Abstract
Objectives
Clinicians have long noted that infected cholesteatomas are more aggressive than uninfected ones without data to support these observations. The purpose of this study is to determine the etiological role of biofilm forming P. aeruginosa (PA) and the virulence factor, type IV pili, in the pathogenesis of experimental cholesteatomas.
Design
We evaluated three different PA strains and one E. coli strain in cholesteatoma progression: PA14, a well characterized wound isolate, OPPA8, an otopathogenic strain from a human cholesteatoma, OPPA8-NP, an isogenic type IV pili deletion mutant and DH5α, an E. coli strain.
Methods
Cholesteatomas were induced in gerbils. We inoculated the right ear with bacteria and the left with vehicle. After 6 weeks their cholesteatomas were evaluated by micro-CT scanning. Cholesteatoma size and bone resorption were analyzed digitally.
Results
Results demonstrate that PA infection increases cholesteatoma size when compared to uninfected controls: OPPA8 a showed an 8.9 fold increase, PA14 a 2.6 fold increase, OPPA8-NP a 1.9 fold increase while DH5α was not increased over controls. Additionally, infected bullae showed 10 to 50% more cholesteatoma-induced bone resorption.
Conclusions
In this model, PA infected cholesteatomas enlarge more rapidly and are more destructive than un-infected controls. OPPA8, the strain from a human cholesteatoma, showed the greatest enlargement and bone destruction. Additionally, we demonstrate that type IV pili is a virulence factor in this model since the non-piliated isogenic mutant, OPPA8-NP, was significantly less aggressive than the wild-type OPPA8 indicating that Type IV pili may be a virulence factor in this disease.
Keywords: Cholesteatoma, P. aeruginosa, micro-CT, gerbil, bone resorption
INTRODUCTION
Cholesteatomas are cyst-like epithelial inclusions that develop in the middle ear and mastoid, usually as a consequence of chronic otitis media (COM). While the exact incidence of aural cholesteatoma is unknown, in well documented populations the prevalence of COM is about 1% and approximately 40% of those have cholesteatomas1. The incidence of COM varies widely in population groups. In the US, there are approximately 4000 procedures done for cholesteatomas each year at a cost of approximately 80 million dollars per year.
Aggressive cholesteatomas may also invade intracranially leading to meningitis and brain abscess. In a large reported case series 78% of subjects who had complications secondary to COM were found to have cholesteatomas2. Unlike uncomplicated otitis media, cholesteatomas cannot be eradicated by host defenses or pharmacological means. Since the lining wall of a cholesteatoma is keratinizing epithelium, keratin debris accumulates relentlessly within the lesion leading to gradual enlargement with characteristic destruction of surrounding bone. The only effective intervention is tympanomastoid surgery and removal of the lesion. Many cholesteatomas become chronically infected which tend to enlarge more rapidly and are observed by clinicians to be more destructive locally3.
Most cholesteatomas develop by ingrowth of keratinizing epithelium into the middle ear rapidly contacting and eroding adjacent structures. Nearly all acquired cholesteatomas become chronically infected, most commonly with P. aeruginosa (PA)4–6. Brook and colleagues found that 85% of cholesteatomas contained bacteria4. Fulghum & Chole6 found 93% of spontaneously occurring cholesteatomas in gerbils had bacteria. Cholesteatomas are characterized by increased adherence of bacteria to entrapped keratin and keratinocyte proliferation resulting in a ‘matrix’ of desquamated keratinocytes which forms an expanding mass. As chronically infected cholesteatomas enlarge, osteoclasts are recruited and activated leading to erosion of adjacent bone leading to ossicular destruction and hearing loss. Chronically infected cholesteatomas are more aggressive, recalcitrant to surgical and medical treatment and often recurrent7–9. We have previously demonstrated the presence of bacterial biofilms within cholesteatomas9 and shown that PA isolated from human cholesteatomas form biofilms in vitro10.
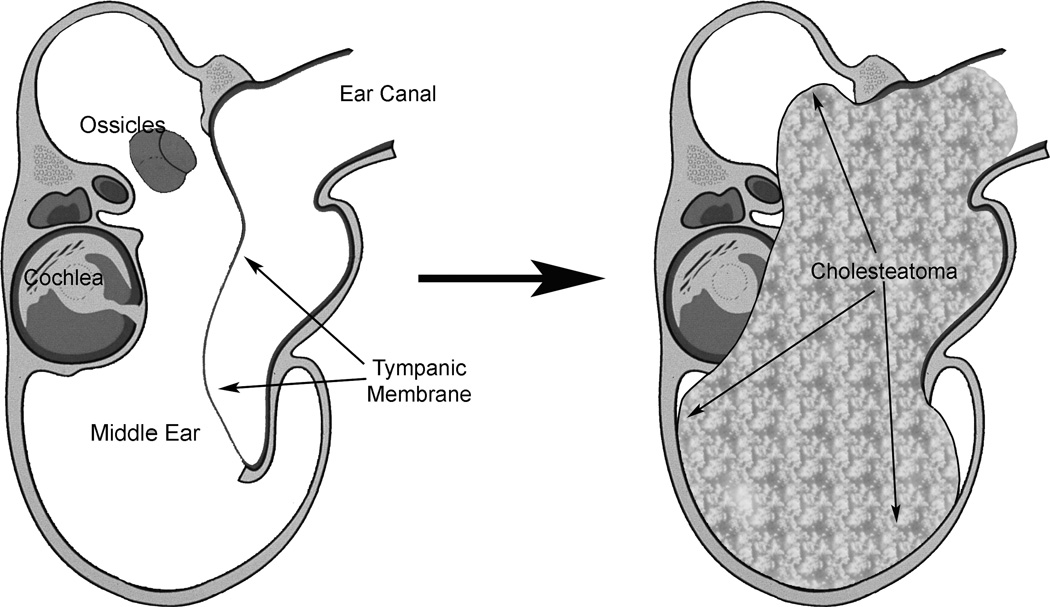
Mongolian gerbils, Meriones unguiculatus, are the only non-human animal known to develop spontaneous cholesteatomas11. These spontaneous cholesteatomas develop slowly and are more prevalent with increasing age12. An experimental model of induced cholesteatomas showed similar characteristics to spontaneous cholesteatomas11. In gerbils, the cholesteatoma develops within the ear canal and as it enlarges, displacing the tympanic membrane medially into the bulla where it erodes into the surrounding bony structures, including the external ear canal, ossicles, cochlea and bulla walls (Fig. 1).
Figure 1.
Diagram of cholesteatoma development in the gerbil model. After surgical ligation of the ear canal, keratin debris fills the canal. As it expands, it erodes into adjacent bone, leading to hearing loss.
In these studies, we used a modified protocol to inoculate the ear canals with PA prior to surgical ligation of the canal to determine the role of these bacteria in the etiology of cholesteatoma development. We evaluated three different PA strains: PA14, a well characterized and fully sequenced wound isolate13, OPPA8, an otopathogenic strain isolated from a human cholesteatoma10 and OPPA8-NP, an isogenic type IV pili deletion mutant generated in our laboratory (see methods) and DH5α, an E. coli laboratory strain. (See methods).
MATERIALS AND METHODS
DESIGN AND RATIONALE
Bilateral cholesteatomas were induced in 27 gerbils. One ear was infected with the indicated bacteria and the other with sterile vehicle only. At six weeks microCT scans were performed and the extent of the cholesteatoma and bone changes were measured. Four strains of bacteria were chosen:
PA14 A laboratory strain of P. aeruginosa was obtained from Alice Prince (Columbia University, NY). This strain, UCBPP-PA14, was originally isolated from a wound by M. Schroth at the University of California, Berkeley.
OPPA8 A strain of otopathogenic P. aeruginosa isolated from a patient with an infected cholesteatoma. We have characterized this strain previously. 10 This strain is a competent biofilm former and we hypothesized that it would be the most virulent in this experimental model.
DH5α A standard laboratory strain of E. coli. Used as a comparison strain of a gram negative bacteria that forms LPS but has not been shown to be virulent in cholesteatomas.
OPPA8-NP A strain devoid of type IV pili derived from OPPA8 This strain lacks type IV pili which are required for colonization of host tissues and for twitching motility. Adherence is thought to be the first step in the formation of microcolonies and hence biofilms. The strain is identical to OPPA8 except for the lack of type IV pili. Our hypothesis is that type IV pili are a virulence factor in chronically infected cholesteatomas.
OPPA8-NP Construction
Primers were designed to 5’ and 3’ flanking regions of the pilA gene containing restriction sites at the ends (5’forward- ccagcggccgccggttttctccgcaggcaagtcg, 5’reverse- ccaggatccccgagcgaatgccgctaatagg, 3’forward- ccaggatccctccattgatatatccaggcctaacgc, 3’reverse- ccagtcgacccacaacggaactactcgatgctgg). PCR products amplified from genomic OPPA8 DNA were digested with the corresponding enzymes and ligated at the SalI sites and simultaneously ligated into a suicide plasmid, pJB4648, at the NotI and BamHI sites. The suicide plasmid contains genes for gentamicin resistance (GentR) and sucrose sensitivity (SacB). The resulting plasmid containing the fragments was transformed into OPPA8 by electroporation. Plasmid integrants were selected by plating on LB with gentamicin (20µg/mL). Colonies were then plated on LB with 4% sucrose to select for loop-outs of the pilA gene. True deletion mutants were confirmed by PCR of sucrose resistant colonies.
Experimental cholesteatoma
Four to six week old Mongolian gerbils, Meriones unguiculatus, were obtained from Charles River Laboratories (Wilmington, MA). Use of these vertebrate animals was approved by the Washington University Animal Studies Committee. Cholesteatoma was induced by surgical ligation of the external auditory canal as described by McGinn, et. al.11 Briefly, the gerbils were anesthetized with a mixture of ketamine HCl (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA, 0.08mg/g)/xylazine HCl (supplied by the Department of Comparative Medicine, Washington University, St. Louis, MO, 0.02mg/g) injected intraperitoneally. Bupivicaine HCl (Marcaine, Hospira, Inc, Lake Forest, IL, 0.1µg/g) was also given for pain relief. A small postauricular incision was made, 20µL sterile PBS was inserted into the left external auditory canal, which was ligated with 4-0 silk suture around the cartilaginous canal. This process was repeated using 20µL of a P. aeruginosa (PA) strain diluted in PBS and inserted into the right auditory canal. For preliminary experiments, PA strain OPPA8 was used at 104–108 cfu/mL. These animals were sacrificed 1 and 2 weeks post operatively for microbiological, histological, and preliminary micro-CT evaluations. For animals that underwent controlled micro-CT evaluation, four groups of six animals were infected with strains PA14, OPPA8, OPPA8-NP, or DH5α at 107 cfu/mL (2 × 105 cfu per ear inoculation), and one group of three animals were used as untreated controls. One animal from the PA14, OPPA8-NP, and control groups and two animals from the OPPA8 group and one from the OPPA8-NP group died before the end of the experiment and were excluded from the analysis. This study was approved by the Washington University Animal Studies Committee.
Micro-computed tomography and histomorphometry
Micro-computed tomography (micro-CT) was used to assess the level of bone erosion and reactive bone formation present in the gerbil ear. Six weeks after surgery, animals were given sodium pentobarbital (Sleepaway, Fort Dodge Animal Health, Fort Dodge, IA, 0.5mg/g) to anesthetize them before perfusing with 2% paraformaldehyde, 2.5% glutaraldehyde in a 0.1M phosphate buffer (pH 7.4). The intact head from each animal was oriented in a precise axial orientation within the 36 mm diameter plastic cylinder. The cylinder with the properly oriented head was filled with agarose for stabilization. The micro CT scanning was performed on a Scanco µCT 40 (Scanco Medical AG, Basserdorf, Switzerland) with point-spread function of 0.036 mm over a period of 2–3 hours per specimen. The scanning parameters in the standard mode consisted of 70 kVp tube potential, 114 mA tube current, 200 ms integration time. The resulting reconstructed volume had 36 µm isotropic voxels. For all analyses, the raw CT image was modified in Adobe Photoshop® (Adobe Systems Inc., San Jose, CA) in a standard manner; levels converted from 0–255 to a contrast range of 0–150 to better differentiate gas from tissue and tissue from bone. The grayscale images are saved as *.tif files for analysis with ImageJ® (Rasband, W.S., U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2006). For histomorphometry, axial microCT images were selected from a level that intersects both external auditory canals and includes the mid-portion of the cochlea. All axial images were coded and blinded for image analysis.
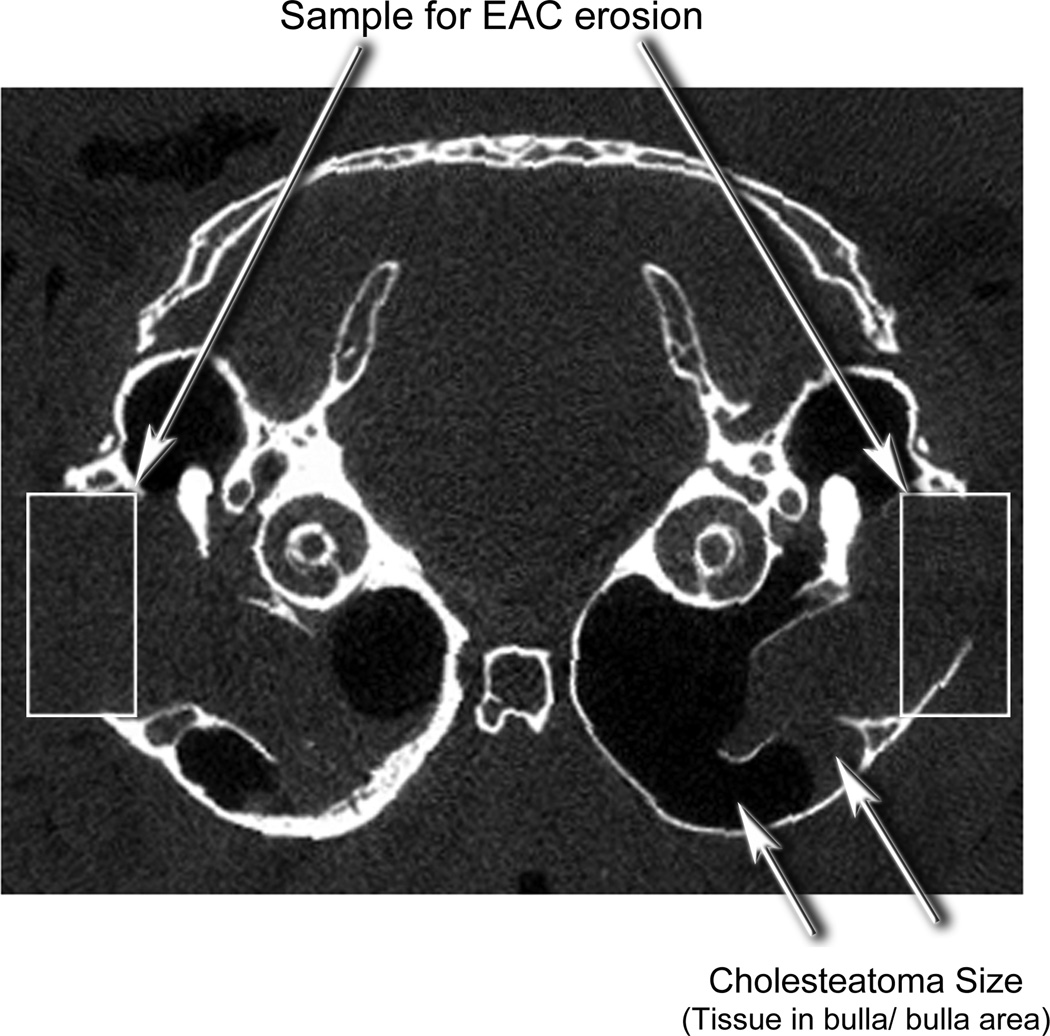
For cholesteatoma size, radiolucent areas, presumably trapped air or gas, were delineated and saved; radio-opaque areas, presumably cholesteatoma, were separately delineated and saved (Fig. 2 and 3.) The separately captured images were converted to black and white images and saved as *.tif files. Using NIH ImageJ®, the area of the gas and the cholesteatoma were measured (calibrated directly from CT image). The proportion of cholesteatoma (radio-opaque area) was expressed as a percent of total: cholesteatoma/(gas + cholesteatoma). Results were recorded as percent of tissue within total bulla area within each axial section.
Figure 2.
An axial microCT scan of a normal gerbil head demonstrates the position of the bullae, ear canals, calvarium, malleus and cochlea.
Figure 3.
An axial microCT scan of a gerbil head with induced cholesteatoma demonstrating morphometric measures. The area of bone resorption was determined by the length of remaining bone in a standardized box. The area occupied by the cholesteatoma was determined by the area of soft tissue (radio-opaque) divided by the area of soft tissue plus area of gas (radiolucent)
For bone erosion measurements, a standard rectangular box was placed over the lateral bulla between the buttresses. (Fig. 3) The image was captured and saved as a *.tif file. Using NIH ImageJ®, the length of the bone was subtracted from the total distance across that area of the bulla and was expressed in mm. The external auditory canal is in this region and is seen as a deficient area of bone of the lateral bulla (rectangle on the right). Erosion of this area is typically seen in gerbil cholesteatomas and is evident by the larger defect on the right side compared to the right (Fig. 3). Results were recorded as length of bone divided by total length between buttresses. All parameters were measured in three reproducible frames.
The outlines were then converted to bitmap images and the number of pixels was calculated using ImageJ software (Rasband, W.S., U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2006). Statistical significance across was evaluated using standard parametric statistics (i.e. paired t-test). An α level of 0.05 was established for significance. All statistical calculations were performed with SigmaStat software (version 3.5, Systat Software, Richmond, CA).
The histomorphometric measurements were highly reproducible. When measurements of cholesteatoma size were analyzed by two separate, blinded observers, the ratio of infected to uninfected cholesteatoma size for OPPA8, OPPA8-NP and PA14 were 9.3, 2.3 and 3.0 from one investigator (D.L.,) and 8.9, 1.9 and 2.6 (R.C).
Microbiology
Swabs were taken from the ears of a subset of the gerbils in the preliminary study at the time of sacrifice. The swabs were streaked onto P. Isolation Agar (PIA, Remel, Lenexa, KS) plates to obtain PA colonies for further PCR identification. The swabs were then sent to the Clinical Microbiology Laboratory of Barnes Jewish Hospital, St. Louis, MO to identify other species present. Four colonies from each swab were selected for colony PCR to recover OPPA8. Colonies were re-suspended in 25µL dH2O and boiled for 10 minutes. The PCR contained 1X buffer, 0.2mM dNTPs, 1.5mM MgCl2, 2µL boiled colony DNA, 5U Taq polymerase, and 0.5µM of each primer, (forward-ccggtgtgactggctctatt, reverse-tgccatcctcctgctatttc). The reaction was carried out at 94°C for 2 minutes followed by 35 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute,
RESULTS
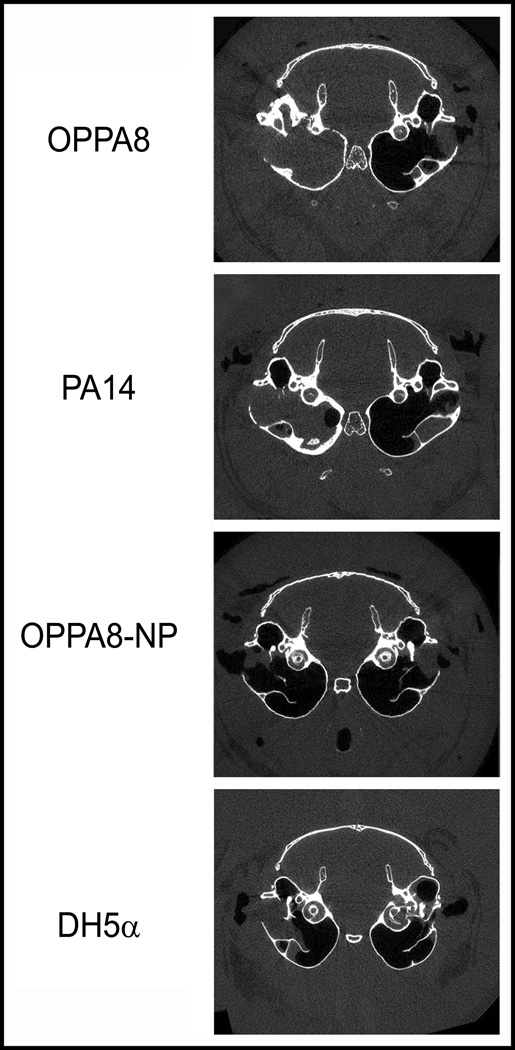
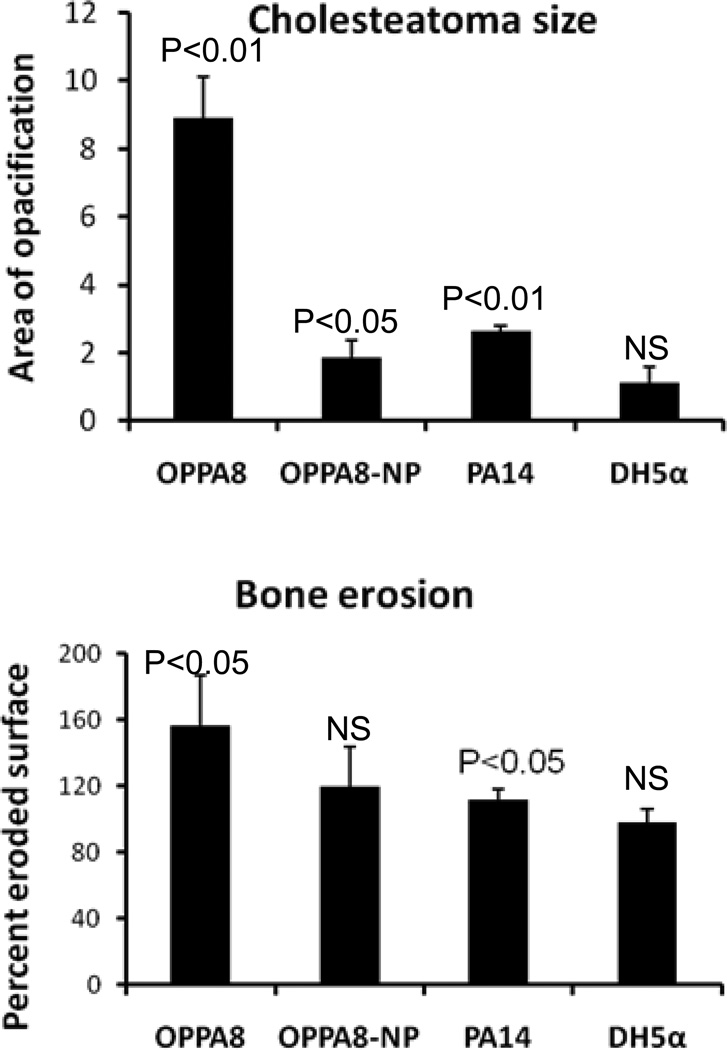
To determine the etiologic role of P. aeruginosa in cholesteatoma development, we inoculated bacteria (right ears) or PBS (left ears) prior to surgical ligation. Animals were sacrificed after 6 weeks, fixed in mixed aldehydes and imaged by micro-CT. For each animal, three reproducible sections were quantitatively analyzed and representative axial images of experimental gerbil cholesteatomas are shown in Figure 4. For cholesteatoma size, results are shown as ratios between experimental and control (mean ± standard deviation). For bone erosion, results are shown as percent eroded surface, bacterial minus control (mean ± standard deviation). The p values above each group represent paired t-tests comparing control and bacteria. (Fig.5) For these experiments, we consistently inoculated the left ear with PBS and the right ear with bacteria, using each animal as its own control. Gerbils were coded at the time of surgery and images were blinded to the individual doing the analysis (RN).
Figure 4.
Representative axial microCT scans of the experimental animals. Indicated bacteria were inoculated into the induced cholesteatomas on the right side (left side of image) and vehicle only was introduced into the left side (right side of image.) Note that OPPA8 and PA14 scans show increased cholesteatoma size and bony change compared to the non-piliated OPPA8 and the E. coli strain DH5α.
Figure 5.
Upper panel, P. aeruginosa infection increases the size and aggressiveness of cholesteatomas. The Otopathogenic strain (OPPA8) causes the most growth. PA14 and OPPA8-NP cause modest growth compared to controls but significantly less than OPPA8. DH5α (E. coli) causes no more enlargement than the control (vehicle only). Lower panel, Significant bone erosion was seen with infection with OPPA8 and PA14 but not with OPPA8-NP or DH5α.
Results demonstrate that inoculation with PA into the ear canal prior to ligation dramatically increases both the size of the cholesteatoma and the amount of bone resorption when compared to the un-infected contralateral ear. OPPA8 showed 8.9-fold, OPPA8-NP 1.9-fold and PA14 2.6-fold increased size compared to the un-infected contralateral ear (Fig. 5). OPPA8, the cholesteatoma-derived PA strain, induced significantly larger cholesteatomas than both the non-piliated strain, OPPA8-NP and the laboratory wound-derived PA strain, PA14 (p<0.05). Importantly, the Escherichia E. coli strain DH5α, did not induce increased expansion of the cholesteatomas formation. (Fig. 5)
Cholesteatoma-induced bone erosion for OPPA8, OPPA8-NP and PA14 were increased 156%, 120% and 120% compared to un-infected controls, respectively and the DH5α E. coli strain did not induce bone erosion (Fig. 5). Interestingly, the cholesteatoma-derived PA strain demonstrated significantly more erosion than the laboratory strain PA14 (Fig. 5 p=0.025).
We verified the presence of the cholesteatoma by gross dissection of the bulla. Additionally, appropriate P. aeruginosa strains were recovered in the cholesteatomas by PCR (data not shown).
DISCUSSION
P. aeruginosa is the most common pathogen associated with nosocomial pneumonia and burn infections 14 and continues to be a leading cause of morbidity and mortality in cystic fibrosis patients 15. It is also a common cause of corneal keratitis16, urinary tract infections17, and external otitis18. Our laboratory has shown that PA is also an important pathogen associated with aural cholesteatomas10, 19. In fact, it is the most common gram negative organism isolated from human cholesteatomas, accounting for 21% to 51% of the organisms cultured6, 10. PA is also the most common gram negative organism cultured from experimental cholesteatomas in gerbils6.
One of the hallmarks of aural cholesteatoma is chronic and recurrent infection, which is highly resistant to eradication, often despite multiple courses of antibiotics. Once a cholesteatoma is infected, chronic otorrhea usually occurs. Although suppression can occur with topical and systemic antibiotics, recurrence is common. The ability of PA to form biofilms may explain many of these clinical observations. Previous studies in our laboratory have demonstrated histological and ultrastructural evidence that bacterial biofilms are common in human and experimentally induced gerbilline cholesteatomas9. We have also demonstrated that a majority of PA isolates from cholesteatoma (otopathogenic PA or OPPA) have increased adhesion to keratinocytes and increased biofilm formation compared to a common environmental strain ( PA01)10.
In our previous study of ligation induced cholesteatomas in gerbils, the rate of cholesteatoma formation is highly variable, with only 40 to 70% of ligated ears developing cholesteatomas and these generally did not expand beyond the confines of the tympanic membrane and external ear until 4–6 months after ligation11, 12. In this current model, 100% of ligated ear canals inoculated with Pseudomonas aeruginosa demonstrated radiographic evidence of cholesteatoma development after only six weeks, suggesting that infection with PA results in highly aggressive cholesteatomas. Quantitative histomorphometric measurements demonstrated increased cholesteatoma size and bone resorption in response to PA infection. The largest and most destructive cholesteatoma was induced by OPPA8, a cholesteatoma-derived PA strain. Interestingly, cholesteatoma size and bone erosion was attenuated in a non-piliated OPPA8 strain, suggesting that this effect is dependent on the presence of type IV pili (TFP), which is considered a virulence factor because of it’s role in twitching motility and surface adhesin.. In cystic fibrosis, TFP have been shown to be an important mediator of adhesion to pulmonary epithelium20, virulence in animal models21, and in immune recognition, with their hypervariable regions involved in antigen formation22, 23. The decreased virulence that we observed using the non-piliated mutants may be due to diminished ability to form biofilms since adherence of these bacteria to keratinocytes is likely impaired.
These results demonstrate an etiologic role for PA in the development of larger, more aggressive cholesteatomas. Since the E. coli strain, DH5αa did not accelerate cholesteatoma expansion, our results are suggestive of a specific interaction of Pseudomonas species within the ear canal resulting in dramatic enlargement and aggressiveness of the cholesteatomas. The complete lack of response to E. coli further suggests that simple presence of bacteria is insufficient to account for the enhanced aggressiveness of these cholesteatomas. Additionally, there was significant attenuation in the non-piliated strain, suggesting a role for type IV pili in the pathogenesis of PA-induced cholesteatomas. Strikingly, medial expansion of cholesteatomas occurred in every PA-infected ear canal much more rapidly than previously reported un-infected cholesteatomas (6 weeks compared to 6 months).
CONCLUSION
When infected with PA, experimental cholesteatomas enlarge more rapidly and are more destructive than un-infected cholesteatomas. An otopathogenic strain, OPPA8, isolated from a human cholesteatoma, was the most aggressive. The virulence of this strain was diminished by deletion of its principal adherence structure, type IV pili. Comparison with laboratory strains, PA14 and E. coli (DH5α) likewise showed less virulence.
Acknowledgments
Supported by: NIDCD R01-DC000263-23 (R.A.C.), T32 DC00022 (E.W.W. and R.N.), P30 DC004665-11 (RAC)
Footnotes
Financial Disclosure: None other than NIH support. RAC served on the Board of Scientific Counselors for the NIDCD at the time the study was performed.
Conflict of Interest: None
REFERENCES
- 1.Podoshin L, Fradis M, Ben-David Y, Margalit A, Tamir A, Epstein L. Cholesteatoma: an epidemiologic study among members of kibbutzim in northern Israel. Ann Otol Rhinol Laryngol. 1986;95:365–368. doi: 10.1177/000348948609500408. [DOI] [PubMed] [Google Scholar]
- 2.Osma U, Cureoglu S, Hosoglu S. The complications of chronic otitis media: report of 93 cases. J Laryngol Otol. 2000;114:97–100. doi: 10.1258/0022215001905012. [DOI] [PubMed] [Google Scholar]
- 3.Chole RA, Nason R. Chronic Otitis Media and Cholesteatoma. In: Snow JB, Wackym PA, editors. Ballenger's Otorhinolaryngology and head and Neck Surgery. Shelton, CT: B. C. Decker; 2009. pp. 217–227. [Google Scholar]
- 4.Brook I. Aerobic and anaerobic bacteriology of cholesteatoma. Laryngoscope. 1981;91:250–253. doi: 10.1288/00005537-198102000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Brook I. The role of anaerobic bacteria in otitis media: microbiology, pathogenesis, and implications on therapy. Am J Otolaryngol. 1987;8:109–117. doi: 10.1016/s0196-0709(87)80033-9. [DOI] [PubMed] [Google Scholar]
- 6.Fulghum RS, Chole RA. Bacterial flora in spontaneously occurring aural cholesteatomas in Mongolian gerbils. Infect Immun. 1985;50:678–681. doi: 10.1128/iai.50.3.678-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albino AP, Kimmelman CP, Parisier SC. Cholesteatoma: a molecular and cellular puzzle. Am J Otol. 1998;19:7–19. [PubMed] [Google Scholar]
- 8.Attallah MS. Microbiology of chronic suppurative otitis media with cholesteatoma. Saudi Med J. 2000;21:924–927. [PubMed] [Google Scholar]
- 9.Chole RA, Faddis BT. Evidence for microbial biofilms in cholesteatomas. Arch Otolaryngol Head Neck Surg. 2002;128:1129–1133. doi: 10.1001/archotol.128.10.1129. [DOI] [PubMed] [Google Scholar]
- 10.Wang EW, Jung JY, Pashia ME, Nason R, Scholnick S, Chole RA. Otopathogenic P. aeruginosa strains as competent biofilm formers. Arch Otolaryngol Head Neck Surg. 2005;131:983–989. doi: 10.1001/archotol.131.11.983. [DOI] [PubMed] [Google Scholar]
- 11.McGinn MD, Chole RA, Henry KR. Cholesteatoma. Experimental induction in the Mongolian Gerbil, Meriones Unguiculatus. Acta Otolaryngol. 1982;93:61–67. doi: 10.3109/00016488209130853. [DOI] [PubMed] [Google Scholar]
- 12.Chole RA, Henry KR, McGinn MD. Cholesteatoma: spontaneous occurrence in the Mongolian gerbil Meriones unguiculatis. Am J Otol. 1981;2:204–210. [PubMed] [Google Scholar]
- 13.Choi JY, Sifri CD, Goumnerov BC, Rahme LG, Ausubel FM, Calderwood SB. Identification of virulence genes in a pathogenic strain of P. aeruginosa by representational difference analysis. J Bacteriol. 2002;184:952–961. doi: 10.1128/jb.184.4.952-961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison AJ, Jr., Wenzel RP. Epidemiology of infections due to P. aeruginosa. Rev Infect Dis. 1984;6(Suppl 3):S627–S642. doi: 10.1093/clinids/6.supplement_3.s627. [DOI] [PubMed] [Google Scholar]
- 15.Prince AS. Biofilms, antimicrobial resistance, and airway infection. N Engl J Med. 2002;347:1110–1111. doi: 10.1056/NEJMcibr021776. [DOI] [PubMed] [Google Scholar]
- 16.Cowell BA, Weissman BA, Yeung KK, et al. Phenotype of P. aeruginosa isolates causing corneal infection between 1997 and 2000. Cornea. 2003;22:131–134. doi: 10.1097/00003226-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Johansen TE, Cek M, Naber KG, Stratchounski L, Svendsen MV, Tenke P. Hospital acquired urinary tract infections in urology departments: pathogens, susceptibility and use of antibiotics. Data from the PEP and PEAP-studies. Int J Antimicrob Agents. 2006;28(Suppl 1):S91–S107. doi: 10.1016/j.ijantimicag.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Rubin Grandis J, Branstetter BFt, Yu VL. The changing face of malignant (necrotizing) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis. 2004;4:34–39. doi: 10.1016/s1473-3099(03)00858-2. [DOI] [PubMed] [Google Scholar]
- 19.Chole RA, Faddis BT. Anatomical evidence of microbial biofilms in tonsillar tissues: a possible mechanism to explain chronicity. Arch Otolaryngol Head Neck Surg. 2003;129:634–636. doi: 10.1001/archotol.129.6.634. [DOI] [PubMed] [Google Scholar]
- 20.Chi E, Mehl T, Nunn D, Lory S. Interaction of P. aeruginosa with A549 pneumocyte cells. Infect Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farinha MA, Conway BD, Glasier LM, et al. Alteration of the pilin adhesin of P. aeruginosa PAO results in normal pilus biogenesis but a loss of adherence to human pneumocyte cells and decreased virulence in mice. Infect Immun. 1994;62:4118–4123. doi: 10.1128/iai.62.10.4118-4123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forest KT, Bernstein SL, Getzoff ED, et al. Assembly and antigenicity of the Neisseria gonorrhoeae pilus mapped with antibodies. Infect Immun. 1996;64:644–652. doi: 10.1128/iai.64.2.644-652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KK, Doig P, Irvin RT, Paranchych W, Hodges RS. Mapping the surface regions of P. aeruginosa PAK pilin: the importance of the C-terminal region for adherence to human buccal epithelial cells. Mol Microbiol. 1989;3:1493–1499. doi: 10.1111/j.1365-2958.1989.tb00135.x. [DOI] [PubMed] [Google Scholar]