Abstract
Purinergic signaling plays a unique role in the brain by integrating neuronal and glial cellular circuits. The metabotropic P1 adenosine receptors and P2Y nucleotide receptors and ionotropic P2X receptors control numerous physiological functions of neuronal and glial cells and have been implicated in a wide variety of neuropathologies. Emerging research suggests that purinergic receptor interactions between cells of the central nervous system (CNS) have relevance in the prevention and attenuation of neurodegenerative diseases resulting from chronic inflammation. CNS responses to chronic inflammation are largely dependent on interactions between different cell types (i.e., neurons and glia) and activation of signaling molecules including P2X and P2Y receptors. Whereas numerous P2 receptors contribute to functions of the CNS, the P2Y2 receptor is believed to play an important role in neuroprotection under inflammatory conditions. While acute inflammation is necessary for tissue repair due to injury, chronic inflammation contributes to neurodegeneration in Alzheimer’s disease and occurs when glial cells undergo prolonged activation resulting in extended release of proinflammatory cytokines and nucleotides. This review describes cell-specific and tissue-integrated functions of P2 receptors in the CNS with an emphasis on P2Y2 receptor signaling pathways in neurons, glia, and endothelium and their role in neuroprotection.
Keywords: P2Y2 receptor, CNS, P2X and P2Y receptors
Introduction
It has become apparent that P2 receptors for extracellular nucleotides are ubiquitously expressed in a wide variety of tissues, and the complexity of responses to nucleotides is due in large part to the presence of multiple subtypes of P2X receptor ligand-gated ion channels and G protein-coupled P2Y receptors [1–7]. This functional complexity is well manifested in the central nervous system (CNS) where 7 P2X and 8 P2Y receptor subtypes are expressed under a range of conditions in several different interacting cell types [6, 8–12]. Accordingly, studies on P2 receptor functions in the brain must consider the combined contributions of P2X and P2Y receptors expressed in neurons, microglial cells, astrocytes, and endothelium [10, 13–15]. In addition, the major ligand that activates many of these P2 receptor subtypes is ATP, released in the course of neurotransmission or under proinflammatory or cell apoptotic conditions [1, 16–18]. Since ATP or its degradative products activate most of the P2 nucleotide and P1 adenosine receptor subtypes identified in the CNS [1], unraveling the effects of ATP in vivo is difficult. This analysis can be simplified using animal models with selective knockout of specific P2 receptor subtypes or subtype-selective agonists/antagonists when available. Another approach is to analyze the effects of uridine nucleotides (i.e., UTP/UDP) that only activate Gq-coupled P2Y2, P2Y4, and P2Y6 receptors among the known P2 receptor subtypes [2, 4, 19, 20]. Among these three receptors, the P2Y2 receptor (P2Y2R) has unique motifs that promote interactions with integrins and growth factor receptors, thereby enabling activation of signaling pathways beyond Gq-dependent phospholipase C (PLC) [21–24]. Furthermore, the contribution of the P2Y2R subtype to CNS functions is becoming better understood [14, 15, 25] and appears to be most important under pathophysiological conditions, such as inflammation and bacterial infection [15, 26]. In addition, the availability of the P2Y2R knockout mouse has provided a valuable tool to dissect functional interactions between P2Y2Rs and other P2 receptors expressed in cell types of the CNS under various conditions [27, 28].
This review focuses on cell-specific and tissue-integrated functions of P2Y2Rs in the CNS with an emphasis on P2Y2R signaling pathways in neurons, glia, and endothelium that comprise the structure of the brain. In addition, we describe mechanisms whereby the P2Y2R activates cellular responses under proinflammatory conditions associated with neurodegenerative diseases, such as Alzheimer’s disease (AD), and postulate a role for these receptors in the regulation of neuroprotective responses.
P2 receptors in the CNS
Purinergic receptors are expressed in many mammalian cell types and are activated by extracellular adenine and uridine nucleotides or nucleosides [6, 29–31]. Both P1 receptors for adenosine and P2 receptors (P2Rs) for adenine and/or uridine nucleotides are expressed in cells comprising the CNS and have been shown to regulate important physiological and pathophysiological functions, including neurotransmission, inflammation, cell growth, and apoptosis [11, 31–33]. The P2R agonist ATP is a neuro- and gliotransmitter released by exocytosis from neurons and by diffusion through hemichannels, pannexins, and voltage-gated channels in various cell types [12, 17, 34–37]. P2Rs (both P2X ligand-gated cation channels and P2Y G protein-coupled receptors) [1, 4, 6] are expressed in neuroglia (astrocytes, oligodendrocytes) and microglia of the CNS [14], where they regulate differentiation, nociceptive transmission, cytokine release, apoptosis, and metalloprotease-dependent degradation of amyloid precursor protein (APP) [16, 27, 31, 38–40]. Among cell types that comprise the CNS, mRNAs for P2X1–7 and P2Y1,2,4,6,11,12,13,14 receptor subtypes have been identified in primary rat astrocytes [4, 41–44], and their expression patterns can vary with the age of the animal [45, 46]. Neurons express mRNAs for P2X3, 5–7 and P2Y1,2,4,6,12,13 receptors [12, 47–49]. Multiple subtypes of P2Rs are expressed in monocytes (P2X1,4,7 and P2Y1,2,4,6,11,12,13 receptors) [50] and human endothelial cells (P2X4 and P2Y1,2,4,6,11 receptors) [51]. The role of P1 and P2 receptors in the function of immune cells (e.g., neutrophils, eosinophils, monocytes, macrophages, mast cells, and lymphocytes) has been well described [26, 52–57], and the studies suggest that these receptors regulate cellular responses associated with inflammatory diseases. P2Rs are expressed at presynaptic nerve terminals where P2X1, P2X2, and P2X3 receptors have facilitatory, whereas P2Y1, P2Y2, and P2Y4 receptors have inhibitory roles in synaptic transmission [10, 58–62]. Studies also have shown that postsynaptic P2 receptors including P2X3, P2Y4, and P2Y1 receptors are involved in neuromodulation [10, 63–65], where they regulate either transmitter release or postsynaptic sensitivity to other neurotransmitters.
Among the G protein-coupled P2YRs, the Gq-coupled P2Y2R subtype is expressed in neurons and glial cells [13, 15, 48, 66–68]. Our studies using in situ hybridization and reverse transcriptase polymerase chain reaction with rodent brain slices have shown high levels of P2Y2R expression in the hippocampus and cerebellum [20], and P2Y2R expression can be significantly upregulated in mouse cortical neurons by the proinflammatory cytokine interleukin-1β (IL-1β) [48]. P2YRs have been shown to be coupled either directly or indirectly to Gq, Gi, Go, and G12 protein activation and downstream signaling pathways associated with alterations in PLC or adenylyl cyclase activities [1, 20, 69–71]. The agonist selectivity of P2YR subtypes varies widely, in contrast to P2X receptors [1]; for example, the P2Y2, P2Y4, P2Y6, and P2Y14 receptor subtypes can be activated by uridine nucleotides or UDP-glucose that are ineffective agonists of all P2X and four P2Y receptor subtypes [1, 4, 19, 20, 56, 70, 72–74].
Interactions have been reported between different P2 receptor subtypes in cells of the CNS. For example, activation of both astrocytic P2YRs and P2X7Rs occurs in brain lesions during the functional remodeling that accompanies astrogliosis and neuroinflammation [72]. Interactions between P2X7R and P2Y2R signaling pathways mediate glial cell-dependent neuroprotective responses [15, 75, 76] in which P2X7R activation in microglial cells leads to the release of nucleotides and cytokines, including IL-1β [75], that enhance the functional expression of P2Y2Rs in neurons [48]. P2Y1R activation in astrocytes of hippocampal cultures also provides neuroprotection from oxidative stress by increasing IL-6 release [77]. P2Y2 and P2Y6 receptors have been suggested to play complementary roles in the regulation of apoptosis, since P2Y6R activation inhibits tumor necrosis factor-α (TNF-α) receptor signaling [78] and P2Y2R activation upregulates anti-apoptotic proteins [79] to promote survival mechanisms in astrocytic cells.
P2YR functions associated with the pathogenesis of inflammation in the CNS, a process involving astrocyte and microglial cell proliferation and migration to a site of injury (i.e., gliosis), can be induced by a variety of conditions (e.g., oxidative stress or excessive β-amyloid (Aβ) peptide production) that stimulate the release of proinflammatory mediators, including cytokines [15, 75, 76, 80–86]. Among these mediators, ATP and other nucleotides also can be released into the extracellular space due to cell damage, oxidative stress, hypoxia, ischemia, or mechanical stress [17, 81–84], whereupon the nucleotides activate P2X and P2Y receptors expressed in surrounding cells. Several studies have proposed the involvement of P2Rs, including the P2X7R and P2Y2R, in proinflammatory responses mediated by glial cells that are associated with neurodegenerative diseases [15, 20, 57, 75, 76, 85]. In the absence of inflammation, P2Y2R expression levels are low in neurons, but the presence of IL-1β upregulates P2Y2R expression [48]. As mentioned above, we will focus on the role of P2Y2Rs in the regulation of neuroprotective responses associated with inflammation.
P2Y2 receptor signaling pathways
Activation of the Gq-coupled P2Y2R by ATP or UTP (EC50 ∼ 1–6 μM) [7, 74] is linked to the stimulation of PLC leading to an increase in the production of inositol 1,4,5 trisphosphate (IP3) and diacylglycerol that elevates the intracellular Ca2+ concentration, [Ca2+]i, and activates protein kinase C (PKC), respectively [73, 87]. Recent studies have demonstrated that the P2Y2R can activate signaling pathways independent of coupling to Gq protein. Although P2Y2R-mediated activation of mitogen-activated protein (MAP) kinases is stimulated by Gq-dependent increases in [Ca2+]i, P2Y2R activation also stimulates epidermal growth factor receptor (EGFR) phosphorylation and significantly enhances the activities of the MAP kinases ERK1/2 and related adhesion focal tyrosine kinase (RAFTK) via a mechanism involving Src and Shc/Grb2 [21, 88]. Other studies have shown that the P2Y2R contains 2 Src-homology-3 (SH3) binding domains in the intracellular C terminus that facilitate the binding of Src and the association of the P2Y2R with the EGFR, thereby enabling nucleotides to induce Src-dependent phosphorylation of the EFGR [22, 89]. This P2Y2R-mediated transactivation of growth factor receptors has implications in the regulation of cell growth, motility, differentiation, and cytoskeleton-associated morphological changes [22, 89]. Studies also have shown that P2Y2Rs in the presence of nerve growth factor co-localize with tyrosine receptor kinase A via a Src-dependent event that promotes neurite outgrowth and cell division [27]. This pathway leads to the activation of p38 and ERK1/2 MAP kinases and is inhibited by siRNA directed against P2Y2R mRNA. P2Y2R-mediated activation of PI3-kinase/Akt and MAP kinases has been shown to inhibit apoptosis in PC12 pheochromocytoma cells and dorsal root ganglion neurons [90]. In smooth muscle cells of human chorionic arteries, transactivation of the EGFR by the P2Y2R can activate RhoA and Rac1, a pathway that is dependent on clustering of these molecules in lipid rafts and internalization of the P2Y2R [91].
P2Y2Rs in endothelial cells can activate vascular endothelial growth factor receptor-2 (VEGFR-2) that has been shown to lead to the upregulation of vascular cell adhesion molecule-1 (VCAM-1) and an increase in the binding of monocytic cells to endothelium [89]. Deletion of the SH3-binding domains in the P2Y2R prevented nucleotides from activating VEGFR-2-dependent VCAM-1 upregulation [22, 89]. VCAM-1 expression in endothelial cells also was found to be dependent on increases in [Ca2+]i and p38 and Rho kinase activation but was independent of ERK1/2 activity [92]. Similarly, lymphocyte binding to epithelium is stimulated by P2Y2R activation in epithelial cells via EGFR-dependent VCAM-1 upregulation [93]. However, this pathway was found to be Src-independent and required the release of growth factors by P2Y2R-dependent activation of matrix metalloproteases (MMPs) [94].
The human and mouse P2Y2Rs contain an integrin-binding Arg-Gly-Asp (RGD) motif in the first extracellular loop that enables nucleotides to activate integrin signaling pathways [23, 24, 95]. In contrast, the rat P2Y2R homolog contains Gln-Gly-Asp (QGD) instead of RGD [96], although this is considered to be a conservative substitution that maintains integrin-binding affinity [23, 97]. Although the presence of a RGD motif in a G protein-coupled receptor is rare, its functional significance has not been extensively investigated. Studies have shown that the RGD sequence in the P2Y2R promotes its interaction with αvβ3/5 integrins [23], and following P2Y2R activation by UTP, there is an increase in the activation of monomeric Go and G12 proteins and the subsequent stimulation of the small GTPases Rho and Rac [24, 95]. Results indicate that mutation of the RGD sequence to Arg-Gly-Glu (RGE), a motif that does not bind well to integrins [98], prevented the binding of the P2Y2R to αvβ3/5 integrins and inhibited nucleotide-induced Go, G12, Rho, and Rac activation [24, 95]. Go-dependent Rac and G12-dependent Rho activation are known to mediate cytoskeletal rearrangements and cell migration through a mechanism involving the activation of LIM kinase-dependent cofilin phosphorylation, a key regulator of actin polymerization [99], and studies indicate that activation of the P2Y2R promotes cytoskeletal rearrangements and cell migration that are abolished by mutation of the RGD motif to RGE [24, 95]. Thus, it appears that the ability of the P2Y2R to increase cell chemokinesis is dependent upon P2Y2R association with αvβ3/5 integrins that stimulates signaling pathways involved in cytoskeletal reorganization required for cell motility.
The C-terminal domain of the P2Y2R has been shown to bind filamin A (FLNa), an actin-binding protein that regulates cytoskeletal dynamics [100]. Using the yeast 2-hybrid system, an 11-amino acid stretch including the SH3-binding domains in the C-terminal tail of the P2Y2R was found to regulate FLNa binding to the P2Y2R and nucleotide-induced increases in cell migration and spreading [100]. Since both P2Y2R-mediated transactivation of growth factor receptors and integrins contribute to cell migration [21, 24, 91], it is intriguing to postulate that FLNa binding to the SH3-binding domains of the P2Y2R links nucleotide-induced EGFR transactivation to the RGD-dependent integrin signaling pathway that regulates cytoskeletal rearrangements required to increase cell motility. In other studies, P2Y2R-mediated monocyte diapedesis (i.e., transendothelial migration) has been shown to occur by disruption of intercellular adherens junctions, suggesting that cytoskeletal rearrangements promoted by the P2Y2R also can affect cell polarization [89, 93, 95, 101–103]. Therefore, these data suggest a mechanism whereby the tropism of monocytic cells (e.g., microglia) into damaged areas of the CNS can be induced by activation of P2Y2Rs.
Activation of P2Y2Rs expressed in human astrocytoma cells or rat primary cortical neurons (rPCNs) stimulates α-secretases, i.e., the MMPs adamalysin 10/17 (ADAM10/17), that mediate the proteolytic processing of APP to generate the non-amyloidogenic soluble APPα (sAPPα) peptide [38, 48]. P2Y2R-mediated α-secretase activity is dependent on activation of the PI3-kinase/Akt pathway and partially dependent on activation of PKC and ERK1/2. Recent data with rat cortical astrocytes indicate that both P2Y2 and P2Y4 receptor activation can increase the production and release of APP via activation of ERK and p38 [40]. In rPCNs, P2Y2R expression is relatively low but is significantly upregulated by IL-1β via a pathway that involves the activation of the transcription factor NF-κB [48]. Indeed, it has been shown that the P2Y2R promoter contains a NF-κB binding sequence that is required for inflammation-induced P2Y2R upregulation, a pathway that can be blocked by Bay-11-7085, a specific inhibitor of the phosphorylation of IκB-α, the endogenous regulator of NF-κB activity [104]. Thus, it seems likely that the co-release of IL-1β and nucleotides mediated by ATP-induced P2X7R activation in microglia [16, 75, 105] provides an in vivo mechanism for both the upregulation and activation of P2Y2Rs in neurons and other cell types. ATP release also occurs from activated microglia and astrocytes under oxidative stress [15] and following neuronal excitation [17, 106] via volume-activated anion channels [106], P2X7Rs [107], and pannexin hemichannels [37, 108] or upon exposure to fibrillar or oligomeric Aβ1–42 [14, 75, 109]. Clearly, the proinflammatory effects of cytokine and ATP release in the CNS can be coordinately regulated by the P2X7 and P2Y2 receptors.
The P2Y2R is known to desensitize and internalize following activation [110, 111], which can be inhibited by deletion of segments of the C terminus of the receptor [110]. Depletion of intracellular calcium stores is another mechanism by which further G protein-coupled receptor (GPCR)-induced elevations in [Ca2+]i in microglia can be desensitized [112]. Following agonist-induced GPCR desensitization, receptor internalization occurs, a process that is regarded both as a resensitization step and as a means to link a GPCR to intracellular signaling pathways [113]. GPCR internalization often requires arrestin binding to the desensitized receptor that provides a scaffold for multiple protein–protein interactions [113, 114]. A role for arrestin-2 in cell migration has been reported [115], and arrestin has been shown to associate with LIM kinase/cofilin [116] and FLNa [117] providing a mechanism for activation of arrestins by a GPCR, such as the P2Y2R. The β1-adrenergic receptor-mediated transactivation of the EGFR is mediated by arrestin 1 and 2 following G protein receptor kinase 5/6-dependent phosphorylation of the β1 receptor [118]. These interactions lead to Src-dependent activation of MMPs and consequent release of the HB-EGF ligand to enable autocrine activation of the EGFR [119]. Since Gq-coupled P2Y2R activation can induce Src-dependent activation of the EGFR [21, 22, 88, 89], activation of the MMPs α-secretases [38], and integrin-dependent increases in cell motility [24, 95], it is intriguing to speculate that arrestins and receptor internalization play a role in these processes. Signaling pathways known to be coupled to P2Y2R activation are shown in Fig. 1.
Fig. 1.
P2Y2R signaling pathways. Upon activation with ATP or UTP, the Gq-coupled P2Y2R stimulates Gqα-dependent phospholipase C (PLC) activity which generates inositol 1,4,5 trisphosphate (IP3) and diacylglycerol (DAG) resulting in an elevation in the intracellular calcium concentration via IP3-dependent calcium release from intracellular stores and DAG-dependent activation of protein kinase C (PKC), respectively. The P2Y2R via an extracellularly oriented RGD domain can interact with αVβ3/5 integrins to regulate the activities of G12-dependent Rho, Go-dependent Rac, LIM kinase, and cofilin, proteins that regulate actin cytoskeletal rearrangements. Src-homology-3 binding domains (PXXP) within the C terminus of the P2Y2R bind Src to enable ATP or UTP to transactivate growth factor receptors and related adhesion focal tyrosine kinase (RAFTK; also known as Pyk2) and downstream MAP kinases. P2Y2R-mediated transactivation of growth factor receptors leads to upregulation of vascular cell adhesion molecule 1 (VCAM-1). The C terminus of the P2Y2R also has been shown to interact with the actin-binding protein filamin A (FLNa). P2Y2R activation can stimulate the activity of matrix metalloproteases (e.g., ADAM10 and ADAM17) leading to the α-secretase-dependent processing of APP to the non-amyloidogenic peptide sAPPα [38, 48] and release of growth factors (e.g., NRG1) [94]
P2Y2 receptors in glial cells
The major glial cells in the brain are astrocytes, oligodendrocytes, and microglia. Astrocytes are derived from the ectoderm and contribute to the maintenance of the blood–brain barrier (BBB) [120–122], which prevents invasion of pathogenic substances into the brain from the circulation [123]. Astrocytes also release neurotrophic factors that play an important role in neuronal survival and sprouting and supply energy substrates to neurons [124]. Oligodendrocytes are involved in the insulation of axons in the CNS, and it has been shown that oligodendrocyte precursor cells express P2Y1, P2Y2, and P2Y4 receptors [12], but these cells do not appear to have a significant role in glial cell activation due to brain injury [123]. P2Y2R interactions with integrins have been shown to promote migration of astrocytes [68]. P2Y1Rs and P2Y2Rs mediate astroglial calcium signals at the gliovascular interface by two distinct forms of P2R-dependent negative feedback mechanisms that differentially control Ca2+ signaling in astrocytes, suggesting divergent roles for these receptor subtypes in downstream signal transduction [125]. Functional studies with astrocytes and oligodendrocytes have demonstrated a role for both P2Y1 and P2Y2 receptors in mediating ATP-evoked and IP3-dependent increases in [Ca2+]i [20, 80]. P2Y2Rs are upregulated in reactive astrocytes of the cortex and nucleus accumbens in rat due to mechanical injury, suggesting a role in modulating responses to trauma [126]. Furthermore, studies with astrocytic cells also suggest that P2Y2Rs play a role in astrocyte survival after injury [13, 79].
Microglial cells, in contrast to astrocytes and oligodendrocytes, belong to the myelomonocytic lineage and become parenchymal cells in the CNS at early stages of embryonic development [127]. Microglial cells are originally derived from the mesoderm and possess functions similar to peripheral monocytes/macrophages [120, 128]. Microglia have important immunoregulatory functions in the CNS. Injury or other disturbances to the CNS trigger rapid transformation of ramified (quiescent) microglia into activated phenotypes that further develop into phagocytic macrophages [129, 130]. Activated microglia can be either neuroprotective [130–134] or neurotoxic [131, 135–137]. Although the CNS is considered to be an immune-privileged site because the BBB limits entry of blood-borne cells and proteins, recent findings indicate that peripheral leukocytes have important physiological and pathophysiological functions in the CNS [138]. Discrete populations of blood-borne leukocytes are recruited into the CNS by traversing the BBB under normal conditions or in response to injury or disease [139]. Similarly, hematopoietic cells can cross the BBB and enter the CNS whereupon they differentiate into microglia [140]. In fact, these peripheral macrophages have been shown to be more adept than resident microglia in the phagocytosis of neurotoxic Aβ in animal models of AD [138]. Peripheral macrophages have a dynamic life cycle and can enter and exit the CNS [141] to engulf and digest significant amounts of cellular debris and pathogens [138].
Microglial cell activation by the proinflammatory cytokines TNF-α, IL-1β, and IL-6 is accompanied by partial rounding and increased cell motility and proliferation [142]. The P2Y2R agonists UTP and ATP, released from apoptotic cells as a result of caspase 3/7 activation, also have been shown to induce cell migration of phagocytic cells [143]. In an in vivo model of cell migration, supernatants from apoptotic cells produced a three-fold greater recruitment of monocytes and macrophages than supernatants from control cells and depletion of nucleotides in the apoptotic cell supernatants diminished cell migration [143]. Extracellular nucleotides also have been shown to promote human monocyte migration in vitro by co-activation of P2Y2 and P2Y6 receptors that enhances TLR1/2-induced IL-8 release, whereas the innate immune response resulting from P2Y2R and P2Y6R activation in vivo was shown to be dependent upon TLR2 [56]. It has been reported that following injury or neuroinflammation, P2Y6Rs are functionally upregulated in microglial cells and their activation by UDP triggers phagocytosis [144]. Our data with mouse primary microglial cells indicate that P2Y2R activation enhances microglial cell migration (Table 1) and their uptake and degradation of neurotoxic oligomeric Aβ1–42 (Table 2), responses that were markedly decreased in microglia from P2Y2R−/− cells [145].
Table1.
Role of the P2Y2R in cell migration
| Control | Wild type | P2Y2R−/− | |||||
|---|---|---|---|---|---|---|---|
| oAβ1–42 | – | + | – | – | + | – | – |
| ATP | – | – | + | – | – | + | – |
| UTP | – | – | – | + | – | – | + |
| Cell motility (fold over controls) | 3-fold increase** | 6-fold increase** | 7-fold increase** | ∼ same as control | ∼ same as control | ∼ same as control | |
Briefly, primary microglial cells (1 × 106) from WT or P2Y2R−/− mice were seeded in the upper chamber of Transwell inserts that were then placed in six-well plates, and cells were treated for 6 h with or without oligomeric Aβ1–42 (1 μM) or ATP or UTP (100 μM) in the lower chamber. Cells that migrated across the membrane were counted under a microscope, and cell motility was expressed as fold increase over untreated control. Data represent means ± SEM (n = 4)
**p < 0.01 indicates a significant difference from untreated control
Table 2.
Role of the P2Y2R in Aβ1–42 uptake and degradation
| Control | Wild type | P2Y2R−/− | ||||
|---|---|---|---|---|---|---|
| ATP 100 μM | – | – | + | – | + | – |
| UTP 100 μM | – | – | – | + | – | + |
| Aβ1–42 uptake (pg/mg protein) | 1800 | 2000 | 3800** | 4500** | 2000 | 2100 |
| Aβ1–42 degradation | 47 % | 78 %* | 80 %* | 50 % | 48 % | |
Primary microglial cells from WT or P2Y2R−/− mice were treated with ATP or UTP (100 μM) followed by oligomeric Aβ1–42 (1 μM) for 1 h. Control cells from WT P2Y2R−/− were treated with oligomeric Aβ1–42 without ATP or UTP, and the values represent average of two control groups. Cell lysates were analyzed for intracellular Aβ1–42 uptake by ELISA. Data represent means ± SEM (n = 3). To determine P2Y2R-mediated Aβ1–42 degradation, cells were incubated with Aβ1–42 for 1 h, the medium was removed, cells were washed, and fresh media containing ATP or UTP (100 μM) was added. After 24 h, cell lysates were analyzed for intracellular Aβ1–42 levels by ELISA. Levels of Aβ1–42 remaining in the cell lysates after 24 h were divided by Aβ1–42 levels in cell lysates after 1 h to calculate the percentage of Aβ1–42 degradation. Data represent means ± SEM (n = 4)
*p < 0.05 indicates a significant difference from untreated control; **p < 0.01 indicates a significant difference from untreated control
Microglial cell migration at early stages of local CNS injury has been suggested to be regulated by P2Y12R expression, which is robust in the resting stage, but decreases upon microglial cell activation [146]. It also has been shown that activated microglia attach to and engulf myelinated axons in the dorsal horn after a peripheral nerve injury, and P2Y12R inhibition suppresses engulfment of these myelinated axons by activated microglia [147]. P2Y12 and P2X4 receptor activation has been shown to induce migration of ramified microglia, attracting them to regions of high ATP concentrations [148, 149]. Studies using peritoneal macrophages in mice have shown that stimulation of P2Y2 and P2Y12 receptors induces the formation of lamellipodial membrane protrusions that leads to cell spreading and efficient directional motility of cells [150]. Taken together, these findings support the hypothesis that extracellular nucleotides serve as endogenous danger signals that activate microglia during the innate immune response.
P2Y2 receptors in neurons
P2Y2Rs are expressed in neurons of the central and peripheral nervous systems [1], but expression levels are relatively low, as compared to other tissues [48, 151, 152]. P2Y2R expression in mouse primary cortical neurons can be upregulated in response to the proinflammatory cytokine IL-1β [16, 48], the levels of which are elevated in the AD brain [153]. Activation of the P2X7R in microglia promotes the release of IL-1β, TNF-α, and ATP [16, 75, 105, 154–156], suggesting a mechanism whereby the P2X7R regulates functional P2Y2R expression in neurons and other cells. The finding that P2Y2R expression under proinflammatory conditions is regulated by NF-κB binding to the P2Y2R promoter [104] is consistent with the established role of NF-κB activation in the induction of inflammation [157].
Other studies suggest a role for P2Y2Rs in the regulation of neuroprotective responses. As discussed above, P2Y2R/αv integrin interaction enables nucleotides to stimulate Rac and Rho and induce cytoskeletal rearrangements [24, 95], well-established signaling pathways that regulate the outgrowth and stabilization of dendritic spines [99, 158]. The P2Y2R agonist UTP has been shown to increase levels of neurofilament M and neurofilaments that promote neurite outgrowth [159]. In neural progenitor cells isolated from the subventricular zone of adult mouse brain, P2Y2R activation was shown to induce rapid and transient activation of the EGFR, ERK1/2, and CREB [160]. P2Y2R mRNA levels also were shown to increase during the acute and chronic stages of spinal cord injury [161] and with brain ischemia, mechanical injury to the nucleus accumbens, and brain trauma, an acute inflammatory response suggested to provide neuroprotection [126]. Other potential neuroprotective responses linked to P2Y2R function in primary cortical neurons include the activation of non-amyloidogenic APP processing [48]. The P2Y2R also has been suggested to contribute to synaptic transmission through the regulation of intracellular calcium waves in astrocytes [162].
P2Y2Rs in peripheral neurons can regulate the sensation of bladder distension in response to stretch-induced ATP release [163]. P2Y1, P2Y2, and A2 adenosine receptors have been suggested to regulate smell via the plasma membrane localization of olfactory receptor M71 in olfactory neurons [164]. Interactions between activated P2Y2Rs and the capsaicin-sensitive TRPV1 channel in peripheral neurons have been suggested to modulate pain sensation [165]. P2Y2R activation in retinal neurons has been shown to stimulate subretinal fluid reabsorption, inhibit retinal folding and apoptosis, and increase the rate of retinal reattachment in rat and rabbit models of experimental retinal detachment [166]. Thus, the P2Y2R appears to play a neuroprotective and/or reparative function under a variety of conditions associated with tissue injury, such as inflammation, pain, and mechanical damage.
Glial–neuronal interactions involving P2Y2 receptors
Over the past three decades, our understanding of intercellular communication in the CNS has evolved, and it has become widely accepted that glial cells act as organized networks rather than single cells. In vitro studies indicate that neurons require help from glia to form and maintain proper synaptic connections, and it is hypothesized that development of neuronal synapses is influenced by the differentiation of surrounding glial cells [167]. For example, astrocytes release gliotransmitters, including glutamate, ATP, and d-serine that exert direct effects on synaptic plasticity [168–170]. Microglial cells also have relevance to neuronal function in various types of brain injury and disease, such as ischemic trauma and AD. Activation of microglia under pathological conditions results in their transformation to amoeboid morphology, migration toward the site of injury/damage, and release of neuroactive compounds that can have either neurotoxic or neuroprotective effects [171, 172]. In vivo two-photon imaging revealed that resting microglia make brief but direct contacts with synapses without undergoing complete transformation/activation associated with a pathological phenotype [173, 174]. In contrast, prolonged microglial cell contact with neurons can initiate a cascade of events that results in synaptic stripping and functional impairment of neuronal circuits [175, 176]. Ca2+ waves in astrocytes can extend hundreds of micrometers from their site of initiation (e.g., an injury) and have been suggested to serve as a long range signal for recruitment of microglia from uninjured to injured areas of the brain [177]. Ca2+ waves initiated in astrocytes can propagate into microglia by an ATP-dependent pathway [177, 178]. Microglia–neuronal interactions are known to be mediated by release of a variety of cell signaling molecules from microglia or neurons that activate cell surface receptors [179–183]. Some of the chemoattractant signals released at synapses include glutamate, nucleosides, nucleotides, brain-derived growth factor, dopamine, noradrenaline, and chemokines [146, 184–186]. Both adenine and uridine nucleotides increase the motility of microglial cells [146, 187], and P2Y2 and P2Y12 receptors have been shown to mediate these effects [146, 184, 187]. Extracellular ATP release significantly increases process extension toward an injury site for resting or activated microglia [148]. Astrocytes under pathological conditions also can release ATP to activate P2Rs in neighboring cells [18, 188, 189], and inflammation in vivo can elevate extracellular ATP levels sufficiently to activate P2 receptors [18].
The specific contributions of individual P2R subtypes to functional responses in tissues are difficult to discern, particularly when multiple P2R subtypes are co-expressed at different levels and since individual subtypes can be activated at different agonist concentrations [190, 191]. Also, activation of individual P2R subtypes can increase [Ca2+]i to differing extents [80], and there can be significant divergence in the intracellular signaling pathways coupled to each P2R subtype. Additional sources of complexity include interactive effects of nucleotides with other ligands and variability of P2Y2R-mediated responses with cell type or experimental condition. Microglia express both ionotropic P2X and metabotropic P2Y receptors [60, 66], and stimulation of these P2X and P2Y receptors by ATP increases [Ca2+]i via extracellular Ca2+ influx or release of Ca2+ from intracellular stores, respectively [191]. However, responses downstream of P2X and P2Y receptor activation can vary widely. For example, Ca2+ influx and associated changes in membrane conductance accompanying activation of P2XRs trigger the opening of voltage-gated and Ca2+-dependent K+ channels [192], whereas P2Y receptor activation is coupled to a variety of G protein-dependent and G protein-independent signaling pathways, as described above.
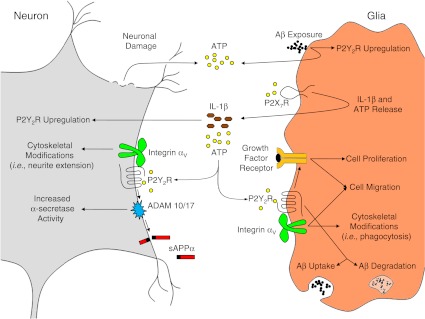
Accumulation of proinflammatory cytokines and neurotoxic oligomeric Aβ peptide is associated with the progression of AD [193]. As shown in Fig. 2, Aβ exposure in glial cells causes ATP release which activates the P2X7R to increase the release of nucleotides and cytokines, including IL-1β [75], which enhances the functional expression of P2Y2Rs in neurons [48] and glial cells (unpublished results). P2Y2R activation in glial cells increases their proliferation and migration by transactivation of growth factor receptors and integrins [20, 68]. We postulate that under proinflammatory conditions, IL-1β-dependent P2Y2R upregulation in neurons and P2Y2R activation by released ATP or UTP regulates neuroprotective responses, such as the non-amyloidogenic processing of APP, rather than Aβ generation, and the stimulation of integrin-dependent dendritic spine growth (see Fig. 2). Furthermore, P2Y2R activation in microglial cells in response to released nucleotides is postulated to provide neuroprotection by increasing microglial cell migration toward sites of Aβ release followed by P2Y2R-mediated Aβ phagocytosis and degradation by the activated microglia (see Fig. 3). Thus, P2Y2R upregulation in response to inflammation likely serves a neuroprotective function in the CNS that requires contributions from both glial and neuronal P2Y2Rs.
Fig. 2.
Neuronal–glial cell interactions involving the P2Y2R in the CNS. Extracellular ATP, released via neuronal damage, microglial cell exposure to Aβ, or tissue inflammation or injury, activates P2X7Rs on microglial cells that stimulate the release of IL-1β and additional ATP. Exposure of neurons to IL-1β upregulates the P2Y2R, whereas subsequent P2Y2R activation increases ADAM10/17 activity to increase non-amyloidogenic APP processing. P2Y2R interaction with integrins induces cytoskeletal rearrangements involved in neurite extension. P2Y2R activation in microglia increases their proliferation and migration by transactivation of growth factor receptors and integrins. P2Y2R activation in microglia also increases Aβ phagocytosis and degradation (see Fig. 3)
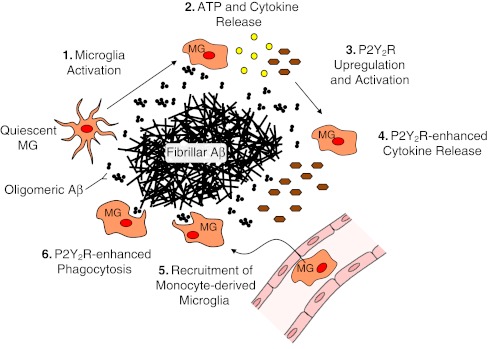
Fig. 3.
The P2Y2R contributes to microglia-mediated uptake and degradation of Aβ. In response to oligomeric Aβ1–42 exposure, microglial cells (MG) become activated (1), release cytokines and ATP (2), and the P2Y2R is upregulated (3). Subsequent P2Y2R activation by released ATP can enhance Aβ-induced cytokine release (4), which increases monocyte-derived microglial cell recruitment from the blood (5). The P2Y2R also plays a role in phagocytosis and degradation of fibrillar and oligomeric Aβ1–42 (6)
P2Y2Rs in CNS inflammation
Inflammation is an early response to injury, although it remains controversial whether the inflammatory response is beneficial or detrimental to brain tissue [194, 195]. Chronic inflammation damages cells and is thought to be a key player in neurodegenerative disorders, such as AD [193]. The point at which acute inflammation turns chronic is unclear. However, it has been suggested that sustained oxidative stress on cells of the CNS, associated with activation of NADPH oxidase and production of reactive oxygen species, leads to amyloidogenic Aβ production and cell death in AD [196–199]. Early neuroinflammation is thought to have a protective effect in the brain by activating glial cells that secrete cytokines, chemokines, and growth factors at the site of injury [200], which fits well with our model on the role of ATP release and P2Y2R activation as a neuroprotective response in inflammation (see Fig. 2). In CNS injury, the upregulation and activation of MMPs, a known component of the P2Y2R signaling pathway [38], can be either beneficial or detrimental depending on the length of time after the injury, the profile of the inflammatory cells at the injury site, and the substrates present [201]. During neuroinflammation, astrocytes undergo morphological and functional changes (i.e., reactive gliosis) characterized by hypertrophy, proliferation, upregulation of the intermediate filament protein glial fibrillary acidic protein, accumulation of activated glial cells around plaques, adhesion of cells to Aβ peptides, internalization and degradation of Aβ peptides by activated glial cells, expression of proteinases required for Aβ peptide catabolism, production of arachidonic acid and related proinflammatory substances in the vicinity of plaques, and regulation of regenerative processes in the brain [202–206]. Activated glia have been shown to produce neurotrophic factors [207, 208] and stimulate neuronal outgrowth during development and repair of damaged brain cells in the adult [209]. P2Y2Rs regulate many of these responses associated with reactive gliosis [13, 15, 20, 68, 79, 126].
Purinergic signaling has been shown to influence the initiation, progression, and downregulation of an inflammatory response [210], and the P2Y2R is an important mediator of neuroinflammation [15]. As described above, IL-1β regulates the expression of the P2Y2R in neurons [48] and other proinflammatory mediators in the AD brain [134, 211], and overexpression of IL-1β has been associated with head trauma, epilepsy, genetic polymorphisms, and age-related damage [212, 213]. In AD, IL-1β increases with Aβ plaque accumulation and dystrophic neurite formation [200]. Although the endogenous expression of P2Y2Rs has been reported in mouse microglia [67, 214], it seems likely that increased levels of proinflammatory cytokines should further increase P2Y2R expression in glial cells in vivo. Our recent in vitro data show that treatment of mouse primary oligomeric/oligomeric Aβ1–42 upregulates P2Y2R expression [145] via a pathway likely involving P2X7R-mediated IL-1β release [16, 75, 105, 154–156]. It also has been determined that P2X7R activation increases P2Y2R expression in rat astrocytes [215]. IL-1β has been shown to stimulate neuronal synthesis of APP, leading to the increased production of neurotoxic Aβ, which in turn activates microglia and further enhances IL-1β production [216]. Since IL-1β also upregulates P2Y2R expression in neurons to promote non-neurotoxic APP processing [48], we postulate that P2Y2R upregulation can counteract the deleterious effects of increased APP synthesis induced by IL-1β. Accordingly, we suggest that a major effect of P2Y2R upregulation in the CNS is to delay the progression of neurodegeneration that occurs with chronic inflammation.
It is known that activated astrocytes and microglia internalize and degrade Aβ [217–221], a mechanism that reduces Aβ toxicity in neurons, which is postulated to cause neuronal death in AD. Recruitment of activated microglia to sites of inflammation enhances the phagocytosis of aggregated Aβ via the Fc receptor [222, 223]. Studies have shown that microglial cells exposed to Aβ release ATP [75, 224]. Our recent data indicate that primary microglial cells exposed to oligomeric Aβ1–42 have 3–4-fold increased levels of TNF-α and IL-1β release, as compared to control cells without Aβ1–42 treatment (Table 3). In addition, there was a significant reduction in cytokine release in response to Aβ1–42 in microglial cells from P2Y2R−/− mice (Table 3) or in apyrase-treated microglial cells (not shown).
Table 3.
Role of the P2Y2R in proinflammatory cytokine release
| oAβ1–42 (1 μM) | TNF-α (pg/ml) | IL-1β (pg/ml) | |
|---|---|---|---|
| Control | – | 20 | 28 |
| Wild type | + | 95** | 115** |
| P2Y2R−/− | + | 35 | 41 |
WT and P2Y2R−/− mouse primary microglial cells were treated with oAβ1–42 (1 μM), incubated for 24 h, and supernatants were collected and analyzed for TNF-α and IL-1β by ELISA. Data represent means ± SEM (n = 3)
**p < 0.01 indicates a significant difference from untreated control
Figure 3 summarizes the postulated role of the P2Y2R in microglial cell-mediated phagocytosis and degradation of Aβ. Phagocytosis also plays a major role in controlling inflammation and antigen-cross presentation via uptake of apoptotic bodies from dying cells. The P2R agonists ATP, ADP, α,β-methylene ATP, 3′-O-(4-benzoyl) benzoyl ATP, UTP, and UDP have been shown to increase phagocytosis in macrophages [225], suggesting that multiple P2Rs contribute to phagocytosis in the myelomonocytic lineage. We postulate that P2Y2Rs can contribute to the phagocytosis of apoptotic debris generated due to ATP release and cell apoptosis previously linked to P2X7R activation [226, 227].
Proinflammatory P2Y2R functions in endothelium
It has been shown that monocytic cell infiltration across the BBB augments the resident microglial cell population of the AD brain due to differentiation of the infiltrating monocytes into microglia [109]. Our previous studies provide strong evidence that P2Y2Rs in endothelial cells regulate the binding and the transendothelial migration (i.e., diapedesis) of monocytic cells. As described above, P2Y2Rs mediate the Src-dependent transactivation of the vascular endothelial growth factor receptor-2 (VEGFR-2) in endothelial cells that promotes upregulation of monocyte-binding proteins (e.g., VCAM-1) and a decrease in endothelial adherens junction integrity [22, 89, 92, 101, 102]. Other studies have shown that microglia are attracted to and surround Aβ plaques in both human AD brain and rodent transgenic models that develop AD-like symptoms [228–237]. Although the role of microglial cells in AD (i.e., neurotoxic vs. neuroprotective) is controversial, recent work has shown that the majority of microglia that surround amyloid plaques in an AD mouse model are derived from monocytes originating in bone marrow [233, 238] and thus must pass from the bone marrow into the bloodstream through the vasculature and across the BBB to reach sites of plaque formation in the brain. Furthermore, these bone marrow-derived microglia (to a greater extent than resident microglia in the brain) were shown to eliminate Aβ deposits by phagocytosis in AD mice [238], strongly suggesting that bone marrow-derived microglia serve a neuroprotective role in restricting AD progression. Previous in vivo work by us and others indicates that the P2Y2R is important for the recruitment of leukocytes (monocytes, neutrophils, and eosinophils) to sites of sterile surgical injury [101] and tissue infected with allergens or bacteria [239–241]. The process of leukocyte recruitment involves several steps: the emigration of leukocytes from bone marrow into the circulation, adhesion of circulating leukocytes to vascular endothelial cells, and diapedesis of leukocytes towards chemoattractants released at the site of injury or infection. Although the leukocyte P2Y2R is important for controlling the leukocyte migration step [143, 239, 240], other studies indicate that the endothelial P2Y2R promotes both the leukocyte adhesion step, by increasing the expression of VCAM-1 in endothelial cells [22, 89, 92] and the diapedesis step [102]. A postulated pathway for the regulation of leukocyte diapedesis by the P2Y2R is shown in Fig. 4.
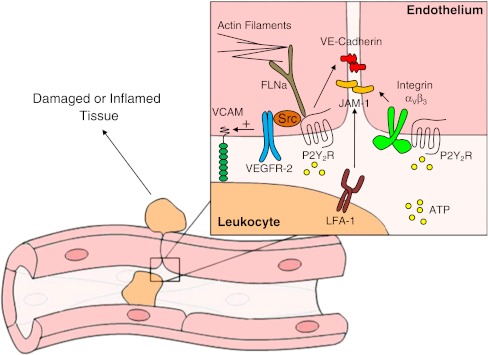
Fig. 4.
Proposed role of the endothelial P2Y2R in paracellular leukocyte diapedesis. Tissue damage or infection causes the local release of inflammatory signals that upregulate the endothelial P2Y2R as well as adhesion molecules required for leukocyte adhesion to vascular endothelium. These leukocytes begin migrating toward chemoattractants released from the damaged/infected site and, in so doing, release a burst of ATP from their leading edge [239]. We hypothesize that ATP released from adherent leukocytes activates the endothelial P2Y2R causing trafficking or relocation of this receptor to endothelial adherens junctions, possibly through interaction of the P2Y2R with Src, VEGFR-2, and the actin-binding protein, filamin A [22, 89, 100]. We also hypothesize that endothelial P2Y2R relocation to adherens junctions assists in the transient disruption of endothelial junctions and escorts leukocytes to endothelial borders for paracellular diapedesis, possibly through association of the P2Y2R with the αVβ3 integrin. This integrin is known to form a complex with the P2Y2R [23] and with JAM-1 [276], a junctional adhesion molecule in endothelial cells that interacts with the leukocyte integrin, LFA-1, and is important for both leukocyte adhesion and diapedesis [277]
It is known that signal transduction in endothelial cells (e.g., increases in [Ca2+]i, phosphorylation of myosin light chain, and RhoA activation) occurs in response to adhesion of activated leukocytes and that these events are required for leukocyte diapedesis [242, 243]. However, mechanisms by which endothelial cells promote leukocyte diapedesis are less clear. In a recent in vitro diapedesis study, expression of the endothelial P2Y2R was found to be important for the transendothelial migration of neutrophils toward lipopolysaccharide (LPS), a chemoattractive component of gram-negative bacteria [102]. This study also demonstrated that diapedesis of neutrophils toward LPS required Rho kinase activity and was potentiated by treatment with UTP [102]. Nucleotides are released from leukocytes migrating toward chemoattractants [239], including Aβ1–42 [224], and increased expression of P2Y2Rs in vascular endothelium of damaged tissue [244] regulates nucleotide-induced increases in [Ca2+]i, phosphorylation of myosin light chain, and activation of Rho kinases [24, 95]. Thus, it seems plausible that the P2Y2R in microvascular endothelium of the brain should contribute to monocyte binding, diapedesis, and accumulation of bone marrow-derived microglia around brain tissue burdened with Aβ plaques whereupon the loss of this mechanism should limit Aβ clearance and proinflammatory neuroprotection in the AD brain.
Leukocyte diapedesis and the increase in microvascular permeability to macromolecules that occurs during an inflammatory event are controlled by the formation and dissociation of endothelial cell adhesion structures comprised of adherens junctions, tight junctions, and gap junctions [245]. It is well-known that Rho family GTPases regulate the endothelial permeability barrier by affecting the stability of these junctional structures [246]. For example, endothelial Rho GTPase and Rho kinase promote the sealing of intercellular junctions by controlling phosphorylation of myosin light chain [247], whereas dominant negative Rac enhances thrombin-induced permeability of macromolecules [248]. Many compounds, including thrombin, VEGF, TNF-α, and histamine, have been found to alter the endothelial permeability barrier due to modulation of the activities of Rho GTPases and protein distribution in adherens junctions [249–256]. Extracellular nucleotides also have been shown to play a role in regulating blood vessel permeability properties [257, 258], and the P2Y2R, in particular, has been shown to stimulate leukocyte recruitment [101, 239] and Rho GTPase activity through a mechanism involving P2Y2R interaction with αvβ3/5 integrins [24, 95]. In addition, the endothelial P2Y2R has been linked to other proinflammatory responses, including vasodilation of rat cerebral arteries through a Ca2+-dependent mechanism involving the production of nitric oxide and endothelium-derived hyperpolarizing factor [259]; production of prostacyclin, an effective vasodilator and inhibitor of platelet activation [73]; and the upregulation of tissue factor, an initiator of platelet aggregation [260]. Recently, we found that activation of the P2Y2R in human coronary artery endothelial cells causes a rapid translocation of the receptor to cell–cell junctional zones where it interacts with VE-cadherin (unpublished results), a protein found specifically in endothelial adherens junctions that is important for maintaining the vascular permeability barrier. Furthermore, we found that luminal application of UTP increases microvascular permeability of albumin in wild type, but not P2Y2R−/−, mice (unpublished results), indicating that the P2Y2R controls the microvascular barrier function in vivo. Therefore, we hypothesize that the endothelial P2Y2R assists in monocyte diapedesis by interacting with VE-cadherin and disrupting endothelial junctions localized at the site of leukocyte passage. VE-cadherin has been well recognized for its role in regulating the endothelial permeability barrier and leukocyte recruitment [261–267]. The N-terminal extracellular domain of VE-cadherin provides tight adhesion between endothelial cells through Ca2+-dependent homophilic interaction, whereas the cytoplasmic domain interacts with various intracellular binding partners, including α-, β-, and p120 catenins, providing a linkage to the actin cytoskeleton [268]. Modulation of cell–cell contacts that regulate cell adhesion and cell motility likely requires interactions between cadherins and catenins, and it has been shown that p120 catenin regulates actin cytoskeletal organization and cell motility by activation of Rho GTPases [269–271]. In addition, VE-cadherin associates with VEGFR-2, known to be transactivated by the P2Y2R [20, 89], and also with Src, Shc, Csk [272, 273], and the vascular endothelial protein tyrosine phosphatase, VE-PTP [274]. VEGFR-2 activation in endothelial cells has been shown to stimulate the tyrosine phosphorylation of VE-cadherin, β- and p120-catenins, plakoglobin, and PECAM-1 [254]. These interactions may be important for regulating cell–cell contacts, cell adhesion, and growth factor signaling [275].
Conclusion
This review evaluates the role of P2Y2Rs in the CNS with an emphasis on brain functions. P2Y2Rs are expressed in glial cells (i.e., astrocytes and microglia), neurons, and endothelium, primary cell types comprising the CNS. The P2Y2R has been shown to play a role in the activation of astrocytes and microglia and the phagocytosis of apoptotic cell debris. P2Y2R expression is upregulated under proinflammatory conditions in neurons and glial cells. The Gq-coupled P2Y2R has structural motifs that have been shown to facilitate interactions with growth factor receptors, integrins, and filamin A that activate signaling pathways beyond those regulated by Gq protein activation alone. Results indicate that under proinflammatory conditions associated with neurodegenerative diseases, such as AD, the release of cytokines, including IL-1β, upregulates P2Y2R expression through activation of NF-κB and its binding to the P2Y2R promoter. Upregulation of the P2Y2R by proinflammatory cytokines in neurons enables P2Y2R activation to promote neuroprotective responses, such as the metalloprotease-dependent non-amyloidogenic processing of APP and integrin-dependent neurite outgrowth. In addition, activation of P2Y2Rs expressed in glial cells can increase cell migration and phagocytosis and degradation of neurotoxic Aβ. Furthermore, P2Y2Rs in endothelium promote the binding of monocytes and their diapedesis, which is postulated to increase the neuroprotective microglial cell population in the brain. Taken together, current research suggests that the P2Y2R plays a neuroprotective role during inflammation in the CNS and indicates mechanisms that should be further investigated as promising targets for the treatment of neurodegenerative diseases, including AD.
Acknowledgments
This work was supported by NIH grants AG018357, DE017591, and DE07389.
References
- 1.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 2.Sak K, Webb TE. A retrospective of recombinant P2Y receptor subtypes and their pharmacology. Arch Biochem Biophys. 2002;397(1):131–136. doi: 10.1006/abbi.2001.2616. [DOI] [PubMed] [Google Scholar]
- 3.Inbe H, Watanabe S, Miyawaki M, Tanabe E, Encinas JA. Identification and characterization of a cell-surface receptor, P2Y15, for AMP and adenosine. J Biol Chem. 2004;279(19):19790–19799. doi: 10.1074/jbc.M400360200. [DOI] [PubMed] [Google Scholar]
- 4.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner JT, Weisman GA, Landon LA, Park M, Camden JM. Salivary gland nucleotide receptors: evidence for functional expression of both P2X and P2Y subtypes. Eur J Morphol. 1998;36(Suppl):170–175. [PubMed] [Google Scholar]
- 6.Weisman GA, Yu N, Liao Z, Gonzalez F, Erb L, Seye CI. P2 receptors in health and disease. Biotechnol Genet Eng Rev. 2006;22:171–195. doi: 10.1080/02648725.2006.10648070. [DOI] [PubMed] [Google Scholar]
- 7.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch. 2006;452(5):552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6(4):526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 9.Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19(1):13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 10.Illes P, Alexandre Ribeiro J. Molecular physiology of P2 receptors in the central nervous system. Eur J Pharmacol. 2004;483(1):5–17. doi: 10.1016/j.ejphar.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32(4):189–198. doi: 10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Koles L, Leichsenring A, Rubini P, Illes P. P2 receptor signaling in neurons and glial cells of the central nervous system. Adv Pharmacol. 2011;61:441–493. doi: 10.1016/B978-0-12-385526-8.00014-X. [DOI] [PubMed] [Google Scholar]
- 13.Burgos M, Neary JT, Gonzalez FA. P2Y2 nucleotide receptors inhibit trauma-induced death of astrocytic cells. J Neurochem. 2007;103(5):1785–1800. doi: 10.1111/j.1471-4159.2007.04872.x. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K. Purinergic systems in microglia. Cell Mol Life Sci. 2008;65(19):3074–3080. doi: 10.1007/s00018-008-8210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson TS, Camden JM, Wang Y, Seye CI, Wood WG, Sun GY, Erb L, Petris MJ, Weisman GA. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol. 2010;41(2–3):356–366. doi: 10.1007/s12035-010-8115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24(1):1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26(8–9):959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 18.Butt AM. ATP: a ubiquitous gliotransmitter integrating neuron–glial networks. Semin Cell Dev Biol. 2011;22(2):205–213. doi: 10.1016/j.semcdb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Molliver DC, Cook SP, Carlsten JA, Wright DE, McCleskey EW. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci. 2002;16(10):1850–1860. doi: 10.1046/j.1460-9568.2002.02253.x. [DOI] [PubMed] [Google Scholar]
- 20.Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, Gonzalez FA, Seye CI, Erb L. Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol. 2005;31(1–3):169–183. doi: 10.1385/MN:31:1-3:169. [DOI] [PubMed] [Google Scholar]
- 21.Soltoff SP. Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. Phorbol ester or [Ca2+]i elevation can substitute for receptor activation. J Biol Chem. 1998;273(36):23110–23117. doi: 10.1074/jbc.273.36.23110. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem. 2004;279(9):8212–8218. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- 23.Erb L, Liu J, Ockerhausen J, Kong Q, Garrad RC, Griffin K, Neal C, Krugh B, Santiago-Perez LI, Gonzalez FA, Gresham HD, Turner JT, Weisman GA. An RGD sequence in the P2Y2 receptor interacts with αvβ3 integrins and is required for Go-mediated signal transduction. J Cell Biol. 2001;153(3):491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor interacts with αv integrins to activate Go and induce cell migration. J Biol Chem. 2005;280(47):39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- 25.Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther. 2006;109(3):297–324. doi: 10.1016/j.pharmthera.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3(125):ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci U S A. 2005;102(52):19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Homolya L, Watt WC, Lazarowski ER, Koller BH, Boucher RC. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y2 receptor−/− mice. J Biol Chem. 1999;274(37):26454–26460. doi: 10.1074/jbc.274.37.26454. [DOI] [PubMed] [Google Scholar]
- 29.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 2010;199(2):93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 30.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4(3):237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnstock G, Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9. doi: 10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnstock G. Purinergic signalling: past, present and future. Braz J Med Biol Res. 2009;42(1):3–8. doi: 10.1590/s0100-879x2008005000037. [DOI] [PubMed] [Google Scholar]
- 33.Burnstock G, Dumsday B, Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol. 2007;129(6):485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278(2):1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 36.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19(2):520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwabuchi S, Kawahara K. Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem Int. 2011;58(3):376–384. doi: 10.1016/j.neuint.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280(19):18696–18702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- 39.Villa G, Fumagalli M, Verderio C, Abbracchio MP, Ceruti S. Expression and contribution of satellite glial cells purinoceptors to pain transmission in sensory ganglia: an update. Neuron Glia Biol. 2010;6(1):31–42. doi: 10.1017/S1740925X10000086. [DOI] [PubMed] [Google Scholar]
- 40.Tran MD. P2 receptor stimulation induces amyloid precursor protein production and secretion in rat cortical astrocytes. Neurosci Lett. 2011;492(3):155–159. doi: 10.1016/j.neulet.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 41.Fumagalli M, Brambilla R, D’Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43(3):218–303. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- 42.Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol. 2008;153(7):1528–1537. doi: 10.1038/sj.bjp.0707692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandenburg LO, Jansen S, Wruck CJ, Lucius R, Pufe T. Antimicrobial peptide rCRAMP induced glial cell activation through P2Y receptor signalling pathways. Mol Immunol. 2010;47(10):1905–1913. doi: 10.1016/j.molimm.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Ko H, Das A, Jacobson KA, Lazarowski ER, Harden TK. Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol Pharmacol. 2009;76(6):1341–1348. doi: 10.1124/mol.109.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenz G, Gottfried C, Luo Z, Avruch J, Rodnight R, Nie WJ, Kang Y, Neary JT. P2Y purinoceptor subtypes recruit different mek activators in astrocytes. Br J Pharmacol. 2000;129(5):927–936. doi: 10.1038/sj.bjp.0703138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacques-Silva MC, Rodnight R, Lenz G, Liao Z, Kong Q, Tran M, Kang Y, Gonzalez FA, Weisman GA, Neary JT. P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol. 2004;141(7):1106–1117. doi: 10.1038/sj.bjp.0705685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong Q, Wang M, Liao Z, Camden JM, Yu S, Simonyi A, Sun GY, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2X7 nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signal. 2005;1(4):337–347. doi: 10.1007/s11302-005-7145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong Q, Peterson TS, Baker O, Stanley E, Camden J, Seye CI, Erb L, Simonyi A, Wood WG, Sun GY, Weisman GA. Interleukin-1β enhances nucleotide-induced and α-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y2 receptor. J Neurochem. 2009;109(5):1300–1310. doi: 10.1111/j.1471-4159.2009.06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Espada S, Ortega F, Molina-Jijon E, Rojo AI, Perez-Sen R, Pedraza-Chaverri J, Miras-Portugal MT, Cuadrado A. The purinergic P2Y13 receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic Biol Med. 2010;49(3):416–426. doi: 10.1016/j.freeradbiomed.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40(6):841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Grassi F. Purinergic control of neutrophil activation. J Mol Cell Biol. 2010;2(4):176–177. doi: 10.1093/jmcb/mjq014. [DOI] [PubMed] [Google Scholar]
- 53.Sun WC, Berghaus LJ, Moore JN, Hurley DJ, Vandenplas ML, Thompson R, Linden J. Lipopolysaccharide and TNF-alpha modify adenosine A2A receptor expression and function in equine monocytes. Vet Immunol Immunopathol. 2010;135(3–4):289–295. doi: 10.1016/j.vetimm.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-α production by cord blood monocytes: the role of adenosine receptors. Pediatr Res. 2009;65(2):203–208. doi: 10.1203/PDR.0b013e31818d66b1. [DOI] [PubMed] [Google Scholar]
- 55.Rizzo R, Ferrari D, Melchiorri L, Stignani M, Gulinelli S, Baricordi OR, Virgilio F. Extracellular ATP acting at the P2X7 receptor inhibits secretion of soluble HLA-G from human monocytes. J Immunol. 2009;183(7):4302–4311. doi: 10.4049/jimmunol.0804265. [DOI] [PubMed] [Google Scholar]
- 56.Ben Yebdri F, Kukulski F, Tremblay A, Sevigny J. Concomitant activation of P2Y2 and P2Y6 receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur J Immunol. 2009;39(10):2885–2894. doi: 10.1002/eji.200939347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu GD, Ding JQ, Xiao Q, Chen SD. P2Y6 receptor and immunoinflammation. Neurosci Bull. 2009;25(3):161–164. doi: 10.1007/s12264-009-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papp L, Balazsa T, Kofalvi A, Erdelyi F, Szabo G, Vizi ES, Sperlagh B. P2X receptor activation elicits transporter-mediated noradrenaline release from rat hippocampal slices. J Pharmacol Exp Ther. 2004;310(3):973–980. doi: 10.1124/jpet.104.066712. [DOI] [PubMed] [Google Scholar]
- 59.Sperlagh B, Vizi ES. Effect of presynaptic P2 receptor stimulation on transmitter release. J Neurochem. 1991;56(5):1466–1470. doi: 10.1111/j.1471-4159.1991.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 60.Sperlagh B, Illes P. Purinergic modulation of microglial cell activation. Purinergic Signal. 2007;3(1–2):117–127. doi: 10.1007/s11302-006-9043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunha RA, Ribeiro JA. ATP as a presynaptic modulator. Life Sci. 2000;68(2):119–137. doi: 10.1016/s0024-3205(00)00923-1. [DOI] [PubMed] [Google Scholar]
- 62.Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci. 2005;25(27):6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hussl S, Boehm S. Functions of neuronal P2Y receptors. Pflugers Arch. 2006;452(5):538–551. doi: 10.1007/s00424-006-0063-8. [DOI] [PubMed] [Google Scholar]
- 64.Fischer W, Krugel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem. 2007;14(23):2429–2455. doi: 10.2174/092986707782023695. [DOI] [PubMed] [Google Scholar]
- 65.Gerevich Z, Muller C, Illes P. Metabotropic P2Y1 receptors inhibit P2X3 receptor-channels in rat dorsal root ganglion neurons. Eur J Pharmacol. 2005;521(1–3):34–38. doi: 10.1016/j.ejphar.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005;48(2):144–156. doi: 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Crain JM, Nikodemova M, Watters JJ. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. J Neuroinflammation. 2009;6:24. doi: 10.1186/1742-2094-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. P2Y nucleotide receptor interaction with αv integrin mediates astrocyte migration. J Neurochem. 2005;95(3):630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- 69.Czajkowski R, Lei L, Sabala P, Baranska J. ADP-evoked phospholipase C stimulation and adenylyl cyclase inhibition in glioma C6 cells occur through two distinct nucleotide receptors, P2Y1 and P2Y12. FEBS Lett. 2002;513(2–3):179–183. doi: 10.1016/s0014-5793(02)02255-x. [DOI] [PubMed] [Google Scholar]
- 70.Nicholas RA, Lazarowski ER, Watt WC, Li Q, Boyer J, Harden TK. Pharmacological and second messenger signalling selectivities of cloned P2Y receptors. J Auton Pharmacol. 1996;16(6):319–323. doi: 10.1111/j.1474-8673.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 71.Boyer JL, Zohn IE, Jacobson KA, Harden TK. Differential effects of P2-purinoceptor antagonists on phospholipase C- and adenylyl cyclase-coupled P2Y-purinoceptors. Br J Pharmacol. 1994;113(2):614–620. doi: 10.1111/j.1476-5381.1994.tb17034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39(3):190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 73.Lustig KD, Erb L, Landis DM, Hicks-Taylor CS, Zhang X, Sportiello MG, Weisman GA. Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim Biophys Acta. 1992;1134(1):61–72. doi: 10.1016/0167-4889(92)90028-a. [DOI] [PubMed] [Google Scholar]
- 74.Erb L, Lustig KD, Sullivan DM, Turner JT, Weisman GA. Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc Natl Acad Sci U S A. 1993;90(22):10449–10453. doi: 10.1073/pnas.90.22.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Virgilio F. Activation of microglia by amyloid β requires P2X7 receptor expression. J Immunol. 2009;182(7):4378–4385. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- 76.Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32(2):79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Fujita T, Tozaki-Saitoh H, Inoue K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia. 2009;57(3):244–257. doi: 10.1002/glia.20749. [DOI] [PubMed] [Google Scholar]
- 78.Kim SG, Soltysiak KA, Gao ZG, Chang TS, Chung E, Jacobson KA. Tumor necrosis factor alpha-induced apoptosis in astrocytes is prevented by the activation of P2Y6, but not P2Y4 nucleotide receptors. Biochem Pharmacol. 2003;65(6):923–931. doi: 10.1016/s0006-2952(02)01614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem. 2004;91(1):119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- 80.James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447(2–3):247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- 81.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26(1):40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 82.Ostrom RS, Gregorian C, Drenan RM, Gabot K, Rana BK, Insel PA. Key role for constitutive cyclooxygenase-2 of MDCK cells in basal signaling and response to released ATP. Am J Physiol Cell Physiol. 2001;281(2):C524–C531. doi: 10.1152/ajpcell.2001.281.2.C524. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74(5):1951–1960. [PubMed] [Google Scholar]
- 84.Ciccarelli R, Iorio P, Giuliani P, D’Alimonte I, Ballerini P, Caciagli F, Rathbone MP. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25(1):93–98. [PubMed] [Google Scholar]
- 85.Pineau I, Lacroix S. Endogenous signals initiating inflammation in the injured nervous system. Glia. 2009;57(4):351–361. doi: 10.1002/glia.20763. [DOI] [PubMed] [Google Scholar]
- 86.Inoue K. UDP facilitates microglial phagocytosis through P2Y6 receptors. Cell Adh Migr. 2007;1(3):131–132. doi: 10.4161/cam.1.3.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weisman GA, Garrad RC, Erb LJ, Santos-Berrios C, Gonzalez FA. P2Y receptors in the nervous system: molecular studies of a P2Y2 receptor subtype from NG108-15 neuroblastoma x glioma hybrid cells. Prog Brain Res. 1999;120:33–43. doi: 10.1016/s0079-6123(08)63544-x. [DOI] [PubMed] [Google Scholar]
- 88.Soltoff SP, Avraham H, Avraham S, Cantley LC. Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J Biol Chem. 1998;273(5):2653–2660. doi: 10.1074/jbc.273.5.2653. [DOI] [PubMed] [Google Scholar]
- 89.Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279(34):35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- 90.Arthur DB, Akassoglou K, Insel PA. P2Y2 and TrkA receptors interact with Src family kinase for neuronal differentiation. Biochem Biophys Res Commun. 2006;347(3):678–682. doi: 10.1016/j.bbrc.2006.06.141. [DOI] [PubMed] [Google Scholar]
- 91.Norambuena A, Palma F, Poblete MI, Donoso MV, Pardo E, Gonzalez A, Huidobro-Toro JP. UTP controls cell surface distribution and vasomotor activity of the human P2Y2 receptor through an epidermal growth factor receptor-transregulated mechanism. J Biol Chem. 2010;285(5):2940–2950. doi: 10.1074/jbc.M109.081166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, Erb L, Gonzalez FA, Weisman GA. The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem. 2003;278(27):24960–24965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- 93.Baker OJ, Camden JM, Rome DE, Seye CI, Weisman GA. P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecule-1 expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol Immunol. 2008;45(1):65–75. doi: 10.1016/j.molimm.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ratchford AM, Baker OJ, Camden JM, Rikka S, Petris MJ, Seye CI, Erb L, Weisman GA. P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. J Biol Chem. 2010;285(10):7545–7555. doi: 10.1074/jbc.M109.078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with αv integrins to access and activate G12. J Cell Sci. 2007;120(Pt 9):1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rice WR, Burton FM, Fiedeldey DT. Cloning and expression of the alveolar type II cell P2u-purinergic receptor. Am J Respir Cell Mol Biol. 1995;12(1):27–32. doi: 10.1165/ajrcmb.12.1.7811468. [DOI] [PubMed] [Google Scholar]
- 97.Gresham HD, Adams SP, Brown EJ. Ligand binding specificity of the leukocyte response integrin expressed by human neutrophils. J Biol Chem. 1992;267(20):13895–13902. [PubMed] [Google Scholar]
- 98.Hautanen A, Gailit J, Mann DM, Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989;264(3):1437–1442. [PubMed] [Google Scholar]
- 99.Jang DH, Han JH, Lee SH, Lee YS, Park H, Kim H, Kaang BK. Cofilin expression induces cofilin-actin rod formation and disrupts synaptic structure and function in Aplysia synapses. Proc Natl Acad Sci U S A. 2005;102(44):16072–16077. doi: 10.1073/pnas.0507675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu N, Erb L, Shivaji R, Weisman GA, Seye CI. Binding of the P2Y2 nucleotide receptor to filamin A regulates migration of vascular smooth muscle cells. Circ Res. 2008;102(5):581–588. doi: 10.1161/CIRCRESAHA.107.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106(21):2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- 102.Kukulski F, Ben Yebdri F, Bahrami F, Fausther M, Tremblay A, Sevigny J. Endothelial P2Y2 receptor regulates LPS-induced neutrophil transendothelial migration in vitro. Mol Immunol. 2010;47(5):991–999. doi: 10.1016/j.molimm.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, Weisman GA. Proinflammatory cytokines tumor necrosis factor-α and interferon-γ alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol. 2008;295(5):C1191–C1201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Degagne E, Grbic DM, Dupuis AA, Lavoie EG, Langlois C, Jain N, Weisman GA, Sevigny J, Gendron FP. P2Y2 receptor transcription is increased by NF-κB and stimulates cyclooxygenase-2 expression and PGE2 released by intestinal epithelial cells. J Immunol. 2009;183(7):4521–4529. doi: 10.4049/jimmunol.0803977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Seguela P. ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. 2002;22(8):3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fields RD. Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin Cell Dev Biol. 2011;22(2):214–219. doi: 10.1016/j.semcdb.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26(5):1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46(5):731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 109.El Khoury J, Luster AD. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends Pharmacol Sci. 2008;29(12):626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 110.Garrad RC, Otero MA, Erb L, Theiss PM, Clarke LL, Gonzalez FA, Turner JT, Weisman GA. Structural basis of agonist-induced desensitization and sequestration of the P2Y2 nucleotide receptor. Consequences of truncation of the C terminus. J Biol Chem. 1998;273(45):29437–29444. doi: 10.1074/jbc.273.45.29437. [DOI] [PubMed] [Google Scholar]
- 111.Sromek SM, Harden TK. Agonist-induced internalization of the P2Y2 receptor. Mol Pharmacol. 1998;54(3):485–494. doi: 10.1124/mol.54.3.485. [DOI] [PubMed] [Google Scholar]
- 112.Toescu EC, Moller T, Kettenmann H, Verkhratsky A. Long-term activation of capacitative Ca2+ entry in mouse microglial cells. Neuroscience. 1998;86(3):925–935. doi: 10.1016/s0306-4522(98)00123-7. [DOI] [PubMed] [Google Scholar]
- 113.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62(2):305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53(1):1–24. [PubMed] [Google Scholar]
- 115.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99(11):7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007;282(28):20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]
- 117.Scott MG, Pierotti V, Storez H, Lindberg E, Thuret A, Muntaner O, Labbe-Jullie C, Pitcher JA, Marullo S. Cooperative regulation of extracellular signal-regulated kinase activation and cell shape change by filamin A and beta-arrestins. Mol Cell Biol. 2006;26(9):3432–3445. doi: 10.1128/MCB.26.9.3432-3445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. beta-Arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117(9):2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 120.Sudo S, Tanaka J, Toku K, Desaki J, Matsuda S, Arai T, Sakanaka M, Maeda N. Neurons induce the activation of microglial cells in vitro. Exp Neurol. 1998;154(2):499–510. doi: 10.1006/exnr.1998.6911. [DOI] [PubMed] [Google Scholar]
- 121.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barreto GE, Gonzalez J, Torres Y, Morales L. Astrocytic-neuronal crosstalk: implications for neuroprotection from brain injury. Neurosci Res. 2011;71:107–113. doi: 10.1016/j.neures.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Sugama S, Takenouchi T, Cho BP, Joh TH, Hashimoto M, Kitani H. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets. 2009;8(4):277–284. doi: 10.2174/187152809789352249. [DOI] [PubMed] [Google Scholar]
- 124.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11(2):87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 125.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23(27):9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Franke H, Krugel U, Grosche J, Heine C, Hartig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127(2):431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 127.Streit WJ. Microglia and macrophages in the developing CNS. Neurotoxicology. 2001;22(5):619–624. doi: 10.1016/s0161-813x(01)00033-x. [DOI] [PubMed] [Google Scholar]
- 128.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 129.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 130.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 131.Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol Cell Neurosci. 2005;29(3):381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 132.Turrin NP, Rivest S. Tumor necrosis factor alpha but not interleukin 1 beta mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci. 2006;26(1):143–151. doi: 10.1523/JNEUROSCI.4032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27(10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 135.Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184(1–2):92–99. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 136.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 137.Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29(9):506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer’s disease: the blood-borne identity. J Neural Transm. 2010;117(8):961–970. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106(4):1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7(12):1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 141.Dickson DW. Microglia in Alzheimer’s disease and transgenic models. How close the fit? Am J Pathol. 1999;154(6):1627–1631. doi: 10.1016/S0002-9440(10)65416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41(2–3):242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 143.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446(7139):1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim HJ, Ajit D, Peterson TS, Wang Y, Camden JM, Wood GW, Sun GY, Erb L, Petris M, Weisman GA (2012) Nucleotides released from Aβ1–42-treated microglial cells increase cell migration and Aβ1–42 uptake through P2Y2 receptor activation. J Neurochem. doi:10.1111/j.1471-4159.2012.07700.x [DOI] [PMC free article] [PubMed]
- 146.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 147.Maeda M, Tsuda M, Tozaki-Saitoh H, Inoue K, Kiyama H. Nerve injury-activated microglia engulf myelinated axons in a P2Y12 signaling-dependent manner in the dorsal horn. Glia. 2010;58(15):1838–1846. doi: 10.1002/glia.21053. [DOI] [PubMed] [Google Scholar]
- 148.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 149.Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55(6):604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 150.Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schon P, Schwab A, Hanley PJ. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3(132):ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 151.Loesch A, Glass R. Electron microscopy and in situ hybridization: expression of P2Y2 receptor mRNA in the cerebellum. Methods Mol Biol. 2006;326:151–162. doi: 10.1385/1-59745-007-3:151. [DOI] [PubMed] [Google Scholar]
- 152.Cheung KK, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228(2):254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- 153.Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer’s disease. Arch Pharm Res. 2010;33(10):1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 154.Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-induced release and processing of IL-1beta in microglial cells. Crit Rev Immunol. 2009;29(4):335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- 155.Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27(18):4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mingam R, Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, Laye S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav Immun. 2008;22(2):234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wullaert A, Bonnet MC, Pasparakis M. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21(1):146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bamburg JR, Bloom GS. Cytoskeletal pathologies of Alzheimer disease. Cell Motil Cytoskeleton. 2009;66(8):635–649. doi: 10.1002/cm.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pooler AM, Guez DH, Benedictus R, Wurtman RJ. Uridine enhances neurite outgrowth in nerve growth factor-differentiated PC12. Neuroscience. 2005;134(1):207–214. doi: 10.1016/j.neuroscience.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 160.Grimm I, Messemer N, Stanke M, Gachet C, Zimmermann H. Coordinate pathways for nucleotide and EGF signaling in cultured adult neural progenitor cells. J Cell Sci. 2009;122(Pt 14):2524–2533. doi: 10.1242/jcs.044891. [DOI] [PubMed] [Google Scholar]
- 161.Rodriguez-Zayas AE, Torrado AI, Miranda JD. P2Y2 receptor expression is altered in rats after spinal cord injury. Int J Dev Neurosci. 2010;28(6):413–421. doi: 10.1016/j.ijdevneu.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57(4):343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen X, Molliver DC, Gebhart GF. The P2Y2 receptor sensitizes mouse bladder sensory neurons and facilitates purinergic currents. J Neurosci. 2010;30(6):2365–2372. doi: 10.1523/JNEUROSCI.5462-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bush CF, Jones SV, Lyle AN, Minneman KP, Ressler KJ, Hall RA. Specificity of olfactory receptor interactions with other G protein-coupled receptors. J Biol Chem. 2007;282(26):19042–19051. doi: 10.1074/jbc.M610781200. [DOI] [PubMed] [Google Scholar]
- 165.Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol. 2005;25(5):819–832. doi: 10.1007/s10571-005-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nour M, Quiambao AB, Peterson WM, Al-Ubaidi MR, Naash MI. P2Y2 receptor agonist INS37217 enhances functional recovery after detachment caused by subretinal injection in normal and rds mice. Invest Ophthalmol Vis Sci. 2003;44(10):4505–4514. doi: 10.1167/iovs.03-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Rev. 2010;63(1–2):39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 168.Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26(10):536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 169.Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 170.Barker AJ, Ullian EM. Astrocytes and synaptic plasticity. Neuroscientist. 2010;16(1):40–50. doi: 10.1177/1073858409339215. [DOI] [PubMed] [Google Scholar]
- 171.Vilhardt F. Microglia: phagocyte and glia cell. Int J Biochem Cell Biol. 2005;37(1):17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 172.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘on’ and ‘off’ signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 173.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 174.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Graeber MB, Bise K, Mehraein P. Synaptic stripping in the human facial nucleus. Acta Neuropathol. 1993;86(2):179–181. doi: 10.1007/BF00334886. [DOI] [PubMed] [Google Scholar]
- 176.Yamada J, Hayashi Y, Jinno S, Wu Z, Inoue K, Kohsaka S, Nakanishi H. Reduced synaptic activity precedes synaptic stripping in vagal motoneurons after axotomy. Glia. 2008;56(13):1448–1462. doi: 10.1002/glia.20711. [DOI] [PubMed] [Google Scholar]
- 177.Schipke CG, Boucsein C, Ohlemeyer C, Kirchhoff F, Kettenmann H. Astrocyte Ca2+ waves trigger responses in microglial cells in brain slices. FASEB J. 2002;16(2):255–257. doi: 10.1096/fj.01-0514fje. [DOI] [PubMed] [Google Scholar]
- 178.Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol. 2001;166(10):6383–6391. doi: 10.4049/jimmunol.166.10.6383. [DOI] [PubMed] [Google Scholar]
- 179.Haas AH, Weering HR, Jong EK, Boddeke HW, Biber KP. Neuronal chemokines: versatile messengers in central nervous system cell interaction. Mol Neurobiol. 2007;36(2):137–151. doi: 10.1007/s12035-007-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55(3):233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 181.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30(10):527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 182.Nakajima K, Kikuchi Y, Ikoma E, Honda S, Ishikawa M, Liu Y, Kohsaka S. Neurotrophins regulate the function of cultured microglia. Glia. 1998;24(3):272–289. doi: 10.1002/(sici)1098-1136(199811)24:3<272::aid-glia2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 183.Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20(1):251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109(1–2):210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 185.Farber K, Kettenmann H. Physiology of microglial cells. Brain Res Brain Res Rev. 2005;48(2):133–143. doi: 10.1016/j.brainresrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 186.Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69(5):341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 187.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21(6):1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hamilton N, Vayro S, Wigley R, Butt AM. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia. 2010;58(1):66–79. doi: 10.1002/glia.20902. [DOI] [PubMed] [Google Scholar]
- 189.Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC, Butt AM. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia. 2008;56(7):734–749. doi: 10.1002/glia.20649. [DOI] [PubMed] [Google Scholar]
- 190.Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17(11):2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- 191.McLarnon JG. Purinergic mediated changes in Ca2+ mobilization and functional responses in microglia: effects of low levels of ATP. J Neurosci Res. 2005;81(3):349–356. doi: 10.1002/jnr.20475. [DOI] [PubMed] [Google Scholar]
- 192.Norenberg W, Langosch JM, Gebicke-Haerter PJ, Illes P. Characterization and possible function of adenosine 5′-triphosphate receptors in activated rat microglia. Br J Pharmacol. 1994;111(3):942–950. doi: 10.1111/j.1476-5381.1994.tb14830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 195.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9(6):429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 196.Pratico D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J. 2002;16(9):1138–1140. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- 197.Zhu X, Raina AK, Lee HG, Casadesus G, Smith MA, Perry G. Oxidative stress signalling in Alzheimer’s disease. Brain Res. 2004;1000(1–2):32–39. doi: 10.1016/j.brainres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 198.Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004;1742(1–3):81–87. doi: 10.1016/j.bbamcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 199.Jensen MD, Sheng W, Simonyi A, Johnson GS, Sun AY, Sun GY. Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes. Neurochem Int. 2009;55(6):362–368. doi: 10.1016/j.neuint.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Griffin WS. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83(2):470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- 201.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9(6):481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 202.Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23(35):11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Alarcon R, Fuenzalida C, Santibanez M, Bernhardi R. Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J Biol Chem. 2005;280(34):30406–30415. doi: 10.1074/jbc.M414686200. [DOI] [PubMed] [Google Scholar]
- 204.Mouser PE, Head E, Ha KH, Rohn TT. Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer’s disease brain. Am J Pathol. 2006;168(3):936–946. doi: 10.2353/ajpath.2006.050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee JM. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26(43):10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ziabreva I, Perry E, Perry R, Minger SL, Ekonomou A, Przyborski S, Ballard C. Altered neurogenesis in Alzheimer’s disease. J Psychosom Res. 2006;61(3):311–316. doi: 10.1016/j.jpsychores.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 207.Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25(2–4):116–126. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- 208.Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev. 2005;48(2):234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 209.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Virgilio F. P2 receptors and extracellular ATP: a novel homeostatic pathway in inflammation. Front Biosci (Schol Ed) 2011;3:1443–1456. doi: 10.2741/235. [DOI] [PubMed] [Google Scholar]
- 211.Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184(1–2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 212.Campbell SJ, Deacon RM, Jiang Y, Ferrari C, Pitossi FJ, Anthony DC. Overexpression of IL-1beta by adenoviral-mediated gene transfer in the rat brain causes a prolonged hepatic chemokine response, axonal injury and the suppression of spontaneous behaviour. Neurobiol Dis. 2007;27(2):151–163. doi: 10.1016/j.nbd.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 213.Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64(9):721–728. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- 214.Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78(6):1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- 215.D’Alimonte I, Ciccarelli R, Iorio P, Nargi E, Buccella S, Giuliani P, Rathbone MP, Jiang S, Caciagli F, Ballerini P. Activation of P2X7 receptors stimulates the expression of P2Y2 receptor mRNA in astrocytes cultured from rat brain. Int J Immunopathol Pharmacol. 2007;20(2):301–316. doi: 10.1177/039463200702000210. [DOI] [PubMed] [Google Scholar]
- 216.Walker DG, Kim SU, McGeer PL. Complement and cytokine gene expression in cultured microglial derived from postmortem human brains. J Neurosci Res. 1995;40(4):478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- 217.Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia. 2008;56(2):154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- 218.Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29(13):4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7(5):612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 220.Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer’s amyloid beta-peptide by microglial cells. J Biol Chem. 1999;274(45):32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- 221.Kong Y, Ruan L, Qian L, Liu X, Le Y. Norepinephrine promotes microglia to uptake and degrade amyloid beta peptide through upregulation of mouse formyl peptide receptor 2 and induction of insulin-degrading enzyme. J Neurosci. 2010;30(35):11848–11857. doi: 10.1523/JNEUROSCI.2985-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 223.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 224.Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem Int. 2001;39(5–6):333–340. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 225.Marques-da-Silva C, Burnstock G, Ojcius DM, Coutinho-Silva R. Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology. 2011;216(1–2):1–11. doi: 10.1016/j.imbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 226.Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447(1):71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 227.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Collo G, Buell G, Virgilio F. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997;36(9):1295–1301. doi: 10.1016/s0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 228.Wegiel J, Wang KC, Imaki H, Rubenstein R, Wronska A, Osuchowski M, Lipinski WJ, Walker LC, LeVine H. The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol Aging. 2001;22(1):49–61. doi: 10.1016/s0197-4580(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 229.Wegiel J, Imaki H, Wang KC, Wronska A, Osuchowski M, Rubenstein R. Origin and turnover of microglial cells in fibrillar plaques of APPsw transgenic mice. Acta Neuropathol. 2003;105(4):393–402. doi: 10.1007/s00401-002-0660-3. [DOI] [PubMed] [Google Scholar]
- 230.Wegiel J, Imaki H, Wang KC, Rubenstein R. Cells of monocyte/microglial lineage are involved in both microvessel amyloidosis and fibrillar plaque formation in APPsw tg mice. Brain Res. 2004;1022(1–2):19–29. doi: 10.1016/j.brainres.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 231.Sheng JG, Mrak RE, Griffin WS. Neuritic plaque evolution in Alzheimer’s disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 1997;94(1):1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- 232.Perlmutter LS, Scott SA, Barron E, Chui HC. MHC class II-positive microglia in human brain: association with Alzheimer lesions. J Neurosci Res. 1992;33(4):549–558. doi: 10.1002/jnr.490330407. [DOI] [PubMed] [Google Scholar]
- 233.Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18(1):134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 234.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 235.Haga S, Akai K, Ishii T. Demonstration of microglial cells in and around senile (neuritic) plaques in the Alzheimer brain. An immunohistochemical study using a novel monoclonal antibody. Acta Neuropathol. 1989;77(6):569–575. doi: 10.1007/BF00687883. [DOI] [PubMed] [Google Scholar]
- 236.Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152(1):307–317. [PMC free article] [PubMed] [Google Scholar]
- 237.Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH. Alzheimer’s disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol. 1988;132(1):86–101. [PMC free article] [PubMed] [Google Scholar]
- 238.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49(4):489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 239.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 240.Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Virgilio F, Boeynaems JM, Virchow JC, Idzko M. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65(12):1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 241.Vanderstocken G, Bondue B, Horckmans M, Pietrantonio L, Robaye B, Boeynaems JM, Communi D. P2Y2 receptor regulates VCAM-1 membrane and soluble forms and eosinophil accumulation during lung inflammation. J Immunol. 2010;185(6):3702–3707. doi: 10.4049/jimmunol.0903908. [DOI] [PubMed] [Google Scholar]
- 242.Buul JD, Hordijk PL. Signaling in leukocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2004;24(5):824–833. doi: 10.1161/01.ATV.0000122854.76267.5c. [DOI] [PubMed] [Google Scholar]
- 243.Huang AJ, Manning JE, Bandak TM, Ratau MC, Hanser KR, Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol. 1993;120(6):1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, Desgranges C. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler Thromb Vasc Biol. 1997;17(12):3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
- 245.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 246.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39(4–5):187–199. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 247.Saito H, Minamiya Y, Saito S, Ogawa J. Endothelial Rho and Rho kinase regulate neutrophil migration via endothelial myosin light chain phosphorylation. J Leukoc Biol. 2002;72(4):829–836. [PubMed] [Google Scholar]
- 248.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114(Pt 7):1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 249.Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176(1):150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 250.Vouret-Craviari V, Boquet P, Pouyssegur J, Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell. 1998;9(9):2639–2653. doi: 10.1091/mbc.9.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Soga N, Namba N, McAllister S, Cornelius L, Teitelbaum SL, Dowdy SF, Kawamura J, Hruska KA. Rho family GTPases regulate VEGF-stimulated endothelial cell motility. Exp Cell Res. 2001;269(1):73–87. doi: 10.1006/excr.2001.5295. [DOI] [PubMed] [Google Scholar]
- 252.Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol. 1996;16(3):488–496. doi: 10.1161/01.atv.16.3.488. [DOI] [PubMed] [Google Scholar]
- 253.Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273(24):15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- 254.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111(Pt 13):1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 255.Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol. 1999;19(10):2286–2297. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- 256.Wong RK, Baldwin AL, Heimark RL. Cadherin-5 redistribution at sites of TNF-alpha and IFN-gamma-induced permeability in mesenteric venules. Am J Physiol. 1999;276(2 Pt 2):H736–H748. doi: 10.1152/ajpheart.1999.276.2.H736. [DOI] [PubMed] [Google Scholar]
- 257.Noll T, Holschermann H, Koprek K, Gunduz D, Haberbosch W, Tillmanns H, Piper HM. ATP reduces macromolecule permeability of endothelial monolayers despite increasing [Ca2+]i. Am J Physiol. 1999;276(6 Pt 2):H1892–H1901. doi: 10.1152/ajpheart.1999.276.6.H1892. [DOI] [PubMed] [Google Scholar]
- 258.Tanaka N, Kawasaki K, Nejime N, Kubota Y, Nakamura K, Kunitomo M, Takahashi K, Hashimoto M, Shinozuka K. P2Y receptor-mediated Ca2+ signaling increases human vascular endothelial cell permeability. J Pharmacol Sci. 2004;95(2):174–180. doi: 10.1254/jphs.fpj03036x. [DOI] [PubMed] [Google Scholar]
- 259.Marrelli SP. Mechanisms of endothelial P2Y1- and P2Y2-mediated vasodilatation involve differential [Ca2+]i responses. Am J Physiol Heart Circ Physiol. 2001;281(4):H1759–H1766. doi: 10.1152/ajpheart.2001.281.4.H1759. [DOI] [PubMed] [Google Scholar]
- 260.Ding L, Ma W, Littmann T, Camp R, Shen J. The P2Y2 nucleotide receptor mediates tissue factor expression in human coronary artery endothelial cells. J Biol Chem. 2011;286:27027–27038. doi: 10.1074/jbc.M111.235176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wallez Y, Vilgrain I, Huber P. Angiogenesis: the VE-cadherin switch. Trends Cardiovasc Med. 2006;16(2):55–59. doi: 10.1016/j.tcm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 262.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778(3):794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 263.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, Huber P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26(7):1067–1077. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 264.Matsuyoshi N, Toda K, Horiguchi Y, Tanaka T, Nakagawa S, Takeichi M, Imamura S. In vivo evidence of the critical role of cadherin-5 in murine vascular integrity. Proc Assoc Am Physicians. 1997;109(4):362–371. [PubMed] [Google Scholar]
- 265.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110(Pt 5):583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 266.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96(17):9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol. 1995;15(8):1229–1239. doi: 10.1161/01.atv.15.8.1229. [DOI] [PubMed] [Google Scholar]
- 268.Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm’s length. Am J Physiol Cell Physiol. 2004;286(5):C987–C997. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- 269.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150(3):567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell–cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114(Pt 4):695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 271.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2(9):637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 272.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002;13(4):1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Association of Csk to VE-cadherin and inhibition of cell proliferation. EMBO J. 2005;24(9):1686–1695. doi: 10.1038/sj.emboj.7600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21(18):4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Dejana E, Bazzoni G, Lampugnani MG. The role of endothelial cell-to-cell junctions in vascular morphogenesis. Thromb Haemost. 1999;82(2):755–761. [PubMed] [Google Scholar]
- 276.Naik MU, Mousa SA, Parkos CA, Naik UP. Signaling through JAM-1 and alphavbeta3 is required for the angiogenic action of bFGF: dissociation of the JAM-1 and alphavbeta3 complex. Blood. 2003;102(6):2108–2114. doi: 10.1182/blood-2003-04-1114. [DOI] [PubMed] [Google Scholar]
- 277.Aurrand-Lions M, Johnson-Leger C, Imhof BA. The last molecular fortress in leukocyte trans-endothelial migration. Nat Immunol. 2002;3(2):116–118. doi: 10.1038/ni0202-116. [DOI] [PubMed] [Google Scholar]