Abstract
Replacement of lost or dysfunctional tissues by stem cells has recently raised many investigations on therapeutic applications. Purinergic signaling has been shown to regulate proliferation, differentiation, cell death, and successful engraftment of stem cells originated from diverse origins. Adenosine triphosphate release occurs in a controlled way by exocytosis, transporters, and lysosomes or in large amounts from damaged cells, which is then subsequently degraded into adenosine. Paracrine and autocrine mechanisms induced by immune responses present critical factors for the success of stem cell therapy. While P1 receptors generally exert beneficial effects including anti-inflammatory activity, P2 receptor-mediated actions depend on the subtype of stimulated receptors and localization of tissue repair. Pro-inflammatory actions and excitatory tissue damages mainly result from P2X7 receptor activation, while other purinergic receptor subtypes participate in proliferation and differentiation, thereby providing adequate niches for stem cell engraftment and novel mechanisms for cell therapy and endogenous tissue repair. Therapeutic applications based on regulation of purinergic signaling are foreseen for kidney and heart muscle regeneration, Clara-like cell replacement for pulmonary and bronchial epithelial cells as well as for induction of neurogenesis in case of neurodegenerative diseases.
Keywords: ATP, Adenosine nucleotides, Purinergic signaling, Tissue injury, Differentiation, Immune system
An overview of purinergic signaling
Receptors for purines and pyrimidines are classified based on their agonist specificity. P1 receptors subtypes are selective for adenosine and are classical 7-transmembrane metabotropic receptors coupled to several families of Gi, Go, and Gs proteins. There are four types of adenosine receptors (A1, A2A, A2B, and A3) differing in their pharmacological and functional properties [1]. P2 receptors are divided into P2X and P2Y subtypes based on their structural characteristics. P2X receptors are ATP-activated, ligand-gated cationic (Na+/K+/Ca2+) channels [2, 3], assembled in trimeric form from P2X1 to P2X7 subunits [1, 3]. Metabotropic P2Y purinoceptors expressed by mammalians are divided into P2Y1,2,4,6,11,12,13,14 subtypes based on phylogenetic similarity and are stimulated by ATP, ADP, UTP, UDP, or UDP glucose [1]. Purinergic receptors are expressed by almost every cell type and are one of the first expressed neurotransmitter receptors in development [4–6]. The extracellular nucleotide/nucleoside availability is controlled by a highly efficient enzymatic cascade, which includes the members of the ectonucleoside triphosphate diphosphohydrolase (E-NTPDases, NTPDase1–8), ectonucleotide pyrophosphatase/phosphodiesterase (E-NPPs), ecto-alkaline phosphatases, and ecto-5′-nucleotidase/CD73. These enzymes catalyze the complete nucleotide hydrolysis (e.g., ATP) to nucleosides (e.g., adenosine) and represent a powerful tool for controlling the effects mediated by extracellular purines [7–9].
Stem cells and purinergic signaling
Replacement of lost or dysfunctional tissues has recently raised many investigations on possible therapeutic application of stem cells. An impressive number of clinical trials and animal studies have already been performed to determine the therapeutic potential of various stem cells models [10, 11]. The first isolation of embryonic stem (ES) cells from mouse goes back to 1981 followed by human ES cell isolation and culture in 1998 [12, 13]. Organ-specific stem cells were isolated from embryonic and adult tissues including brain, bone marrow, umbilical cord, skeletal and cardiac muscles, and adipose tissue [14]. Pluripotent ES cells are capable to originate any somatic cell type, while tissue-specific stem cells are mostly multipotent and subsequently originate cell types found in these specific tissues. Both, ES and tissue-specific stem cells can proliferate symmetrically replicating themselves for self-renewal or asymmetrically giving rise to a stem cell and another more differentiated cell type. The most promising and recently discovered stem cell model for basic research and even therapy is the induced pluripotent stem cell (iPS cell), reprogrammed in 2006 from differentiated mouse cells and in 2007 from human cells [15, 16]. The recent described capacity of genetically reprogrammed somatic cells towards pluripotent ones could bypass obstacles, such as the lack of histocompatibility and ethical concerns, by allowing the generation of autologous cells from the patient. This new pluripotent cell source initially obtained by overexpression of the genes Klf-4, Oct4, Sox2, and c-Myc responsible for pluripotency [reviewed by 17] has opened expectations for treatment of many diseases. Importantly, iPS cells derived from different species demonstrated the potential to differentiate into tissues derived from the three germ layers, such as known from ES cells. However, care must be taken on using these cells as well as ES cells for transplantation purposes due to their possible tumorigenic potential.
Therapeutic application of stem cells in patients is particularly promising for treatment of heart disease, where new cardiomyocytes could restore contractile function after myocardial infarction. Cell regeneration therapy could be also relevant for repair of pancreatic function in diabetes with the replacement of β insulin-secreting cells [18]. Further possible applications are foreseen for the treatment of the damaged neuronal system and neurodegenerative diseases. For instance, efforts are being made to replace dopaminergic neurons in Parkinson’s disease [19] or to use the stem cell therapy to restore motorneuron function in patients suffering from spinal cord injuries [16]. However, the little obtained progress in many cases did not satisfy the high expectations made. Moreover, observed functional improvements observed in the treated tissues did not often result from the integration of stem cells into existing tissue architectures. It is evident that transplanted cells contribute to endogenous tissue repair through paracrine mechanisms more than by differentiating themselves. For instance, the success of neural progenitor cell (NPC) engrafting into the spinal cord of Sprague–Dawley rats, subjected to contusion at T8–T9 levels, was limited by allodynia due to the death of transplanted cells [7]. However, injection of conditioned media recovered from cultured stem cells promoted arteriogenesis and functional improvement when injected into the damaged heart [20]. Therefore, it has been postulated that trophic factors represent the principle mechanism responsible for tissue repair.
Usual strategies for cell replacement therapy are based on the isolation of a stem cell source from a donor or the patient, followed by induction to proliferate and/or differentiate into tissue types which shall be repaired. Cell death and rejection of transplanted cells are mostly due to immune responses and the absence of adequate stem cell niches at the localization of transplantation. Although mechanisms by which the local milieu influences stem cell differentiation and tissue engraftment need yet to be elucidated, it seems that the fate of bone marrow stem cells is determined by the environment in which they engraft rather than by an intrinsically programmed fate. As support for such hypothesis, positive inotropic (pharmacologic augmentation of contractility) or chronotropic stimuli (heart rate increase by exercise) promoted and intensified the differentiation of bone marrow-derived stem cells into cardiomyocyte phenotypes [21]. Furthermore, stem cells secret trophic and immunomodulatory factors controlling local and systematic inflammatory responses. Such factors, liberated by, i.e., bone marrow stem cells are therapeutically important, since they stimulate local tissue regeneration and/or recruitment of endogenous stem or progenitor cells. Moreover, some studies have demonstrated that mesenchymal stem cells (MSC) can diminish the apoptosis degree and infarct size of the damaged areas by secreting a wide range of cytoprotective molecules like vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor 1, stromal cell-derived factor-1, platelet-derived growth factor, interleukin-1 beta, or hepatocyte growth factor [22].
Other factors with such therapeutic potential are UTP, UDP, ADP, and adenosine acting through purinergic receptors. Nucleotides, released after tissue injury and cell death and hydrolyzed by ectonucleotidases, also regulate immune cell function induced by damage-associated molecular pattern molecules [23]. Moreover, ATP released from immune cells participates in autocrine as well as in paracrine feedback loops with regulatory functions during T-cell activation in the immune synapse (junction between T cell and antigen-presenting cell) [24]. During the inflammatory process following cell transplantation and hindering repair, purines exert trophic functions and keep several immune functions under control, including the release of prostanoids, activation of matrix metalloproteinase-9, cytokines and chemokines, proliferation, differentiation/maturation and stimulation of immune cells, endothelial adhesion, free radical production, degranulation, phagocytosis, fusion, and cell death [25]. Depending on the involved purinergic receptor subtype, ATP often exerts proinflammatory effects while adenosine induces mainly anti-inflammatory effects [25]. Several studies demonstrated that the absence or inhibition of the P2X7 receptor (a mediator of the pro-inflammatory effects of ATP) results in less severe outcomes in chronic inflammatory diseases and enhanced functional recovery [23, 26, 27].
Besides importance of purinergic receptor agonists in differentiated immune cells, these compounds also modulate hematopoietic stem cell (HSC) self-renewal, expansion, and differentiation with implications not only in hematopoiesis, but also in tissue repair and regenerative medicine [28, 29]. For instance, ATP induces the proliferation of human HSC and contributed through P2X receptor activation during inflammation process [29, 30]. UTP also induces proliferation and migration of HSCs [30, 31] while adenosine potentiates the stimulatory effect of growth factors and cytokines on HSC proliferation and differentiation [8]. Moreover, human MSCs at early stages of culture (P0–P5) spontaneously release ATP reducing cell proliferation. Increased human MSC proliferation is induced by the unselective P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonate (PPADS) and by the selective P2Y1 receptor antagonist 2′-deoxy-N6-methyladenosine-3′,5′-bisphosphate (MRS 2179). In summary, ATP modulates HSC and MSC proliferation and likely acts as one of the early factors determining their cell fate [32]. Furthermore, nucleotides also contribute to inflammatory responses and cell fate decisions occurring in the brain. P2X7 receptors expressed by NPCs are responsible for cell death, being in agreement with observations that high levels of extracellular ATP in inflammatory central nervous system (CNS) lesions hinder successful NPC engraftment [33].
The extracellular nucleotide/nucleoside availability is controlled by a highly efficient enzymatic cascade, which includes the members of the E-NTPDases (NTPDase1–8), E-NPPs, ecto-alkaline phosphatases, and ecto-5′-nucleotidase/CD73. These enzymes are responsible for nucleotide hydrolysis (e.g. ATP) into nucleosides (e.g., adenosine) and represent a powerful mechanism for controlling the effects mediated by extracellular purines [9, 34]. Although purinergic signaling has been extensively studied, only few studies are found in the literature demonstrating the involvement of extracellular nucleotide metabolizing enzymes in stem cell biology. Expression and activities of members of ectonucleotidase families as well as purinergic receptor subtypes have been detected in different types of stem and progenitor cells. Recent works have identified the presence of NTPDase2 in adult mouse hippocampal progenitors [35] and in type B cells of the subventricular zone (SVZ) [36], two neurogenic regions of the adult mammalian brain. In accordance, neurospheres cultured from the adult mouse SVZ express NTPDase2, the tissue nonspecific isoform of alkaline phosphatase (TNAP) and functional P2 receptors in synergism with growth factors for enhancing cell proliferation [37]. In addition, deletion of TNAP expression or inhibition of its enzymatic activity in neural progenitors reduces cell proliferation and differentiation into neurons or oligodendrocytes [38]. These published data corroborate the importance of NTPDase2 and TNAP, two potential ATP scavengers, as novel markers for progenitor cells both in the adult and developing brain [39]. Reinforcing these results, spontaneous ATP release was observed in murine NPCs and, interestingly, purinergic receptors antagonists were able to suppress progenitor cell proliferation [40]. Moreover, neuronal differentiation was accompanied by a decrease in ATP release and a loss of functional P2Y receptors, suggesting that purine nucleotides act as proliferation-inducing factors for NPCs and downregulators of neuronal differentiation, once again pointing at the importance of purinergic signaling and involved enzymes for neurogenesis in the adult brain [40]. These data are in agreement with results of our laboratory [41], showing down-regulation of P2Y1 receptor expression and activity in differentiating P19 mouse embryonal carcinoma cells. This observation is in line with functions of the P2Y1 subtype in promoting proliferation of undifferentiated cells, but not induction of neuronal differentiation. Finally, the studies presented here demonstrate the potential participation of ectonucleotidases in the biology of stem or progenitor cells from different tissues. Initial results on roles of these ecto-enzymes will encourage more studies for better understanding of their importance in stem cell biology, differentiation, and tissue repair. In the following, we will discuss new trends of stem cell research related to purinergic signaling and the perspectives of using these discoveries as tools for future tissue repair in clinical trials as this new approach develops (see Fig. 1 for a scheme of the possible therapeutic use of purines in combination with stem cells).
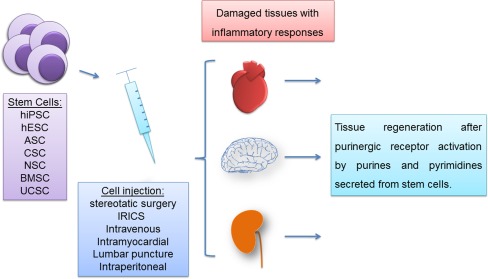
Fig. 1.
Therapeutic potential of stem cells and supposed effects of purinergic signaling. Stem cells of diverse origins, such as from adipose, cardiac, and neural tissues can restore and regenerate damaged tissues by secreting paracrine factors including purines and pyrimidines. ATP and adenosine interfere with tissue reactions following transplantation of stem cells of various origins in different ways. (1) Nucleotides modulate the immune response and thereby reduce inflammation processes and the risk of transplant rejection and cell death. (2) Purines and pyrimidines promote proliferation and differentiation of transplanted and endogenous stem cells by providing adequate stem cell niches. (3) Purines and pyrimidines promote migration of endogenous stem cells to the site of injury and increase engraftment rates. Stem cell types with therapeutic applications are human induced-pluripotent stem cells (hiPSC), human embryonic stem cells (hESC), adipose stem cells (ASC), cardiac stem cells (CSC), neural stem cells (NSC), bone marrow stem cells (BMSC), and umbilical cord stem cells (UCSC) which are transplanted by using stereotaxic surgery (SS), intracoronary retrograde infusion through coronary sinus (IRICS) or intravenous, intramyocardial, or intraperitoneal injection or lumbar puncture
Purinergic signaling and perspectives in tissue regeneration
Implications of the purinergic system in stem cell biology and tissue regeneration will be discussed with emphasis on the recent hypothesis that paracrine effects present the most important mechanisms in this process. Since this idea is very recent, few data are available directly relating the purinergic system with stem cell differentiation and tissue regeneration; however, the authors of this review are confident that the present article will encourage research in order to better understand the participation of purinergic signaling in this context.
Heart injury
The heart is an organ composed basically of fibroblasts and cardiomyocytes, terminal-differentiated cells which give the heart the pumping ability. During ischemia and other injuries, the most affected cells are the cardiomyocytes because they die and a scar is formed due to the inability of renewing these cells. The scar stiffens the heart, decreasing its capability and efficiency in pumping the blood. Therefore, intense efforts are being made for the restoration of lost cells by cell therapy and maintenance of cardiac function in patients with heart injury. Many stem cells types have been studied in order to select the best model for cardiac cell therapy. ES cells, iPS cells as well as adult stem cells (bone marrow, adipose tissue-derived, and cardiac stem cells) are already tested in animal models and humans with often promising results [42, 43].
The most promising model is provided by cardiac stem cells (CSCs) that reside in small populations in the adult mammalian myocardium and have the potential to differentiate into cardiomyocytes and other cell types, such as endothelial and vascular smooth muscle cells [44–47]. However, differentiation of these cells is rare under physiological conditions [48]. For therapeutic purposes, CSCs can be generated by expanding autologous cells ex vivo or stimulating the regeneration capacity of these cells in vivo. Nevertheless, one of the biggest problems hindering the therapeutic use of stem cells lies still in the difficulty of keeping stem cells alive following transplantation. Cell death occurs before cells can engraft in their environment due to inflammation-signaling responses, or cells do not even identify the injured tissue site for engraftment. Therefore, signaling factors necessary for cell establishment at the location of transplantation are being investigated. Such paracrine factors include ATP and adenosine and their respective receptor subtypes. P2Y14 receptors expressed by bone marrow HSCs induce migration of these cells to the localization of injury followed by induction of differentiation at the site mediated by activation of other purinergic receptors [49] (Fig. 2). Adenosine plays many roles in the heart including regulation of growth, differentiation, angiogenesis, coronary blood flow, cardiac conduction and heart rate, substrate metabolism, and sensitivity to adrenergic stimulation [50], and also functions as an endogenous determinant of ischemic tolerance [50]. The two A2 receptor subtypes (A2A and A2BA) possess important anti-inflammatory and immunomodulatory functions, and probably control the impact of inflammatory processes during ischemic and post-ischemic damage. Vinten-Johansen and colleagues confirm protective functions of A2A receptors in cardiac tissue by inhibition of neutrophil activation and neutrophil–vascular interactions as seen in Fig. 2 [51, 52].
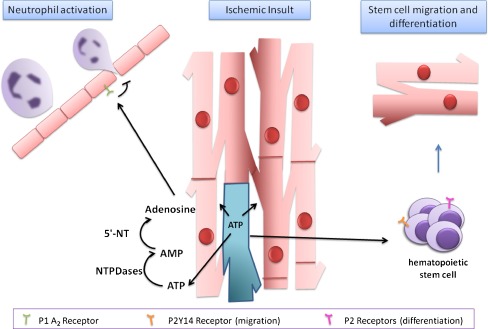
Fig. 2.
ATP-and adenosine-induced actions following cardiac ischemic insult. After myocardial injury following an ischemic insult, dead cells release ATP into the extracellular space. The released ATP stimulates P2Y14 receptors expressed by hematopoietic stem cells and possibly purinergic receptors on cardiac stem cells. NTPDases (ecto-nucleoside triphosphate diphosphohydrolases) dephosphorylate ATP via ADP to AMP, and 5-nucleotidase (5′-NT) catalyzes the hydrolysis of AMP to adenosine inducing anti-inflammatory responses by activation of A2A and A2B receptors, blocking neutrophil activation and migration
Extracellular pyridoxal-5′-phosphate (PLP), a synthesis precursor of PPADS, is considered a P2 receptor antagonist. When this compound is used in the micromolar concentration range, it prevents ATP-induced calcium influx in isolated rat cardiomyocytes, inhibiting the positive inotropic effects of ATP on isolated perfused hearts and blocking ATP binding to the cardiac sarcolemma. Recent research suggests that at least part of the protective effect observed during reperfusion by PLP may be mediated through its inhibitory action on purinergic receptors. The possible receptors expressed in cardiomyocytes and subject to inhibition by PLP are P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 subtypes [53]. Taking together, the strategy of cell therapy following a heart attack could base on activation of P2Y14 purinergic receptors expressed by bone marrow stem cells which then would induce migration to the site of injury and thus could restore heart tissue before the formation of a scar. Furthermore, concomitant activation of A2 receptors would decrease the damage caused by ischemia due to the anti-inflammatory activity of these receptors in preventing the activation of neutrophils which may cause further damage tissue. However, reservations remain regarding stimulation of P2Y receptors in cardiomyocytes due to their involvement in apoptosis induction. Taken together, fundamental roles exist for the purinergic system in cardiac protection and preconditioning suggesting possible applications together with stem cell therapy.
Bladder dysfunction and glomerular injury
Much effort has been spent for establishing a stem cell therapy for the regeneration of tissues, including nephron and bladder. The urinary system is composed basically by kidneys, ureters, bladder, and urethra, and disorders in any of these structures can cause much pain and suffering for the patient. Hemodialysis and implementation of tubes are usually used for the treatment of patients with urogenital diseases; however, unfortunately, there is no cure for many diseases. Different stem cell types have been tested for therapeutic applications with varying success. For therapy of bladder dysfunction, Nishijima et al. transplanted bone marrow stem cells by intrabladder injection resulting in restored bladder contraction in rats [54]. Huang et al. transplanted adipose-derived stem cells by intrabladder or intravenous injection resulting in improved tissue parameters and urodynamics in a rat model of overactive bladder [55]. Interestingly, De Coppi et al. showed that intrabladder transplantation of amniotic fluid or bone marrow stem cells promoted post-injury bladder remodeling by a paracrine mechanism [56]. According to Hallman et al., the repair of injured renal epithelium is thought to be mediated by surviving renal proximal tubular cells that must dedifferentiate to allow for proliferation and migration necessary for epithelial regeneration. ATP and its intracellular signaling have also crucial functions in this regeneration process. Kartha et al. showed that adenine nucleotides stimulate migration of kidney epithelial cells in an in vitro culture resembling wounded kidney. In these experiments, cells were treated with 10 μM of different adenine nucleotides, and the number of cells that migrated into the leasioned area of 1 mm2 in size was counted 24 h later [57]. Increases in migration were induced by cAMP, adenosine, AMP, and ATP suggesting purinergic receptors activation; however, P1 receptors may promote contrary functions in this context, as adenosine can induce apoptosis in glomerular mesangial cells causing glomerular injury [58]. Babelova et al. showed that the secretion of the pro-inflammatory master cytokine interleukin (IL)-1β during inflammatory renal injury interacts with purinergic P2X4/P2X7 receptors [59]. Moreover, P2X7 receptor expression in glomeruli was augmented tenfold in diabetic and hypertensive rat models when compared to that of healthy rat glomeruli [60]. Purinergic signaling has also been related to renal protection via A2a adenosine receptor activation in conditions of reperfusion injury [61]. In summary, for treatment of renal epithelium injury, transplantation of bone marrow or adipose tissue stem cells are promising. Migration to the injured sites can be induced by injecting cAMP, adenosine, AMP, and ATP suggesting purinergic receptor activation. On the other site, P1 receptor inhibition is indicated due to the contribution of these receptors to apoptosis under these conditions.
Parkinson’s disease
Probably most effort has been put into the study of the applicability of cellular therapy in the nervous system due to its enormous impact on patient’s life and a lack of therapeutic strategies to cure neurodegenerative diseases and spinal cord injuries. We describe here some recent discoveries related to purinergic signaling with impact on tissue repair in the neuronal system.
Parkinson’s disease (PD) is a neurodegenerative illness caused by death of dopaminergic neurons in the substantia nigra pars, but the underlying mechanisms of neuronal death remain largely unknown. Dopaminergic neurons are responsible for dopamine neurotransmitter secretion and control body movements. The absence of this molecule in patients with PD generates tremor rigidity, postural instability, and loss of motor coordination affecting writing capability among other disturbances. Increased survival is achieved by surgical therapies and medications, principally based on administration of l-DOPA, but its prolonged use may generate uncontrollable movements known as dyskinesia [63, 64]. Purinergic signaling has implications in PD, since treatment with ATP enhances the release of dopamine from dopaminergic neurons of the substantia nigra. However, at the same time, ATP release may activate P2X7 receptors expressed by neighboring cells thereby promoting cell death and contributing to an increase of the necrotic volume [62–65]. Furthermore, Feuvre et al. [66] provided evidence that P2X7 receptor activation following ATP release induces expression of proteins involved in the inflammatory response followed by liberation of cytokines. In addition, ATP together with glutamate released in neurodegenerative disorders may change intracellular Ca2+ homeostasis, mainly in neurons, with major importance for the disease progress [67].
Primary cultures of rat dopaminergic neurons express P2X1-7 and P2Y1 receptors together with D1 and D2 dopamine receptors [68]. P2Y receptor antagonists are potent neuroprotecting agents in the brain cortex, hippocampus, and cerebellum by modulating excessive neurotransmitter release in brain disorders [69, 70]; however, these effects would be undesirable in PD, since even PPADS blocking P2 receptors was shown to decrease dopamine secretion [71, 72]. A wide range of different strategies is under investigation for PD treatment, with a major focus research on stem cell therapy applications. Exogenous molecules are known to guide neural differentiation and are responsible for the high grade of phenotype specification, including induction of axonal growth and establishment of synaptic contact [73]. Milosevic et al. [74] detected P2Y4, P2Y6, and P2X4 receptor expression in cultured human NPCs from human fetal midbrain. UTP and UDP are known as agonists of the P2Y2/P2Y4 and P2Y6 receptors, respectively [75]. The treatment of hNPCs with UTP, in the presence of EGF and FGF2, increases cell proliferation. Moreover, UTP and UDP in the presence of specific culture medium enhance dopaminergic cell differentiation, and these effects are reduced by antagonists of P2 receptors.
Adenosine A2A receptors are selectively located on striatopallidal neurons and are capable of forming functional heteromeric complexes with dopamine D2 and metabotropic glutamate mGlu5 receptors. A2A receptor antagonists have emerged as an attractive nondopaminergic target to improve the motor deficits that characterize PD, based on the regional and unique cellular distribution of this receptor, being in agreement with data showing that A2A receptor antagonists improve motor symptoms in animal models of Parkinson’s disease and in initial clinical trials. Some experimental data also indicate that A2A receptor antagonists do not induce neuroplasticity phenomena which complicate long-term dopaminergic treatments [76].
These data suggest the involvement of purinergic signaling in dopaminergic cell differentiation and possible applications for purinergic receptors in in vitro differentiation cultures for posterior PD cell therapy [74]. However, more studies are needed to clarify whether extracellular nucleotides may contribute to favorable endogenous niches for stem cell transplantation or even recruit endogenous NPCs for dopaminergic differentiation.
Alzheimer’s disease
The pathogenicity of Alzheimer’s disease (AD) involves amyloids plaques and neurofibrillary tangle formation in the neuron extracellular medium. AD patients present an elevated production and secretion of the amyloid β peptide (Aβ) by neurons into the extracellular medium with progressive deposit of fibrils with high-grade toxicity, generating neuronal dysfunction and cell death [77–79]. This initial deposit triggers an inflammatory process with microglia and astrocyte recruitment to the injury site. Then, elevating cytokine secretion promotes Aβ internalization by neurons elevating neuronal damage [80–82]. ATP is released in high concentrations as result of cell death and enhances the local inflammatory effects besides increasing vulnerability of neurons by Aβ [61, 81]. Microglial cells recruited to the injury site showed elevated increased P2X7 receptor expression, as observed in animal models and human patients [83, 84]. P2X7 receptor activation by elevated ATP concentration promotes the secretion of the cytokines by microglial cells and activated oxygen species, increasing inflammation and stimulating Aβ-plaque formation, which also stimulates ATP liberation [82, 85, 86]. Furthermore, the P2Y1 subtype is expressed in AD typical structures such as Aβ plaques and neurofibrillary tangles, and receptor immunostaining was notably high in AD brain suggesting that P2Y1 receptors may participate in signaling events triggering neurodegenerative processes [61, 81, 82]. A1 and A2A adenosine receptor subtypes are expressed in the cortex, hippocampus, and microglia in the brain of patients suffering from AD. The A2A receptor was suggested to contribute to memory deficits. The administration of caffeine, an antagonist of A1 and A2A receptors, promoted the protection against Aβ-induced neurotoxicity. Moreover, in vivo studies with A2A antagonists resulted in reduced Aβ production and still protected against Aβ toxicity [87].
Hippocampus and the subventricular zone are the brain structures most affected in AD and are also the main sites of NPC localization. Increased NPC proliferation was observed in different illness stages; however, subsequent differentiation of these cells was not detected [88, 89]. NPCs implanted into the brain of a rat model of migrated to the disease site. Moreover, the presence of NPCs decreased microgliosis and the expression and secretion of pro-inflammatory cytokines, both characteristic conditions for AD. Elevated neuroprotection was also observed together with augmented expression of MAP-2, a marker protein for mature neurons. However, NPCs were nestin–positive and negative for expression of neuronal marker proteins in immunostaining assays, indicating that neuronal differentiation did not occur [90]. Secreted Aβ 1–42, a more toxic form of the amyloid peptide causing cell death, evoked a reduction of NPC proliferation [91]. Nowadays, acetylcholinesterase inhibitors are being used to enhance cholinergic function and induce a temporary cognition improvement. Implants of NPCs derived from the cholinergic regions of the forebrain, appear to be a valid approach for cell therapy. ATP, a natural cotransmitter of acetylcholine, may gain importance in this context for helping to reestablish defective cholinergic transmission.
Several cell lines and animal models are used to assess mechanisms of neural differentiation and the inter-relationship of action of various metabotropic and ionotropic receptors in this process. Trujillo et al. [62] suggested the intrinsic regulation between purinergic, cholinergic, and kallikrein–kinin systems for phenotype determination during neural differentiation. Using P19 embryonal carcinoma cells as in vitro model for neuronal differentiation, our group observed that functional purinergic receptors are essential for cell differentiation into neurons with functional cholinergic receptors [41].
According to Delarasse et al. [92], activation of P2X7 receptors stimulates soluble amyloid precursor protein α release from mouse neuroblastoma cells. In view of that, a possible treatment for AD could include inhibition of P2 receptors to decrease inflammatory responses, together with NPC injection secreting factors for reduction of inflammatory responses. Further studies will also reveal whether stimulation with ATP will help restoring cholinergic functions.
Epilepsy
Epilepsy is a brain disturbance manifested by frequent seizures with constant neural activation. It may be accompanied by massive glial cell proliferation, initiating following neurodegenerative processes. Several anti-epileptic agents inhibit the ability of astrocytes in transmitting intracellular Ca2+ waves. In view of that, purinergic receptor antagonists should offer a novel treatment for blocking Ca2+ wave propagation stimulated by ATP [93]. As further proof for such mechanism, injection of high doses of ATP into rat cortex promoted an increase in seizure occurrence, which could be antagonized by suramin [94]. Hippocampi from chronic epileptic rats demonstrated elevated P2X7 receptor expression and abnormal responses to ATP, suggesting a possible participation of this system in the pathophysiology of epilepsy [95]. Potent drugs are administrated in high doses in rats and mice to promote sequential seizures and behavioral and electrographic changes [62]. In a rat epilepsy model, kainate application elevated microglial purinergic receptor expression, mainly P2X7 and P2Y12 receptor subtypes. Both receptors are associated with the active state of microglia, inducing inflammatory responses and microglia migration, respectively [95]. In a temporal lobe epilepsy model induced by pilocarpine injection, P2X4 receptor expression was significantly reduced in pyramidal neurons reflecting a neuronal loss in a chronic status, while elevated P2X7 receptor expression was observed in glial cells suggesting again its participation in the inflammatory response [95]. Oses [96] observed a decrease in P2X receptor expression in rat hippocampus following convulsive periods which may be associated with progressing neurodegeneration and seizure worsening during epilepsy. However, adenosine acting through A1 receptors in an epilepsy model induced by pilocarpine promoted significant protection against seizures [95, 97]. As a possible mechanism, adenosine participates in cell proliferation regulation and apoptosis eliminating useless and damaged cells during repair, without the necessity of neurotoxic mediators or immunomodulators. Furthermore, adenosine can control astrocyte proliferation triggered by other purine nucleotides [98].
Cell transplantation strategies have been employed for the treatment of epileptic disorders, but the effect of exogenous neural stem cells is unknown. Chua et al. evaluated possible anti-epileptogenic effect of NSCs in adult rats with status epilepticus and showed that NSCs differentiate into inhibitory interneurons and decrease neuronal excitability, preventing spontaneous recurrent seizure formation in adult rats with pilocarpine-induced temporal lobe epilespy [99]. Therefore, a novel cellular source for the local therapeutic delivery of adenosine, a stem cell-based delivery system for adenosine, was generated by disruption of both alleles of adenosine kinase (AK) in mouse ES cells. These Ak−/− ES cells were differentiated into glial precursor cells and released significant amounts of adenosine. Rats with adenosine releasing Ak−/− ES cell-derived implants displayed transient protection against convulsive seizures and a profound reduction of after-discharge activity in EEG recordings, providing a proof-of-principle evidence that Ak−/− ES cell-derived brain implants suppress seizure activity by a paracrine mode of action [100]. In summary, stem cell therapy may be successful for epilepsy if transplanted NSCs feature a paracrine effect by releasing adenosine, which decreases the number of seizures, besides their ability to differentiate into inhibitory interneurons.
Trauma, ischemia, and hypoxia in the CNS
During several injury conditions such as trauma, ischemia, and hypoxia, ATP secretion is an important signaling molecule involved in repair of damaged tissue. After spinal cord injury, a large peritraumatic region sustains pathological processes to keep high ATP concentrations in the extracellular medium [61] involving P2X7 receptor activation and cell death as already discussed in this review. For instance, P2X7 receptors are expressed in neurons, astrocytes, and microglia of brain tissue suffering from ischemic conditions [82, 101]. Accordingly, administration of P2 receptor antagonists improved cell function and reduced cell death in the peritraumatic zone [81]. Moreover, after lesions in the peripheral nervous system. P2X3 receptor expression in intact neurons, suggesting a role for this receptor in post-traumatic repair [102]. Following trauma, astrocytes increased expression of P2X4 receptors thereby inducing trombospondin-1 secretion, which constitutes an extracellular molecule for synapse formation contributing to CNS remodeling [81, 103]. However, as already said, ATP also promotes neuronal apoptosis, necrosis and astrocytic death after traumatic events. P2 receptors promote the recruitment of microglial cells from distal areas to the traumatic core [104]. In vivo studies showed that P2X4 and P2Y12 receptors stimulated migration of microglial cells to the injury area after trauma, followed by expression of P2Y6 receptors favoring the secondary damage moment, the debris phagocytosis [82]. Experimental evidence indicates liberation of adenosine into the extracellular medium after tissue damage together with down-regulation of AK expression, leading to adenosine accumulation and neuroprotection following injury [105]. ATP in the extracellular medium may attract astrocytes and microglia to the site of injury in order to assist tissue repair [106]. In the normal adult brain, ATP secreted by astrocytes stimulates NPC proliferation and migration, while P2Y receptor antagonists reversed this effect by inhibiting proliferation. During the neurogenesis process, NPCs revealed NTPDase activity for controlling ATP concentration and subsequently directing neuronal and glial differentiation [40]. In summary, treatment of spinal cord injury and other traumatic and ischemic disorders of the CNS would benefit from P2 receptor inhibition in order to reduce cell death, followed by activation of P2X3 and P2X4 receptors for induction of synapse formation. Thereby, extracellular adenosine accumulation leads to neuroprotection during injury while ATP may attract astrocytes and microglia to the site of injury to assist tissue repair. In vitro NPC differentiation is directed by P2Y receptors; such mechanism should be further validated in animal models.
Skin injury
Skin is a stratified epithelium, where the epidermis is the outermost part of this tissue and dermis is innermost. Epidermis is mainly constituted by keratinocytes (90–95%) and these cells are arranged in continuous layers from the inside towards outer layers: the basal layer, the stratum spinosum, the granular layer and stratum corneum. The epidermis is capable of self-renewal by presenting adult stem cells, which proliferate and can originate a new epidermis to cover all body surfaces. These stem cells are located in a portion of the follicle hair known as bulge migrating upwards to the proliferative basal layer. Keratinocytes migrate from the basal layer to the skin surface with concomitant differentiation [107–109]. In physiological and in pathological conditions, many kind of cells related to nervous and immune systems, can generate ATP extravasation and accumulation in the extracellular medium of keratinocytes. Two important functions are attributed to this nucleotide such as modulation of keratinocyte proliferation and differentiation [110]. Many studies have shown that these effects are mediated by ATP action through P2X and P2Y receptor subtypes, and it is known that the epidermis expresses P2X5, P2X7, P2Y1, and P2Y2 subtypes with diverse functions [111–113].
P2Y1 and P2Y2 receptors expressed in basal layers of the fetal and adult epidermis [111, 113] were immune colocalized with the cell proliferation markers Ki67 and PCNA (proliferation cell nuclear antigen) [111]. The P2Y1 receptor agonist 2-methylthio-ADP (P2Y1 agonist) and UTP activating P2Y2 receptors induced proliferation in cell cultures of basal keratinocytes [113]. P2Y1 and P2Y2 subtypes are coupled to Phospholipase C via Gq/11 proteins with generation of Inositol 3-phosphate and, in sequence, induce intracellular calcium mobilization [113] leading to Cl− conductance and starting keratinocyte differentiation [112]. In an in vivo wound-healing model, the P2Y1 receptor is expressed in epidermal basal layers and the wound edge, while the P2Y2 subtype is expressed in basal and suprabasal layers, but is not expressed in the wound edge. Alterations of distribution patterns of purinergic receptors occur during phenotype changes as keratinocytes become migratory cells in the wound-healing process [110]. P2X5 receptors are expressed in undifferentiated basal and intermediate layers of fetal epidermis with high immunoreactivity for cytokeratin-10, an initial differentiation marker [111]. In wounded epidermis, keratinocytes of the wound edge increase P2X5 receptor expression [111]. P2X7 receptor expression was detected together with labeling for caspase-3 and TUNEL, markers for terminal differentiation and apoptosis, respectively, suggesting that this receptor eliminates not any more needed cells during final epidermis development [111]. Furthermore, the P2X7 subtype is also expressed in corneum stratum in adult epidermis suggesting its participation in apoptotic control [111, 113]. During wound healing processes, P2X7 receptor expression was not detected [111]. ATP is released by keratinocytes into the extracellular space by mechanical stress and external damage and achieves elevated extracellular, cytotoxic levels. Elevated ATP concentrations (300 μM) were applied together with UV radiation as external damage model in cultured human epidermal keratinocytes. Both situations augmented significantly P2X7 receptor and reduced P2Y2 receptor expression while P2X5 and P2Y1 subtype expression levels were not altered. These events associated with elevated extracellular ATP concentration result in skin inflammation, demonstrating the role of purinergic signaling in skin physiology and disease induction [112]. Purinergic signaling could promote skin injury therapy by selective activation of P2Y1 and P2Y2 receptors favoring the phenotype of migratory cells without induction of inflammatory responses.
Pulmonary epithelium injury
The airway epithelium is exposed to environmental pollutants, allergens and pathogens that might lead to tissue damage or the development of a variety of infectious and inflammatory diseases such as chronic bronchitis, chronic obstructive pulmonary disease, asthma, and fibrosis. In this context, stem and progenitor cells are involved in lung regeneration. They are located within the basal layer of the upper airways, within or near pulmonary neuroendocrine cell rests, at the bronchoalveolar junction, and within the epithelial surface [114–116]. The airway epithelium represents the first barrier to inhaled particles and pathogens and because of this, it suffers constant damages. Thus, the mechanism of the repair of damaged epithelium has been widely studied. Epithelial progenitors termed Clara cells (transit-amplifying cells) are broadly distributed and after injury differentiate into ciliated cells [117, 118]. In addition to Clara cells, bronchiolar airways have also rare stem cells that contribute to repair of the tissue [119]. Both Clara and stem cells present the CD45neg CD31neg CD34neg Scal low phenotype. However, it is possible to distinguish between the two cell types based on high (AFhigh) and low autofluorescence (AFlow), respectively [120]. Clara-like cells are another cell type that exhibits many features of pluripotent stem cells and apparently contributes to epithelial regeneration [120–122]. They can be discriminated from Clara cells by their resistance to naphthalene and their close association with pulmonary neuroepithelial bodies (NEBs) [123, 124]. ATP released from secretory vesicles of rodent NEBs [125] in response to depolarization in lung slices promotes paracrine effects on surrounding Clara-like cells by activation of P2Y2 receptors. Considering the stem cell-like characteristics of Clara-like cells, this purinergic signaling might be of great importance for airway epithelial repair after injury [123].
Furthermore, ATP regulates diverse processes involved in host defense such as anion transport, ciliary function and mucin expression and is also suggested to function in wound repair [126–128]. ATP-mediated P2 purinergic receptor activation promotes bronchial epithelial migration and epithelial repair. This is suggested to occur after activation of dual oxidase 1 mediated by release of ATP during injury [123]. In addition, adenosine also stimulates cell migration, proliferation, and angiogenesis [129, 130]. Experimental evidence suggests that adenosine evokes wound closure via A2A receptor activation, since A2A agonists promote early wound closure while A2A antagonists impede the healing process [131]. The continuous denudation and repair of airway epithelium occurs especially in inflammatory airways diseases such as asthma [132]. Asthma is a chronic inflammatory airway disease orchestrated by eosinophils, mast cells, Th2 lymphocytes, and dendritic cells (DCs) [133]. ATP is reported to be important for the genesis and maintenance of this disease. For instance, ATP triggers and maintains asthmatic inflammation by activating DCs and enhancing its Th2-priming capacity [134, 135]. Another study demonstrated that this allergic inflammation in humans and mice is associated with the functional up-regulation of P2X7 receptor expression on immune cells (macrophages and eosinophils) and that P2X7 receptor signaling (e.g., via modulating of DC function) is involved in ATP-mediated pro-asthmatic effects [136]. P2X7 receptor −/− knock-out animals or animals treated with a selective P2X7 receptor antagonist showed a strong reduction in all cardinal features of acute allergic airway inflammation including airway eosinophilia, goblet cell hyperplasia, and bronchial hyper-responsiveness to methacholine [137]. Thus, P2X7 receptor antagonists might be a new therapeutic option for the treatment of severe asthma. Moreover, adenosine is also important in asthmatic inflammation. Inhaled adenosine induced bronchoconstriction in patients suffering from chronic asthma or obstructive pulmonary disorder (COPD), and adenosine receptor blockade prevented this bronchoconstriction [138]. Adenosine-mediated effects through A2B and A3 receptor activation play key roles in mast cells producing pro-inflammatory mediators (histamine, IL-8, and degranulation) [139, 140]. Therefore, CVT-6883, an A2B receptor antagonist, is being evaluated in phase I clinical studies for the management of asthma and COPD in human patients. Mobilization of hematopoietic progenitor cells from the bone marrow comprises also a feature of asthmatic inflammation [141–143]. However, in the airway, these progenitor cells have the potential to generate in situ mature inflammatory cells, principally eosinophils [142, 144]. Moreover, it has been suggested that purinergic signaling in HSCs is important for genesis of asthma. Some studies indicate that this allergy is transferable and curable with allogeneic hematopoietic cell transplantation, but more studies are still necessary [144, 145]. In summary, for pulmonary epithelium repair, promotion of P2 purinergic receptor-mediated effects inducing bronchial epithelial migration and epithelial repair would be a valid strategy, while adenosine stimulates migration, proliferation and angiogenesis. Hematopoietic progenitor cells from the bone marrow have the potential to generate in situ mature inflammatory cells; therefore, it would be necessary to inhibit this effect while the epithelium is regenerating.
Conclusions
Stem cell transplantation and engraftment depends on the secretion of anti-inflammatory molecules, in addition to extrinsic and endogenous factors promoting differentiation into distinct cell types depending on the injury site. While adenosine receptors often, but not every time, exert beneficial effects in providing adequate stem cell niches, functions of P2Y and P2X receptors depend very much on the tissue and the expression pattern of these receptors (see Table 1). Therapeutic applications based on activation of purinergic signaling are foreseen for kidney and heart muscle regeneration, while other disease conditions will yet need further investigation. While nucleotides have been shown to promote differentiation of dopaminergic neurons destroyed in Parkinson’s disease, other neuronal diseases involve excitatory cell damage mostly due to P2X7 receptor action. Therapeutic inhibition of such receptor activity would be required for improving disease conditions. Finally, the need of P2Y2 and A2A receptor activation during Clara-like cell differentiation into pulmonary and bronchial epithelial cells just corroborates the fact that purinergic signaling is well involved in tissue repair, specially mediated by stem cells. More work need to be done for elucidation of crucial concepts which could revolutionize cell therapy.
Table 1.
Functions of purinergic receptor in stem cells and tissue repair
| Tissue/cell | Purinergic receptors | Action |
|---|---|---|
| Kidney epithelial cells | ↑P2R | Induction of cell migration in wounded kidney |
| Kidney | ↑P1 A2aR | Protection during reperfusion (ischemia) |
| Heart | ↑P1 A2A and A2BR | Anti-inflammatory function (ischemia) |
| Heart | ↓P2Y1, P2Y2, P2Y4, P2Y6,P2Y11R | Protection during reperfusion (ischemia) |
| Substantia nigra | ↑P2X7R | Induction of cell death (Parkinson’s Disease) |
| Brain cortex, hippocampus and cerebellum | ↑P2YR | Modulation of neurotransmitter release (healthy tissue) |
| Human neural progenitor cell | ↑P2Y4, P2Y6R | Induction of proliferation/dopaminergic differentiation of NPCs |
| Brain | ↑P2X7R | Cytokine secretion by microglial (increasing inflammation) |
| Brain | ↓A1, A2AR | Protection against Αβ plaque-mediated neurotoxicity (Alzheimer’s disease) |
| Skin | ↑P2Y1, P2Y2R | Induction of proliferation / migration of basal keratinocytes (wounded tissue) |
| Epidermis | ↑P2X5R | Induction of differentiation to keratinocytes (wounded tissue) |
| Epithelial pulmonary cells | ↑P2Y2R | Activation of Clara-like cells for tissue repair (tissue damage) |
| Bronchial epithelial cells | ↑P1 A2AR | Activation of cell migration and wound repair |
↑ upregulation and ↓ downregulation of purinergic receptor expression
Acknowledgments
HU acknowledges grant support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil, project no. 2006/61285-9. TG, PDN, and CL are supported by fellowships from FAPESP. ARC, MMP, and IC are grateful for fellowships from CNPq. Grant support by FAPERGS/CNPq - PRONEX, Brazil, is also acknowledged.
References
- 1.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39(3):190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36(9):1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 3.North RA. P2X receptors: a third major class of ligand-gated ion channels. Ciba Found Symp. 1996;198:91–105. doi: 10.1002/9780470514900.ch5. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. 1970. Br J Pharmacol. 1997;120(4 Suppl):337–357. doi: 10.1111/j.1476-5381.1997.tb06815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. The changing face of autonomic neurotransmission. Acta Physiol Scand. 1986;126(1):67–91. doi: 10.1111/j.1748-1716.1986.tb07790.x. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G, Ulrich H. Purinergic signaling in embryonic and stem cell development. Cell Mol Life Sci. 2011;68(8):1369–1394. doi: 10.1007/s00018-010-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8(3):346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 8.Hofer M, Vacek A, Pospisil M, Weiterova L, Hola J, Streitova D, Znojil V. Adenosine potentiates stimulatory effects on granulocyte-macrophage hematopoietic progenitor cells in vitro of IL-3 and SCF, but not those of G-CSF, GM-CSF and IL-11. Physiol Res. 2006;55(5):591–596. doi: 10.33549/physiolres.930854. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev Res. 2001;52(1–2):44–56. [Google Scholar]
- 10.Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;10:9–52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;17(30):9. doi: 10.1186/1756-9966-30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 13.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 14.Pelacho B, Mazo M, Gavira JJ, Prosper F. Adult stem cells: from new cell sources to changes in methodology. J Cardiovasc Transl Res. 2011;4(2):154–160. doi: 10.1007/s12265-010-9245-z. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465(7299):704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passier R, Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453(7193):322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 19.Dambrot C, Passier R, Atsma D, Mummery CL. Cardiomyocyte differentiation of pluripotent stem cells and their use as cardiac disease models. Biochem J. 2011;434(1):25–35. doi: 10.1042/BJ20101707. [DOI] [PubMed] [Google Scholar]
- 20.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20(6):661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 21.Laflamme MA, Myerson D, Saffitz JE. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 22.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 23.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442(7102):527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 24.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11(3):201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virgilio F, Falzoni S, Mutini C, Sanz JM, Chiozzi P. Purinergic P2X7 receptor: a pivotal role in inflammation and immunomodulation. Drug Dev Res. 1998;45(3–4):doi:10.1002/(SICI)1098-2299(199811/12)45:3/4<207::AID-DDR18>3.0.CO;2-N. [Google Scholar]
- 26.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 27.Carroll WA, Donnelly-Roberts D, Jarvis MF. Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signal. 2009;5(1):63–73. doi: 10.1007/s11302-008-9110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sak K, Boeynaems JM, Everaus H. Involvement of P2Y receptors in the differentiation of haematopoietic cells. J Leukoc Biol. 2003;73(4):442–447. doi: 10.1189/jlb.1102561. [DOI] [PubMed] [Google Scholar]
- 29.Casati A, Frascoli M, Traggiai E, Proietti M, Schenk U, Grassi F. Cell-autonomous regulation of hematopoietic stem cell cycling activity by ATP. Cell Death Differ. 2011;18(3):396–404. doi: 10.1038/cdd.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemoli RM, Ferrari D, Fogli M, Rossi L, Pizzirani C, Forchap S, Chiozzi P, Vaselli D, Bertolini F, Foutz T, Aluigi M, Baccarani M, Virgilio F. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood. 2004;104(6):1662–1670. doi: 10.1182/blood-2004-03-0834. [DOI] [PubMed] [Google Scholar]
- 31.Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, Salati S, Salvestrini V, Gulinelli S, Adinolfi E, Ferrari S, Virgilio F, Baccarani M, Lemoli RM. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109(2):533–542. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
- 32.Coppi E, Pugliese AM, Urbani S, Melani A, Cerbai E, Mazzanti B, Bosi A, Saccardi R, Pedata F. ATP modulates cell proliferation and elicits two different electrophysiological responses in human mesenchymal stem cells. Stem Cells. 2007;25(7):1840–1849. doi: 10.1634/stemcells.2006-0669. [DOI] [PubMed] [Google Scholar]
- 33.Delarasse C, Gonnord P, Galante M, Auger R, Daniel H, Motta I, Kanellopoulos JM. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J Neurochem. 2009;109(3):846–857. doi: 10.1111/j.1471-4159.2009.06008.x. [DOI] [PubMed] [Google Scholar]
- 34.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2(2):409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla V, Zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sevigny J, Robson SC, Braun N. Functional expression of the ecto-ATPase NTPDase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J Neurosci Res. 2005;80(5):600–610. doi: 10.1002/jnr.20508. [DOI] [PubMed] [Google Scholar]
- 36.Braun N, Sevigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003;17(7):1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- 37.Mishra SK, Braun N, Shukla V, Fullgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sevigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133(4):675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- 38.Kermer V, Ritter M, Albuquerque B, Leib C, Stanke M, Zimmermann H. Knockdown of tissue nonspecific alkaline phosphatase impairs neural stem cell proliferation and differentiation. Neurosci Lett. 2010;485(3):208–211. doi: 10.1016/j.neulet.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Langer D, Ikehara Y, Takebayashi H, Hawkes R, Zimmermann H. The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience. 2007;150(4):863–879. doi: 10.1016/j.neuroscience.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 40.Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol. 2007;302(1):356–366. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resende RR, Britto LR, Ulrich H. Pharmacological properties of purinergic receptors and their effects on proliferation and induction of neuronal differentiation of P19 embryonal carcinoma cells. Int J Dev Neurosci. 2008;26(7):763–777. doi: 10.1016/j.ijdevneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Xie X, Sun A, Huang Z, Zhu W, Wang S, Zou Y, Ge J. Another possible cell source for cardiac regenerative medicine: reprogramming adult fibroblasts to cardiomyocytes and endothelial progenitor cells. Med Hypotheses. 2011;76(3):365–367. doi: 10.1016/j.mehy.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 43.Martinez EC, Kofidis T. Adult stem cells for cardiac tissue engineering. J Mol Cell Cardiol. 2011;50(2):312–319. doi: 10.1016/j.yjmcc.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 45.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265(1):262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13(8):970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee BC, Cheng T, Adams GB, Attar EC, Miura N, Lee SB, Saito Y, Olszak I, Dombkowski D, Olson DP, Hancock J, Choi PS, Haber DA, Luster AD, Scadden DT. P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev. 2003;17(13):1592–1604. doi: 10.1101/gad.1071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Headrick JP, Hack B, Ashton KJ. Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am J Physiol Heart Circ Physiol. 2003;285(5):H1797–H1818. doi: 10.1152/ajpheart.00407.2003. [DOI] [PubMed] [Google Scholar]
- 51.Jordan JE, Zhao ZQ, Sato H, Taft S, Vinten-Johansen J. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther. 1997;280(1):301–309. [PubMed] [Google Scholar]
- 52.Zhao ZQ, Todd JC, Sato H, Ma XL, Vinten-Johansen J. Adenosine inhibition of neutrophil damage during reperfusion does not involve K(ATP)-channel activation. Am J Physiol. 1997;273(4 Pt 2):H1677–H1687. doi: 10.1152/ajpheart.1997.273.4.H1677. [DOI] [PubMed] [Google Scholar]
- 53.Millart H, Alouane L, Oszust F, Chevallier S, Robinet A. Involvement of P2Y receptors in pyridoxal-5′-phosphate-induced cardiac preconditioning. Fundam Clin Pharmacol. 2009;23(3):279–292. doi: 10.1111/j.1472-8206.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 54.Nishijima S, Sugaya K, Miyazato M, Kadekawa K, Oshiro Y, Uchida A, Hokama S, Ogawa Y. Restoration of bladder contraction by bone marrow transplantation in rats with underactive bladder. Biomed Res. 2007;28(5):275–280. doi: 10.2220/biomedres.28.275. [DOI] [PubMed] [Google Scholar]
- 55.Huang YC, Shindel AW, Ning H, Lin G, Harraz AM, Wang G, Garcia M, Lue TF, Lin CS. Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol. 2010;183(3):1232–1240. doi: 10.1016/j.juro.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coppi P, Callegari A, Chiavegato A, Gasparotto L, Piccoli M, Taiani J, Pozzobon M, Boldrin L, Okabe M, Cozzi E, Atala A, Gamba P, Sartore S. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol. 2007;177(1):369–376. doi: 10.1016/j.juro.2006.09.103. [DOI] [PubMed] [Google Scholar]
- 57.Kartha S, Toback FG. Adenine nucleotides stimulate migration in wounded cultures of kidney epithelial cells. J Clin Invest. 1992;90(1):288–292. doi: 10.1172/JCI115851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Z, Kapoian T, Shepard M, Lianos EA. Adenosine-induced apoptosis in glomerular mesangial cells. Kidney Int. 2002;61(4):1276–1285. doi: 10.1046/j.1523-1755.2002.00256.x. [DOI] [PubMed] [Google Scholar]
- 59.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284(36):24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vonend O, Turner CM, Chan CM, Loesch A, Dell’Anna GC, Srai KS, Burnstock G, Unwin RJ. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int. 2004;66(1):157–166. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 61.Lee HT, Emala CW. Systemic adenosine given after ischemia protects renal function via A(2a) adenosine receptor activation. Am J Kidney Dis. 2001;38(3):610–618. doi: 10.1053/ajkd.2001.26888. [DOI] [PubMed] [Google Scholar]
- 62.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7(7):575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 63.Trujillo CA, Schwindt TT, Martins AH, Alves JM, Mello LE, Ulrich H. Novel perspectives of neural stem cell differentiation: from neurotransmitters to therapeutics. Cytometry A. 2009;75(1):38–53. doi: 10.1002/cyto.a.20666. [DOI] [PubMed] [Google Scholar]
- 64.Morley JF, Hurtig HI. Current understanding and management of Parkinson disease: five new things. Neurology. 2010;75(18 Suppl 1):S9–S15. doi: 10.1212/WNL.0b013e3181fb3628. [DOI] [PubMed] [Google Scholar]
- 65.Jun D, Kim K (2004) ATP-mediated necrotic volume increase (NVI) in sustancia nigra pars compacta dopaminergic neuron. Abstract Viewer/Itinerary Planner; Program no. 222.18. Society for Neuroscience, Washington, DC
- 66.Feuvre R, Brough D, Rothwell N. Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol. 2002;447(2–3):261–269. doi: 10.1016/s0014-2999(02)01848-4. [DOI] [PubMed] [Google Scholar]
- 67.Heine C, Wegner A, Grosche J, Allgaier C, Illes P, Franke H. P2 receptor expression in the dopaminergic system of the rat brain during development. Neuroscience. 2007;149(1):165–181. doi: 10.1016/j.neuroscience.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 68.Scheibler P, Pesic M, Franke H, Reinhardt R, Wirkner K, Illes P, Norenberg W. P2X2 and P2Y1 immunofluorescence in rat neostriatal medium-spiny projection neurones and cholinergic interneurones is not linked to respective purinergic receptor function. Br J Pharmacol. 2004;143(1):119–131. doi: 10.1038/sj.bjp.0705916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zona C, Marchetti C, Volonte C, Mercuri NB, Bernardi G. Effect of P2 purinoceptor antagonists on kainate-induced currents in rat cultured neurons. Brain Res. 2000;882(1–2):26–35. doi: 10.1016/s0006-8993(00)02781-5. [DOI] [PubMed] [Google Scholar]
- 70.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58(1):58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 71.Krugel U, Kittner H, Illes P. Adenosine 5′-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Neurosci Lett. 1999;265(1):49–52. doi: 10.1016/s0304-3940(99)00206-2. [DOI] [PubMed] [Google Scholar]
- 72.Krugel U, Kittner H, Franke H, Illes P. Accelerated functional recovery after neuronal injury by P2 receptor blockade. Eur J Pharmacol. 2001;420(2–3):R3–R4. doi: 10.1016/s0014-2999(01)01001-9. [DOI] [PubMed] [Google Scholar]
- 73.Gaspard N, Vanderhaeghen P. From stem cells to neural networks: recent advances and perspectives for neurodevelopmental disorders. Develop Med Child Neurol. 2011;53(1):13–17. doi: 10.1111/j.1469-8749.2010.03827.x. [DOI] [PubMed] [Google Scholar]
- 74.Milosevic J, Brandt A, Roemuss U, Arnold A, Wegner F, Schwarz SC, Storch A, Zimmermann H, Schwarz J. Uracil nucleotides stimulate human neural precursor cell proliferation and dopaminergic differentiation: involvement of MEK/ERK signalling. J Neurochem. 2006;99(3):913–923. doi: 10.1111/j.1471-4159.2006.04132.x. [DOI] [PubMed] [Google Scholar]
- 75.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 76.Morellia M, Paolob T, Wardasc J, Calonb F, Xiaod D, Schwarzschildd MA. Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog Neurobiol. 2007;83(5):293–309. doi: 10.1016/j.pneurobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Haughey NJ, Mattson MP. Alzheimer’s amyloid beta-peptide enhances ATP/gap junction-mediated calcium-wave propagation in astrocytes. Neuromolecular Med. 2003;3(3):173–180. doi: 10.1385/NMM:3:3:173. [DOI] [PubMed] [Google Scholar]
- 78.Thathiah A, Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat Rev Neurosci. 2011;12(2):73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- 79.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 80.Zhang YX, Yamashita H, Ohshita T, Sawamoto N, Nakamura S. ATP increases extracellular dopamine level through stimulation of P2Y purinoceptors in the rat striatum. Brain Res. 1995;691(1–2):205–212. doi: 10.1016/0006-8993(95)00676-h. [DOI] [PubMed] [Google Scholar]
- 81.Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther. 2006;109(3):297–324. doi: 10.1016/j.pharmthera.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Virgilio F. Activation of microglia by amyloid beta requires P2X7 receptor expression. J Immunol. 2009;182(7):4378–4385. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- 83.McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol. 2006;65(11):1090–1097. doi: 10.1097/01.jnen.0000240470.97295.d3. [DOI] [PubMed] [Google Scholar]
- 84.Rampe D, Wang L, Ringheim GE. P2X7 receptor modulation of beta-amyloid- and LPS-induced cytokine secretion from human macrophages and microglia. J Neuroimmunol. 2004;147(1–2):56–61. doi: 10.1016/j.jneuroim.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Majumder P, Trujillo CA, Lopes CG, Resende RR, Gomes KN, Yuahasi KK, Britto LR, Ulrich H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007;3(4):317–331. doi: 10.1007/s11302-007-9074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83(6):1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 87.Ballard C, Gauthier S, Corbett A, Brayne C, Jones AD, Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 88.Resende RR, Majumder P, Gomes KN, Britto LR, Ulrich H. P19 embryonal carcinoma cells as in vitro model for studying purinergic receptor expression and modulation of N-methyl-d-aspartate-glutamate and acetylcholine receptors during neuronal differentiation. Neuroscience. 2007;146(3):1169–1181. doi: 10.1016/j.neuroscience.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 89.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders—time for clinical translation? J Clin Invest. 2010;120(1):29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ryu JK, Cho T, Wang YT, McLarnon JG. Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed AD brain. J Neuroinflammation. 2009;6:39. doi: 10.1186/1742-2094-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chuang TT. Neurogenesis in mouse models of Alzheimer’s disease. Biochim Biophys Acta. 2010;1802(10):872–880. doi: 10.1016/j.bbadis.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 92.Delarasse C, Auger R, Gonnord P, Fontaine B, Kanellopoulos JM. The purinergic receptor P2X7 triggers α-secretase-dependent processing of the amyloid precursor protein. J Bio Chem. 2011;286:2596–2606. doi: 10.1074/jbc.M110.200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knutsen LJ, Murray TF. Adenosine and ATP epilepsy. In: Jacobson KA, Jarvis MF, editors. Purinergic approaches in experimental therapeutics. New York: Wiley; 1997. pp. 423–447. [Google Scholar]
- 94.Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. 2008;28(37):9133–9144. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dona F, Ulrich H, Persike DS, Conceicao IM, Blini JP, Cavalheiro EA, Fernandes MJ. Alteration of purinergic P2X4 and P2X7 receptor expression in rats with temporal-lobe epilepsy induced by pilocarpine. Epilepsy Res. 2009;83(2–3):157–167. doi: 10.1016/j.eplepsyres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Oses JP. Modification by kainate-induced convulsions of the density of presynaptic P2X receptors in the rat hippocampus. Purinergic Signalling. 2006;2:252–253. [Google Scholar]
- 97.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 98.Ciccarelli R, Ballerini P, Sabatino G, Rathbone MP, D’Onofrio M, Caciagli F, Iorio P. Involvement of astrocytes in purine-mediated reparative processes in the brain. Int J Dev Neurosci. 2001;19(4):395–414. doi: 10.1016/s0736-5748(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 99.Chua K, Kima M, Junga K, Jeond D, Leea S, Kima J, Jeongf S, Kimg SU, Leea SK, Shind H, Roha J. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004;1023(2):213–221. doi: 10.1016/j.brainres.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 100.Guttinger M, Fedele D, Koch P, Padrun V, Pralong WF, Brustle O, Boison D. Suppression of kindled seizures by paracrine adenosine release from stem cell-derived brain implants. Epilepsia. 2005;46(8):1162–1169. doi: 10.1111/j.1528-1167.2005.61804.x. [DOI] [PubMed] [Google Scholar]
- 101.Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63(7):686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 102.Tsuzuki K, Kondo E, Fukuoka T, Yi D, Tsujino H, Sakagami M, Noguchi K. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91(3):351–360. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- 103.Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Virgilio F, Abbracchio MP, Verderio C. A role for P2X7 in microglial proliferation. J Neurochem. 2006;99(3):745–758. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- 104.Ceruti S, Villa G, Genovese T, Mazzon E, Longhi R, Rosa P, Bramanti P, Cuzzocrea S, Abbracchio MP. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain. 2009;132(Pt 8):2206–2218. doi: 10.1093/brain/awp147. [DOI] [PubMed] [Google Scholar]
- 105.Cunha RA. Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1(2):111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nedeljkovic N, Bjelobaba I, Subasic S, Lavrnja I, Pekovic S, Stojkov D, Vjestica A, Rakic L, Stojiljkovic M. Up-regulation of ectonucleotidase activity after cortical stab injury in rats. Cell Biol Int. 2006;30(6):541–546. doi: 10.1016/j.cellbi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 107.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3(3):199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 108.Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002;12(4):390–399. [PubMed] [Google Scholar]
- 109.Fujishita K, Koizumi S, Inoue K. Upregulation of P2Y2 receptors by retinoids in normal human epidermal keratinocytes. Purinergic Signal. 2006;2(3):491–498. doi: 10.1007/s11302-005-7331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holzer AM, Granstein RD. Role of extracellular adenosine triphosphate in human skin. J Cutan Med Surg. 2004;8(2):90–96. doi: 10.1007/s10227-004-0125-5. [DOI] [PubMed] [Google Scholar]
- 111.Greig AV, James SE, McGrouther DA, Terenghi G, Burnstock G. Purinergic receptor expression in the regeneration epidermis in a rat model of normal and delayed wound healing. Exp Dermatol. 2003;12(6):860–871. doi: 10.1111/j.0906-6705.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 112.Greig AV, Linge C, Cambrey A, Burnstock G. Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation, and apoptosis in human fetal epidermis. J Invest Dermatol. 2003;121(5):1145–1149. doi: 10.1046/j.1523-1747.2003.12567.x. [DOI] [PubMed] [Google Scholar]
- 113.Inoue K, Denda M, Tozaki H, Fujishita K, Koizumi S. Characterization of multiple P2X receptors in cultured normal human epidermal keratinocytes. J Invest Dermatol. 2005;124(4):756–763. doi: 10.1111/j.0022-202X.2005.23683.x. [DOI] [PubMed] [Google Scholar]
- 114.Burnstock G, Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9. doi: 10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Volonte C, Amadio S, D’Ambrosi N, Colpi M, Burnstock G. P2 receptor web: complexity and fine-tuning. Pharmacol Ther. 2006;112(1):264–280. doi: 10.1016/j.pharmthera.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 116.Snyder JC, Teisanu RM, Stripp BR. Endogenous lung stem cells and contribution to disease. J Pathol. 2009;217(2):254–264. doi: 10.1002/path.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chistiakov DA. Endogenous and exogenous stem cells: a role in lung repair and use in airway tissue engineering and transplantation. J Biomed Sci. 2010;17:92. doi: 10.1186/1423-0127-17-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sueblinvong V, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res. 2010;156(3):188–205. doi: 10.1016/j.trsl.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest. 1978;38(6):648–653. [PubMed] [Google Scholar]
- 120.Stripp BR. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell? Proc Am Thorac Soc. 2008;5(6):695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teisanu RM, Lagasse E, Whitesides JF, Stripp BR. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells. 2009;27(3):612–622. doi: 10.1634/stemcells.2008-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24(6):662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 123.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156(1):269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Proost I, Pintelon I, Wilkinson WJ, Goethals S, Brouns I, Nassauw L, Riccardi D, Timmermans JP, Kemp PJ, Adriaensen D. Purinergic signaling in the pulmonary neuroepithelial body microenvironment unraveled by live cell imaging. FASEB J. 2009;23(4):1153–1160. doi: 10.1096/fj.08-109579. [DOI] [PubMed] [Google Scholar]
- 125.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol. 2000;278(6):L1256–L1263. doi: 10.1152/ajplung.2000.278.6.L1256. [DOI] [PubMed] [Google Scholar]
- 126.Brouns I, Oztay F, Pintelon I, Proost I, Lembrechts R, Timmermans JP, Adriaensen D. Neurochemical pattern of the complex innervation of neuroepithelial bodies in mouse lungs. Histochem Cell Biol. 2009;131(1):55–74. doi: 10.1007/s00418-008-0495-7. [DOI] [PubMed] [Google Scholar]
- 127.Conway JD, Bartolotta T, Abdullah LH, Davis CW. Regulation of mucin secretion from human bronchial epithelial cells grown in murine hosted xenografts. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L945–L954. doi: 10.1152/ajplung.00410.2002. [DOI] [PubMed] [Google Scholar]
- 128.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615(1–2):7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 129.Wesley UV, Bove PF, Hristova M, McCarthy S, Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem. 2007;282(5):3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 130.Ethier MF, Chander V, Dobson JG., Jr Adenosine stimulates proliferation of human endothelial cells in culture. Am J Physiol. 1993;265(1 Pt 2):H131–H138. doi: 10.1152/ajpheart.1993.265.1.H131. [DOI] [PubMed] [Google Scholar]
- 131.Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160(6):2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Allen-Gipson DS, Wong J, Spurzem JR, Sisson JH, Wyatt TA. Adenosine A2A receptors promote adenosine-stimulated wound healing in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L849–L855. doi: 10.1152/ajplung.00373.2005. [DOI] [PubMed] [Google Scholar]
- 133.Erjefalt JS, Persson CG. Airway epithelial repair: breathtakingly quick and multipotentially pathogenic. Thorax. 1997;52(11):1010–1012. doi: 10.1136/thx.52.11.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 135.Idzko M, Hammad H, Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Virgilio F, Virchow JC, Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13(8):913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 136.Sala A, Sebastiani S, Ferrari D, Virgilio F, Idzko M, Norgauer J, Girolomoni G. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood. 2002;99(5):1715–1722. doi: 10.1182/blood.v99.5.1715. [DOI] [PubMed] [Google Scholar]
- 137.Muller T, Vieira RP, Grimm M, Durk T, Cicko S, Zeiser R, Jakob T, Martin SF, Blumenthal B, Sorichter S, Ferrari D, Virgillio F, Idzko M. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol. 2011;44(4):456–464. doi: 10.1165/rcmb.2010-0129OC. [DOI] [PubMed] [Google Scholar]
- 138.Polosa R, Holgate ST. Adenosine receptors as promising therapeutic targets for drug development in chronic airway inflammation. Curr Drug Targets. 2006;7(6):699–706. doi: 10.2174/138945006777435236. [DOI] [PubMed] [Google Scholar]
- 139.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003;171(1):338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 140.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Dikov MM, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J Immunol. 2008;180(11):7212–7220. doi: 10.4049/jimmunol.180.11.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gibson PG, Manning PJ, O’Byrne PM, Girgis-Gabardo A, Dolovich J, Denburg JA, Hargreave FE. Allergen-induced asthmatic responses. Relationship between increases in airway responsiveness and increases in circulating eosinophils, basophils, and their progenitors. Am Rev Respir Dis. 1991;143(2):331–335. doi: 10.1164/ajrccm/143.2.331. [DOI] [PubMed] [Google Scholar]
- 142.Dorman SC, Efthimiadis A, Babirad I, Watson RM, Denburg JA, Hargreave FE, O’Byrne PM, Sehmi R. Sputum CD34+IL-5Ralpha+ cells increase after allergen: evidence for in situ eosinophilopoiesis. Am J Respir Crit Care Med. 2004;169(5):573–577. doi: 10.1164/rccm.200307-1004OC. [DOI] [PubMed] [Google Scholar]
- 143.Catalli AE, Thomson JV, Babirad IM, Duong M, Doyle TM, Howie KJ, Newbold P, Craggs RI, Foster M, Gauvreau GM, O’Byrne PM, Sehmi R. Modulation of beta1-integrins on hemopoietic progenitor cells after allergen challenge in asthmatic subjects. J Allergy Clin Immunol. 2008;122(4):803–810. doi: 10.1016/j.jaci.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 144.Southam DS, Widmer N, Ellis R, Hirota JA, Inman MD, Sehmi R. Increased eosinophil-lineage committed progenitors in the lung of allergen-challenged mice. J Allergy Clin Immunol. 2005;115(1):95–102. doi: 10.1016/j.jaci.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 145.Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20(2):349–360. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]