Tonani JA, Magnuson NS. Pim-1 negatively regulates the activity of PTP-U2S phosphatase and influences terminal differentiation and apoptosis of monoblastoid leukemia cells. Arch Biochem Biophys 2001;390:9–18 [DOI] [PubMed] [Google Scholar]
- 57.Rainio EM, Sandholm J, Koskinen PJ. Cutting edge: transcriptional activity of NFATc1 is enhanced by the Pim-1 kinase. J Immunol 2002;168:1524–1527 [DOI] [PubMed] [Google Scholar]
- 58.Patra AK, Drewes T, Engelmann S, Chuvpilo S, Kishi H, Hunig T, Serfling E, Bommhardt UH. PKB rescues calcineurin/NFAT-induced arrest of Rag expression and pre-T cell differentiation. J Immunol 2006;177:4567–4576 [DOI] [PubMed] [Google Scholar]
- 59.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 2003;111:450–463 [DOI] [PubMed] [Google Scholar]
- 60.Mullarkey MF, Blumenstein BA, Andrade WP, Bailey GA, Olason I, Wetzel CE. Methotrexate in the treatment of corticosteroid-dependent asthma: a double-blind crossover study. N Engl J Med 1988;318:603–607 [DOI] [PubMed] [Google Scholar]
- 61.Alexander AG, Barnes NC, Kay AB. Trial of cyclosporin in corticosteroid-dependent chronic severe asthma. Lancet 1992;339:324–328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.