Abstract
Low angle x-ray diffraction patterns were obtained from resting and activated frog sartorius muscles by means of a position-sensitive detector. Although the intensity ratio I10/I11 decreased many-fold upon activation, it was nearly the same during isometric and isotonic contraction. Thus, motion has a much smaller effect on the low order equatorial pattern than the transition from rest to activity. Analysis of the 10 and 11 reflections separately showed that I10 and I11 change reciprocally upon activation, and that they both increase by a small amount in the transition from isometric to isotonic contraction. If the intensity ratio can be taken as a measure of cross-bridge number, the results provide evidence that the drop in force in an actively shortening muscle is due primarily to the influence of motion on the configuration, rather than the number, of cross-bridges.
Full text
PDF
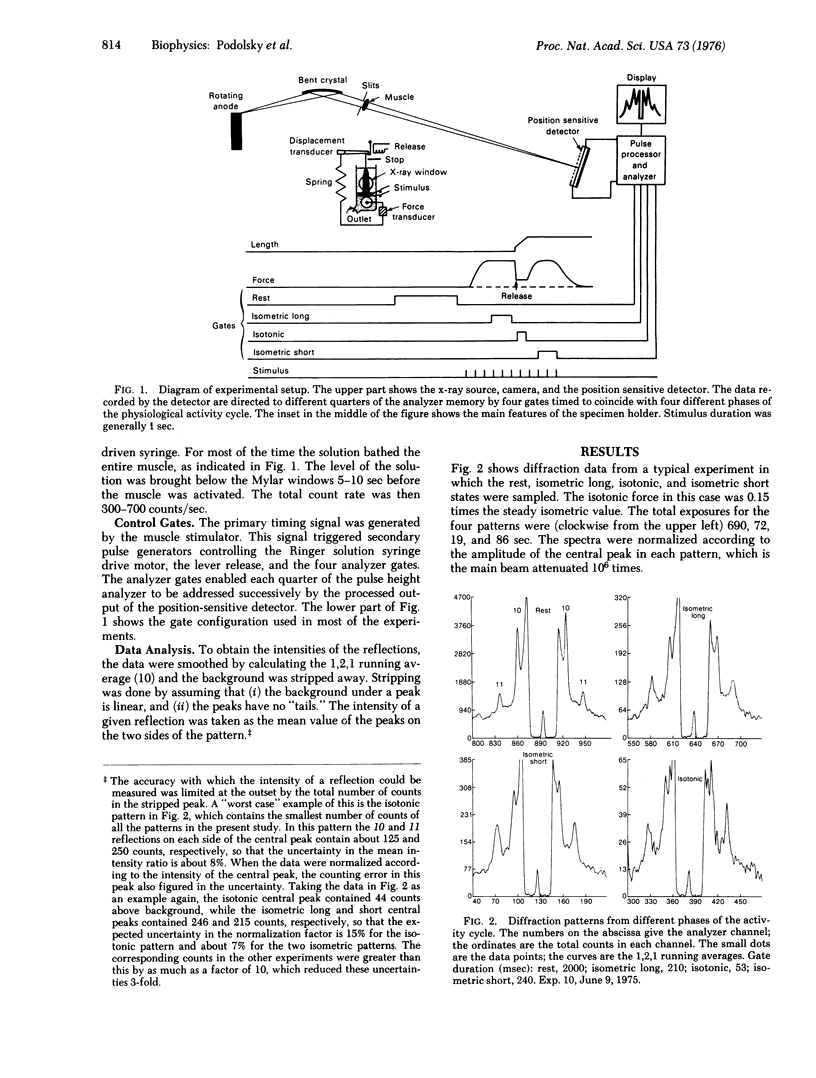
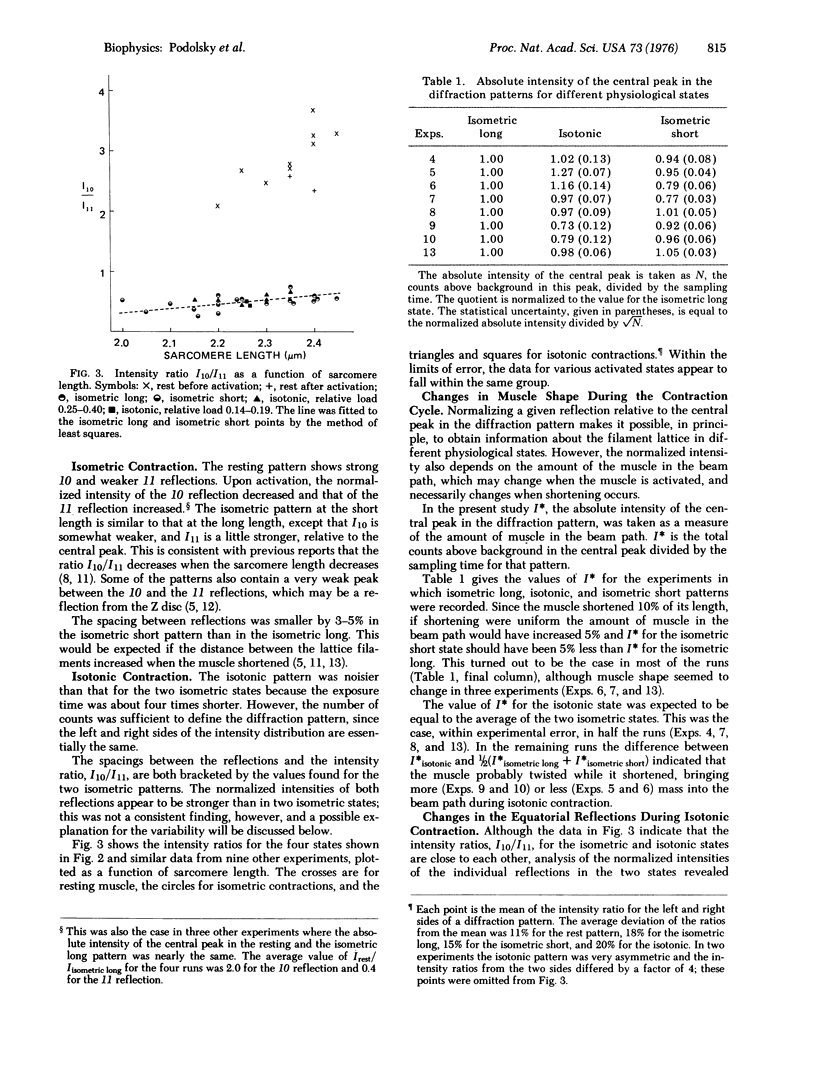
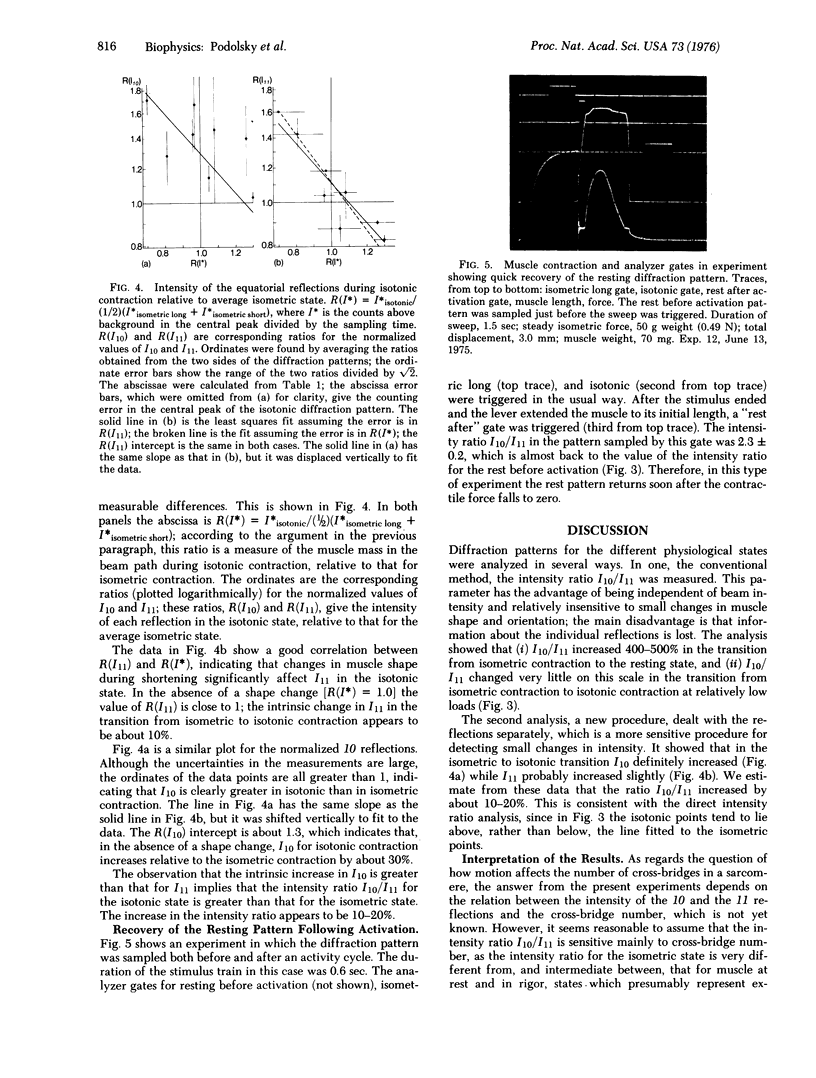

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elliott G. F., Lowy J., Millman B. M. Low-angle x-ray diffraction studies of living striated muscle during contraction. J Mol Biol. 1967 Apr 14;25(1):31–45. doi: 10.1016/0022-2836(67)90277-x. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Chen Y. D., Podolsky R. J. Some self-consistent two-state sliding filament models of muscle contraction. Biophys J. 1975 Apr;15(4):335–372. doi: 10.1016/S0006-3495(75)85823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W. Equatorial X-ray reflections and cross arm movement in skeletal muscle. Nature. 1975 Dec 25;258(5537):770–772. doi: 10.1038/258770a0. [DOI] [PubMed] [Google Scholar]
- Nihel T., Mendelson R. A., Botts J. The site of force generation in muscle contraction as deduced from fluorescence polarization studies. Proc Natl Acad Sci U S A. 1974 Feb;71(2):274–277. doi: 10.1073/pnas.71.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., Nolan A. C., Zaveler S. A. Cross-bridge properties derived from muscle isotonic velocity transients. Proc Natl Acad Sci U S A. 1969 Oct;64(2):504–511. doi: 10.1073/pnas.64.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]