Abstract
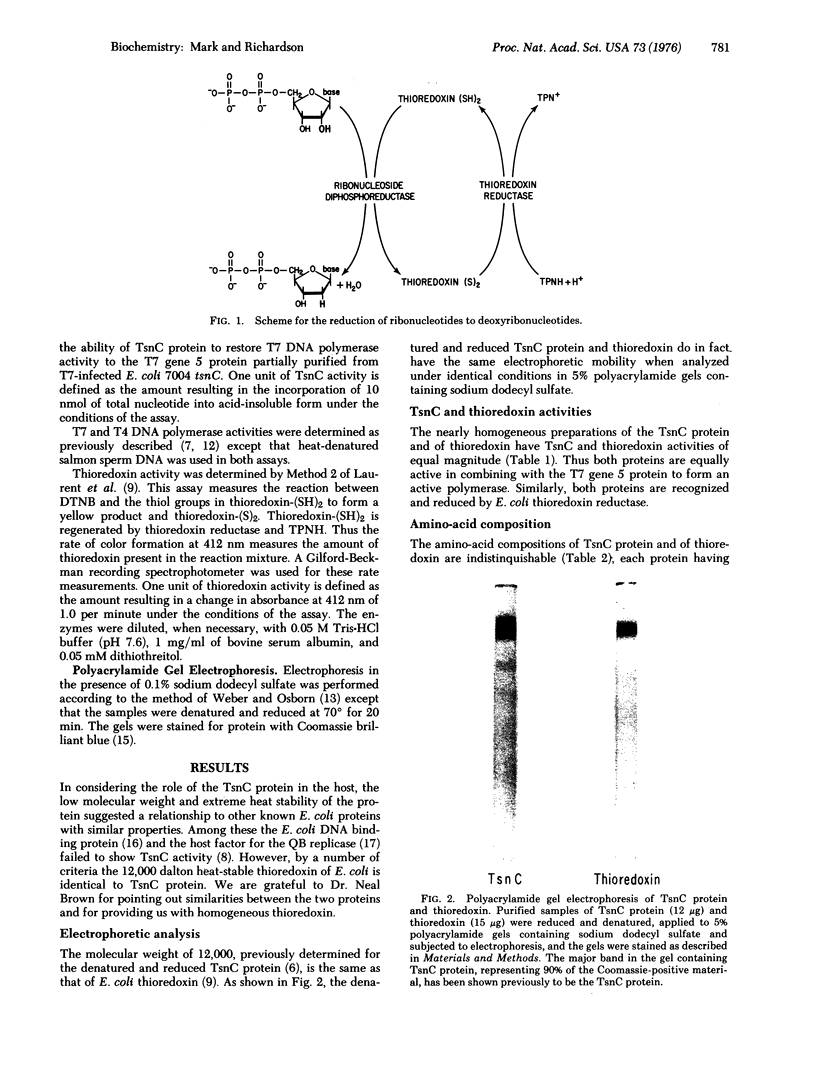
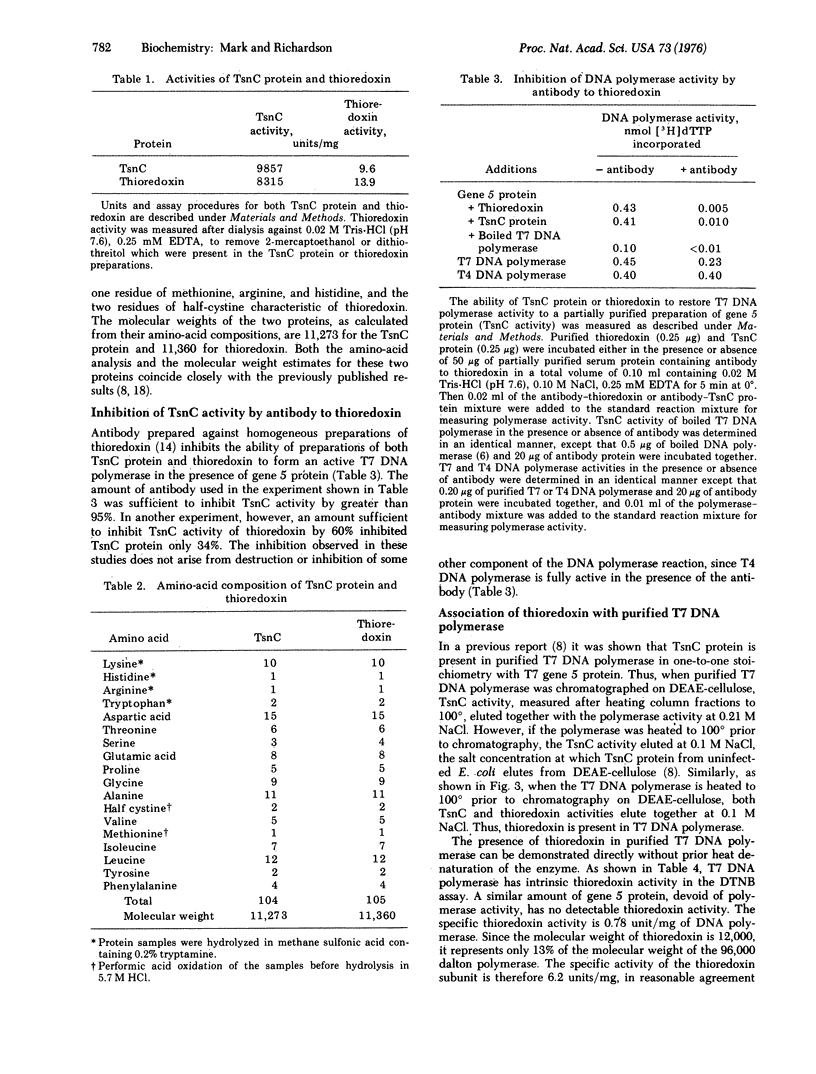
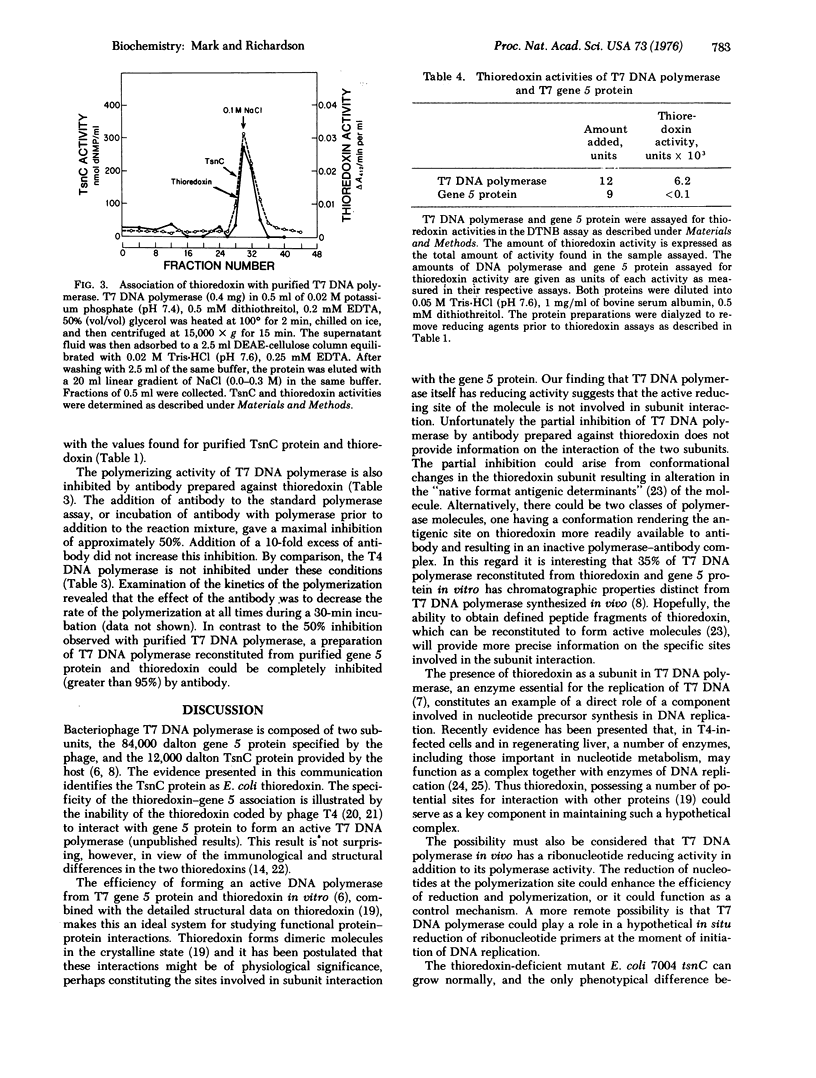
T7 DNA polymerase (DNA nucleotidyltransferase; deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase, EC 2.7.7.7) is composed of an 84,000 dalton protein specified by the gene 5 of the phage and a 12,000 dalton protein (TsnC protein) specified by the tsnC gene of E. coli [Modrich, P. & Richardson, C. C. (1975) J. Biol. Chem. 250 5515-5522]. Both proteins are necessary for T7 DNA polymerase activity and for the replication of T7 DNA. The TsnC protein is identical to thioredoxin of E. coli by the following criteria: (1) Homogeneous preparations of both proteins have TsnC and thioredoxin activity. (2) Both proteins show similar stability to heat. (3) They have identical mobilities, corresponding to a molecular weight of 12,000, on polyacrylamide gels containing sodium dodecyl sulfate. (4) Their amino-acid compositions are indistinguishabe. (5) Antibody prepared against thioredoxin inhibits TsnC activity. (6) TsnC protein isolated from purified T7 DNA polymerase has thioredoxin activity. In addition, preparations of T7 DNA polymerase itself exhibit thioredoxin activity and are partially inhibited by antibody to thioredoxin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baril E. F., Jenkins M. D., Brown O. E., Laszlo J., Morris H. P. DNA polymerases I and II in regenerating rat liver and Morris hepatomas. Cancer Res. 1973 Jun;33(6):1187–1193. [PubMed] [Google Scholar]
- Berglund O., Holmgren A. A thioredoxin induced by bacteriophage T4. 3. Amino acid sequence around the active center disulfide. J Biol Chem. 1970 Nov 25;245(22):6036–6038. [PubMed] [Google Scholar]
- Berglund O., Sjöberg B. M. A thioredoxin induced by bacteriophage T4. II. Purification and characterization. J Biol Chem. 1970 Nov 25;245(22):6030–6035. [PubMed] [Google Scholar]
- Chamberlin M. Isolation and characterization of prototrophic mutants of Escherichia coli unable to support the intracellular growth of T7. J Virol. 1974 Sep;14(3):509–516. doi: 10.1128/jvi.14.3.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Franze de Fernandez M. T., Hayward W. S., August J. T. Bacterial proteins required for replication of phage Q ribonucleic acid. Pruification and properties of host factor I, a ribonucleic acid-binding protein. J Biol Chem. 1972 Feb 10;247(3):824–831. [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Sjöberg B. M. Immunochemistry of thioredoxin. I. Preparation and cross-reactivity of antibodies against thioredoxin from Escherichia coli and bacteriophage T4. J Biol Chem. 1972 Jul 10;247(13):4160–4164. [PubMed] [Google Scholar]
- Holmgren A., Söderberg B. O., Eklund H., Brändén C. I. Three-dimensional structure of Escherichia coli thioredoxin-S2 to 2.8 A resolution. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2305–2309. doi: 10.1073/pnas.72.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from escherichia coli B. Eur J Biochem. 1968 Dec 5;6(4):475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 Deoxyribonucleic acid replication in vitro. A protein of Escherichia coli required for bacteriophage T7 DNA polymerase activity. J Biol Chem. 1975 Jul 25;250(14):5508–5514. [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication invitro. Bacteriophage T7 DNA polymerase: an an emzyme composed of phage- and host-specific subunits. J Biol Chem. 1975 Jul 25;250(14):5515–5522. [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby I., Holmgren A. Reconstitution of Escherichia coli thioredoxin from complementing peptide fragments obtained by cleavage at methionine-37 or arginine-73. J Biol Chem. 1975 Feb 25;250(4):1340–1347. [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yeh Y. C., Tessman I. Control of pyrimidine biosynthesis by phage T4. II. In vitro complementation between ribonucleotide reductase mutants. Virology. 1972 Mar;47(3):767–772. doi: 10.1016/0042-6822(72)90567-3. [DOI] [PubMed] [Google Scholar]




