Abstract
ATP, acting via P2 purinergic receptors, is a known mediator of inflammatory and neuropathic pain. There is increasing evidence that the ATP-gated P2X4 receptor (P2X4R) subtype is a locus through which activity of spinal microglia and peripheral macrophages instigate pain hypersensitivity caused by inflammation or by injury to a peripheral nerve. The present article highlights the recent advances in our understanding of microglia–neuron interactions in neuropathic pain by focusing on the signaling and regulation of the P2X4R. We will also develop a framework for understanding converging lines of evidence for involvement of P2X4Rs expressed on macrophages in peripheral inflammatory pain.
Keywords: Microglia, P2X4 purinoceptors, BDNF, Neuropathic pain, Inflammatory pain
Introduction
Chronic pain, unlike acute pain, has no known defensive or beneficial purpose and is a reflection of aberrant functioning of pathologically altered peripheral and/or central nociceptive neural networks [46, 62, 84]. Chronic pain can be broadly categorized as chronic inflammatory pain and neuropathic pain. Chronic inflammatory pain arises as a consequence of inflammatory responses mounted by the immune system following tissue damage and generally abates after such damage is repaired [48, 58]. Neuropathic pain, on the other hand, arises because of injury to a nerve caused by trauma, infection, or pathology, and is characterized by pain that persists long after the initiating event has healed [62, 89]. In both chronic inflammatory and neuropathic pain, pain may arise spontaneously in the absence of an overt stimulus, or it can be evoked, such as in the case of allodynia (pain resulting from an innocuous stimulus) and hyperalgesia (an exaggerated pain response to a noxious stimulus). The symptoms of neuropathic pain are difficult to treat and often resistant to the current available treatments, including the potent analgesic effects of opioid drugs, which is in stark contrast to acute and chronic inflammatory pain for which there are many effective therapies. Neuropathic pain is therefore considered among the most debilitating and difficult to manage chronic pain conditions.
Previously, the predominant view was that neuropathic pain following peripheral nerve injury is the direct result of alterations in neurons and neuronal function in the peripheral and central nervous systems. Indeed, injury to a peripheral nerve instigates a series of cellular and molecular changes that directly affect neuronal plasticity, leading ultimately to altered synaptic connectivity and the reorganization of peripheral and central nociceptive circuitry [62, 83, 84, 89]. Such changes produce a dichotomous affect on pain transmission: they suppress mechanisms that inhibit pain and enhance mechanisms that facilitate pain. The net effect is a pathological amplification of sensory input and output from the spinal cord that manifests as pain hypersensitivity. The basis of these neuronal adaptations is critical to understanding the pathophysiology of neuropathic pain. However, pharmacological therapies that have been directed against cellular targets in neurons have not produced optimal therapeutic effects in patients suffering from chronic pain, in particular, neuropathic pain.
Although it is not disputed that neurons are fundamentally involved in neuropathic pain, it is now apparent that a neuron-centric approach to understanding pain is an oversimplification and does not account for the diverse network of cell types within the central nervous system. This realization is in response to a rapidly growing body of evidence that glia–neuron interactions are critical in establishing and maintaining neuropathic pain [1, 31, 36, 50]. Microglia, in particular, have emerged as key players in the etiology of neuropathic pain [36, 71, 75, 81, 82]. Microglia–neuron communication is bidirectional and considerable evidence implicates purinergic signaling as a critical molecular component [17, 23, 39, 47]. In particular, evidence is accruing that adenosine triphosphate (ATP), acting via P2 purinergic receptors, is involved in pain hypersensitivity associated with neuropathic pain and peripheral inflammation [12, 23, 24, 39]. ATP released from damaged and/or inflamed tissue directly modulates microglial functioning, and microglia in turn release a myriad of cytokines, chemokines, and neurotrophic factors that have a profound effect on neuronal function [15, 21, 36, 71, 82, 86]. In vivo imaging studies show that upon traumatic injury microglia rapidly extend processes by an ATP-dependent mechanism and converge on the site of injury [19, 32, 45, 53]. Microglia express a range of P2 purinergic receptors: of the metabotropic P2YRs, microglia express P2Y1, 2, 4, 6, and 12Rs [7, 25, 36, 61], with P2Y12R signaling mediating chemotaxis and tactile allodynia associated with nerve injury [44, 69]. By contrast, microglial expression of the ionotropic P2XR is restricted to the P2X4 and P2X7R subtypes [16, 28, 36, 75].
Activation of P2X7Rs is implicated in the microglia response to inflammation [16, 37], microglial proliferation [4, 51] and release of proinflammatory cytokines [10, 13, 15, 26, 27]. A role for P2X7Rs in neuropathic pain has also been suggested on the basis that P2X7R mRNA and protein expression are upregulated in spinal microglia follow peripheral nerve injury [43], and pharmacological blockade or genetic deletion of this receptor subtype ameliorates the development of pain hypersensitivity [9, 14, 22, 33, 34, 49, 54]. Recently, genetic variation in P2X7R function has been linked to variations in pain in mice and humans [65]. Despite accruing evidence that P2X7Rs are critically involved in both inflammatory and neuropathic pain, conclusions about the specific role microglial P2X7Rs play in these chronic pain conditions is confounded by the fact that P2X7Rs, in addition to being expressed on microglia, are localized on astrocytes and neurons in the spinal cord. Thus, involvement of P2X7Rs in chronic pain may not be specific to microglia and could instead involve activation of P2X7Rs expressed on other cell types in the spinal cord. The P2X4R, on the other hand, is emerging as a core signaling pathway in microglia that underlies the sequelae of peripheral nerve injury.
The first demonstration that P2X4Rs are involved in neuropathic pain was reported by Tsuda et al. [75]. It was found that intrathecal injection of TNP-ATP, an antagonist of P2X1-4Rs, produced a rapid and transient reversal of mechanical allodynia in rats that sustained a peripheral nerve injury 1 to 2 weeks prior. By contrast, treatment with PPADS, an antagonist of P2X1-3,5,7Rs but not the P2X4R, had no effect on mechanical allodynia when injected at the same time point after nerve injury. Based on the pharmacological profiles of these two antagonists, it was deduced that mechanical allodynia depends upon tonic activity of spinal P2X4Rs. Moreover, the time course of development of mechanical hypersensitivity was correlated with a progressive increase in spinal P2X4R protein expression and this occurred specifically in microglia. This observation was recently confirmed in the CX3CR1+/GFP mice, in which induction of spinal P2X4R expression resulting from peripheral nerve lesion was restricted to activated eGFP expressing microglia [79]. Directly targeting P2X4Rs with intrathecal administration of a P2X4R antisense oligodeoxynucleotide prevented the increase in P2X4R protein expression and suppressed the development of mechanical allodynia [75]. An important line of evidence for the necessity of P2X4Rs comes from findings that mice lacking the P2X4R do not develop mechanical allodynia after peripheral nerve injury [72, 79]. Although the behavioral pain responses in these mice were absent, the proliferative response of microglia to peripheral nerve injury was undiminished [79], suggesting that while tonic P2X4R activation is required for maintaining peripheral nerve injury-induced allodynia, the proliferation and upregulation of microglial P2X4Rs in the spinal dorsal horn are independent. These experiments demonstrated the necessity of P2X4Rs as an active component in neuropathic pain, but do not preclude the possibility of intermediary factors being required (i.e., sufficiency had not been demonstrated). Sufficiency of P2X4R stimulation in microglia for development of allodynia is supported by evidence that direct injection of P2X4R-stimulated cultured microglia into the spinal cords of otherwise naïve animals elicits robust mechanical allodynia that is blocked by TNP-ATP [17, 75]. Collectively, the biochemical and behavioral findings indicate that activity of P2X4Rs expressed on microglia residing in the spinal dorsal horn is both necessary and sufficient to induce tactile allodynia after peripheral nerve injury.
The above results strongly support upregulation of P2X4Rs in microglia as being a critical process in neuropathic pain. Significant advances have been made towards understanding how injury to a nerve signals to the dorsal horn causing increased P2X4R expression in microglia. One such neuron–microglia signaling molecule is CCL21, a microglia-activating chemokine released from injured neurons [5, 20]. A recent study by Biber et al. [5] reported that following injury to a peripheral nerve, CCL21 expression was significantly increased in small diameter neurons in the dorsal root ganglia and in primary afferent nerve terminals in the spinal dorsal horn, but not in microglia or in astrocytes. Early intervention with intrathecal delivery of CCL21 neutralizing antibody before and for the first 3 days after nerve injury significantly attenuated tactile allodynia throughout the 14-day experiment in wild-type mice; this early preemptive effect argues for involvement of CCL21 in the initiation of neuropathic pain. In accord, mice that lack neuronal CCL21 did not develop tactile allodynia following nerve injury and failed to upregulate microglial P2X4R expression. Stimulation of microglia in culture with recombinant CCL21 induced a rapid and concentration dependent increase in P2X4R expression, suggesting a direct effect of CCL21 on the microglia. This notion is corroborated by observations that intrathecal injection of CCL21 to nerve injured CCL21 deficient mice induced long lasting allodynia and upregulated P2X4R expression. The pronociceptive actions of CCL21 are strictly dependent on P2X4R function because injection of this chemokine in P2X4R knockout mice had no effect on tactile allodynia. Taken together, these findings reveal CCL21 as a key neuronal molecule that functions as an upstream activator of P2X4R expression in spinal microglia that is required for the induction of neuropathic pain.
Several other signaling elements have been implicated in the upregulation of P2X4Rs following peripheral nerve injury. For example, the cytokine interferon γ has been identified as a signaling molecule that drives P2X4R expression and through which resting spinal microglia transform into an activated state [73]. Release of tryptase from activated mast cells has also been found to increase P2X4R in microglia [85]. Likewise, microglia grown in culture in the presence of the extracellular matrix molecule fibronectin show a marked increase in P2X4R levels and a consequent enhanced Ca2+ response to ATP stimulation [52]. Fibronectin expression was elevated in the spinal cord of nerve injured animals and blockade of fibronectin receptors suppressed both the increase in P2X4Rs and tactile allodynia [52, 76–78]. Fibronectin induced increase in microglial P2X4R requires activity of the tyrosine kinase Lyn and downstream activation of intracellular signaling pathways involving phosphatidylinositol 3-kinase (PI3K)-Akt and mitogen-activated protein kinase kinase (MAPK kinase, MEK)-extracellular signal-regulated kinase (ERK), which have distinct roles in the up-regulation of P2X4R expression in microglia at the transcriptional and post-transcriptional levels, respectively [77]. Thus, several critical signaling elements necessary for the upregulation of P2X4R in microglia following nerve injury have been identified, but how these elements are causally connected and whether they encompass the entirety of the necessary pathways remains an open question.
P2X4Rs are known to undergo rapid constitutive internalization and reinsertion to the plasma membrane [6, 30, 59, 67]. In microglia, internalized P2X4Rs are targeted to lysosomes and constitutive retrieval of these receptors from the plasma membrane regulates the proportion of P2X4Rs on the cell surface [56]. Lysosome exocytosis, stimulated by incubating microglia with the Ca2+ ionophore ionomycin, leads to an accumulation of P2X4Rs on the cell surface and enhances P2X4R-mediated currents [56]. Cell surface targeting of P2X4Rs by lysosomes has recently been shown to involve the chemokine CC2 [68]. It is well established that injury to a cell induces lysosome exoctyosis [35, 38, 57, 66]; thus, it is conceivable that cell damage resulting from injury to a nerve might mobilize P2X4Rs from lysosomes to the microglial cell surface.
P2X4Rs expressed on the cell surface are capable of being stimulated by ATP. Activation of these receptors instigates a series of conformational changes that allow cations, such as Ca2+ and Na+, entry into the cell through a non-selective channel [11]. The recent isolation of the P2X4R crystal structure has illuminated many atomic details about its extracellular domain structure, the putative ATP binding site, transmembrane regions, and ion permeation pathway [41]. The C-terminus of the P2X4R in particular appears important in agonist-induced desensitization [29], binding of phosphoinositide PIP2 [3], and constitutive internalization [30, 56, 59]. Time-lapse imaging of ATP induced structural changes in the P2X4R has revealed two distinct activated conformations: in the presence of extracellular Ca2+, ATP stimulation caused the P2X4R to open a non-selective cation-permeable channel, but in the absence of extracellular Ca2+, the receptor formed a transmembrane pore (i.e., pore dilation) that allowed passage of large molecules [64]. P2X4R expressed on microglia and macrophages have recently been reported to possess this ability to function as both an ion channel and a pore [2, 63]. Although the biochemical and biophysical ion channel properties of the P2X4R are well characterized and widely investigated in microglia, the significance of this dual function, in particular the role of P2X4R pore dilation in the context of neuropathic pain remains to be elucidated.
The first clues that activity of P2X4Rs expressed on microglia alters fundamental spinal nociceptive processing stem from a series of experiments by Coull et al. [17], who demonstrated that intrathecal administration of ATP stimulated microglia produced a depolarizing shift in Eanion and converting GABA-evoked responses from hyperpolarizing to depolarizing in spinal lamina I neurons. The altered inhibitory responses initiated by P2X4R stimulation in microglia unmasks low threshold input to lamina I, thereby driving the phenotypic switch in their response properties. This phenotypic switch caused spinal lamina I neurons to relay innocuous mechanical input, increase discharge to a noxious stimulus, and display spontaneous activity [18, 42]. The changes in output of the lamina I neurons may be interpreted as providing a neural substrate for neuropathic pain. Because microglia are not directly involved in the spinal neural network that process and relay nociceptive information, Coull et al. [17] reasoned that microglia must signal to lamina I neurons to affect neuronal hyperexcitability. Indeed, several key findings pointed to brain-derived neurotrophic factor (BDNF) as being the critical microglia–neuron signaling molecule: (1) function blocking antibody against trkB, the cognate receptor for BDNF, and the BDNF-sequestering TrkB-Fc fusion protein prevented mechanical allodynia evoked by administering P2X4R-stimulated microglia; (2) treatment of cultured microglia with siRNA against BDNF prevented the effects of intrathecally administered microglia on lamina I neurons and on the microglia-elicited pain behaviors; and (3) P2X4R stimulation by ATP caused release of BDNF from microglia in culture, a response prevented by TNP-ATP or by knocking down expression of BDNF with siRNA [17]. Taken together, the most parsimonious explanation for these findings is that P2X4R stimulated microglia signal to spinal lamina I dorsal horn neurons causing aberrant nociceptive output and that the critical microglia–neuron signaling molecule is BDNF. The conclusion that P2X4Rs control BDNF release from microglia is congruent with observations in P2X4R-deficient mice that BDNF accumulates in spinal dorsal horn microglia following nerve injury and that microglia isolated from these mice show impaired ATP-evoked BDNF release [79]. However, it has yet to be shown that resident microglia in the spinal cord respond to nerve injury by releasing BDNF. In mice, where BDNF was genetically eliminated from small diameter sensory neurons, inflammatory pain was significantly attenuated but development of allodynia following nerve injury was unperturbed [87]. This finding is consistent with primary afferent derived BDNF not having a causal role in the sequelae of neurpathic pain arising from injury to a peripheral nerve.
We recently addressed the gap in understanding how activation of P2X4R leads to the release of BDNF from microglia [70]. We demonstrated in cultured microglia that influx of Ca2+ through the P2X4R is a critical intracellular step linking stimulation of these receptors to the activation of p38 MAPK. We discovered that p38 MAPK is required for the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-dependent vesicular release of pre-made BDNF and also increased transcription and translation of BDNF. By demonstrating that p38-MAPK is a cellular intermediary in the release and synthesis of BDNF evoked by stimulating microglial P2X4R, we provided a unifying mechanism for observations that ongoing expression of neuropathic pain behaviors requires both the activation of P2X4R [17, 75, 79] and p38-MAPK [40, 74, 88]. These findings place p38 MAPK in the core P2X4R-BDNF signaling cascade and implicate p38 MAPK activation and BDNF release as an essential step in microglia–neuron communication leading to nerve injury induced pain hypersensitivity. Likewise, activation of P2X7Rs or P2Y12Rs leading to the development of pain hypersensitivity engages distinct intracellular signaling pathways in microglia that converge onto p38 MAPK [15, 44]. Thus, p38 MAPK is a cellular intermediary and its activation is a critical point of convergence for P2X4R, P2X7R, and P2Y12R signaling in neuropathic pain. The significance of this convergence in microglia signaling and the upstream, as well as, downstream components of these signaling pathways in aberrant spinal nociceptive processing following nerve injury remains to be fully elucidated.
A similar link between P2X4R and p38 MAPK signaling has recently been uncovered in peripheral macrophages [80], which like microglia are immune cells that constitutively express functional P2X4Rs [8, 80] targeted to the cell surface by lysosome trafficking [56]. Rassendren and colleagues demonstrated that stimulating P2X4R expressed in macrophages triggers Ca2+ influx and p38 MAPK phosphorylation that drives the production and release of prostaglandin E2 [80], a principle substrate for inflammation that elicits pain hypersensitivity through sensitization of peripheral nociceptors [55, 60]. Genetic deletion of the P2X4R abrogates P2X4R-mediated release of prostaglandin E2 from macrophages [80] and prevents pain hypersensitivity in response to inflammatory challenges [72]. Thus, these discoveries highlight a novel role for P2X4R in inflammation and represent a significant conceptual advance that extends involvement of P2X4R in chronic pain beyond neuropathic pain to encompass the pathoetiology of inflammatory pain.
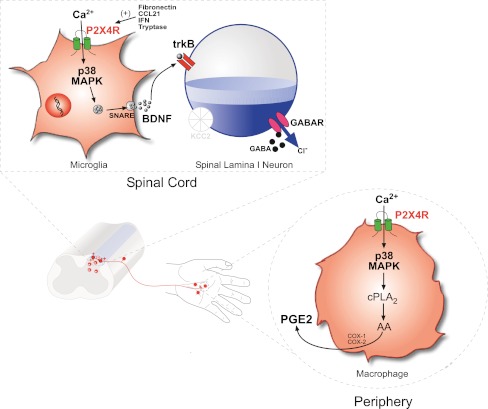
In conclusion, the P2X4R has emerged as a core microglia–neuron signaling pathway that is necessary for ongoing tonic expression of tactile allodynia following nerve injury. Converging lines of evidence also point to activity of P2X4Rs as being a locus through which spinal microglia and peripheral macrophages contribute to neuropathic pain and inflammatory pain, respectively. In both cell types, the cellular and physiological consequences of P2X4R activation are mediated by influx of Ca2+ through the P2X4R and activation of p38 MAPK (Fig. 1). This mechanistically identical signaling step results in the release of distinct signaling molecules from microglia (BDNF) and peripheral macrophages (prostaglandin E2) that alter neuronal nociceptive output and translates into pain hypersensitivity. Thus, identification of the upstream and downstream components of the P2X4R signaling pathway has provided important new information about the fundamental central and peripheral mechanisms that underlie pain hypersensitivity subsequent to nerve injury and inflammation. The P2X4R is therefore a promising therapeutic target for the treatment of neuropathic pain and inflammatory pain.
Fig. 1.
P2X4R-p38 MAPK is a core signaling pathway through which spinal microglia and peripheral macrophages contribute to neuropathic pain and inflammatory pain. a Following injury to a peripheral nerve, expression of P2X4R is upregulated in microglia that reside in the spinal dorsal horn. Several factors have recently been implicated in the upregulation of microglial P2X4R: the fibronectin-Lyn kinase signaling cascade, cytokine IFN-γ, tryptase released from mast cells, and the chemokine CCL21. Influx of Ca2+ through ATP stimulated P2X4R activates p38-MAPK and drives the synthesis and SNARE-dependent release of BDNF. Acting on its cognate receptor, trkB, BDNF released from microglia signals to dorsal horn lamina I neurons to downregulate the K+-Cl− cotransporter KCC2, resulting in aberrant nociceptive output that underlies pain hypersensitivity characterized by hyperalgesia, allodynia, and spontaneous pain. b Like microglia, peripheral macrophages constitutively express P2X4R. Inflammatory challenges that activate P2X4R on macrophages trigger Ca2+ influx and p38 MAPK phosphorylation. In macrophages, the P2X4R-p38 MAPK pathway is a signaling hub that activates cytosolic PLA2 which liberates arachidonic acid (AA) resulting in a cyclooxygenase (COX) dependent synthesis and release of prostaglandin E2 (PGE2), a key substrate that sensitizes primary sensory neurons and leads to hyperexcitability of peripheral nociceptive pathways that is a hallmark of inflammatory pain
Acknowledgments
The work of the authors is supported by grants from the Canadian Institutes of Health Research (CIHR; grant number MT-11219), the Krembil Foundation, and the Ontario Research Fund Research Excellence Program. MWS holds a Canada Research Chair (Tier I) in Neuroplasticity and Pain, and is the Anne and Max Tanenbaum Chair in Molecular Medicine at the Hospital for Sick Children. TT was supported by a CIHR fellowship. We thank Ms. Janice Hicks and Dr. Simon Beggs for helpful comments and revisions on the manuscript.
References
- 1.Beggs S, Salter MW. Microglia–neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol. 2010;20:474–480. doi: 10.1016/j.conb.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernier LP, Ase AR, Boue-Grabot E, Seguela P (2012) P2X4 receptor channels form large noncytolytic pores in resting and activated microglia. Glia 60:728–737 [DOI] [PubMed]
- 3.Bernier LP, Ase AR, Chevallier S, Blais D, Zhao Q, Boue-Grabot E, Logothetis D, Seguela P. Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J Neurosci. 2008;28:12938–12945. doi: 10.1523/JNEUROSCI.3038-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 5.Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T, Boddeke H, Inoue K (2011) Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J 30(9):1864–1873 [DOI] [PMC free article] [PubMed]
- 6.Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22:4814–4824. doi: 10.1523/JNEUROSCI.22-12-04814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17:2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- 8.Brone B, Moechars D, Marrannes R, Mercken M, Meert T. P2X currents in peritoneal macrophages of wild type and P2X4−/− mice. Immunol Lett. 2007;113:83–89. doi: 10.1016/j.imlet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Broom DC, Matson DJ, Bradshaw E, Buck ME, Meade R, Coombs S, Matchett M, Ford KK, Yu W, Yuan J, Sun SH, Ochoa R, Krause JE, Wustrow DJ, Cortright DN. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-amino-pyrrolidin-1-yl)methyl]-2-chloro-ben zamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther. 2008;327:620–633. doi: 10.1124/jpet.108.141853. [DOI] [PubMed] [Google Scholar]
- 10.Brough D, Feuvre RA, Iwakura Y, Rothwell NJ. Purinergic (P2X7) receptor activation of microglia induces cell death via an interleukin-1-independent mechanism. Mol Cell Neurosci. 2002;19:272–280. doi: 10.1006/mcne.2001.1054. [DOI] [PubMed] [Google Scholar]
- 11.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Seguela P. ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. 2002;22:3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Clark AK, Wodarski R, Guida F, Sasso O, Malcangio M. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia. 2010;58:1710–1726. doi: 10.1002/glia.21042. [DOI] [PubMed] [Google Scholar]
- 16.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/S0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 17.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De KY. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 18.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De KP, De KY. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 19.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 20.Jong EK, Dijkstra IM, Hensens M, Brouwer N, Amerongen M, Liem RS, Boddeke HW, Biber K. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/S0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 22.Dell'Antonio G, Quattrini A, Cin ED, Fulgenzi A, Ferrero ME. Relief of inflammatory pain in rats by local use of the selective P2X7 ATP receptor inhibitor, oxidized ATP. Arthritis Rheum. 2002;46:3378–3385. doi: 10.1002/art.10678. [DOI] [PubMed] [Google Scholar]
- 23.Di VF. Purinergic signalling between axons and microglia. Novartis Found Symp. 2006;276:253–258. doi: 10.1002/9780470032244.ch20. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J Pharmacol Exp Ther. 2008;324:409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- 25.Farber K, Kettenmann H. Physiology of microglial cells. Brain Res Brain Res Rev. 2005;48:133–143. doi: 10.1016/j.brainresrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari D, Chiozzi P, Falzoni S, Dal SM, Melchiorri L, Baricordi OR, Di VF. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 27.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di VF. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di VF. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- 29.Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281:15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- 30.Fujii K, Young MT, Harris KD (2011) Exploiting powder X-ray diffraction for direct structure determination in structural biology: the P2X4 receptor trafficking motif YEQGL. J Struct Biol (in press) [DOI] [PMC free article] [PubMed]
- 31.Grace PM, Rolan PE, Hutchinson MR (2011) Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav Immun (in press) [DOI] [PubMed]
- 32.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 33.Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, Zhu C, Carroll W, Perez-Medrano A, Iwakura Y, Jarvis MF. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1alphabeta knockout mice. Behav Brain Res. 2009;204:77–81. doi: 10.1016/j.bbr.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- 35.Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue K, Tsuda M. The role of microglia and ATP receptors in a mechanism of neuropathic pain. Nippon Yakurigaku Zasshi. 2006;127:14–17. doi: 10.1254/fpj.127.14. [DOI] [PubMed] [Google Scholar]
- 37.Itoh K, Chiang CY, Li Z, Lee JC, Dostrovsky JO, Sessle BJ. Central sensitization of nociceptive neurons in rat medullary dorsal horn involves purinergic P2X7 receptors. Neuroscience. 2011;192:721–731. doi: 10.1016/j.neuroscience.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller AF, Beggs S, Salter MW, De KY. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett. 2011;504:57–61. doi: 10.1016/j.neulet.2011.08.058. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28:2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurpius D, Nolley EP, Dailey ME. Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia. 2007;55:873–884. doi: 10.1002/glia.20509. [DOI] [PubMed] [Google Scholar]
- 46.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda M, Tsuda M, Tozaki-Saitoh H, Inoue K, Kiyama H. Nerve injury-activated microglia engulf myelinated axons in a P2Y12 signaling-dependent manner in the dorsal horn. Glia. 2010;58:1838–1846. doi: 10.1002/glia.21053. [DOI] [PubMed] [Google Scholar]
- 48.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 49.McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146:1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 50.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nasu-Tada K, Koizumi S, Tsuda M, Kunifusa E, Inoue K. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X4, a key molecule for mechanical allodynia. Glia. 2006;53:769–775. doi: 10.1002/glia.20339. [DOI] [PubMed] [Google Scholar]
- 53.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 54.Perez-Medrano A, Donnelly-Roberts DL, Honore P, Hsieh GC, Namovic MT, Peddi S, Shuai Q, Wang Y, Faltynek CR, Jarvis MF, Carroll WA. Discovery and biological evaluation of novel cyanoguanidine P2X(7) antagonists with analgesic activity in a rat model of neuropathic pain. J Med Chem. 2009;52:3366–3376. doi: 10.1021/jm8015848. [DOI] [PubMed] [Google Scholar]
- 55.Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120:3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- 57.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/S0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 58.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Royle SJ, Bobanovic LK, Murrell-Lagnado RD. Identification of a non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem. 2002;277:35378–35385. doi: 10.1074/jbc.M204844200. [DOI] [PubMed] [Google Scholar]
- 60.Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390–396. doi: 10.1016/S1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44:242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- 62.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 63.Seil M, El OM, Fontanils U, Etxebarria IG, Pochet S, Dal MG, Marino A, Dehaye JP. Ivermectin-dependent release of IL-1beta in response to ATP by peritoneal macrophages from P2X(7)-KO mice. Purinergic Signal. 2010;6:405–416. doi: 10.1007/s11302-010-9205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shinozaki Y, Sumitomo K, Tsuda M, Koizumi S, Inoue K, Torimitsu K. Direct observation of ATP-induced conformational changes in single P2X4 receptors. PLoS Biol. 2009;7:e103. doi: 10.1371/journal.pbio.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Meulen HV, Costigan M, Herbert TA, Yarkoni-Abitbul M, Tichauer D, Livneh J, Gershon E, Zheng M, Tan K, John SL, Slade GD, Jordan J, Woolf CJ, Peltz G, Maixner,W, Diatchenko L, Seltzer Z, Salter MW, Mogil JS (2012) Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med doi:10.1038/nm.2710 [DOI] [PMC free article] [PubMed]
- 66.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol. 2010;189:1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toulme E, Soto F, Garret M, Boue-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol. 2006;69:576–587. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]
- 68.Toyomitsu E, Tsuda M, Yamashita T, Tozaki-Saitoh H, Tanaka Y, Inoue K (2012) CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal (in press) [DOI] [PMC free article] [PubMed]
- 69.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- 75.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 76.Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Nasu-Tada K, Koizumi S, Yamamoto K, Ando J, Inoue K. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- 77.Tsuda M, Toyomitsu E, Kometani M, Tozaki-Saitoh H, Inoue K. Mechanisms underlying fibronectin-induced up-regulation of P2X4R expression in microglia: distinct roles of PI3K-Akt and MEK-ERK signalling pathways. J Cell Mol Med. 2009;13:3251–3259. doi: 10.1111/j.1582-4934.2009.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsuda M, Tozaki-Saitoh H, Masuda T, Toyomitsu E, Tezuka T, Yamamoto T, Inoue K. Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia. 2008;56:50–58. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- 79.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 82.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/S0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 83.Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: implications for diagnosis and therapy. Life Sci. 2004;74:2605–2610. doi: 10.1016/j.lfs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 85.Yuan H, Zhu X, Zhou S, Chen Q, Zhu X, Ma X, He X, Tian M, Shi X. Role of mast cell activation in inducing microglial cells to release neurotrophin. J Neurosci Res. 2010;88:1348–1354. doi: 10.1002/jnr.22304. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J, De KY. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- 87.Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN (2006) Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci 31(3):539–548 [DOI] [PubMed]
- 88.Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/S0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]