Abstract
In the last decades, the discovery that glial cells do not only fill in the empty space among neurons or furnish them with trophic support but are rather essential participants to the various activities of the central and peripheral nervous system has fostered the search for the signalling pathways controlling their functions. Since the early 1990s, purines were foreseen as some of the most promising candidate molecules. Originally just a hypothesis, this has become a certainty as experimental evidence accumulated over years, as demonstrated by the exponentially growing number of articles related to the role of extracellular nucleotides and nucleosides in controlling glial cell functions. Indeed, as new functions for already known glial cells (for example, the ability of parenchymal astrocytes to behave as stem cells) or new subtypes of glial cells (for example, NG2+ cells, also called polydendrocytes) are discovered also, new actions and new targets for the purinergic system are identified. Thus, glial purinergic receptors have emerged as new possible pharmacological targets for various acute and chronic pathologies, such as stroke, traumatic brain and spinal cord injury, demyelinating diseases, trigeminal pain and migraine, and retinopathies. In this article, we will summarize the most important and promising actions mediated by extracellular purines and pyrimidines in controlling the functions, survival, and differentiation of the various “classical” types of glial cells (i.e., astrocytes, oligodendrocytes, microglial cells, Müller cells, satellite glial cells, and enteric glial cells) but also of some rather new members of the family (e.g., polydendrocytes) and of other cells somehow related to glial cells (e.g., pericytes and spinal cord ependymal cells).
Keywords: Astrocytes, Microglial cells, Oligodendrocytes, Müller cells, Reactive gliosis, Myelination
Introduction
In recent years, the role of the purinergic system in the modulation of cell-to-cell communication in the central (CNS) and peripheral nervous system (PNS) has clearly emerged, as extensively reviewed elsewhere in this issue. Not only neurons but also many types of glial cells (mainly, astrocytes and microglia) release and respond to ATP and other purinergic molecules under both physiological and pathological conditions [1]. As shown in Fig. 1, P1 and P2 receptors are expressed on neurons and astrocytes, as well as on all other types of CNS and PNS glia, including oligodendrocytes (OLs) and Schwann cells (SCs) (that myelinate nerve terminals, thus ensuring rapid impulse conduction and axonal protection) and microglia (the brain’s and spinal cord’s immunocytes that continuously patrol the nervous tissue and react to damaging insults). Purinergic signalling also plays an important role in regulating the activity and function of both satellite glial cells (SGCs), the type of glia found in sensory ganglia, and of Müller cells, the retinal glial cells crucially involved in the transmission of visual inputs. In addition, ATP is an important signalling substance at the gliovascular interface and exerts a crucial control in the regulation of microvascular blood flow. This complex and highly integrated glial purinergic network acts in an autocrine and paracrine manner to regulate nervous system function under physiological conditions and to ensure response and foster repair under acute and chronic pathological conditions. Here, we aim at summarizing the role played by the purinergic system in astrocytes, OLs, SCs, microglia, SGCs, and Müller cells. We also briefly revise the current literature on the involvement of purinergic signalling in some other cells that are not typically glia but are somehow related to the glial lineages, such as the ependymal cells lining spinal cord central canal, and pericytes, connective tissue cells that participate to the formation of the blood–brain barrier (BBB) and express typical glial markers, such as the proteoglycan NG2. We focus on the purinergic control of the physiological functions of these different cell types, as well as on the effects on their proliferation, differentiation, and death under both normal and pathological conditions.
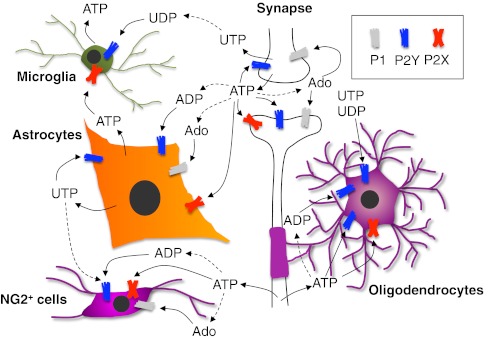
Fig. 1.
Schematic representation of the role played by extracellular nucleosides and nucleotides in modulating cell-to-cell communication between neurons and glial cells in the central nervous system. ATP is exocytotically released from nerve terminals as a co-transmitter during synaptic transmission, and activates its pre- and post-synaptic receptors but also receptors on surrounding astrocytes and microglial cells. ADP and adenosine are generated through the activity of ecto-nucleotidases and further contribute to neurotransmission and to the modulation of glial cell functions. Astrocytes and microglia themselves can release ATP, which acts autocrinally or paracrinally on surrounding cells. An axonal non-vesicular ATP release has been recently identified, which plays a fundamental role in controlling oligodendrocyte myelinating functions, and, possibly, also NG2+ cell activity and maturation. No clear evidence for a vesicular release of uracil nucleotides is currently available, but pyrimidinergic receptors have been identified on any of the cell types shown here, thus indicating their important role in controlling glial activity. For the sake of clarity, only generic receptor families (i.e., P1, P2Y, and P2X) are shown. See text for details
Astrocytes
Purines and pyrimidines in the physiological regulation of astrocyte functions
Under physiological conditions, astrocytes are exposed to extracellular nucleotides released during synaptic transmission. Astrocytes themselves can release ATP through a highly controlled vesicle system [2] and various membrane channels [3]. Interestingly, ATP release from astrocytes is regulated by circadian clock genes and IP3 signalling, suggesting that extracellular nucleotides can contribute to the observed daily oscillation in brain functions [4]. In turn, extracellular nucleotides and the corresponding nucleosides deriving from their metabolism act paracrinally on surrounding cells or autocrinally on astrocytes themselves. In fact, virtually all purinergic receptor P1 and P2 subtypes have been identified in astrocytes in vivo and in vitro by means of different approaches. As reviewed in details by Verkhratsky and colleagues [3], some receptor subtypes have been only identified at the mRNA level or by immunocytochemistry, with no clear indication of their role in controlling astrocyte functions (i.e., the P2X subtypes). Indeed, some receptor subtypes are expressed but not functional under basal conditions and are recruited and activated only following specific physiopathological situations ([5]; see also below). Finally, the lack of really selective agonists/antagonists for each purinoceptors has greatly contributed to the paucity of information on the role of specific receptor subtypes in the observed nucleotide-mediated effects on astrocytes. Apart from the above-mentioned difficulties in identifying the individual receptor subtypes responsible for specific functions, it has been clearly shown that extracellular ATP is responsible for short-term communication among astrocytes through the generation of [Ca2+]i transients, which in turn can further modulate exocytotic glutamate and ATP release from the same cells (for details, see [6]; see also below, “The discovery of a novel, long-range cell-to-cell signalling system: ATP-dependent glial calcium waves”). Thanks to the activity of ecto-nucleotidases, the subsequent extracellular generation of adenosine is then further involved in the modulation of the amplitude and kinetics of calcium waves among astrocytes and can control glutamate uptake by inhibiting the specific astrocyte transporter [3]. Astrocyte-derived extracellular nucleotides and nucleosides can indeed modulate synaptic transmission by activating their pre- and post-synaptic receptors (Fig. 1). Thus, based on its emerging specific role in controlling astrocyte functions and cell-to-cell communication, ATP has been correctly defined as the major “gliotransmitter” in the brain.
Purines and pyrimidines in the modulation of reactive astrogliosis
Substantial evidence indicates a key role for the purinergic system in controlling and promoting reactive astrogliosis, the typical response of astrocytes to any harmful event affecting the nervous tissue. This is particularly important in the light of the exciting recent discovery that reactive astrocytes do not only contribute to isolating the damaged area from the surrounding healthy or poorly injured tissue and release growth factors and trophic substances to promote tissue recovery, but they can themselves behave as stem-like cells, giving rise to the whole progeny of CNS cells at least in vitro ([7]; see also below). In detail, after traumatic or ischemic injury, resident CNS astrocytes become activated and start re-expressing neural cell stem markers, thus constituting a potential source of newborn neurons and glial cells for reparative purposes [7]. Therefore, early studies demonstrating synergistic effects of purinergic ligands and classical growth factors in the formation of “reactive” astrocytes [8, 9] need to be re-interpreted and indeed represented the first demonstration that, upon injury, purinergic ligands can induce astrocytes to de-differentiate to multipotent stem-like cells.
In this respect, it is now recognized that following pathological events, astrocytes exposure to extracellular nucleotides and nucleosides is dramatically increased, due to both overactivated excitatory synaptic transmission, and to massive cell death, leading to membrane disruption and extracellular leakage of the cytosolic purines and pyrimidines pool. As already mentioned in the previous paragraph, the elucidation of the specific contribution of the different purinergic receptor subtypes to the observed functional effects on astrocytes has been greatly slowed down by various factors. Nevertheless, it can be now undoubtedly stated that extracellular nucleotides are key players in modulating astrocytic reaction to injury. Here, we shall summarize some key facts in the purinergic regulation of reactive astrogliosis. For more details on this phenomenon with special reference to the signalling systems involved, the reader is referred to a more detailed review on this topic in this same issue.
The role of extracellular nucleotides in promoting reactive astrogliosis has been identified and studied since the early 1990s. The extensive work from both Neary’s and our laboratories has clearly shown that extracellular ATP promotes astrocyte hypertrophy, elongation of glial fibrillary acidic protein (GFAP)-positive processes and proliferation, through the activation of the ERK1/2 and of the pro-inflammatory COX-2 signalling pathways [10–12]. ATP-induced effects closely resemble growth factor modulation of reactive astrogliosis, and the two signalling systems closely collaborate together [9]. These effects seem to be mostly mediated by P2Y receptors, although the exact contribution of specific receptor subtypes has not been clearly defined yet. ADP-sensitive P2Y1 subtype promotes astrocytic reaction to traumatic injury in vivo through the PI3K/Akt signalling pathway [13] and controls astrocytic cytokine/chemokine transcription thus worsening cerebral damage in vivo [14]. Activation of the P2Y2 subtype promotes cytoskeletal changes and astrocyte migration through direct interaction with integrins ([15]; reviewed in [16]). Interestingly, this receptor subtype can also directly transactivate growth factor receptors, providing an additional possible explanation for nucleotide-growth factors interaction in controlling astrocyte functions [17]. Indeed, activation of the P2Y2 and P2Y4 subtypes promotes the astrocytic production and secretion of amyloid precursor protein [18]. This observation, together with the known ability of activated astrocytes to release various pro-inflammatory mediators (as reviewed in [19]), opens entirely new avenues on the role of astrocytes and of the purinergic system in the onset and development of Alzheimer’s disease, which could unveil new pharmacological targets to this devastating pathology. It is also worth mentioning that, at the BBB level, astrocytic end feet express predominantly the P2Y2 and P2Y4 receptor subtypes [20], which could be paracrinally or autocrinally activated by uracil nucleotides released by astrocytes following exposure to thrombin, as occurs in vivo as a consequence of plasma extravasation due to BBB rupture (for review, see [21]). Thus, a role for these receptors in controlling BBB integrity and permeability can be envisaged, which could have important implications during inflammatory and traumatic events but also for cancer cell migration and metastatization to the nervous system.
Although the exact contribution of P2X receptors to reactive astrogliosis is still unclear, a specific role for the P2X7 subtype has emerged. In fact, it seems that a clear expression of this receptor subtype in astrocytes can be detected only following traumatic or ischemic events, or when cells are activated in vitro by the culturing procedure [3]. Opening of this receptor channel leads to the sustained release of glutamate and other pro-inflammatory mediators (reviewed in [3]), thus highlighting the important role played by the P2X7 receptor subtype in the general frame of events leading to tissue remodeling and response to acute and chronic harmful events.
Following any ischemic and/or traumatic event, the massive increase in the extracellular nucleotides concentration bears as a logical consequence a delayed exposure to high adenosine concentrations, which in turn contributes to the modulation of reactive astrogliosis. In this respect, the observation that the astrocytic expression of ecto-nucleotidases, the nucleotide metabolizing enzymes, is dynamically regulated following brain damage (e.g., stab wound) with upregulation in the injured area [22] suggests that the contribution of adenosine receptors to reactive astrogliosis could become increasingly important with time after injury. The A1 adenosine receptors play a protective and anti-apoptotic role for astrocytes exposed to oxygen–glucose deprivation [23]. Activation of the A2A subtype in vivo has been shown to induce signs of gliosis [24], and its selective blockade completely prevented bFGF-mediated gliotic reaction in vitro [25]. A cross-talk between the pro-inflammatory cytokine TNFα and the A2B receptor subtype has been also demonstrated, with TNFα inhibiting A2B receptor desensitization and contributing to A2B-mediated elongation of astrocytic processes, a typical hallmark of reactive astrocytes in vitro [26]. An interesting double-edged sword activity for the A3 receptor subtype was also shown both in vivo and in vitro, with low agonist concentration promoting astrocytic survival, elongation of GFAP-positive processes [27], and increasing animals’ survival following ischemia [28]. Conversely, high micromolar agonist concentrations were responsible for induction of apoptosis in vitro [27] and worsened animals’ outcome following ischemia in vivo [28].
Although still incomplete, the picture that emerges from the above-mentioned and other experimental evidence highlights a central role for the purinergic system in controlling and fine-tuning astrocytic response to acute and chronic events. A renewed interest in this field can be foreseen in the next years, based on the already above-mentioned new discoveries that parenchymal reactive astrocytes possess stem cell-like properties. In fact, during their reaction to damage, normally quiescent parenchymal astrocytes undergo a dedifferentiation process, revealing many of the molecular traits of progenitor or stem cells (for review, see [7]). Although they can generate both astrocytes and neurons in vitro, they only give rise to astrocytes in vivo, thus suggesting that local inhibitory signals to neurogenesis may exist [19]. In this respect, the elucidation of the permissive or inhibitory role played by the various purinergic agonists, and receptor subtypes could contribute to unveil new strategies to promote tissue recovery following brain injury. Indeed, also stem cells in the neurogenic subventricular zone (SVZ) display typical astrocytic features [29]; these cells normally divide slowly, but following experimental depletion of SVZ cells, they are capable of reconstituting the principal SVZ cell populations [30]. Since these cells retain an apical contact with the lateral ventricle and a basal process that extends towards local vasculature [31], they are possibly exposed to multiple sources of extracellular nucleotides and nucleosides, which can modulate their self-renewal and differentiative abilities.
Schwann cells and oligodendrocytes
The role of the activity-dependent axonal signal ATP and its metabolites on Schwann cell and oligodendrocyte proliferation and differentiation
In the mammalian nervous system, most large-diameter axons are wrapped by a myelin sheath, which ensures the rapid conduction of nerve impulses and protects axons from damage. As mentioned in the “Introduction,” during development, myelin formation is provided by SCs in the PNS and by OLs in the CNS and requires a highly coordinated series of events in which multiple factors regulate precursor cell proliferation and their timely differentiation into myelinating glia [32, 33]. Myelin has recently received a lot of interest from the scientific community. One reason at the basis of this interest is because different diseases target myelin, including hereditary demyelinating neuropathies in the PNS, and multiple sclerosis (MS) and hereditary leukodystrophies in the CNS [34]. In recent years, myelin has also generated new interest for its involvement in normal cognitive function, learning and intelligence quotient, and as an unexpected contributor to a wide range of psychiatric disorders, including depression and schizophrenia (for review see: [35]). Moreover, it is now becoming clear that myelination continues into adulthood, affects information processing by regulating the velocity and synchrony of impulse conduction between distant cortical regions and is modifiable by experience and likely by drugs [36]. On this basis, it is obvious that an in-depth knowledge of the factors and mechanisms regulating myelination in the adult brain would greatly advance our understanding of brain function under both physiological and pathological conditions.
Purinergic signalling is one of the main pathways that regulate myelination in both the PNS and CNS. Extracellular ATP has been identified as an important, activity-dependent axonal signal that, when non-synaptically released from electrically stimulated axons, activates purinergic P2 receptors on neighboring SCs and OLs [37].
Non-vesicular ATP release has been indicated to occur from premyelinated axons of dorsal root ganglion (DRG) neurons upon electrical stimulation and to cause calcium elevation in perinatal SCs, which is blocked by P2Y receptor antagonists or by the application of apyrase, one of the enzymes that breaks down ATP to ADP, AMP, and adenosine [38]. In accordance to this, exogenous application of ATP induces an increase of intracellular calcium concentrations by the activation of purinergic receptors on cells acutely isolated from both adult and neonatal sciatic nerves [39, 40]. Of note, some P2 receptor subtypes (e.g., P2X7, P2Y1, and P2Y2) have been localized so far on myelinating and non-myelinating SC membranes [38, 41–43]. In particular, P2Y1 receptor stimulation upon activity-dependent release of ATP from DRG neurons or by the exogenous selective agonist 2-methylthio ADP [44] has been described to inhibit both SC proliferation and differentiation, as indicated by the analysis of the O4 immature SC/OL marker in co-culture. It has been postulated that this inhibitory mechanism is meant at preventing premature SC differentiation, increasing the pool of pre-differentiated SCs ready to respond to appropriate myelination signals [37]. However, it has been reported by other studies [45], that, in adult nerves, SCs fail to respond to action potential firing, even if functional P2 receptors have been detected ([42]; see also above). Although technical limitations cannot be ruled out, these data suggest that activity-dependent ATP release (whose mechanisms are still unknown) might be restricted to specific early phases of development, when these responses are elicited in SCs by premyelinated axons [37]. It has been reported that SCs are also able to respond with intracellular calcium transients to ATP released from ear vestibular neurons, acting through purinergic receptors. In turn, purinergic activation of SCs induces release of neurotrophin brain-derived neurotrophic factor (BDNF, mainly acting on P2X7 receptors), which is required by vestibular neurons to support their survival and growth [46]. These data represent a clear example of a functional cross-talk between neurons and SCs.
Similarly to ATP, adenosine, a product of ATP metabolism by extracellular ecto-nucleotidases [47] inhibits SC proliferation induced by platelet-derived growth factor (PDGF), by activating ERK/MAPK through stimulation of cAMP-linked A2A receptors [43, 44]. However, in contrast to ATP, adenosine fails to inhibit the differentiation of SCs to the O4+ stage, suggesting that, besides ATP, adenosine is an activity-dependent signalling molecule between axons and premyelinating SCs, acting through distinct intracellular signalling pathways in response to neural impulse activity [44].
Also in the CNS, neuronal activity influences myelination by activating purinergic signalling. During development, OLs are generated by cells of the embryonic ventricular zone in the brain and spinal cord, giving rise to oligodendrocyte precursor cells (OPCs) that proliferate and migrate throughout the CNS [48]. These OPCs can then terminally differentiate into mature OLs during a step-by-step process characterized by the downregulation of precocious markers (such as the membrane chondroitin sulfate proteoglycan, NG2, and the PDGF receptor-alpha (PDGFRα)) and the acquisition of immature OL antigens such as O4, followed by myelin specific structural proteins (i.e., myelin basic protein (MBP)). Furthermore, early undifferentiated bipolar OPCs acquire a more complex morphology with many branched processes, culminating in the formation of membrane sheaths that wrap around axons [49]. Also in CNS, myelination is regulated by extracellular signals and intracellular factors acting on both the various steps of OL maturation and axonal activity [33].
In detail, adenosine has been reported to inhibit OPC proliferation in the presence of the mitogenic agent PDGF and to promote cell differentiation towards premyelinating oligodendrocytes, thus globally increasing myelination, as shown by the rise of MBP+ oligodendrocytes in DRG/OPC-treated co-cultures. Of note, the percentage of myelinating MBP+ oligodendrocytes was lower in co-cultures treated with the adenosine receptor antagonists alone, suggesting that endogenous sources of adenosine are sufficient to promote differentiation effects [50]. In addition, activation of A1 receptors has been reported to induce OPC migration ([51]). In line with these functional data, all four P1 adenosine receptors have been detected by RT-PCR in cultured OPCs [50]. Functional purinergic P2 receptors (i.e., P2Y1, P2Y2, P2Y6, P2Y12, P2Y13) have been also identified in OPCs, by assessing intracellular calcium transients elicited by some relatively selective purinergic agonists [50, 52–54]. In line with these data, ATP and ADP are able to inhibit OPC proliferation in vitro, both in purified cultures and in cerebellar tissue slices. ATP and ADP, but not UTP, have been also shown more effective than PDGF in stimulating OPC migration and in promoting oligodendrocyte differentiation acting mainly on P2Y1 receptor [53]. More recently, purinergic signalling has been found to stimulate myelination at later stages of OL development, through an indirect mechanism involving astrocyte release of the promyelinating cytokine, leukemia inhibitory factor (LIF). It has been shown that ATP, 2-methylthio ATP, and α,β−methylene ATP, but not UTP, increase myelination when DRG, co-cultured with immunopurified O1+ stage oligodendrocytes, are supplemented with astrocytes [55]. In these culture conditions, upon ATP treatment, astrocytes are induced to produce LIF, which, as previously reported [56], stimulates myelination.
The opposite effects of ATP in OPC and SC differentiation have been related with differences in the timing of myelination of the CNS and PNS axonal branches of DRG neurons with respect to the onset of electrical activity during development. Myelination of the peripheral axon branch being delayed with respect to central one, the blockade of SC differentiation by activity-dependent release of ATP may serve to prevent premature SC differentiation at times when axonal ATP has not yet induced the differentiation of OPCs [50].
Specific P2 receptor subtypes as promising targets for myelin repair in the CNS
A contribution to oligodendrocyte function has been recently postulated for the P2Y12 receptor subtype, being this receptor found to be expressed in mature OLs throughout the corticospinal tract [57]. Interestingly, the analysis of postmortem samples of the cerebral cortex from healthy human subjects and MS patients has shown that P2Y12 protein expression is reduced in proximity to the lesions. In particular, its reduction was found to be inversely proportional to the extent of demyelination found in gray matter cortical plaques and subcortical white matter, suggesting that this reduction may represent an additional marker of the development of the lesions in the disease [58].
A number of recent studies have demonstrated the existence of pyrimidinergic signalling pathways mediated by uracil nucleotides (i.e., UTP and UDP) and their sugar conjugates (i.e., UDP-glucose and UDP-galactose), which have been also reported to modulate OPC proliferation and differentiation [21]. In this respect, UDP, UDP–glucose, and UDP–galactose have been shown to act as endogenous agonists at the P2Y-like receptor GPR17 [59, 60], recently identified as an important regulator of OL maturation [54, 61–63]. Interestingly, GPR17 is also activated by cysteinyl–leukotrienes (cysLTs; a family of pro-inflammatory arachidonic acid-derived molecules that also markedly increase at CNS injury sites, see also below), representing one of the first example of G protein-coupled receptor showing a dual pharmacology [59, 64]. We were indeed the first to show that, in the adult rodent brain, GPR17 is expressed by a subpopulation of NG2+ OPCs (see also below), whereas the receptor was not found in mature OLs expressing the classical mature myelin markers such as MBP and the proteolipid protein PLP [54, 61, 63]. Similarly, in vitro, GPR17 has been found to decorate two distinct subsets of slowly proliferating NG2+ OPCs: (1) morphologically immature cells expressing other early proteins like Olig2, A2B5, PDGFRα, and the immature DM-20 isoform of the PLP1 myelin protein, and (2) ramified pre-OLs, already loosing NG2 and PDGFRα immunoreactivity and acquiring expression of more mature factors like O4 and O1 (for details on the more general role of NG2 cells in the CNS, see also the following section). After the O4/O1 differentiation stage, GPR17 is progressively turned down and never found in fully mature MBP+ oligodendrocytes [54], indicating that GPR17 expression is restricted to very early differentiation stages and completely segregated from that of mature myelin. We also reported that GPR17 activation by its endogenous ligands (i.e., UDP–glucose and LTD4), results in a marked inhibition of adenylyl cyclase and cAMP production [54], an effect counteracted by GPR17 antagonists (e.g., AR-C69931MX, also named cangrelor, and montelukast, [59]) and by receptor silencing with siRNAs [54]. By means of pharmacological and biotechnological approaches, we also showed that uracil nucleotides promote OL maturation. Conversely, the GPR17 antagonist cangrelor [59] significantly reduced the number of mature MBP+ or CNPase+ cells, indicating a shift of cells toward a less differentiated stage, as also confirmed by the prevalence, in culture, of a morphologically undifferentiated phenotype. The detected delay in OPC maturation in response to cangrelor could be ascribed to blockade of the binding of endogenous ligands (i.e., UDP–glucose, UDP) to GPR17, suggesting that, even in culture, GPR17 is extrinsically regulated by its physiological ligands that accumulate in the extracellular milieu [54]. Interestingly, suppression of GPR17 expression at early differentiation stages by siRNA significantly reduced the number of terminally differentiated MBP+ cells, suggesting that GPR17 is initially necessary to commit progenitors towards mature OLs. However, the absence of GPR17 expression in mature MBP+ cells suggest that, at later differentiation stages, the receptor has to be turned down to allow cell maturation. In this respect, starting at postnatal week 2, transgenic mice overexpressing GPR17 under the control of the promoter of CNPase, a relatively late marker of OPC differentiation, displayed generalized tremors, hind limb paralysis, with a parallel reduction of MBP and PLP1 expressing cells in brain white matter starting at postnatal day 14, followed by precocious death at 3 weeks of age. These data confirm that the forced GPR17 over-expression at late differentiation stages, when OPCs are already irreversibly committed to become mature OLs, inhibits cells’ terminal maturation [62]. Interestingly, recent data have shown that the presence of both bFGF and EGF is required to maintain a low expression of GPR17, suggesting that the two growth factors act in synergy in keeping OPCs at a less differentiated, proliferating state and that they do so by restraining GPR17 expression. GPR17 protein expression was instead abundantly induced in a subpopulation of O4+ cells when cultures were grown in the absence of growth factors and in the presence of a differentiating medium [65]. Globally, these data suggest that GPR17 is specifically involved in the differentiation rather than in the self-maintenance of the pool of undifferentiated proliferating OPCs. In line with this hypothesis, in vivo maximal GPR17 expression was found in post-mitotic NG2+ cells undergoing morphological differentiation [66]. Of note, O4+ cells were also shown to undergo massive cell death in the presence of 100 μM ATP only when coexpressing GPR17, whereas cells that did not express the receptor were partially spared [63]. This suggests that different levels of GPR17 might dictate susceptibility to ATP-induced cell death, an event that may prove important for both regulating cell number during development and for determining myelin damage under traumatic/hypoxic conditions (see also below). A cytotoxic effect exerted on mature OLs by high ATP concentrations has been also previously demonstrated by a mechanism involving activation of the P2X7 receptor subtype. MBP-expressing cells from the rat optic nerve exposed to 1 mM ATP undergo massive cell death, and an increased expression of P2X7 in myelinating OLs was also observed preceding the onset of experimental autoimmune encephalomyelitis (EAE), suggesting that this receptor subtype might represent a risk factor for the development of MS. Accordingly, the P2X7 receptor antagonist Brilliant Blue G has been shown to exhibit beneficial effects in the same animal model of MS [67]. The P2X7 receptor has been also demonstrated to mediate ischemic damage to OLs in vitro. Indeed, OLs release high concentrations of ATP during ischemia, which activate P2X7 receptors, inducing massive myelin destruction, in addition to OLs death [68]. It may therefore well be that both GPR17 and P2X7 cooperate in modulating OL survival and death depending upon the extracellular ATP concentrations, with GPR17 playing a predominant role at lower ATP levels and the P2X7 subtype being recruited only when higher extracellular nucleotide concentrations are reached [63]. In conclusion, it is anticipated that the full elucidation of the complex interplay between specific P2 receptors in OL proliferation, differentiation, and death may unveil novel targets to combat demyelination and promote myelin repair.
Purinergic signalling in adult NG2+ cells
As discussed above, the demonstration that NG2+ OPCs are still present in the adult CNS has generated a lot of interest on these cells as a reservoir of OLs to repair demyelinated lesions [69, 70]. However, recent reports on the lineage and the electrophysiological properties of these cells have questioned the notion that all NG2 expressing cells (also called polydendrocytes) simply act as progenitors committed to the OL lineage. It has been indeed reported that, under specific conditions, NG2 cells can give rise to neurons and astrocytes [71]. This indicates that polydendrocytes exhibit additional properties apart from their assumed functions as OPCs, although this concept is currently a highly debated topic. NG2 cells have been also demonstrated to make multiple contacts with the axonal membrane at nodes of Ranvier [72] and with synaptic terminals [73], suggesting a role in surveillance of neuronal activity and raising the question of whether adult NG2 cells may be capable of responding to or influencing neuronal activity [74–76]. In this respect, several data indicate that, similarly to mature OLs, these cells respond to neuronal activity and to neurotransmitters (for review, see [76]. In particular, by confocal calcium imaging on optic nerves from a transgenic mouse line in which NG2 glia are identified by the expression of the red fluorescent protein DsRed, it has been recently shown that ATP, released during axonal action potential propagation and mechanical stimulation of astrocytes, evokes calcium signal in these cells, mainly through the activation of P2Y1 and P2X7 receptors [77]. These results strongly favor the view of a close interdependence among polydendrocytes, astrocytes, and neurons in synaptic transmission, supporting the existence of the “tetrapartite synapse” proposed by Butt and coworkers in 2005 [76]. As well as responding to ATP, polydendrocytes have also been shown to respond to GABAergic inputs, glutamate and probably to other neurotransmitters and neuromodulators, since they also express receptors for glycine, acetylcholine, monoamines, and substance P [78].
Highly relevant, NG2+ cells also react to many pathological conditions by active proliferation, hypertrophy, NG2 upregulation, and contribution to glial scar formation, suggesting that they actively participate in the neuroinflammatory events of the injured nervous system [79]. Of note, GPR17 receptor has been found to be expressed by adult NG2+ OPCs that take part in the remodelling and repair after ischemia. Starting from 48 to 72 h after ischemia induction, GPR17-expressing OPCs start to proliferate in the peri-lesion area and to differentiate into more mature oligodendrocytes, indicating an attempt of remyelination [61]. A similar time-dependent induction of GPR17 in OPCs was found in a mechanical spinal cord injury model in mice [65]. In line with our data, Chen and coworkers [62] found the receptor to be highly expressed during development, to undergo downregulation at adulthood and to be upregulated upon induction of EAE in mice, a model of MS. In line with these findings, as already stated above, the forced expression of GPR17 at advanced OPC differentiation stages impairs physiological myelination [54] and increases susceptibility to death [63]. These initial data in MS models suggest that GPR17 may be pathologically upregulated in demyelinating conditions, suggesting the hypothesis that excessive, chronic, non-physiological activation of GPR17 may prevent OPCs from repairing lesions. In this respect, our recent in vitro data showing that GPR17 pharmacological or biotechnological modulation has important effects on the final destiny of OPCs suggests a key role for endogenous GPR17 ligands in the normal differentiating program of these cells and in its dysregulation under pathological conditions [54]. Of note, both cysLTs and nucleotides accumulate at the site of injury in the ischemic damaged brain [80, 81]. These data point to uracil nucleotides and cysLTs as main extrinsic local regulators of these cells under physiological conditions and during myelin repair [54].
Microglia
Involvement of purinergic signalling in microglia-mediated immunosurveillance of the CNS
Microglia are the immunocompetent cells of the CNS. They have crucial functions in surveillance and homeostasis of CNS, reacting to injury, trauma, or toxins, removing cellular debris and actively participating to neural tissue remodeling after injury. In normal conditions, they are widespread in the whole CNS in a resting state, as a network of small cells with a ramified morphology. Under pathological conditions, they can rapidly acquire an activated phenotype, assuming a different morphological (amoeboid) biochemical and immunological (phagocytic) state. Activated microglia upregulate their surface expression of immunomodulatory proteins and become efficient in producing a variety of cytokines, chemokines, reactive oxygen species, and proteases [82, 83]. While the surveillance properties of microglia are essential for the maintenance of CNS integrity, excessive or uncontrolled microglial activation has severe and deleterious consequences [19]. Microglia differentially regulate their “trophic” and “toxic” effectors depending from the neurotransmitter signal released from injured neurons, which is dependent on the degree of injury [84].
Microglial activation is heavily implicated in the pathogenesis of virtually all CNS diseases, including brain and spinal cord injury, stroke, and degenerative diseases [85]. ATP released in large amounts at the sites of CNS injury contributes to microglial activation through both ionotropic P2X and metabotropic P2Y receptors ([81, 86, 87]). Expression of several P1 and P2 receptors and the ability of purinergic molecules to profoundly affect multiple microglia responses (such as proliferation, process motility, migration, phagocytosis, cytokine, and chemokine release) make this cell type a useful model for studying the role of purines in inflammation [88].
Purinergic regulation of membrane ruffling and chemotaxis
The trophic effects of extracellular nucleotides on microglia were demonstrated several years ago by Honda and colleagues. They demonstrated that ATP induced rapid microglial membrane ruffling after 5 min through P2Y receptors [89]. A significant morphological change was also observed after exposure to ADP and UTP. However, only ATP and ADP induced chemotaxis of microglia, whereas UTP was ineffective. Moreover, membrane ruffling and chemotaxis were completely abolished by pre-incubation with cangrelor, a P2Y12-13 antagonist [89]. Considering that, in microglia, the transcript for P2Y13 has been detected only at low levels, and the encoded receptor protein has not been observed at all [90], it’s likely that P2Y12 signalling is one of the most relevant pathways activated in these cells in response to injury. Purified microglia from wild-type mice demonstrated a strong polarization and chemotaxis toward a nucleotide source (i.e., ATP or ADP). Instead, microglia from P2Y12-deficient mice showed no evidence of membrane ruffling, polarization, or directed movement [90]. The same phenotype has been observed in a more physiological environment, in which nucleotides have been diffused in hippocampal slices from postnatal mice. The same authors analyzed P2Y12-mediated chemotaxis in intact living brain of adult mice, placing a microelectrode containing ATP into the neocortex of Cx3cr1+/GFP mice. In these animals, microglial cells are fluorescent due to specific expression of the green fluorescence protein (GFP), allowing following changes of microglial morphology and function using a two-photon time-lapse microscopy. As expected, GFP+ microglia showed a very active extension of cellular processes toward the nucleotide source. In contrast, GFP+ microglia from P2Y12-deficient mice showed greatly reduced responses [90]. These data demonstrate that P2Y12 is essential for microglial chemotaxis to a site of injury. Interestingly, P2Y12 is expressed in resting ramified microglia, whereas it almost disappears during activation, suggesting that prolonged exposure to ATP and ADP desensitizes this receptor. This indicates that P2Y12 may have a role in the early phases of microglial response to injury, and it has to be subsequently downregulated [90]. Real-time confocal microscopy also demonstrated that release of ATP, ADP, and UTP participates to the rapid microglia response to laser-induced or mechanical brain lesions, when microglial processes respond to and move towards the site of injury. Application of the P2Y receptor inhibitors reactive blue 2 or PPADS markedly reduced the number and motility of microglial processes towards the ablations [87]. These data confirm that, upon nucleotide release from these or other surrounding cells, activation of a P2Y receptor on microglia is necessary for this rapid microglial response to injury. ATP itself could be the chemoattractant responsible for the directional extension of microglial processes. Alternatively, ATP could trigger the release of yet unidentified chemoattractant(s) from the surrounding tissue. In fact, in the presence of apyrase, the application of ATP from a point source is not sufficient per se to attract microglial processes [87].
Although all neural cells are likely to release large amounts of ATP upon injury and contribute to microglial activation, it has been demonstrated that astrocytes are the main players in inducing widespread microglial activation. Application of connexin channel blockers before laser ablation abolished the movement of microglial processes towards an electrode releasing ATP. Since connexins are highly expressed in astrocytes but not microglia in a resting state [91], both the rapid baseline dynamics and the injury-induced response of microglial processes could be mediated by extracellular ATP released from astrocytes [87].
The effect of ATP on cell shape change is transient, since, when kept in culture, microglia return to a baseline ramified morphology after 90 min. Continued exposure of the cells to ATP does not cause further shifts, probably due to desensitization [92]. Therefore, it has been suggested that ATP is only an initiator, whereas additional factors are needed during an inflammatory process in vivo for sustained amoeboid morphology. TNFα has been previously shown to be released from cultured microglia in response to high concentrations of ATP [83]. However, there is no TNFα production when microglia are exposed to low ATP concentrations [92]. Other evidence demonstrated that ATP is not sufficient to trigger cytokine release in vitro in the absence of an LPS priming [93, 94]. It is therefore possible that membrane ruffling and cytokine release belong to two distinct signalling pathways, and ATP is only responsible for the microglia morphological changes [92].
Purinergic signalling and microglial phagocytosis
Extracellular nucleotides do not only play a key role in attracting microglial cells to the site of injury but can also promote microglial phagocytosis through the activation of the P2Y6 receptor subtype. UDP-treated rat microglial cells rapidly changed their morphology through actin reorganization, leading to a strong phagocytosis of zymosan particles in the culture medium. UDP-induced phagocytosis was significantly reduced by MRS2578, a selective antagonist at the P2Y6 receptor subtype and was nearly abolished by the treatment with P2Y6 antisense oligonucleotides [95].
The expression and function of microglial P2Y6 receptors in vivo was demonstrated by intraperitoneal administration of kainic acid (KA) to rats. Neuronal injury caused after KA injection induced a strong increase in UTP concentrations with respect to untreated animals. UTP was subsequently degraded to UDP, the endogenous ligand of P2Y6 receptors, which are also upregulated after injury [95]. The definitive evidence of the involvement of the P2Y6 receptor subtype in the modulation of microglial phagocytosis came from the injection of fluorescent microspheres in the hippocampal CA3 regions after KA administration. The selective knocking down of the P2Y6 receptor by specific antisense oligonucleotides significantly inhibited the number of phagocyted particles, clearly demonstrating that P2Y6-mediated signals are important for microglial phagocytosis even in vivo [95].
Purinergic regulation of microglial process retraction
Local application of ATP onto LPS-activated microglia caused a chemorepulsive migration away from ATP and a rapid process retraction. Whereas process extension has been hypothesized to play an important role in the active surveillance of brain parenchyma, process retraction is evident in activated microglia and considered a hallmark of inflammation in the brain [96]. Considering that the Gi-coupled P2Y12 receptor mediates processes extension toward ATP/ADP in resting microglia, it is likely that a Gs-coupled signalling might mediate process retraction in response to ATP in activated microglia. The application of forskolin caused process retraction in both LPS-treated and untreated microglia, thus confirming this hypothesis [96]. The same authors proposed that this effect was a consequence of the rapid ATP breakdown to adenosine and that this process was mediated by the adenosine A2A receptor. In fact, during microglial activation, P2Y12 is downregulated [90], whereas the A2A receptor is rapidly upregulated. However, the effects of adenosine in cytokine secretion, phagocytosis, and other microglial functions are still poorly understood and need to be further investigated.
The peculiar role of P2X7 on microglia: beyond cell death
The P2X7 receptor plays a pivotal role in the hierarchy of neuroinflammation [97]. P2X7 is a purinergic ATP-gated channel mainly expressed in cells with immune function, including microglia [98]. Its contribution to proinflammatory events such as release of IL-1β and TNFα has been widely described [82, 83]. P2X7 also regulates the release of chemokines. ATP induces mRNA expression and release of CXCL2, a potent chemoattractant for neutrophils with an important role in various CNS pathologies [99]. In BV-2 microglial cells, this induction is dependent on the activation of a NFAT and a MAPK pathway downstream of P2X7 [100]. It is reported that both ERK and JNK are involved in the regulation of ATP-induced expression of TNF-alpha in microglia [83]. Interestingly, enhanced P2X7 expression has been reported in pathological conditions such as MS, amyotrophic lateral sclerosis, Huntington’s disease, Alzheimer’s disease, and brain ischemia [101, 102]. It is likely that ATP released from neurons, astrocytes, or microglia themselves (Fig. 1) relays information to resting microglia, which sense this danger signal through P2X7 receptors. According to this view, this receptor could be one of the first players in neuroinflammation, even before P2Y12.
It has long been known that the transient stimulation of P2X7 with ATP or with the synthetic agonist BzATP opens a channel permeable to small cations, whereas prolonged exposure leads to the formation of a large membrane pore permeable to hydrophilic molecules up to 900 Da. This high stimulation leads to excessive Ca2+ influx, depletion of intracellular ions and metabolites, and eventually cell death [82]. However, P2X7 is not to be considered a receptor merely associated to cytolysis. Back in 2002, ATP was indeed demonstrated to induce proliferation in lymphoid cells through the activation of P2X7 [103]. A few years later, a role for P2X7 in the proliferation of microglia was established for the first time [104]. In detail, a strong decrease in cell growth was observed after exposing either primary hippocampal microglia or N9 microglial cells to the P2X7 receptor antagonists oxidized ATP and Brilliant Blue G. In N9 cells, LPS exposure inhibited both P2X7 expression and cell growth, mainly due to a decrease in intracellular calcium concentrations [104]. The same result was obtained by treatment with the ATP-hydrolyzing enzyme apyrase or by transfection of a siRNA specifically designed to knock-down P2X7 [104]. Finally, flow cytometric analysis indicated that both oxidized ATP and LPS treatment reversibly decreased cell cycle progression, without increasing the percentage of apoptotic cells. Overall, these data showed that P2X7 plays an important role in controlling microglial proliferation by supporting cell cycle progression.
More recently, the role of the P2X7 pore in microglial proliferation was confirmed by showing that overexpression of this receptor in primary microglial cultures is sufficient to drive both microglia activation and proliferation and that these effects were significantly inhibited by pre-incubation with the specific P2X7 antagonist oxATP [105]. Following transfection of a mutant P2X7 receptor (P2X7RG345Y), with normal channel function but in which the capacity to form the pore was ablated, the observed microglial activation was impaired and proliferation was reduced [105]. The mechanism by which ATP supports microglial proliferation is not fully understood, but it is likely that a tonic stimulation increases basal calcium concentrations and generates an intracellular stimulating signal. By contrast, sustained activation can lead to either cell death, or trophic effects mediated by pore formation.
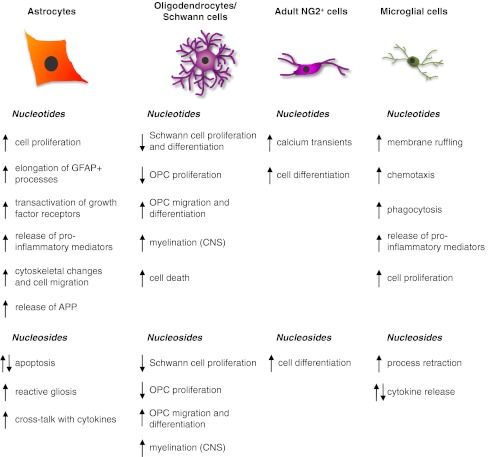
The various effects mediated by extracellular nucleotides and nucleosides on CNS glial cells and Schwann cells described so far are summarized in Fig. 2.
Fig. 2.
Schematic summary of the main effects exerted by extracellular nucleotides and nucleosides on the four main types of central nervous system glial cells and on Schwann cells. See text for details and for the identification (when known) of the receptor subtypes involved in the different actions
Retinal Müller cells
Retinal Müller glial cells release ATP and express various purinergic receptors
Müller cells are the predominant glial cell responsible for preserving the health and activity of retinal neurons. These cells crucially regulate the volume of the extracellular space, ion and water homeostasis, neurovascular coupling, and the release of neuro- and glio-transmitters inside the retina [106]. Reactive Müller cells are neuroprotective but may also contribute to neuronal degeneration under dysregulated pathological conditions ([107]; see also below).
In the retina, purines (mainly ATP) are tonically released from neurons in a calcium-dependent manner and from glial and pigment epithelial cells by both calcium-independent and calcium-dependent mechanisms [108]. Adenosine is formed in the extracellular space from released ATP as a result of the action of ecto-nucleotidases but may also be released by Müller cells via nucleoside transporters [108]. Purine release is also regulated by glutamate (see also below). All P1 receptors together with various P2X and P2Y receptors are expressed on Müller cells.
From a functional point of view, the A1 receptor represents the best characterized P1 receptor in these cells, being its activation by endogenously released adenosine implicated in the purinergic signalling cascade which inhibits the swelling of the cells under hypo-osmotic conditions (see also next section).
Among P2X receptors, human Müller cells (but, importantly, not Müller cells from other mammalian species) express functional ionotropic P2X7 receptors [109] linked to membrane depolarization, calcium influx, sustained activation of calcium-activated, big-conductance potassium (BK) channels, and calcium release from intracellular stores. The P2X7-induced depolarization of the cells is balanced by the hyperpolarization mediated by BK channels. In contrast to the P2X7 of immune cells such as retinal microglia [110], prolonged activation of P2X7 in Müller cells does not induce the formation of large plasma membrane pores [109]. Müller cells also express various P2Y receptors, with P2Y1 representing the most common functional subtype. However, marked species differences in the expression of P2Y receptors and in their coupling to calcium mobilization have been reported (excellently reviewed in [108]). These differences have to be taken into account when translating data from animals to man.
Purinergic regulation of Müller cell volume: a fundamental mechanism for maintaining an appropriate neuronal function
Neuronal activity is associated with rapid ion shifts causing changes of water fluxes, swelling of synapses, and decreases in the volume of the extracellular space. To avoid excessive detrimental decreases in extracellular space volume that would cause neuronal hyperexcitation, Müller cells inhibit their swelling and take up neuron-derived potassium and other osmolytes, which both favor water influx. This important action involves both glutamatergic and purinergic signalling (for a detailed description, the reader is referred to [108]). Briefly, glutamate derived from both Müller cells and neurons and acting at its metabotropic receptors, evokes release of ATP from Müller cells. Ecto-ATPases catabolize ATP to ADP, which activates P2Y1, which, in turn, triggers the release of adenosine from Müller cells [111–113]. Adenosine then activates A1 receptors, leading to the opening of potassium and chloride channels in Müller cell membranes and ion efflux compensating the osmotic gradient across the plasma membrane and thus preventing hypo-osmotic swelling. In the retinal parenchyma of diabetic rats, NTPDase1 (which hydrolyzes ATP and ADP about equally well) is upregulated and therefore extracellularly formed adenosine contributes to the swelling-inhibitory effect of glutamate and ATP (Fig. 3).
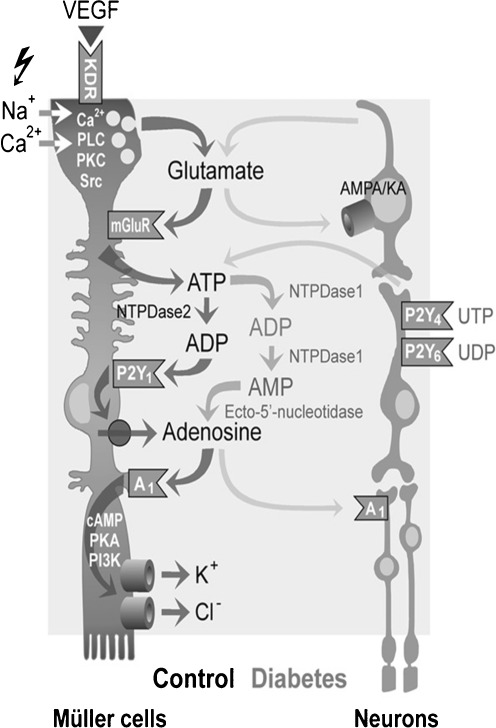
Fig. 3.
Modulation of retinal Müller glial cell functions and of their cross-talk with neurons by extracellular nucleotides and adenosine under control conditions and following diabetic retinopathy. Vascular endothelial growth factor (VEGF) evokes the exocytotic release of glutamate, which in turn activates metabotropic glutamate receptors (mGluRs) leading to the calcium-independent release of ATP from Müller cells. ATP is extracellularly converted by the nucleoside triphosphate diphosphohydrolase-2 to ADP that activates P2Y1, resulting in nucleoside transporter-mediated release of adenosine. Activation of A1 adenosine receptors causes the opening of potassium and chloride channels; the ion efflux equalizes the osmotic gradient across the plasma membrane and thus prevents water influx and cellular swelling under hypo-osmotic stress conditions. In swollen cells, the ion efflux is associated with a water efflux, resulting in decreased cell volume. Neuron-derived glutamate and ATP modulate the volume-regulatory signaling cascade depending upon neuronal activity. In the murine retina, activation of P2Y4,6 by UTP and UDP, respectively, might result in a release of neuronal ATP that activates glial P2Y1. Muller cell-derived glutamate and adenosine may also activate neuronal AMPA/KA and A1 receptors, resulting in stimulation and inhibition, respectively, of neuronal activity. In the retinal parenchyma of diabetic rats, increased extracellular adenosine concentrations further contributes to the swelling-inhibitory effect of glutamate and ATP. Reprinted and modified from Wurm et al. [108] with permission from Elsevier
Müller cells can also release ATP upon mechanical stimulation. This may be important even under physiological conditions, since membrane stretch induced by osmotic perturbations during intense neuronal activation may trigger release of ATP from Müller cells, which, via autocrine activation of P2Y1, prevents cells’ osmotic swelling. Thus, two different mechanisms trigger ATP release from Müller cells: glutamate receptor-dependent release and/or receptor-independent release induced by osmotic membrane stretching.
The discovery of a novel, long-range cell-to-cell signalling system: ATP-dependent glial calcium waves
In some seminal studies at the end of the 1990s, while studying the potential roles of P2 receptors in Müller glial cells, it was noted that the calcium responses mediated by P2Y receptors were restricted to the inner half of the Müller cells [111, 114, 115], as was the distribution of both spontaneous calcium transients [115] and of the intercellular calcium waves induced in astrocytes and Müller cells by various stimuli (including neurotransmitters such as glutamate, or electrical and mechanical stimulation). Under constant illumination conditions, these waves occur at a frequency of 4.6 per cell per 1,000 s, display a duration of 2.5–6 s and propagate at 20–30 μm/s up to 180 μm from the site of initiation [116]. It was also soon appreciated that calcium waves propagation depended upon the release and extracellular diffusion of ATP, with consequent activation of P2Y receptors and release of calcium from internal stores [116]. The discovery of calcium waves in the retina represented the first demonstration of a new form of cell-to-cell communication that was later demonstrated to also occur in the brain and to allow long-distance homotypic astrocyte–astrocyte communication, as well as heterotypic signalling with other cells such as neurons, oligodendrocytes, and microglia (see above; for review, see [6]). In the retina, glial calcium waves have been suggested to mediate an extraneuronal long-range signalling system influencing neuronal activity, neurovascular coupling, and regulation of Müller cell volume. Under pathological conditions, e.g., after local retinal detachment, glial calcium waves may cause gliosis and retinal degeneration to spread toward larger retinal areas, via the release of ATP and growth factors.
Purinergic signalling in retinal Müller cells under pathological conditions: induction of proliferation and reactive gliosis
In a similar way to astrocytes in other parts of the CNS, during acute or chronic insults, Müller cells undergo gliotic changes (i.e., cellular hypertrophy, increased proliferation, and migration). Gliosis may start as an attempt to repair damage, but its chronicization turns it into a detrimental reaction further enhancing retinal damage. In proliferative retinopathies, massive long-term proliferation results in formation of epiretinal membranes that can protect the retina from pathogenic factors present in the vitreous. However, contraction of the membranes also results in retinal detachment and disease aggravation [107]. Thus, inhibition of glial proliferation is a major clinical aim when attempting to prevent blindness. Commonly, proliferative retinopathy is treated by vitreoretinal surgery, since there are no obvious pharmacological means to control retinal cell proliferation. The purinergic system may represent a new target to achieve inhibition of excessive Müller cells proliferation.
Abnormal increases of P2Y receptor responsiveness with markedly enhanced calcium signalling have been reported in proliferative retinopathies, in transient retinal ischemia and in retinal detachment (summarized in [108]). After partial vitreous detachment from the retina, vitreous fibers adhering to Müller cell endfeet exert tractional forces, which may induce ATP release, resulting in autocrine/paracrine cell activation and proliferation. Stretch-induced autocrine ATP signalling in Müller cells may also stimulate epiretinal membranes contraction [117]. Thus, inhibition of purinergic signalling of Müller cells might prevent early and late stages of epiretinal membrane formation [107]. Müller cells from patients with proliferative vitreo-retinopathy also exhibit enhanced P2X7 receptor function compared with healthy donors [118]. Both these receptor changes may play a role in Müller cells gliosis. Partially in line with this hypothesis, suramin reduced the hypertrophy of Müller cells but not the upregulation of ATP-induced calcium responses [119]. To support a specific role for P2X7 in proliferation, in Müller cells from patients with non-proliferative gliosis, no increases in P2X7 currents were observed, although other signs of gliosis (e.g., cellular hypertrophy) could be observed [118]. A bidirectional cross-talk between P2Y and growth factor receptor tyrosine kinases is involved in the proliferation-stimulatory effect of ATP, as shown by the demonstration that P2Y-induced proliferation of Müller cells depends upon the transactivation of receptor tyrosine kinases [108].
Globally, these findings suggest that P2 receptors represent new targets for innovative pharmacological interventions to reduce excessive proliferative gliosis in several retinal diseases, with a great potential in the prevention of the associated blindness. In this respect, one putative strategy is the blockade of purinergic receptors.
Müller glial cells as potential stem cells for retinal repair
Replacement of dying retinal neurons by endogenous sources is under investigation [120]. Proliferative cells displaying some typical hallmarks of multipotent neural stem cells can be isolated from the ciliary body and Müller glia of the mature rodent and human retina. While the neurogenic capability of ciliary cells has been questioned, Müller glial cells can be induced in vitro to express markers of various mature retinal neurons. Moreover, spontaneously immortalized cell lines with Müller glia characteristics have been isolated from the neural retina of several adult eye donors [121]. When grown in vitro as adherent monolayers, these cells responded to EGF and could be expanded indefinitely without growth factors. In the presence of FGF-2 or retinoic acid, they acquired neural morphology, formed neurospheres, and expressed neural stem cell markers including βIIItubulin, Sox2, Pax6, Chx10, and Notch1. They also expressed markers of postmitotic retinal neurons, including peripherin, recoverin, calretinin, S-opsin, and Brn3 [121]. When grafted into the subretinal space of dystrophic RCS rats susceptible to retinal degeneration, immortalized cells migrated into the retina and started expressing various markers of retinal neurons. Thus, adult human neural retina harbors a population of cells expressing both Müller glial and stem cell markers. Furthermore, it has been reported that Notch and Wnt signalling can stimulate in vivo Müller cell proliferation and regeneration of photoreceptors [122]. While no definitive evidence for the functional integration of newly generated cells has been provided, these initial data suggest that these cells may have a potential use for cell-based therapies to restore retinal function.
Effects of purinergic signalling on other glial cells
Being a universal signalling system, purinergic transmission is expected to contribute to the physiopathological behavior of glial cells other than astrocytes, oligodendrocytes, microglia, and retinal Müller cells. Here, we briefly summarize what is currently known on purinergic modulation of SGCs in sensory ganglia, ependymal cells lining the spinal cord central canal, pericytes, and enteric glial cells.
Purinergic modulation of satellite glial cell (SGC) functions
In sensory and autonomic ganglia, neuronal somata are surrounded and wrapped by SGCs, which modulate neuronal functions, and contribute to the development of pathological states like chronic pain and migraine [123, 124]. ATP and extracellular nucleotides contribute to neuron-to-SGC and SGC-to-SGC communication within dorsal root and trigeminal ganglia [125] and SGCs respond to nucleotide application with increases in the intracellular calcium concentrations. Virtually all P2Y receptor subtypes have been found expressed in DRG and trigeminal SGCs, although some subtypes are not functional under basal conditions [126], whereas conflicting results have been published on the expression of P2X receptors (for review, see [125]). Unlikely neurons in sensory ganglia, SGCs selectively express the P2X7 receptor subtype, which seems to play a still ambiguous role in the modulation of pain transmission (for review, see [124]).
Interestingly, inflammatory or chronic pain states can dramatically modulate P2 receptor expression in SGCs. In fact, the induction of temporomandibular joint inflammation increased trigeminal SGCs sensitivity to ATP by causing a shift from P2Y- to P2X-receptor-mediated responses [127]. Moreover, pro-inflammatory and pro-algogenic mediators (such as the calcitonin gene-related peptide) released from trigeminal neurons potentiated P2Y receptor-mediated functions in surrounding SGCs, which in turn released various cytokines and growth factors [128]. This complex network is overactivated in a genetic mouse model of familial hemiplegic migraine, suggesting that the purinergic system might contribute to the development of migraine-associated pain and that targeting P2 receptors on trigeminal SGCs might represent an innovative analgesic strategy [128].
Purinergic modulation of spinal cord ependymal cell functions
The origin and function of ependymal cells lining the spinal cord central canal have just recently started to be elucidated but many details still remain to be clarified. They cannot be classified as “conventional” glial cells, but they are generally believed to originate from the terminal differentiation of radial glia during the early postnatal period [129]. Originally believed to simply constitute a physical and selective barrier between the cerebrospinal fluid and the brain tissue, it is now known that they are rapidly activated following spinal cord injury (SCI), proliferate, overexpress GFAP, and undergo multi-lineage differentiation to generate new oligodendrocytes and, more abundantly, astrocytes that migrate to the lesion site, building a substantial part of the glial scar. Thus, at least a sub-population of spinal cord ependymal cells has latent neural stem cell properties [130, 131], and they are now believed to represent the true stem cells in the adult spinal cord.
Very few data are available on the role of extracellular nucleotides in controlling ependymal cell functions. Together with spinal cord neurons and oligodendrocyte progenitors, these cells express the P2Y-like receptor GPR17 responding to both uracil nucleotides and cysteinyl leukotrienes that is crucially involved in the development of SCI-associated tissue and functional damage [65]. In fact, the selective inhibition of this receptor by the in vivo delivery of antisense oligonucleotide significantly ameliorated the histological and motor deficits of mice exposed to SCI [65]. Interestingly, following SCI GPR17-expressing ependymal cells start proliferating and upregulate the marker of pluripotency marker GFAP, thus suggesting that this receptor might play a role in the tentative reaction of these cells to traumatic injury [65].
The P2X7 subtype has been also found to be expressed in this type of cells, at least at the mRNA level [132]. Since it is known that blockade of P2X7 receptor activation is protective against spinal cord injury-associated damage [86, 133], it can be speculated that ependymal cells contribute to the neuroprotective action evoked by P2X7 antagonists.
Purinergic modulation of pericyte functions
Pericytes are connective tissue cells located around microvessels and capillaries, and control microvasculature functions and diameter. Pericytes exert their effects on local microcirculation virtually in all organs and tissues (e.g., kidney, retina, brain, etc.; [134]). A clear classification of this particular type of cells is still difficult, mostly due to the lack of selective markers. They are generally believed to derive from bone marrow cells [135] and therefore cannot be classified as glial cells, but since they are fundamental to the integrity and formation of the BBB, it is worth mentioning here the effects exerted by purinergic signalling on them as well. Indeed, brain pericytes express the typical glial progenitor marker NG2 [135], and they have been shown to behave as stem cells, giving rise to neurons and glial cells at least in vitro [136].
Very little but interesting information is currently available on the role of purinergic transmission in controlling brain pericyte functions. mRNAs encoding for all the cloned P2Y receptors as well as for ecto-nucleotidase 1 and 2 have been identified in rat brain pericytes [137]. The in vivo administration of lipopolysaccharide induced a transient change in the morphology and lipid membrane composition of hippocampal pericytes leading to an increased expression and function of ecto-nucleotidase 1 [138]. Indeed, the exposure of an in vitro model of the BBB (consisting of triple co-cultures of pericytes, astrocytes, and endothelial cells) to oxygen–glucose deprivation, which mimics hypoxic events in vivo, leads to the shedding of ecto-nucleotidase containing microvesicles from astrocytes and endothelial cells [137]. It can be speculated that the shed vesicles could contribute to cell-to-cell communication in the BBB by delivering nucleotide-metabolizing enzymes, which could finely tune the extracellular concentrations of purines and pyrimidines at the injury site, with important consequences on the subsequent functional outcome.
Purinergic modulation of enteric glial cells
In the enteric nervous system, ATP is co-released with the two major neurotransmitters controlling enteric functions (i.e., noradrenaline and acetylcholine), but the role of the purinergic component in enteric neurotransmission is not fully understood [139] (for a detailed review, see [140]). Glial cells are abundant in the enteric nervous system (with a ratio to neurons of 2:1), and share electrophysiological and molecular similarities with CNS astrocytes, including expression of GFAP, of the calcium-binding protein S-100, as well as of the P2X7 receptor [141–143]. In 1996, it has been shown for the first time that ATP and UTP are able to increase intracellular calcium concentrations in enteric glia probably via P2Y2 or P2Y4 receptors [144], further supported by the evidence of a Ca2+ release from intracellular stores [145]. Networks of enteric glia can generate propagating waves of Ca2+ that pass via gap junctions and depend on ATP release [146]. It has been also demonstrated that the electrical stimulation of axons in interganglionic nerve fiber bundles releases ATP from neurons that in turn stimulates Ca2+ responses in the surrounding enteric glia. This effect is antagonized by the P2 antagonist PPADS and by the phospholipase C inhibitor U73122, indicating the involvement of P2Y receptors [139]. It has been also suggested that the sympathetic stimulation in the colonic myenteric plexus releases ATP that acts at stimulatory P2Y4 receptors on enteric glia [147]. Interestingly, the nucleotide-metabolizing enzyme NTPDase2 has shown to be exclusively localized at the surface of enteric glial cells, suggesting that these cells control the availability of ATP and UTP in the enteric system [148]. Thus, once its physiological role will be more clearly understood, the purinergic modulation of enteric glia functions (and more, in general, of the whole enteric nervous system) could represent a new target for the development of effective pharmacological strategies to various disorders of the gastrointestinal tract.
Conclusions
The evidence summarized here support a key role for purinergic signalling in all types of glial cells under both physiological and disease conditions. As far as the first are concerned, it has long been known that ATP is one of the main gliotransmitters involved in the neuron–astrocyte and astrocyte–astrocyte communication in the CNS. Long-range ATP-dependent calcium waves involving release of ATP from both neurons and astrocytes and autocrine/paracrine activation of purinergic receptors on both cell types, initially discovered in the retina and later extended to the entire CNS, have been recognized to represent a new form of cell-to-cell communication allowing homotypic and heterotypic signalling between distant cells in both the brain and spinal cord. Evidence has been also accumulating to support a role for purinergic signalling in the neural-to-glia communication allowing maturation of SCs and OLs and myelination in both PNS and CNS. In addition to this, the purinergic system controls the normal patrolling functions of microglial cells and is involved in their activation during CNS perturbations, leading to the acquisition of specific phenotypes that promote lesion remodelling and repair. Under pathological conditions, nucleotides released from either neurons or glia also activate astrocytes that become hypertrophic and proliferative and contribute to initiate repair responses. However, while these responses are initially beneficial, it is now acknowledged that, under chronic inflammatory conditions, these glial responses become detrimental and contribute to the neuronal and functional loss that also occur in several disabling human conditions, such as Alzheimer’s, Parkinson’s disease, MS, and retinal degeneration. In this respect, our analysis has highlighted peculiar roles for the P2X7 receptor, for the P2Y1 and for the A2A receptors. Thus, in principle, the pharmacological blockade of these specific purinergic receptors in chronic diseases may prove effective in reducing damage. However, more recent data demonstrating the presence of P1 and P2 receptors, including the P2Y-like receptor GPR17, on glial-related neural stem cells also underline that the purinergic system may be also exploited to unveil the multipotency of these cells and foster repair in demyelinating diseases, retinal detachment, and ischemia-associated degeneration. This latter approach would also allow targeting and implementing the self-repair abilities of the nervous system that, especially in the case of acute insults, allows a larger and temporally more convenient therapeutic window compared to a classical neuroprotective approach. Therefore, new therapeutic opportunities may derive from the proper stimulation of purinergic signalling during the post-injury reparative phase. In this respect, there is an urgent need for compounds to be developed which are orally bioavailable, stable in vivo, and able to cross the BBB, including purinoceptor subtype agonists and antagonists and inhibitors of ecto-nucleotidases and ATP transport.
Acknowledgments
Part of the work summarized in this article has been sponsored by the Italian Ministero della Salute, by the Italian Ministero dell’ Università e della Ricerca (MIUR; PRIN-COFIN program), by the Fondazione Italiana Sclerosi Multipla (FISM) grant 2010/R/2, and by the Italian Comitato Telethon Fondazione onlus grant #GGP10082A to M.P.A.
Footnotes
Davide Lecca, Stefania Ceruti, and Marta Fumagalli are equally contributing.
References
- 1.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32(1):19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278(2):1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 3.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39(3):190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 4.Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaule C. Circadian regulation of ATP release in astrocytes. J Neurosci. 2011;31(23):8342–8350. doi: 10.1523/JNEUROSCI.6537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fumagalli M, Brambilla R, D’Ambrosi N, Volontè C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43(3):218–230. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- 6.Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 2006;276:91–103. doi: 10.1002/9780470032244.ch8. [DOI] [PubMed] [Google Scholar]
- 7.Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol. 2010;79(2):77–89. doi: 10.1016/j.bcp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59(1):67–76. doi: 10.1016/0306-4522(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 9.Abbracchio MP, Ceruti S, Langfelder R, Cattabeni F, Saffrey MJ, Burnstock G. Effects of ATP analogues and basic fibroblast growth factor on astroglial cell differentiation in primary cultures of rat striatum. Int J Dev Neurosci. 1995;13(7):685–693. doi: 10.1016/0736-5748(95)00064-X. [DOI] [PubMed] [Google Scholar]
- 10.Brambilla R, Burnstock G, Bonazzi A, Ceruti S, Cattabeni F, Abbracchio MP. Cyclo-oxygenase-2 mediates P2Y receptor-induced reactive astrogliosis. Br J Pharmacol. 1999;126(3):563–567. doi: 10.1038/sj.bjp.0702333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brambilla R, Ceruti S, Malorni W, Cattabeni F, Abbracchio MP. A novel gliotic P2 receptor mediating cyclooxygenase-2 induction in rat and human astrocytes. J Auton Nerv Syst. 2000;81(1–3):3–9. doi: 10.1016/S0165-1838(00)00152-1. [DOI] [PubMed] [Google Scholar]
- 12.Neary JT, Kang Y, Shi YF, Tran MD, Wanner IB. P2 receptor signalling, proliferation of astrocytes, and expression of molecules involved in cell-cell interactions. Novartis Found Symp. 2006;276:131–143. doi: 10.1002/9780470032244.ch11. [DOI] [PubMed] [Google Scholar]
- 13.Franke H, Sauer C, Rudolph C, Krugel U, Hengstler JG, Illes P. P2 receptor-mediated stimulation of the PI3-K/Akt-pathway in vivo. Glia. 2009;57(10):1031–1045. doi: 10.1002/glia.20827. [DOI] [PubMed] [Google Scholar]
- 14.Kuboyama K, Harada H, Tozaki-Saitoh H, Tsuda M, Ushijima K, Inoue K (2011) Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J Cereb Blood Flow Metab 31:1930-1941 [DOI] [PMC free article] [PubMed]
- 15.Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, Gonzalez FA, Seye CI, Erb L. Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol. 2005;31(1–3):169–183. doi: 10.1385/MN:31:1-3:169. [DOI] [PubMed] [Google Scholar]
- 16.Abbracchio MP, Ceruti S. Roles of P2 receptors in glial cells: focus on astrocytes. Purinergic Signal. 2006;2(4):595–604. doi: 10.1007/s11302-006-9016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson TS, Camden JM, Wang Y, Seye CI, Wood WG, Sun GY, Erb L, Petris MJ, Weisman GA. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol. 2010;41(2–3):356–366. doi: 10.1007/s12035-010-8115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran MD. P2 receptor stimulation induces amyloid precursor protein production and secretion in rat cortical astrocytes. Neurosci Lett. 2011;492(3):155–159. doi: 10.1016/j.neulet.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 19.Fumagalli M, Lecca D, Abbracchio MP. Role of purinergic signalling in neuro-immune cells and adult neural progenitors. Front Biosci. 2011;17:2326–2341. doi: 10.2741/3856. [DOI] [PubMed] [Google Scholar]
- 20.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23(27):9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecca D, Ceruti S. Uracil nucleotides: from metabolic intermediates to neuroprotection and neuroinflammation. Biochem Pharmacol. 2008;75(10):1869–1881. doi: 10.1016/j.bcp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Bjelobaba I, Parabucki A, Lavrnja I, Stojkov D, Dacic S, Pekovic S, Rakic L, Stojiljkovic M, Nedeljkovic N. Dynamic changes in the expression pattern of ecto-5′-nucleotidase in the rat model of cortical stab injury. J Neurosci Res. 2011;89(6):862–873. doi: 10.1002/jnr.22599. [DOI] [PubMed] [Google Scholar]
- 23.Ciccarelli R, D’Alimonte I, Ballerini P, D’Auro M, Nargi E, Buccella S, Iorio P, Bruno V, Nicoletti F, Caciagli F. Molecular signalling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Mol Pharmacol. 2007;71(5):1369–1380. doi: 10.1124/mol.106.031617. [DOI] [PubMed] [Google Scholar]
- 24.Hindley S, Herman MA, Rathbone MP. Stimulation of reactive astrogliosis in vivo by extracellular adenosine diphosphate or an adenosine A2 receptor agonist. J Neurosci Res. 1994;38(4):399–406. doi: 10.1002/jnr.490380405. [DOI] [PubMed] [Google Scholar]
- 25.Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43(2):190–194. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- 26.Trincavelli ML, Marroni M, Tuscano D, Ceruti S, Mazzola A, Mitro N, Abbracchio MP, Martini C. Regulation of A2B adenosine receptor functioning by tumour necrosis factor a in human astroglial cells. J Neurochem. 2004;91(5):1180–1190. doi: 10.1111/j.1471-4159.2004.02793.x. [DOI] [PubMed] [Google Scholar]
- 27.Abbracchio MP, Ceruti S, Brambilla R, Barbieri D, Camurri A, Franceschi C, Giammarioli A, Jacobson KA, Cattabeni F, Malorni W. Adenosine A3 receptors and viability of astrocytes. Drug Dev Res. 1998;45:379–386. doi: 10.1002/(SICI)1098-2299(199811/12)45:3/4<379::AID-DDR38>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubitz DK, Simpson KL, Lin RC. Right thing at a wrong time? Adenosine A3 receptors and cerebroprotection in stroke. Ann N Y Acad Sci. 2001;939:85–96. doi: 10.1111/j.1749-6632.2001.tb03615.x. [DOI] [PubMed] [Google Scholar]
- 29.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 30.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96(20):11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56(14):1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 33.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330(6005):779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 34.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468(7321):244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 35.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31(7):361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PR, Fields RD. Regulation of myelin genes implicated in psychiatric disorders by functional activity in axons. Front Neuroanat. 2009;3:4. doi: 10.3389/neuro.05.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000;23(12):625–633. doi: 10.1016/S0166-2236(00)01674-X. [DOI] [PubMed] [Google Scholar]
- 38.Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287(5461):2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 39.Ansselin AD, Davey DF, Allen DG. Extracellular ATP increases intracellular calcium in cultured adult Schwann cells. Neuroscience. 1997;76(3):947–955. doi: 10.1016/S0306-4522(96)00370-3. [DOI] [PubMed] [Google Scholar]
- 40.Lyons SA, Morell P, McCarthy KD. Schwann cell ATP-mediated calcium increases in vitro and in situ are dependent on contact with neurons. Glia. 1995;13(1):27–38. doi: 10.1002/glia.440130104. [DOI] [PubMed] [Google Scholar]
- 41.Mayer C, Quasthoff S, Grafe P. Differences in the sensitivity to purinergic stimulation of myelinating and non-myelinating Schwann cells in peripheral human and rat nerve. Glia. 1998;23(4):374–382. doi: 10.1002/(SICI)1098-1136(199808)23:4<374::AID-GLIA9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 42.Grafe P, Mayer C, Takigawa T, Kamleiter M, Sanchez-Brandelik R. Confocal calcium imaging reveals an ionotropic P2 nucleotide receptor in the paranodal membrane of rat Schwann cells. J Physiol. 1999;515(Pt 2):377–383. doi: 10.1111/j.1469-7793.1999.377ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rousse I, Robitaille R. Calcium signaling in Schwann cells at synaptic and extra-synaptic sites: active glial modulation of neuronal activity. Glia. 2006;54(7):691–699. doi: 10.1002/glia.20388. [DOI] [PubMed] [Google Scholar]
- 44.Stevens B, Ishibashi T, Chen JF, Fields RD. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol. 2004;1(1):23–34. doi: 10.1017/S1740925X04000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wachtler J, Mayer C, Grafe P. Activity-dependent intracellular Ca2+ transients in unmyelinated nerve fibres of the isolated adult rat vagus nerve. Pflugers Arch. 1998;435(5):678–686. doi: 10.1007/s004240050569. [DOI] [PubMed] [Google Scholar]
- 46.Verderio C, Bianco F, Blanchard MP, Bergami M, Canossa M, Scarfone E, Matteoli M. Cross talk between vestibular neurons and Schwann cells mediates BDNF release and neuronal regeneration. Brain Cell Biol. 2006;35(2–3):187–201. doi: 10.1007/s11068-007-9011-6. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann H, Braun N, Kegel B, Heine P. New insights into molecular structure and function of ectonucleotidases in the nervous system. Neurochem Int. 1998;32(5–6):421–425. doi: 10.1016/S0197-0186(97)00126-5. [DOI] [PubMed] [Google Scholar]
- 48.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7(1):11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro F, Bribian A. The molecular orchestra of the migration of oligodendrocyte precursors during development. Brain Res Brain Res Rev. 2005;49(2):227–241. doi: 10.1016/j.brainresrev.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 50.Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36(5):855–868. doi: 10.1016/S0896-6273(02)01067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Othman T, Yan H, Rivkees SA. Oligodendrocytes express functional A1 adenosine receptors that stimulate cellular migration. Glia. 2003;44(2):166–172. doi: 10.1002/glia.10281. [DOI] [PubMed] [Google Scholar]
- 52.Takeda M, Nelson DJ, Soliven B. Calcium signaling in cultured rat oligodendrocytes. Glia. 1995;14(3):225–236. doi: 10.1002/glia.440140308. [DOI] [PubMed] [Google Scholar]
- 53.Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonte C, Aloisi F, Visentin S. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia. 2005;50(2):132–144. doi: 10.1002/glia.20160. [DOI] [PubMed] [Google Scholar]
- 54.Fumagalli M, Daniele S, Lecca D, Lee PR, Parravicini C, Fields RD, Rosa P, Antonucci F, Verderio C, Trincavelli ML, Bramanti P, Martini C, Abbracchio MP. Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J Biol Chem. 2011;286(12):10593–10604. doi: 10.1074/jbc.M110.162867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J Neurosci. 2002;22(21):9221–9227. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amadio S, Tramini G, Martorana A, Viscomi MT, Sancesario G, Bernardi G, Volonte C. Oligodendrocytes express P2Y12 metabotropic receptor in adult rat brain. Neuroscience. 2006;141(3):1171–1180. doi: 10.1016/j.neuroscience.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 58.Amadio S, Montilli C, Magliozzi R, Bernardi G, Reynolds R, Volonte C. P2Y12 receptor protein in cortical gray matter lesions in multiple sclerosis. Cereb Cortex. 2010;20(6):1263–1273. doi: 10.1093/cercor/bhp193. [DOI] [PubMed] [Google Scholar]
- 59.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25(19):4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benned-Jensen T, Rosenkilde MM. Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants. Br J Pharmacol. 2010;159(5):1092–1105. doi: 10.1111/j.1476-5381.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lecca D, Trincavelli ML, Gelosa P, Sironi L, Ciana P, Fumagalli M, Villa G, Verderio C, Grumelli C, Guerrini U, Tremoli E, Rosa P, Cuboni S, Martini C, Buffo A, Cimino M, Abbracchio MP. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS One. 2008;3(10):e3579. doi: 10.1371/journal.pone.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, Trapp BD, Karandikar NJ, Hsieh J, Lu QR. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12(11):1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ceruti S, Viganò F, Boda E, Ferrario S, Magni G, Boccazzi M, Rosa P, Buffo A, Abbracchio MP. Expression of the new P2Y-like receptor GPR17 during oligodendrocyte precursor cell maturation regulates sensitivity to ATP-induced death. Glia. 2011;59(3):363–378. doi: 10.1002/glia.21107. [DOI] [PubMed] [Google Scholar]
- 64.Pugliese AM, Trincavelli ML, Lecca D, Coppi E, Fumagalli M, Ferrario S, Failli P, Daniele S, Martini C, Pedata F, Abbracchio MP. Functional characterization of two isoforms of the P2Y-like receptor GPR17: [35S]GTPgammaS binding and electrophysiological studies in 1321N1 cells. Am J Physiol Cell Physiol. 2009;297(4):C1028–C1040. doi: 10.1152/ajpcell.00658.2008. [DOI] [PubMed] [Google Scholar]
- 65.Ceruti S, Villa G, Genovese T, Mazzon E, Longhi R, Rosa P, Bramanti P, Cuzzocrea S, Abbracchio MP. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain. 2009;132(Pt 8):2206–2218. doi: 10.1093/brain/awp147. [DOI] [PubMed] [Google Scholar]
- 66.Boda E, Viganò F, Rosa P, Fumagalli M, Labat-gest V, Tempia F, Abbracchio MP, Dimou L, Buffo A (2011) The GPR17 receptor in NG2 expressing cells: focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia 59:1958–1973 [DOI] [PubMed]
- 67.Matute C, Torre I, Perez-Cerda F, Perez-Samartin A, Alberdi E, Etxebarria E, Arranz AM, Ravid R, Rodriguez-Antiguedad A, Sanchez-Gomez M, Domercq M. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;27(35):9525–9533. doi: 10.1523/JNEUROSCI.0579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia. 2010;58(6):730–740. doi: 10.1002/glia.20958. [DOI] [PubMed] [Google Scholar]
- 69.Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58(11):1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, 2nd, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10(1):93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- 71.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10(1):9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 72.Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26(1):84–91. doi: 10.1002/(SICI)1098-1136(199903)26:1<84::AID-GLIA9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 73.Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92(1):83–95. doi: 10.1016/S0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- 74.Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31(6–7):437–455. doi: 10.1023/A:1025783412651. [DOI] [PubMed] [Google Scholar]
- 75.Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31(6–7):551–565. doi: 10.1023/A:1025751900356. [DOI] [PubMed] [Google Scholar]
- 76.Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207(6):695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamilton N, Vayro S, Wigley R, Butt AM. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia. 2010;58(1):66–79. doi: 10.1002/glia.20902. [DOI] [PubMed] [Google Scholar]
- 78.Belachew S, Gallo V. Synaptic and extrasynaptic neurotransmitter receptors in glial precursors’ quest for identity. Glia. 2004;48(3):185–196. doi: 10.1002/glia.20077. [DOI] [PubMed] [Google Scholar]
- 79.Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13(1):62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- 80.Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol. 2001;133(8):1323–1329. doi: 10.1038/sj.bjp.0704189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47(6):442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 82.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185(3):579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24(1):1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lai AY, Todd KG. Differential regulation of trophic and proinflammatory microglial effectors is dependent on severity of neuronal injury. Glia. 2008;56(3):259–270. doi: 10.1002/glia.20610. [DOI] [PubMed] [Google Scholar]
- 85.Sperlagh B, Illes P. Purinergic modulation of microglial cell activation. Purinergic Signal. 2007;3(1–2):117–127. doi: 10.1007/s11302-006-9043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 87.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 88.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 89.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21(6):1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 91.Rouach N, Calvo CF, Glowinski J, Giaume C. Brain macrophages inhibit gap junctional communication and downregulate connexin 43 expression in cultured astrocytes. Eur J Neurosci. 2002;15(2):403–407. doi: 10.1046/j.0953-816x.2001.01868.x. [DOI] [PubMed] [Google Scholar]
- 92.McIlvain HB, Ma L, Ludwig B, Manners MT, Martone RL, Dunlop J, Kaftan EJ, Kennedy JD, Whiteside GT. Purinergic receptor-mediated morphological changes in microglia are transient and independent from inflammatory cytokine release. Eur J Pharmacol. 2010;643(2–3):202–210. doi: 10.1016/j.ejphar.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 93.Virgilio F. Dr. Jekyll/Mr. Hyde: the dual role of extracellular ATP. J Auton Nerv Syst. 2000;81(1–3):59–63. doi: 10.1016/S0165-1838(00)00114-4. [DOI] [PubMed] [Google Scholar]
- 94.Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Virgilio F. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J Immunol. 2009;182(7):4378–4385. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- 95.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446(7139):1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gyoneva S, Orr AG, Traynelis SF. Differential regulation of microglial motility by ATP/ADP and adenosine. Parkinsonism Relat Disord. 2009;15(Suppl 3):S195–S199. doi: 10.1016/S1353-8020(09)70813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monif M, Burnstock G, Williams DA. Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42(11):1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 98.Virgilio F, Sanz JM, Chiozzi P, Falzoni S. The P2Z/P2X7 receptor of microglial cells: a novel immunomodulatory receptor. Prog Brain Res. 1999;120:355–368. doi: 10.1016/S0079-6123(08)63569-4. [DOI] [PubMed] [Google Scholar]
- 99.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159(7):3595–3602. [PubMed] [Google Scholar]
- 100.Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K. P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem. 2010;114(3):810–819. doi: 10.1111/j.1471-4159.2010.06809.x. [DOI] [PubMed] [Google Scholar]
- 101.Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63(7):686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 102.Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A, Cuneo A, Castoldi G, Virgilio F, Baricordi OR. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99(2):706–708. doi: 10.1182/blood.V99.2.706. [DOI] [PubMed] [Google Scholar]
- 104.Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Virgilio F, Abbracchio MP, Verderio C. A role for P2X7 in microglial proliferation. J Neurochem. 2006;99(3):745–758. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- 105.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29(12):3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Bringmann A, Wiedemann P. Involvement of Muller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol. 2009;247(7):865–883. doi: 10.1007/s00417-009-1082-x. [DOI] [PubMed] [Google Scholar]
- 108.Wurm A, Pannicke T, Iandiev I, Francke M, Hollborn M, Wiedemann P, Reichenbach A, Osborne NN, Bringmann A. Purinergic signaling involved in Muller cell function in the mammalian retina. Prog Retin Eye Res. 2011;30(5):324–342. doi: 10.1016/j.preteyeres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 109.Pannicke T, Fischer W, Biedermann B, Schadlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A. P2X7 receptors in Muller glial cells from the human retina. J Neurosci. 2000;20(16):5965–5972. doi: 10.1523/JNEUROSCI.20-16-05965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Innocenti B, Pfeiffer S, Zrenner E, Kohler K, Guenther E. ATP-induced non-neuronal cell permeabilization in the rat inner retina. J Neurosci. 2004;24(39):8577–8583. doi: 10.1523/JNEUROSCI.2812-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Uckermann O, Wolf A, Kutzera F, Kalisch F, Beck-Sickinger AG, Wiedemann P, Reichenbach A, Bringmann A. Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y. J Neurosci Res. 2006;83(4):538–550. doi: 10.1002/jnr.20760. [DOI] [PubMed] [Google Scholar]
- 112.Wurm A, Pannicke T, Iandiev I, Buhner E, Pietsch UC, Reichenbach A, Wiedemann P, Uhlmann S, Bringmann A. Changes in membrane conductance play a pathogenic role in osmotic glial cell swelling in detached retinas. Am J Pathol. 2006;169(6):1990–1998. doi: 10.2353/ajpath.2006.060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wurm A, Pannicke T, Wiedemann P, Reichenbach A, Bringmann A. Glial cell-derived glutamate mediates autocrine cell volume regulation in the retina: activation by VEGF. J Neurochem. 2008;104(2):386–399. doi: 10.1111/j.1471-4159.2007.04992.x. [DOI] [PubMed] [Google Scholar]
- 114.Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18(11):4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25(23):5502–5510. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275(5301):844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guidry C. The role of Muller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24(1):75–86. doi: 10.1016/j.preteyeres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Bringmann A, Pannicke T, Moll V, Milenkovic I, Faude F, Enzmann V, Wolf S, Reichenbach A. Upregulation of P2X(7) receptor currents in Muller glial cells during proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2001;42(3):860–867. [PubMed] [Google Scholar]
- 119.Uhlmann S, Bringmann A, Uckermann O, Pannicke T, Weick M, Ulbricht E, Goczalik I, Reichenbach A, Wiedemann P, Francke M. Early glial cell reactivity in experimental retinal detachment: effect of suramin. Invest Ophthalmol Vis Sci. 2003;44(9):4114–4122. doi: 10.1167/iovs.03-0183. [DOI] [PubMed] [Google Scholar]
- 120.Bull ND, Martin KR. Concise review: toward stem cell-based therapies for retinal neurodegenerative diseases. Stem Cells. 2011;29(8):1170–1175. doi: 10.1002/stem.676. [DOI] [PubMed] [Google Scholar]
- 121.Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, Khaw PT, Limb GA. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25(8):2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 122.Debbio CB, Balasubramanian S, Parameswaran S, Chaudhuri A, Qiu F, Ahmad I. Notch and Wnt signaling mediated rod photoreceptor regeneration by Müller cells in adult mammalian retina. PLoS One. 2010;5(8):e12425. doi: 10.1371/journal.pone.0012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21(5):592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 124.Jasmin L, Vit JP, Bhargava A, Ohara PT. Can satellite glial cells be therapeutic targets for pain control? Neuron Glia Biol. 2010;6(1):63–71. doi: 10.1017/S1740925X10000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Villa G, Fumagalli M, Verderio C, Abbracchio MP, Ceruti S. Expression and contribution of satellite glial cells purinoceptors to pain transmission in sensory ganglia: an update. Neuron Glia Biol. 2010;6(1):31–42. doi: 10.1017/S1740925X10000086. [DOI] [PubMed] [Google Scholar]
- 126.Ceruti S, Fumagalli M, Villa G, Verderio C, Abbracchio MP. Purinoceptor-mediated calcium signaling in primary neuron-glia trigeminal cultures. Cell Calcium. 2008;43(6):576–590. doi: 10.1016/j.ceca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 127.Kushnir R, Cherkas PS, Hanani M. Peripheral inflammation upregulates P2X receptor expression in satellite glial cells of mouse trigeminal ganglia: a calcium imaging study. Neuropharmacology. 2011;61(4):739–746. doi: 10.1016/j.neuropharm.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 128.Ceruti S, Villa G, Fumagalli M, Colombo L, Magni G, Zanardelli M, Fabbretti E, Verderio C, Maagdenberg AM, Nistri A, Abbracchio MP. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: implications for basic mechanisms of migraine pain. J Neurosci. 2011;31(10):3638–3649. doi: 10.1523/JNEUROSCI.6440-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sevc J, Daxnerova Z, Hanova V, Koval J. Novel observations on the origin of ependymal cells in the ventricular zone of the rat spinal cord. Acta Histochem. 2011;113(2):156–162. doi: 10.1016/j.acthis.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 130.Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131(1):177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 131.Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6(7):e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, Umemura A, Mase M, Yamada K, Shimada S. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 133.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106(30):12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood–brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 135.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58(1):1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 136.Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14(16):1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- 137.Ceruti S, Colombo L, Magni G, Viganò F, Boccazzi M, Deli MA, Sperlagh B, Abbracchio MP, Kittel A. Oxygen-glucose deprivation increases the enzymatic activity and the microvescicle-mediated release of ectonucleotidases in the cells composing the blood–brain barrier. Neurochem Int. 2011;59(2):259–271. doi: 10.1016/j.neuint.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 138.Kittel A, Sperlagh B, Pelletier J, Sevigny J, Kirley TL. Transient changes in the localization and activity of ecto-nucleotidases in rat hippocampus following lipopolysaccharide treatment. Int J Dev Neurosci. 2007;25(5):275–282. doi: 10.1016/j.ijdevneu.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136(4):1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 140.Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20(Suppl 1):8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 141.Hanani M, Francke M, Hartig W, Grosche J, Reichenbach A, Pannicke T. Patch-clamp study of neurons and glial cells in isolated myenteric ganglia. Am J Physiol Gastrointest Liver Physiol. 2000;278(4):G644–G651. doi: 10.1152/ajpgi.2000.278.4.G644. [DOI] [PubMed] [Google Scholar]
- 142.Ruhl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17(6):777–790. doi: 10.1111/j.1365-2982.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 143.Vanderwinden JM, Timmermans JP, Schiffmann SN. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. 2003;312(2):149–154. doi: 10.1007/s00441-003-0716-2. [DOI] [PubMed] [Google Scholar]
- 144.Kimball BC, Mulholland MW. Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J Neurochem. 1996;66(2):604–612. doi: 10.1046/j.1471-4159.1996.66020604.x. [DOI] [PubMed] [Google Scholar]
- 145.Sarosi GA, Barnhart DC, Turner DJ, Mulholland MW. Capacitative Ca2+ entry in enteric glia induced by thapsigargin and extracellular ATP. Am J Physiol. 1998;275(3 Pt 1):G550–G555. doi: 10.1152/ajpgi.1998.275.3.G550. [DOI] [PubMed] [Google Scholar]
- 146.Zhang W, Segura BJ, Lin TR, Hu Y, Mulholland MW. Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia. 2003;42(3):252–262. doi: 10.1002/glia.10215. [DOI] [PubMed] [Google Scholar]
- 147.Gulbransen BD, Bains JS, Sharkey KA. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci. 2010;30(19):6801–6809. doi: 10.1523/JNEUROSCI.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Braun N, Sevigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia. 2004;45(2):124–132. doi: 10.1002/glia.10309. [DOI] [PubMed] [Google Scholar]