Abstract
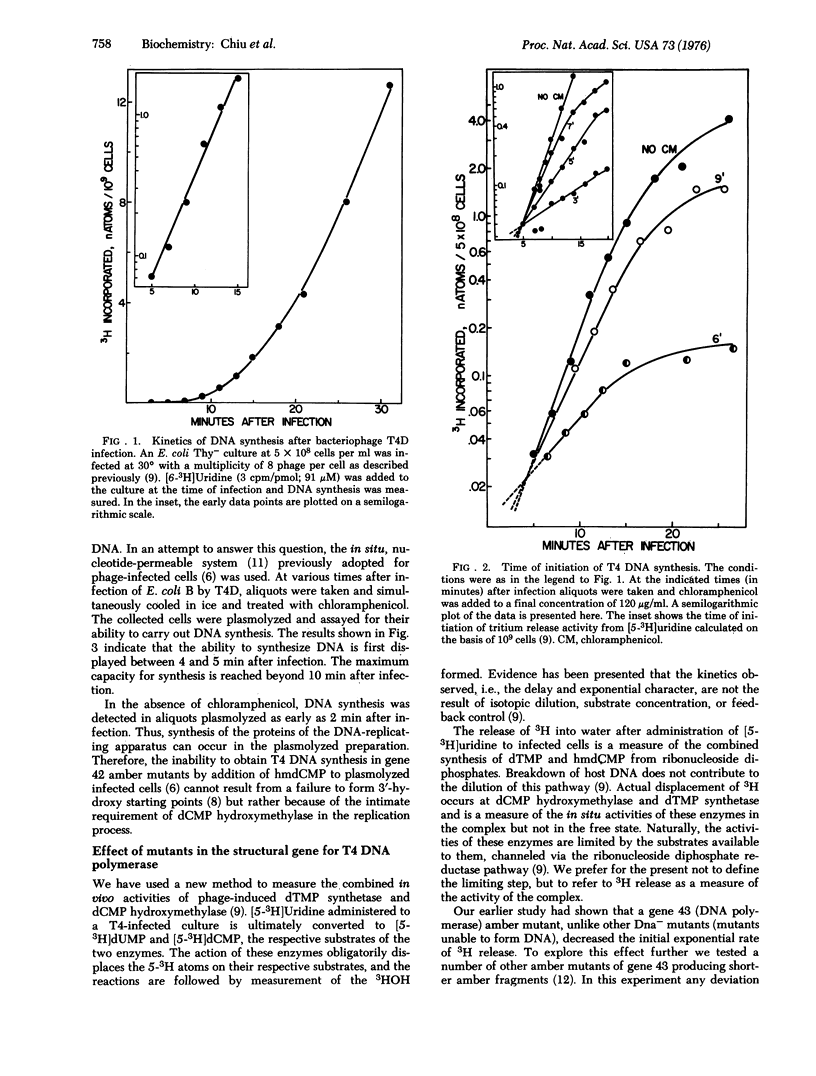
In earlier reports we have suggested that bacteriophate T4 DNA replication occurs in a complex composed of the proteins required for polymerization and the system of enzymes synthesizing the deoxyribonucleoside triphosphate precursors of DNA. T4-induced dCMP hydroxymethylase and dTMP synthetase, though demonstrable in extracts soon after infection, are not active in vivo until about 5 min. The in vivo activities increase exponentially for approximately 15 min and then become constant. We have suggested that the exponential period represents the formation of the complexes. This paper shows that the initiation of DNA synthesis and of the two deoxyribonucleotide-synthesizing activities occurs simultaneously and with coinciding exponential kinetics. The in vivo activities of the two enzymes were tested after infection by a number of T4 amber Dna- mutants. Their activities were essentially unchanged compared to the wild-type phage, except on infection by mutants of gene 43 (T4 DNA nucleotidyltransferase or DNA polymerase). With these mutants the rate of increase of dTMP synthetase and dCMP hydroxymethylase activities was always substantially lower than after infection by wild-type phage. It is proposed that an intimate interaction occurs between T4-induced DNA polymerase and the complex of enzymes forming 5-hydroxymethyl-dCMP and dTMP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. F., Albrecht I., Drake J. W. Properties of bacteriophage T4 mutants defective in DNA polymerase. Genetics. 1970 Jun;65(2):187–200. doi: 10.1093/genetics/65.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLO L. J., BESSMAN M. J. The enzymology of virus-infected bacteria. IV. Purification and properties of the deoxynucleotide kinase induced by bacteriophage T2. J Biol Chem. 1963 May;238:1777–1787. [PubMed] [Google Scholar]
- Barry J., Alberts B. In vitro complementation as an assay for new proteins required for bacteriophage T4 DNA replication: purification of the complex specified by T4 genes 44 and 62. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2717–2721. doi: 10.1073/pnas.69.9.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage T4. II. Allosteric regulation of substrate sepecificity and catalytic activity. J Biol Chem. 1972 Nov 25;247(22):7276–7281. [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage T4. III. Isolation and characterization of proteins B1 and B2. J Biol Chem. 1975 Sep 25;250(18):7450–7455. [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Chung S. T., Greenberg G. R. Loss of an essential function of Escherichia coli by deletions in the thyA region. J Bacteriol. 1973 Dec;116(3):1145–1149. doi: 10.1128/jb.116.3.1145-1149.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinsworth W. L., Mathews C. K. Biochemistry of DNA-defective amber mutants of bacteriophage T4. IV. DNA synthesis in plasmolyzed cells. J Virol. 1974 Apr;13(4):908–915. doi: 10.1128/jvi.13.4.908-915.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- Dicou L., Cozzarelli N. R. Bacteriophage T4-directed DNA synthesis in toluene-treated cells. J Virol. 1973 Dec;12(6):1293–1302. doi: 10.1128/jvi.12.6.1293-1302.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso R. J., Buchanan J. M. Synthesis of early RNA in bacteriophage T4-infected Escherichia coli B. Nature. 1969 Nov 29;224(5222):882–885. doi: 10.1038/224882a0. [DOI] [PubMed] [Google Scholar]
- Guha A., Szybalski W., Salser W., Geiduschek E. P., Pulitzer J. F., Bolle A. Controls and polarity of transcription during bacteriophage T4 development. J Mol Biol. 1971 Jul 28;59(2):329–349. doi: 10.1016/0022-2836(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Homyk T., Jr, Weil J. Deletion analysis of two nonessential regions of the T4 genome. Virology. 1974 Oct;61(2):505–523. doi: 10.1016/0042-6822(74)90286-4. [DOI] [PubMed] [Google Scholar]
- Huang W. M., Buchanan J. M. Synergistic interactions of T4 early proteins concerned with their binding to DNA. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2226–2230. doi: 10.1073/pnas.71.6.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M., Lehman I. R. On the direction of translation of the T4 deoxyribonucleic acid polymerase gene in vivo. J Biol Chem. 1972 Dec 10;247(23):7663–7667. [PubMed] [Google Scholar]
- Karam J. D., O'Donnell P. V. Suppression of amber mutations of bacteriophage T4 gene 43 (DNA polymerase) by translational ambiguity. J Virol. 1973 Jun;11(6):933–945. doi: 10.1128/jvi.11.6.933-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax M. S., Greenberg G. R. Characteristics of the deo operon: role in thymine utilization and sensitivity to deoxyribonucleosides. J Bacteriol. 1968 Aug;96(2):501–514. doi: 10.1128/jb.96.2.501-514.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G. A T4 bacteriophage mutant which lacks deoxyribonucleic acid polymerase but retains the polymerase-associated nuclease. J Biol Chem. 1969 Jan 10;244(1):218–220. [PubMed] [Google Scholar]
- Nossal N. G., Hershfield M. S. Nuclease activity in a fragment of bacteriophage T4 deoxyribonucleic acid polymerase induced by the amber mutant am B22. J Biol Chem. 1971 Sep 10;246(17):5414–5426. [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scocca J. J., Panny S. R., Bessman M. J. Studies of deoxycytidylate deaminase from T4-infected Escherichia coli. J Biol Chem. 1969 Jul 10;244(13):3698–3706. [PubMed] [Google Scholar]
- TOMIZAWA J. I., SUNAKAWA S. The effect of chloramphenicol on deoxyribonucleic acid synthesis and the development of resistance to ultraviolet irradiation in E. coli infected with bacteriophage T2. J Gen Physiol. 1956 Mar 20;39(4):553–565. doi: 10.1085/jgp.39.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Wickner R. B., Hurwitz J. DNA replication in Escherichia coli made permeable by treatment with high sucrose. Biochem Biophys Res Commun. 1972 Apr 14;47(1):202–211. doi: 10.1016/s0006-291x(72)80029-9. [DOI] [PubMed] [Google Scholar]
- Wovcha M. G., Tomich P. K., Chiu C. S., Greenberg G. R. Direct participation of dCMP hydroxymethylase in synthesis of bacteriophage T4 DNA. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2196–2200. doi: 10.1073/pnas.70.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]



