Endothelial and epithelial cells have something in common—they are both the barrier and bridge between different environments. Forming a one-cell layer lining of blood vessels, airways, and urinary tract, they are in constant contact with a wide variety of cells, putting high demands on the communication skills of these cells. The purinergic signaling system offers a dynamic and versatile way for local and rapid intercellular communication and involves three processes: nucleotide release, enzymatic degradation, and activation of purinergic receptors. With an impressive number of molecular members of the system (15 P2 receptor subtypes and in total 16 nucleotide-degrading enzymes), cells have managed to put purines and pyrimidines as well as their derivatives to use in an array of different areas, including regulation of flow, secretion, and vascular tone. Indeed, the purinergic signaling system is an ancient way of intercellular communication that most likely could be found in the first most primitive life form [1]. The scope of this review is to give an overview of exciting features of purinergic signaling and examples of gaps to fill in understanding its role in the cells lining the body—the endothelium and the epithelium.
Purinergic signaling—an overview
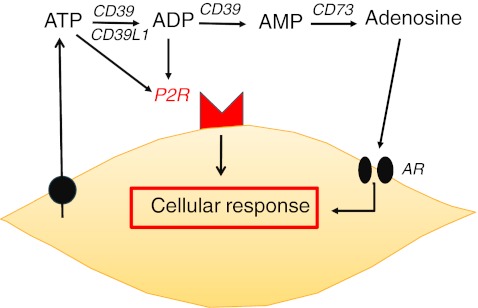
Extracellular nucleotides exert their paracrine signaling by binding to P2 receptors present at the cell surface. The P2 receptor family is divided into two groups: P2X receptors, which are ligand-activated ion channels, and P2Y receptors, which are G protein-coupled receptors (reviewed in [2]). Each receptor is characterized by its affinity pattern for different nucleotides and different cell types display varying receptor profiles. While P2X receptors respond only to ATP, P2Y receptors can be activated by other nucleotides such as ADP, UTP, and UDP. Extracellular nucleotide concentrations are precisely regulated by ecto-enzymes, which cleave nucleotide tri-/diphosphates. The result of signaling by the extracellular nucleotides ATP/ADP, UTP/UDP and the nucleoside adenosine is both acute, for example the rapid platelet aggregation induced by ADP, and chronic, via changes in gene expression induced by P2 receptor stimulation. Ligand availability for P2 receptors is regulated by a group of enzymes termed ectonucleotidases. NTPDase1 (apyrase; CD39) cleaves ATP to AMP, NTPDase2 (CD39L1) cleaves ATP to ADP, and 5′-ectonucleotidase (CD73) generates adenosine from AMP. Adenosine is the end product of purinergic signaling, acting on adenosine receptors (A1, A2a, A2b, and A3; also termed P1 receptors) or recycling by the cell after reuptake through the equilibrating nucleoside transporters ENT1 and 2. UTP is believed to be released simultaneously with ATP via the same mechanism but at a lower concentration (UTP/ATP, 1:10–100). UTP is degraded in the same way as ATP, but the biological function of uridine is poorly understood and no uridine receptor has been identified. Figure 1 shows an outline of nucleotide metabolism.
Fig. 1.
Nucleotide metabolism in a nutshell. UTP is identically released and degraded
Purinergic tone
All investigators that have tried to measure ATP release from cells have experienced the remarkable sensitivity of cells to mechanical stimulation, a phenomenon that also has been scientifically documented [3–7]. Renal epithelial cells, especially Madin–Darby canine kidney (MDCK) cells, a well-differentiated cell line derived from distal tubule/collecting duct, was found to respond to nucleotide signaling via P2 receptor activation already 30 years ago [8]. Subsequent studies revealed the involvement of purinergic signaling in ionic transport [9] and in setting the basal levels of cAMP and arachidonic acid [10]. Insel and colleagues showed that mere tilting of a cell culture plate or changing the medium of MDCK cells is enough to induce auto/paracrine ATP-mediated signaling leading to changes in intracellular second messenger availability [11–13]. Additionally, in non-stimulated MDCK cells, nucleotide release acting back on P2Y receptors during basal conditions has been demonstrated to induce slow spontaneous [Ca2+]i oscillations [14]. Praetorius et al. have subsequently performed several studies that support a strong influence of constitutive P2 receptor activation to steady-state renal function (reviewed in [15]). In addition to the general effect of basal P2 receptor activity on the cell signaling set point, autocrine nucleotide signaling has also been shown to participate in heterologous desensitization of receptors, for example, the sphingosine-1-phosphate receptors of renal mesangial cells [16], thereby further influencing the ability of cells to respond to their environment. Spontaneous nucleotide-mediated signaling contributing to the establishment of the basal level of activity in different cell types has recently been reviewed by Corriden and Insel [17].
General stimuli of purinergic signaling
An interesting characteristic of nucleotide signaling that correlates well with its ability to control the set point of cell activity is the profile of stimuli for nucleotide release. Stimuli that are known to cause purinergic activation through ATP release are general physiological cues such as stretch, osmosis, hypoxia, pH, high glucose, and pathogen interaction [18–20]. Taken together, these features indicate a central role for the purinergic signaling system in the stress response of cells.
The unknown mechanism of release
The mechanism of nucleotide release is subject to great controversy [21]. There are substantial evidence for vesicular release from neuronal [22] and endocrine tissues [23]. Vesicular release has likewise been suggested to occur in non-excitable cells such as endothelial cells [24] and osteoblasts [25], but non-vesicular release is thought to contribute in a major way to ATP release in many cell types. The molecular identity of the ATP release channel is unknown, but potential candidates include large anion channels, cystic fibrosis transmembrane conductance regulator (CFTR), and hemichannels like connexins and pannexin-1, which have been suggested to form ATP-releasing units together with the ATP-gated ion channel P2X7 [26–28]. A detailed discussion regarding this subject is beyond the scope of this review. Please refer to comprehensive review articles on the subject [18, 26, 29].
Fluids and flow
Epithelial transport and clearance
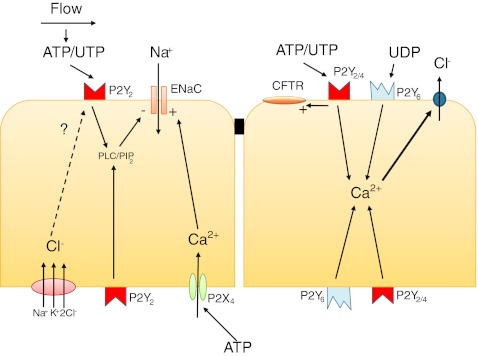
Endothelial and epithelial cells are uniquely exposed to environmental stress and serve to protect the underlying tissues as well as mediate and filter communication over the basal membrane. One such protective mechanism is the ability of epithelial cells to induce luminal fluid accumulation through increased secretion of Cl− and K+ as well as decreased Na+ reabsorption. As described by Leipziger in his comprehensive review on purinergic signaling in epithelial transport [30], the involvement of P2 receptors in Cl− secretion over the epididymis epithelium was one of the first functions found for P2 receptors after their discovery [31]. From this point on, evidence has been presented showing an important role for purinergic signaling in most epithelial tissues. The role for purinergic signaling in the transport of different ions has recently been reviewed in detail by Novak [27]. See Fig. 2 for an overview of the pathways discussed in this section.
Fig. 2.
Examples of pathways employed by purinergic signaling in epithelia in the regulation of ion transport
Purinergic regulation of ENaC
P2Y2-mediated inhibition of electrogenic Na+ absorption by reducing the activity of the epithelial Na+ channel (ENaC) is one of the most well-studied ATP-dependent phenomena and has been found in both gastrointestinal [32], respiratory [33–36], and renal epithelium [37–39]. Since the P2Y2 receptor has equal affinity for both ATP and UTP, both these mediators can activate the receptor. The ENaC passively transports Na+ ions over the epithelial barrier, a process driven by the electrochemical gradient created by the activity of the basolateral Na+/K+-ATPase. The activity of the ENaC can be influenced by affecting expression [40], membrane trafficking, and channel open probability [41].
The most studied mechanism of ENaC activity involves membrane trafficking of the protein [42], but a recent study of the P2Y2 receptor-mediated inhibitory response suggests that purinergic control is conveyed by changes in channel open probability rather than plasma membrane levels of the protein [39]. The way that P2Y2 receptor activation influences ENaC activity has previously been attributed to PLC activation and PIP2 depletion [43]. Recently, a role for increased intracellular Cl− concentration via the basolateral Na+K+2Cl− cotransporter has been suggested in the P2Y2-dependent regulation of ENaC activity [44]. This pathway is only accessible for apically located P2Y2 receptors, while basolateral activation has considerably smaller effect size and involves a different signaling mechanism that also can be employed by apical receptors [44]. There are studies from several epithelial tissues describing increased activation of the ENaC after P2Y receptor stimulation, but this is thought to be due to indirect effects of the purinergic degradation product adenosine and effects on other ion channels [27]. A series of experiments in renal epithelial cell lines have however shown an increased activity in the ENaC via basolateral stimulation of the P2X4 receptor leading to enhanced Na+ reabsorption [45]. This mechanism has been demonstrated to be employed by aldosterone [46] and points out a fascinating versatility of purinergic signaling that need further investigation and perhaps also can be extended to other potentially polarized cells, such as endothelial cells.
Chloride secretion
The predominance of the P2Y2 receptor in the secretory behavior of epithelial cells has been shown also for the gastrointestinal [32] and respiratory tracts [35]. Using a knockout model for the P2Y2 receptor, Cressman et al. demonstrated impaired Cl− secretion in airway and gallbladder epithelium, but not in tissue from the jejunum [47]. Other receptors have also been shown to regulate chloride conductance including P2Y4 in intestinal epithelia [48, 49] and the UDP receptor P2Y6 in bronchial epithelium [50]. P2Y2 receptor-induced Cl− secretion results from increased intracellular Ca2+ concentration leading to activation of Ca2+-activated Cl− channels. The fact that ATP/UTP can stimulate Cl− secretion independently of the CFTR has led to trials using this mechanism to treat cystic fibrosis patients with a stable UTP analog (Defusonol) [51]. Even though ATP/UTP does not depend on CFTR in order to induce Cl− secretion, P2Y receptor signaling has been demonstrated to influence CFTR activity in airway epithelia [50, 52] as well as in submandibular gland ducts [53]. In the latter case, P2Y2 receptor stimulation was found to contribute to chloride absorption via activation of luminal CFTR.
Mucociliary clearance
In respiratory epithelium, nucleotides such as ATP not only stimulate ion and fluid secretion, but also increase ciliary beat frequency [54] and mucin production in goblet cells [55], thereby affecting all parts of the mucociliary clearance system (reviewed by Lazarowski and Boucher [56]). Apical P2Y2 receptor stimulation is involved in the regulation of all processes, with a smaller contribution of P2Y6 and P2X4 receptors, as well as the adenosine receptor A2b. P2Y2 receptor stimulation leads to an increase in intracellular Ca2+ concentrations, which mediate both increased mucin secretion from goblet cells and increased ciliary beat frequency. The concentration of nucleotides in the airway surface liquid has been shown to reach physiological concentrations, and functional studies have confirmed that ATP conveys mucociliary clearance response as a result of increased ATP due to mechanical or pathogenic stress [57–59]. Control of ciliary movement is an example of crosstalk between sodium concentrations and purinergic signaling molecules, since extracellular sodium can regulate ciliary movement by interfering with a P2X receptor (probably P2X4) [60].
In conclusion, purinergic signaling has a wide influence on epithelial ion transport throughout the body. In most tissues, the ATP/UTP receptor P2Y2 has been demonstrated to be the most important contributor. The functional importance of purinergic control of epithelial transport is twofold. Constitutive release of nucleotides out into the surface liquids of different epithelia due to mechanical or chemical stimuli contributes to local balance, while high concentrations of luminal nucleotides elicited by the pathogenic insult caused by a respiratory or gastrointestinal infection lead to increased secretion and motility, thereby supplying epithelial protection.
Integrity of the endothelial barrier function
The protective role of purinergic signaling is valid also for the endothelium where both ATP and adenosine have been demonstrated to stabilize endothelial barrier function by reducing paracellular transport of water and macromolecules—a process that is of great importance to the clinical manifestation of, for example, acute lung injury. Inflammatory stressors mediate increased endothelial permeability by activating G protein-coupled receptors that increase intracellular Ca2+ concentrations. Several lines of evidence suggest that ATP protects from endothelial leakage of water, macromolecules, and immune cells [61–66]. Kolosova et al. showed that the integrity of the endothelial barrier in human pulmonary endothelial cell monolayers was enhanced by ATP acting on P2 receptors leading to cAMP-independent activation of PKA [65]. Also, adenosine protects against endothelial barrier dysfunction by increasing cAMP levels via the A2b receptor. This pathway is activated by hypoxia and also involves upregulation of the ATP-degrading enzymes CD39 and CD73, leading to increased hydrolysis of ATP and AMP to adenosine [67]. The positive effect of ATP on endothelial barrier integrity has been confirmed in vivo where the stable ATP analog ATPγS was shown to protect against vascular leak, immune cells, and gap junction disruption in a mouse model of lipopolysaccharide (LPS)-induced injury [66].
Flow-mediated responses
Both endothelial cells and epithelial cells are in different ways exposed to flow. The endothelial flow response matches blood supply with local need of oxygen and nutrients. Renal epithelial cells have flow-sensing mechanisms that convey changes in epithelial transport to maintain salt and water balance by modulation of urinary excretion. Both endothelial and renal epithelial cells contribute to the regulation of blood pressure, partly as a consequence of their ability to respond to flow.
Flow-sensing mechanisms of renal tubular epithelial cells
Renal epithelial cells are flow-sensing cells that are exposed to fluid moving along the apical membrane. Contrary to endothelial cells, these cells have primary cilia to sense flow changes, but can also react to lateral stretch of the epithelial cell layer [68]. The involvement of nucleotides in both of these mechanisms has been elucidated in a series of experiments by Praetorius and colleagues (reviewed in [15]). Flow causes bending of the cilia leading to influx of Ca2+ via a complex of mechanosensitive transient receptor potential (TRP) channels (TRPV4/TRPP2) [69, 70]. In turn, this induces bilateral release of nucleotides that can activate both apically and basolaterally located P2 receptors in tubular epithelial cells [71]. The flow-mediated response is suggested to create a link between tubular flow and ion transport processes by increasing the inhibitory tone of epithelial transport described above [15, 72]. Nucleotide release into the luminal flow of the renal tubule can also be induced by hormones, such as AVP [73]. The released nucleotides are thought to follow the direction of flow and also affect P2 receptors in more distal parts of the nephron where the signal is finally disrupted by degrading enzymes, f ex CD73 [15, 74, 75].
Direction and mechanism of the endothelial flow response
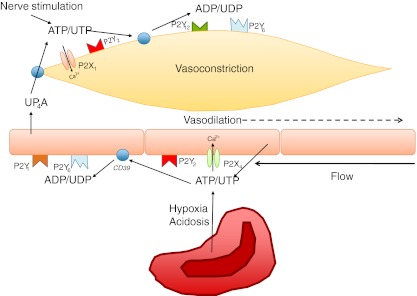
In blood vessels, spreading of the flow-mediated purinergic signal occurs in a different way than in the nephron. Hypoxia and acidosis cause release of dilatory ATP on the venular side of the capillary bed, but to increase blood flow to the tissue the arterioles upstream need to be dilated. Ellsworth et al. have demonstrated that addition of ATP to the venular side binds to purinergic receptors located on the vascular endothelium and induces a vasodilation that is conducted upstream increasing oxygen supply to the region of tissue supplied by the vessel [76].
Winter and Dora developed a system with triple cannulation of isolated arteries to enable focal application of purine and pyrimidine nucleotides to the endothelium, avoiding potential actions of these agents on the smooth muscle. Nucleotides were locally infused through one branch of a bifurcation, causing near maximal local dilatation. Dilatation then spreads rapidly backwards to the adjacent feed artery and upstream against the direction of luminal flow and increases flow into the feed artery. The data demonstrate that direct luminal stimulation of P2Y receptor on the endothelium of rat mesenteric arteries leads to marked spreading retrograde dilatation, suggesting that circulating purines and pyrimidines act as important regulators of blood flow [77]. Since released nucleotides cannot be expected to travel upstream on the luminal side, this retrograde response suggests that the endothelium also has polarized purinergic signaling similar to that of renal epithelial cells where released nucleotides can give opposite effects depending on signal localization.
Shear stress and hypoxia are important stimuli of both ATP and UTP release from endothelial cells [78]. The P2X4 receptor is the highest expressed P2 receptor in endothelium [79, 80]. By using antisense oligonucleotides, the P2X4 receptor was shown to be important for shear stress-dependent Ca2+ influx via an ATP-dependent mechanism [81]. This indicates that ATP and P2 receptors may be of importance for shear stress-mediated effects which is in agreement with the well-established release of ATP from endothelial cells during shear stress [82]. Vessel dilation induced by acute increases in blood flow is markedly suppressed in P2X4−/−mice, indicating that endothelial P2X4 channels are crucial to flow-sensitive mechanisms that regulate blood pressure and vascular remodeling [83]. The importance of the presence of ATP for flow-induced vasodilation has been confirmed in rat mesenteric arteries [84]. If ATP was not present in the lumen, the flow-induced vasodilation was reduced by half. Interestingly, the effect of ATP could be mimicked by UTP, indicating that P2Y receptors could be of similar importance as the P2X4 receptor for shear-induced vasodilatation. P2X4−/− mice have higher blood pressure and excrete smaller amounts of NO products in their urine than do wild-type mice [83].
Nucleotides are released from intracellular compartments during shear stress, but there is also evidence for cell surface ATP synthase to be involved in shear stress-induced ATP release [85]. Immunofluorescence staining of human pulmonary arterial endothelial cells showed that cell surface ATP synthase is distributed in lipid rafts/caveole and critical for shear stress-induced ATP release.
Blood pressure control
The ability of purines to modulate vascular patency as well as their effect on renal Na+ absorption indicates a central role for them in the control of blood pressure. Increased blood pressure is one of the phenotypes found in the P2Y2−/− mouse model and correlated with previous findings of the inhibitory effects of this receptor on electrogenic Na+ reabsorption in the kidney tubuli. Moreover, several in vivo studies have revealed altered P2 receptor expression in the kidney during hyper- and hypovolemia and after ischemic reperfusion injury. Recent advances in the understanding of these mechanisms have elucidated the connection between this local mechanism and hormonal control. The antidiuretic hormone vasopressin has been shown to induce tubular nucleotide secretion from the medullary thick ascending limb of murine nephrons and is suggested to function has a local transport-opposing feedback system in response to transport-activating hormones [73]. As described in a previous chapter, purinergic signaling counteracts aldosterone-mediated Na+ reabsorption via a P2Y2-mediated mechanism. Also, the aldosterone-controlled thiazide-sensitive NaCl cotransporter has been demonstrated to be under purinergic control [86]. In a recent in vivo study, where P2Y2−/− mice were fed a high-Na+ diet, Pochynyuk et al. showed that P2Y2 receptors are important in maintaining NaCl homeostasis and blood pressure regulation. Indeed, a single-nucleotide polymorphism in the P2Y2 gene has been found to be associated with essential hypertension [87].
In the systemic circulation, P2 receptor-mediated blood pressure regulation is the net result of balancing contractile and dilatory effects. ATP and UTP released on the luminal side of endothelial cells and from erythrocytes stimulate vasodilatation in contrast to release from sympathetic nerves on the adventitial side, which results in vasoconstriction. The most important dilatory P2Y receptors on the endothelium are the P2Y1 and P2Y2 receptors, although a small dilatory effect of P2Y6 has also been shown [88].
Diadenosine polyphosphates such as AP4A, AP5A, and AP6A are combinations of two adenosine molecules connected with four to six phosphate groups. They have been identified as vasocontractile agents [89], probably via actions on P2X1 and P2Y2 receptors. AP5A and AP6A are stored at higher levels in platelets from patients with hypertension and may contribute to their increased peripheral vascular resistance [90]. UP4A is a novel endothelium-derived vasoconstrictive factor more potent than endothelin in renal vasoconstriction [91]. It is released upon stimulation of the endothelium by acetylcholine, thrombin, and mechanical stress and can be cleaved into either ATP or UTP to stimulate both P2X1 and P2Y2 receptors on VSMC resulting in increased blood pressure [91]. Figure 3 illustrates and summarizes the influence of purinergic signaling in the vasculature.
Fig. 3.
Overview of receptors and nucleotides involved in modulating vessel patency
Blood pressure control under pathological conditions
Red blood cell release of ATP is important for the regulation of pulmonary resistance [92], and patients with pulmonary hypertension have impaired release of ATP from red blood cells [93]. Endothelium-dependent relaxation to ATP has been shown in human pulmonary arteries [94].
In extreme conditions such as circulatory or septic shock with acidosis and hypoxia, high ATP levels could be deleterious, leading to a drop in blood pressure [95]. At levels above 100 μM, ATP concentrations may exceed the catalytic capacity of ectonucleotidases and, could in fact, stimulate ATP release by increasing permeability of the red blood cell (RBC) [95], probably via P2X7 receptors [96]. ATP can also release ATP from endothelial cells [97]. At high concentrations of ATP, a self-sustaining process may thus be instigated which may contribute to the irreversible stage of circulatory shock that can develop rapidly in severely ill patients.
Intercellular communication in tissue perfusion
Studies of purinergic signaling in red blood cells, especially the work by Sprague and Ellsworth, have revealed an intriguing communication between the cells of the blood and of the vascular wall. The matching of oxygen supply with demand requires a mechanism that increases blood flow in response to decreased tissue oxygen levels. Several reports suggest that the RBC acts as a sensor for hypoxia and different mechanisms have been suggested by which the deoxygenated RBC stimulates vasodilatation [98–101]. RBCs contain millimolar amounts of ATP and possess the membrane-bound glycolytic enzymes necessary for its production [93, 102, 103]. ATP is released in response to reductions in oxygen tension and pH [99, 100]. It has been shown in vitro that vessels dilate in response to low O2 levels only when blood vessels are perfused with RBCs [104]. ATP is released in working human skeletal muscle circulation depending on the number of unoccupied hemoglobin O2-binding sites [99, 100]. The released ATP then binds to P2Y receptors on the endothelium and stimulates vasodilatation. Thus, the RBC may function as an O2 sensor, contributing to the regulation of blood flow and O2 delivery, by releasing ATP depending on the oxygenation state of hemoglobin.
ADP activates a negative feedback pathway for ATP release from human RBCs via P2Y13 receptors [105]. Because blood consists of approximately 40 % RBCs, containing a 1,000-fold higher ATP concentration than plasma (millimole per liter vs. micromole per liter), even a minor release of ATP from the high intracellular concentrations could have major circulatory effects. A negative feedback system may therefore be of great physiological importance to mitigate ATP release.
The tubuloglomerular feedback (TGF) mechanism is important for the regulation of the glomerular filtration rate (GFR) and serves as a beautiful example of purinergic signaling in intercellular communication. In the distal tubule, a specialized epithelial cell type, the macula densa cells, which have the capability to sense the luminal NaCl concentration of the tubule, communicates with the juxtaglomerular cells in the wall of the afferent and efferent arterioles. An increase in NaCl concentration initiates a signal that causes afferent arteriole constriction, which confers decreased GFR. Afferent arteriole constriction can be induced by purinergic signaling by both direct effect of ATP via P2X1 receptors and by adenosine acting on A1 receptors of the vascular smooth muscle [106]. Autoregulatory responses of the afferent arteriole have been shown to involve P2X1 function since this response is impeded in mice deficient of this receptor [107]. While there is no evidence for the involvement of adenosine in this response [107], the relative contribution of TGF-mediated afferent arteriole vasoconstriction has been a matter of controversy [108]. Work done by several groups has pointed out the importance of adenosine in the TGF response. TGF responses were found to be abolished in A1 receptor knockout mice [109, 110] as well as in mice deficient of the AMP-degrading enzyme CD73 that is necessary for formation of ATP-derived adenosine [111, 112]. This leads us to the more detailed mechanism of the TGF response. It has long been known that the specialized epithelial cells of the macula densa are able to convey vasoactive responses in the afferent arteriole via release of one or several substances. Experiments using isolated nephron preparations have demonstrated ATP release from over the basolateral membrane of macula densa cells in response to elevated NaCl concentrations in the tubular fluid [113]. ATP release was detected using biosensor cells that were monitored using patch clamp or Fura-2 loading. Recently, a similar study revealed direct NaCl-independent flow sensing of macula densa cells mediated by bending of primary cilia [114].
Regeneration and inflammation
Another feature of the cellular lining of our body is its capacity to regenerate and respond to injury. Physiological regeneration results from a balance of controlled cell death and cell proliferation. There are several examples of purinergic signaling playing a significant role in this process. In the tips of duodenal villi, the apoptosis-related P2X7 receptor has been found to be expressed, indicating a role in the regeneration process of these cells [30, 115]. In addition to stimulating P2X7 receptor activation leading to cell death, ATP also stimulates P2Y receptor expression in both epithelial and endothelial tissue and subsequent cell proliferation [116, 117]. Inflammatory mediators have also been demonstrated to potentiate proliferation by increasing P2Y2 receptor expression [116], implicating a role of purinergic signaling in the healing process following injury. A recent study elegantly demonstrated that ATP released from injured cells promotes extravasation of neutrophils into the specific area of inflammation, thereby letting them escape through the blood vessels of healthy tissue and find their way to the right location [118].
In mucosal defense, purinergic signaling has been implicated as mediators of the Th2 response to the airborn allergen Alternaria alternata in a murine model of airway inflammation [119]. This response involved release of ATP and activation of P2Y2 receptors on airway epithelial cells.
Chronic vascular inflammation is a hallmark of metabolic cardiovascular disease such as diabetes and atherosclerosis. Nilsson et al. showed that high glucose activates the pro-inflammatory Ca2+-dependent transcription factor nuclear factor of activated T cells (NFAT) in intact murine vessels [120]. Hyperglycemia-induced NFAT activation via extracellular nucleotide signaling has been confirmed in arteries in vivo where it leads to increased expression of the diabetes-related matrix cytokine osteopontin [121]. This mechanism was dependent on nucleotide signaling and mediated by the P2Y6 receptor [120]. Recently, a study investigating the P2 receptor expression response to inflammatory stimuli has revealed selective induction of endothelial P2Y6 receptor shown to promote vascular inflammation [122]. This was confirmed in vivo, as LPS-induced vascular inflammation was attenuated in P2Y6−/− mice [122]. A similar response has been demonstrated in intestinal inflammation [123]. It is noteworthy that one of the most highly expressed P2Y receptors of endothelial cells is the P2Y11 receptor, which has no known function in regulation of vessel patency, but might be involved in the inflammatory response of this tissue. Roles for this receptor have previously been discovered in neutrophils where it acts as a survival factor and in dendritic cell where it mediates release of IL-8 [124].
Conclusion
The many diverse and physiologically relevant functions of purinergic signaling suggest that this system is an ancient way for organisms to maintain homeostasis during stress. Considering that the most common diseases of our time—diabetes, cardiovascular disease, hypertension, and asthma—are not primarily caused by a pathogenic insult to our body, but rather derive from homeostatic disturbances, nucleotide-mediated responses are at the core of both pathogenesis and potential treatment. Much focus has been given to identification and investigations of P2 receptors, but a big part of the purinergic puzzle lies in the degrading ectonucleotidases that regulate which ligand is available for binding. The recent discovery of novel nucleotides, such as Up4A, further widens the signaling possibilities. What this review aims at illustrating, is that epithelial and endothelial cells, although different, share many functions and mechanisms that can serve as inspiration in our continuous work towards understanding purinergic signaling in the pathology and physiology of the human body.
Contributor Information
Jenny Öhman, Email: jenny.ohman@med.lu.se.
David Erlinge, Email: david.erlinge@med.lu.se.
References
- 1.Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195(4):415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 3.Romanello M, et al. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun. 2001;289(5):1275–1281. doi: 10.1006/bbrc.2001.6124. [DOI] [PubMed] [Google Scholar]
- 4.Grierson JP, Meldolesi J. Shear stress-induced [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J Biol Chem. 1995;270(9):4451–4456. doi: 10.1074/jbc.270.9.4451. [DOI] [PubMed] [Google Scholar]
- 5.Sauer H, Hescheler J, Wartenberg M. Mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation. Am J Physiol Cell Physiol. 2000;279(2):C295–C307. doi: 10.1152/ajpcell.2000.279.2.C295. [DOI] [PubMed] [Google Scholar]
- 6.Moerenhout M, Vereecke J, Himpens B. Mechanism of intracellular Ca2+-wave propagation elicited by mechanical stimulation in cultured endothelial CPAE cells. Cell Calcium. 2001;29(2):117–123. doi: 10.1054/ceca.2000.0164. [DOI] [PubMed] [Google Scholar]
- 7.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150(6):1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons NL. Identification of a purine (P2) receptor linked to ion transport in a cultured renal (MDCK) epithelium. Br J Pharmacol. 1981;73(2):379–384. doi: 10.1111/j.1476-5381.1981.tb10432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zegarra-Moran O, Romeo G, Galietta LJ. Regulation of transepithelial ion transport by two different purinoceptors in the apical membrane of canine kidney (MDCK) cells. Br J Pharmacol. 1995;114(5):1052–1056. doi: 10.1111/j.1476-5381.1995.tb13312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Post SR, Jacobson JP, Insel PA. P2 purinergic receptor agonists enhance cAMP production in Madin-Darby canine kidney epithelial cells via an autocrine/paracrine mechanism. J Biol Chem. 1996;271(4):2029–2032. doi: 10.1074/jbc.271.4.2029. [DOI] [PubMed] [Google Scholar]
- 11.Post SR, et al. ATP activates cAMP production via multiple purinergic receptors in MDCK-D1 epithelial cells. Blockade of an autocrine/paracrine pathway to define receptor preference of an agonist. J Biol Chem. 1998;273(36):23093–23097. doi: 10.1074/jbc.273.36.23093. [DOI] [PubMed] [Google Scholar]
- 12.Ostrom RS, et al. Key role for constitutive cyclooxygenase-2 of MDCK cells in basal signaling and response to released ATP. Am J Physiol Cell Physiol. 2001;281(2):C524–C531. doi: 10.1152/ajpcell.2001.281.2.C524. [DOI] [PubMed] [Google Scholar]
- 13.Ostrom RS, Gregorian C, Insel PA. Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem. 2000;275(16):11735–11739. doi: 10.1074/jbc.275.16.11735. [DOI] [PubMed] [Google Scholar]
- 14.Geyti CS, et al. Slow spontaneous [Ca2+] i oscillations reflect nucleotide release from renal epithelia. Pflugers Arch. 2008;455(6):1105–1117. doi: 10.1007/s00424-007-0366-4. [DOI] [PubMed] [Google Scholar]
- 15.Praetorius HA, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol. 2010;72:377–393. doi: 10.1146/annurev-physiol-021909-135825. [DOI] [PubMed] [Google Scholar]
- 16.Xin C, et al. Heterologous desensitization of the sphingosine-1-phosphate receptors by purinoceptor activation in renal mesangial cells. Br J Pharmacol. 2004;143(5):581–589. doi: 10.1038/sj.bjp.0705980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3(104):re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5(4):433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnstock G. Purinergic mechanosensory transduction and visceral pain. Mol Pain. 2009;5:69. doi: 10.1186/1744-8069-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight GE, et al. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282(2):F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- 21.Burnstock G. Unresolved issues and controversies in purinergic signalling. J Physiol. 2008;586(14):3307–3312. doi: 10.1113/jphysiol.2008.155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 23.Karanauskaite J, et al. Quantal ATP release in rat beta-cells by exocytosis of insulin-containing LDCVs. Pflugers Arch. 2009;458(2):389–401. doi: 10.1007/s00424-008-0610-6. [DOI] [PubMed] [Google Scholar]
- 24.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38(6):900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Orriss IR, et al. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol. 2009;220(1):155–162. doi: 10.1002/jcp.21745. [DOI] [PubMed] [Google Scholar]
- 26.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1(4):311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novak I. Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol (Oxf) 2011;202(3):501–522. doi: 10.1111/j.1748-1716.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 28.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103(20):7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarowski ER, et al. Nucleotide release by airway epithelia. Subcell Biochem. 2011;55:1–15. doi: 10.1007/978-94-007-1217-1_1. [DOI] [PubMed] [Google Scholar]
- 30.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284(3):F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 31.Wong PY. Control of anion and fluid secretion by apical P2-purinoceptors in the rat epididymis. Br J Pharmacol. 1988;95(4):1315–1321. doi: 10.1111/j.1476-5381.1988.tb11770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matos JE, et al. Distal colonic Na(+) absorption inhibited by luminal P2Y(2) receptors. Pflugers Arch. 2007;454(6):977–987. doi: 10.1007/s00424-007-0248-9. [DOI] [PubMed] [Google Scholar]
- 33.Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol. 1999;276(4 Pt 1):C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- 34.Inglis SK, et al. Effect of luminal nucleotides on Cl− secretion and Na+ absorption in distal bronchi. Pflugers Arch. 1999;438(5):621–627. doi: 10.1007/s004240051085. [DOI] [PubMed] [Google Scholar]
- 35.Ramminger SJ, et al. P2Y2 receptor-mediated inhibition of ion transport in distal lung epithelial cells. Br J Pharmacol. 1999;128(2):293–300. doi: 10.1038/sj.bjp.0702767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunzelmann K, Schreiber R, Cook D. Mechanisms for the inhibition of amiloride-sensitive Na+ absorption by extracellular nucleotides in mouse trachea. Pflugers Arch. 2002;444(1–2):220–226. doi: 10.1007/s00424-002-0796-y. [DOI] [PubMed] [Google Scholar]
- 37.Lehrmann H, et al. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol. 2002;13(1):10–18. doi: 10.1681/ASN.V13110. [DOI] [PubMed] [Google Scholar]
- 38.Pochynyuk O, et al. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283(52):36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pochynyuk O, et al. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J. 2010;24(6):2056–2065. doi: 10.1096/fj.09-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC) Pflugers Arch. 2009;458(1):111–135. doi: 10.1007/s00424-009-0656-0. [DOI] [PubMed] [Google Scholar]
- 41.Firsov D, et al. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci U S A. 1996;93(26):15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butterworth MB. Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Biochim Biophys Acta. 2010;1802(12):1166–1177. doi: 10.1016/j.bbadis.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pochynyuk O, et al. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol. 2008;294(1):F38–F46. doi: 10.1152/ajprenal.00403.2007. [DOI] [PubMed] [Google Scholar]
- 44.O'Mullane LM, Cook DI, Dinudom A. Purinergic regulation of the epithelial Na+ channel. Clin Exp Pharmacol Physiol. 2009;36(10):1016–1022. doi: 10.1111/j.1440-1681.2009.05256.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. Basolateral P2X4-like receptors regulate the extracellular ATP-stimulated epithelial Na+ channel activity in renal epithelia. Am J Physiol Renal Physiol. 2007;292(6):F1734–F1740. doi: 10.1152/ajprenal.00382.2006. [DOI] [PubMed] [Google Scholar]
- 46.Gorelik J, et al. Aldosterone acts via an ATP autocrine/paracrine system: the Edelman ATP hypothesis revisited. Proc Natl Acad Sci U S A. 2005;102(42):15000–15005. doi: 10.1073/pnas.0507008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cressman VL, et al. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(−) transport. J Biol Chem. 1999;274(37):26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- 48.Ghanem E, et al. The role of epithelial P2Y2 and P2Y4 receptors in the regulation of intestinal chloride secretion. Br J Pharmacol. 2005;146(3):364–369. doi: 10.1038/sj.bjp.0706353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robaye B, et al. Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Mol Pharmacol. 2003;63(4):777–783. doi: 10.1124/mol.63.4.777. [DOI] [PubMed] [Google Scholar]
- 50.Wong AM, et al. Apical versus basolateral P2Y(6) receptor-mediated Cl(−) secretion in immortalized bronchial epithelia. Am J Respir Cell Mol Biol. 2009;40(6):733–745. doi: 10.1165/rcmb.2008-0020OC. [DOI] [PubMed] [Google Scholar]
- 51.Kellerman D, et al. Denufosol: a review of studies with inhaled P2Y(2) agonists that led to phase 3. Pulm Pharmacol Ther. 2008;21(4):600–607. doi: 10.1016/j.pupt.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Faria D, Schreiber R, Kunzelmann K. CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflugers Arch. 2009;457(6):1373–1380. doi: 10.1007/s00424-008-0606-2. [DOI] [PubMed] [Google Scholar]
- 53.Ishibashi K, Okamura K, Yamazaki J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl(−) reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1729–R1736. doi: 10.1152/ajpregu.00758.2007. [DOI] [PubMed] [Google Scholar]
- 54.Wong LB, Yeates DB. Luminal purinergic regulatory mechanisms of tracheal ciliary beat frequency. Am J Respir Cell Mol Biol. 1992;7(4):447–454. doi: 10.1165/ajrcmb/7.4.447. [DOI] [PubMed] [Google Scholar]
- 55.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 56.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9(3):262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Button B, Boucher RC. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163(1–3):189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580(Pt. 2):577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarran R, et al. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem. 2005;280(42):35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma W, et al. Extracellular sodium regulates airway ciliary motility by inhibiting a P2X receptor. Nature. 1999;400(6747):894–897. doi: 10.1038/23743. [DOI] [PubMed] [Google Scholar]
- 61.Gunduz D, et al. ATP antagonism of thrombin-induced endothelial barrier permeability. Cardiovasc Res. 2003;59(2):470–478. doi: 10.1016/S0008-6363(03)00427-9. [DOI] [PubMed] [Google Scholar]
- 62.Gunduz D, et al. Accumulation of extracellular ATP protects against acute reperfusion injury in rat heart endothelial cells. Cardiovasc Res. 2006;71(4):764–773. doi: 10.1016/j.cardiores.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Noll T, et al. ATP reduces macromolecule permeability of endothelial monolayers despite increasing [Ca2+]i. Am J Physiol. 1999;276(6 Pt 2):H1892–H1901. doi: 10.1152/ajpheart.1999.276.6.H1892. [DOI] [PubMed] [Google Scholar]
- 64.Jacobson JR, et al. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L289–L295. doi: 10.1152/ajplung.00343.2005. [DOI] [PubMed] [Google Scholar]
- 65.Kolosova IA, et al. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ Res. 2005;97(2):115–124. doi: 10.1161/01.RES.0000175561.55761.69. [DOI] [PubMed] [Google Scholar]
- 66.Kolosova IA, et al. Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L319–L324. doi: 10.1152/ajplung.00283.2007. [DOI] [PubMed] [Google Scholar]
- 67.Eltzschig HK, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198(5):783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191(1):69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 69.Wu L, et al. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293(5):F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 70.Kottgen M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182(3):437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Praetorius HA, Leipziger J. Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol (Oxf) 2009;197(3):241–251. doi: 10.1111/j.1748-1716.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- 72.Praetorius HA, Leipziger J. Fluid flow sensing and triggered nucleotide release in epithelia. J Physiol. 2008;586(Pt 11):2669. doi: 10.1113/jphysiol.2008.155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Odgaard E, Praetorius HA, Leipziger J. AVP-stimulated nucleotide secretion in perfused mouse medullary thick ascending limb and cortical collecting duct. Am J Physiol Renal Physiol. 2009;297(2):F341–F349. doi: 10.1152/ajprenal.00190.2009. [DOI] [PubMed] [Google Scholar]
- 74.Hir M, Kaissling B. Distribution and regulation of renal ecto-5′-nucleotidase: implications for physiological functions of adenosine. Am J Physiol. 1993;264(3 Pt 2):F377–F387. doi: 10.1152/ajprenal.1993.264.3.F377. [DOI] [PubMed] [Google Scholar]
- 75.Jensen ME, et al. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol. 2007;18(7):2062–2070. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- 76.Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res. 1998;56(1):43–53. doi: 10.1006/mvre.1998.2076. [DOI] [PubMed] [Google Scholar]
- 77.Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582(Pt 1):335–347. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194(Pt 3):335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto K, et al. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279(1):H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- 80.Wang L, et al. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40(6):841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Yamamoto K, et al. Fluid shear stress activates Ca(2+) influx into human endothelial cells via P2X4 purinoceptors. Circ Res. 2000;87(5):385–391. doi: 10.1161/01.res.87.5.385. [DOI] [PubMed] [Google Scholar]
- 82.Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103(1):1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamamoto K, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12(1):133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 84.Liu C, et al. Extracellular ATP facilitates flow-induced vasodilatation in rat small mesenteric arteries. Am J Physiol Heart Circ Physiol. 2004;286(5):H1688–H1695. doi: 10.1152/ajpheart.00576.2003. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto K, et al. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293(3):H1646–H1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, et al. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol. 2011;300(3):F657–F668. doi: 10.1152/ajprenal.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z, et al. The purinergic receptor P2Y, G-protein coupled, 2 (P2RY2) gene associated with essential hypertension in Japanese men. J Hum Hypertens. 2010;24(5):327–335. doi: 10.1038/jhh.2009.67. [DOI] [PubMed] [Google Scholar]
- 88.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4(1):1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schluter H, et al. Diadenosine phosphates and the physiological control of blood pressure. Nature. 1994;367(6459):186–188. doi: 10.1038/367186a0. [DOI] [PubMed] [Google Scholar]
- 90.Hollah P, et al. A novel assay for determination of diadenosine polyphosphates in human platelets: studies in normotensive subjects and in patients with essential hypertension. J Hypertens. 2001;19(2):237–245. doi: 10.1097/00004872-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 91.Jankowski V, et al. Uridine adenosine tetraphosphate: a novel endothelium- derived vasoconstrictive factor. Nat Med. 2005;11(2):223–227. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- 92.Sprague RS, et al. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol. 2003;285(2):H693–H700. doi: 10.1152/ajpheart.01026.2002. [DOI] [PubMed] [Google Scholar]
- 93.Sprague RS, et al. Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med (Maywood) 2001;226(5):434–439. doi: 10.1177/153537020122600507. [DOI] [PubMed] [Google Scholar]
- 94.Greenberg B, Rhoden K, Barnes PJ. Endothelium-dependent relaxation of human pulmonary arteries. Am J Physiol. 1987;252(2 Pt 2):H434–H438. doi: 10.1152/ajpheart.1987.252.2.H434. [DOI] [PubMed] [Google Scholar]
- 95.Trams EJ, Kauffman H, Burnstock GG. A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J Theor Biol. 1980;87:609–621. doi: 10.1016/0022-5193(80)90239-8. [DOI] [PubMed] [Google Scholar]
- 96.Sluyter R, et al. Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem. 2004;279(43):44749–44755. doi: 10.1074/jbc.M405631200. [DOI] [PubMed] [Google Scholar]
- 97.Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol. 1996;27(6):872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 98.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 99.Ellsworth ML, et al. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269(6 Pt 2):H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 100.Yegutkin GG, et al. Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol. 2007;579(Pt 2):553–564. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8(7):711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 102.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26(1):40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 103.Sprague RS, et al. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281(4):C1158–C1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 104.Touchman JW, et al. The genomic region encompassing the nephropathic cystinosis gene (CTNS): complete sequencing of a 200-kb segment and discovery of a novel gene within the common cystinosis-causing deletion. Genome Res. 2000;10(2):165–173. doi: 10.1101/gr.10.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, et al. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res. 2005;96(2):189–196. doi: 10.1161/01.RES.0000153670.07559.E4. [DOI] [PubMed] [Google Scholar]
- 106.Guan Z, Osmond DA, Inscho EW. P2X receptors as regulators of the renal microvasculature. Trends Pharmacol Sci. 2007;28(12):646–652. doi: 10.1016/j.tips.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 107.Inscho EW, et al. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112(12):1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Komlosi P, Fintha A, Bell PD. Renal cell-to-cell communication via extracellular ATP. Physiology (Bethesda) 2005;20:86–90. doi: 10.1152/physiol.00002.2005. [DOI] [PubMed] [Google Scholar]
- 109.Sun D, et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98(17):9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown R, et al. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 111.Castrop H, et al. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114(5):634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thomson S, et al. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest. 2000;106(2):289–298. doi: 10.1172/JCI8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komlosi P, et al. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol. 2004;286(6):F1054–F1058. doi: 10.1152/ajprenal.00336.2003. [DOI] [PubMed] [Google Scholar]
- 114.Sipos A, Vargas S, Peti-Peterdi J. Direct demonstration of tubular fluid flow sensing by macula densa cells. Am J Physiol Renal Physiol. 2010;299(5):F1087–F1093. doi: 10.1152/ajprenal.00469.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Groschel-Stewart U, et al. P2X receptors in the rat duodenal villus. Cell Tissue Res. 1999;297(1):111–117. doi: 10.1007/s004410051338. [DOI] [PubMed] [Google Scholar]
- 116.Hou M, et al. Cytokines induce upregulation of vascular P2Y(2) receptors and increased mitogenic responses to UTP and ATP. Arterioscler Thromb Vasc Biol. 2000;20(9):2064–2069. doi: 10.1161/01.ATV.20.9.2064. [DOI] [PubMed] [Google Scholar]
- 117.Paller MS, Schnaith EJ, Rosenberg ME. Purinergic receptors mediate cell proliferation and enhanced recovery from renal ischemia by adenosine triphosphate. J Lab Clin Med. 1998;131(2):174–183. doi: 10.1016/S0022-2143(98)90161-5. [DOI] [PubMed] [Google Scholar]
- 118.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 119.Kouzaki H, et al. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186(7):4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nilsson J, et al. High glucose activates nuclear factor of activated T cells in native vascular smooth muscle. Arterioscler Thromb Vasc Biol. 2006;26(4):794–800. doi: 10.1161/01.ATV.0000209513.00765.13. [DOI] [PubMed] [Google Scholar]
- 121.Nilsson-Berglund LM, et al. Nuclear factor of activated T cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arterioscler Thromb Vasc Biol. 2010;30(2):218–224. doi: 10.1161/ATVBAHA.109.199299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Riegel AK, et al. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood. 2011;117(8):2548–2555. doi: 10.1182/blood-2010-10-313957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grbic DM, et al. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol. 2008;180(4):2659–2668. doi: 10.4049/jimmunol.180.4.2659. [DOI] [PubMed] [Google Scholar]
- 124.Meis S, et al. NF546 [4,4′-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenyle ne)-carbonylimino))-bis(1,3-xylene-alpha, alpha’-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells. J Pharmacol Exp Ther. 2010;332(1):238–247. doi: 10.1124/jpet.109.157750. [DOI] [PubMed] [Google Scholar]