Abstract
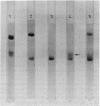
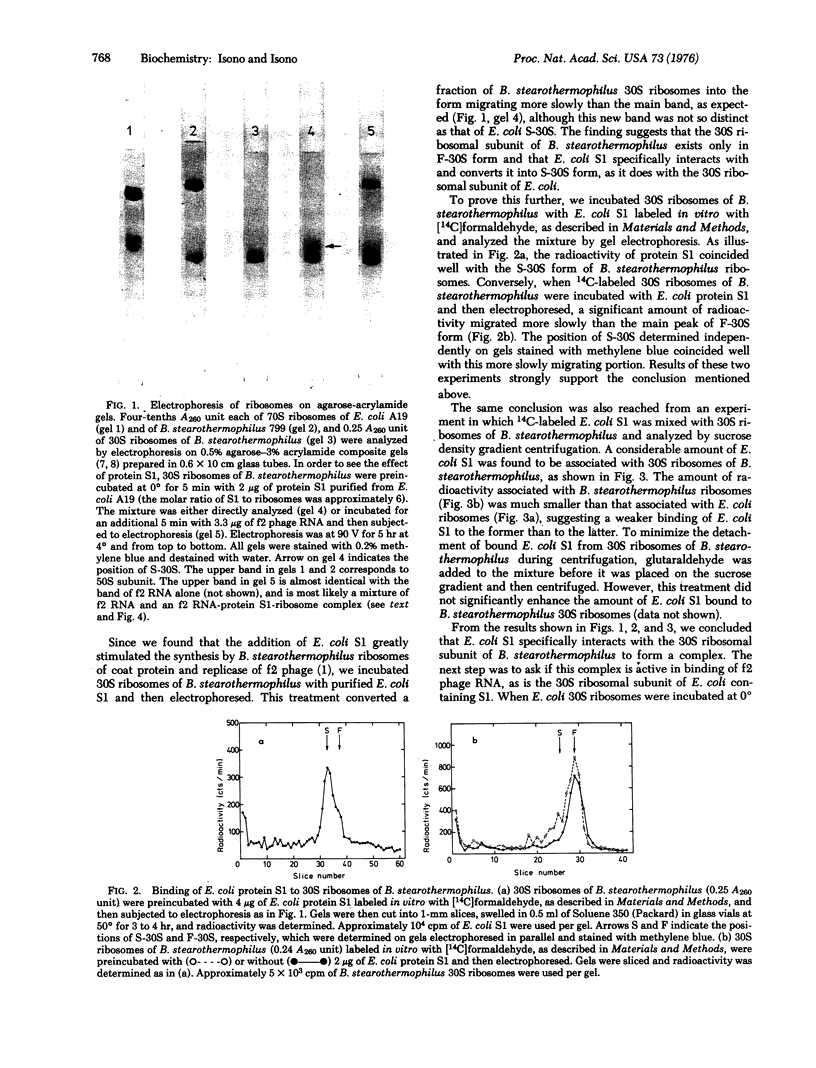
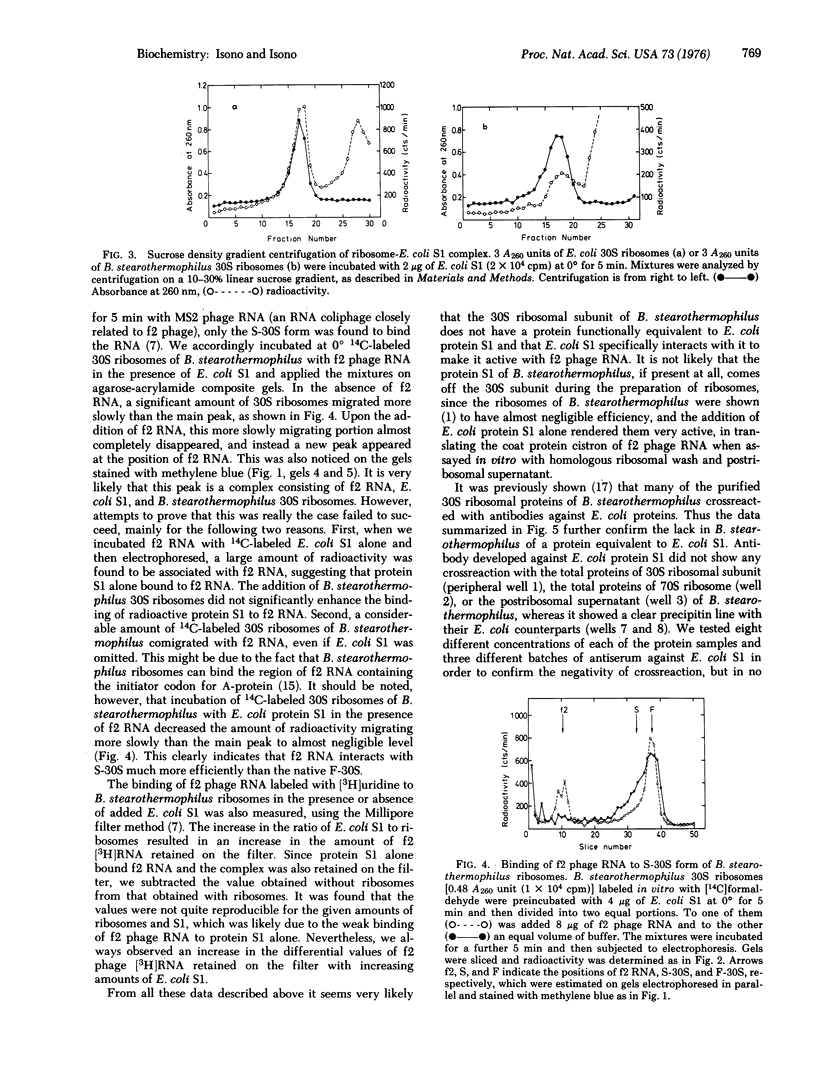
The 30S ribosomal subunit of Bacillus stearothermophilus migrated as a single band when electrophoresed on agarose-acrylamide composite gels. The addition of the ribosomal protein S1 purified from Escherichia coli resulted in the appearance of an additional band migrating more slowly; 14C-labeled S1 of E. coli was shown to be associated only with this form. Antibody against E. coli protein S1 did not crossreact with either the total 30S ribosomal proteins or the postribosomal supernatant from B. stearothermophilus. These results indicate that B. stearothermophilus lacks a protein equivalent to E. coli S1 and may explain our previous finding [Eur. J. Biochem. 56, 15-22 (1975) that E. coli S1 greatly stimulated the translation by B. stearothermophilus ribosomes of f2 phage RNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E. Two forms of the 30 S ribosomal subunit of Escherichia coli. J Biol Chem. 1974 Dec 10;249(23):7673–7678. [PubMed] [Google Scholar]
- Isono K., Isono S., Stöffler G., Visentin L. P., Yaguchi M., Matheson A. T. Correlation between 30S ribosomal proteins of Bacillus stearothermophilus and Escherichia coli. Mol Gen Genet. 1973 Dec 20;127(2):191–195. doi: 10.1007/BF00333666. [DOI] [PubMed] [Google Scholar]
- Isono S., Isono K. Purification and characterization of 30-S ribosomal proteins from Bacillus stearothermophilus. Eur J Biochem. 1975 Jan 15;50(3):483–488. doi: 10.1111/j.1432-1033.1975.tb09886.x. [DOI] [PubMed] [Google Scholar]
- Isono S., Isono K. Role of ribosomal protein S1 in portein synthesis: effects of its addition to Bacillus stearothermophilus cell-free system. Eur J Biochem. 1975 Aug 1;56(1):15–22. doi: 10.1111/j.1432-1033.1975.tb02202.x. [DOI] [PubMed] [Google Scholar]
- Pon C. L., Friedman S. M., Gualerzi C. Studies on the interaction between ribosomes and 14 CH 3 -F 3 initation factor. Mol Gen Genet. 1972;116(2):192–198. doi: 10.1007/BF00582228. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Stallcup M. R., Sharrock W. J., Rabinowitz J. C. Ribsome and messenger specificity in protein synthesis by bacteria. Biochem Biophys Res Commun. 1974 May 7;58(1):92–98. doi: 10.1016/0006-291x(74)90895-x. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Discriminatory ribosome rebinding of isolated regions of protein synthesis initiation from the ribonucleic acid of bacteriophage R17. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2605–2609. doi: 10.1073/pnas.70.9.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler G., Wittmann H. G. Sequence differences of Escherichia coli 30S ribosomal proteins as determined by immunochemical methods. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2283–2287. doi: 10.1073/pnas.68.9.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Leffler S. Interaction of Escherichia coli 30S ribosomal subunits with MS2 phage RNA in the absence of initiation factors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3611–3615. doi: 10.1073/pnas.71.9.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Aviram M., Kanarek A., Weiss A. Polyuridylic acid binding and translating by Escherichia coli ribosomes: stimulation by protein I, inhibition by aurintricarboxylic acid. Biochim Biophys Acta. 1972 Oct 27;281(3):381–392. doi: 10.1016/0005-2787(72)90452-2. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Voynow P., Kurland C. G. Stoichiometry of the 30S ribosomal proteins of Escherichia coli. Biochemistry. 1971 Feb 2;10(3):517–524. doi: 10.1021/bi00779a026. [DOI] [PubMed] [Google Scholar]
- Weber H. J. Stoichiometric measurements of 30S and 50S ribosomal proteins from Escherichia coli. Mol Gen Genet. 1972;119(3):233–248. doi: 10.1007/BF00333861. [DOI] [PubMed] [Google Scholar]
- van Duin J., van Knippenberg P. H. Functional heterogeneity of the 30 S ribosomal subunit of Escherichia coli. 3. Requirement of protein S1 for translation. J Mol Biol. 1974 Mar 25;84(1):185–195. doi: 10.1016/0022-2836(74)90221-6. [DOI] [PubMed] [Google Scholar]