Abstract
The P2X7 receptor is widely recognized to mediate the proinflammatory effects of extracellular ATP. However this receptor in the absence of ATP may have a function unrelated to inflammation. Our data show that P2X7 expressed on the surface of monocyte/macrophages or on epithelial HEK-293 cells greatly augments the engulfment of latex beads and live and heat-killed bacteria by effector phagocyte in the absence of ATP and serum. The expression of P2X7 on the effector also confers the ability to phagocytose apoptotic target cells and an accumulation of P2X7 can be seen at the attachment point to the target. Activation of the P2X7 receptor by ATP causes a slow dissociation (over 10–15 min) of nonmuscle myosin from the P2X7 membrane complex and abolishes further P2X7-mediated phagocytosis of these targets. The recent crystal structure of the homologous zebrafish P2X4 receptor shows an exposed “nose” of the ectodomain (residues 115–162) which contains three of the five disulfide bonds conserved in all P2X receptors. Three short biotin-labeled peptides mimicking sequence of this exposed region bound to apoptotic target cells but not to either viable cells or to other target particles. All three peptides contained one or two cysteine residues and their replacement by alanine abolished peptide binding. These data implicate thiol-disulfide exchange reactions in the initial tethering of apoptotic cells to macrophage and establish P2X7 as one of the scavenger receptors involved in the recognition and removal of apoptotic cells in the absence of extracellular ATP and serum.
Keywords: P2X7 receptor, Scavenger receptor, Phagocytosis, Disulfide bonds, Peptide binding
Phagocytosis is a fundamental aspect of the innate immune system which is preserved in specialized cells of all metazoans and its ancient origins are suggested by the phagocytic ability of a subset of cells within the social amoeba, Dictyostelium discoideum, which feeds on bacteria in the soil [1]. In the zebrafish embryo, primitive macrophages have been shown to clear injected bacteria by phagocytosis [2]. In vertebrates, phagocytosis acts as an immediate innate immune response to invasion by bacteria thereby clearing or limiting the bacterial load until an adaptive immune response can be mounted [3]. Phagocytosis is also a critical physiological process for tissue remodeling which removes apoptotic or aged cells in a regulated manner in the absence of inflammation [4]. Specific recognition of the apoptotic cell by the phagocyte occurs without regard to the species or tissue origin of the target or the nature of the apoptotic stimulus and can occur without the involvement of soluble factors [5].
Macrophage scavenger receptors
Macrophages play a major role in this host innate immune response to invading pathogens by recognizing conserved molecules either expressed on the bacterial cell wall or in soluble form (e.g., lipopolysaccharide, lipoteichoic acid). These pathogen-associated molecular pattern molecules are recognized by Toll-like receptors on the macrophage which activate downstream signaling pathways. Macrophages also express scavenger receptors (SR) which are a large group of structurally diverse cell-surface glycoproteins which recognize and endocytose a wide range of particles and molecules. These targets for macrophage SRs include high density lipoprotein (by SR-B1) [6], hemoglobin-haptoglobin complexes (by CD163) [7], β-amyloid deposits (by CD36) [8] and bacteria (by SR-A, SR-B1, CD36, and CD163) [9–11]. A broad classification of SR into Class A (triple helix collagenous structure) and Class B (two transmembrane domains) down to Class F has been proposed [12] but this classification does not fully capture the diversity of structural motifs. Assays for these receptors use non-selective inhibitors, polyinosinic acid, liposomes containing phosphatidylserine, oxidized low-density lipoprotein etc. to inhibit this pathway. However, at least for SRs which phagocytose particulate matter (0.5–3.0 μm) the most reliable inhibitor is cytochalasin D, a classic inhibitor of F-actin polymerization which prevents the cytoskeletal rearrangements needed for particle uptake.
The search for macrophage receptors responsible for the recognition and clearance of apoptotic cells has focused on molecules which recognize and bind phosphatidylserine (PS) since exposure of this “eat-me” signal on the surface of apoptotic cells is the signal for their removal by professional or non-professional phagocytes [4]. A family of Type-1 single transmembrane proteins, T-cell immunoglobulin, and mucin-domain containing molecules (Tim-1 and Tim-4) have been identified as PS receptors and shown to be important for the binding and engulfment of apoptotic cells by macrophages [13–15]. Other phagocytic receptors with different structure have been shown to bind PS and promote engulfment of apoptotic cells. A seven transmembrane protein belonging to an adhesion-type G-protein coupled receptor family termed brain-specific angiogenesis inhibitor (BAI-1) both binds PS and promotes internalization of apoptotic cells [16]. BAI-1 is expressed in human monocytes and macrophages as well as in the brain and this molecule contributes both to the binding and engulfment of carboxylate-modified beads as well as apoptotic cells. Stabilin-2, a large multi-functional scavenger receptor has also been identified as a PS receptor which can mediate engulfment of apoptotic cells and aged red blood cells [17]. Other PS-dependent receptors for apoptotic cells include Mer receptor tyrosine kinase [18] and αVβ5 integrin both of which bind PS through soluble bridging molecules [19, 20] while a PS independent pathway, the calreticulin/LDL-receptor-related protein (LRP)-dependent pathway has been reported for the removal of tumor targets by dendritic cells [21]. Since redundancy is a prominent feature of apoptotic cell clearance in the body, other pathways are likely to exist.
Flow cytometric assay of phagocytosis
Technical advances in the assessment of phagocytosis have allowed rapid advances in our knowledge of molecular interactions associated with engulfment of particles by phagocytes. Confocal microscopy has shown that internal membranes within the cell fuse with plasma membrane during the course of particle ingestion, and that recycling endosomes are the primary source of membrane for enlargement of phagocytic cup [22]. Flow cytometric assessment of particle engulfment has to some extent replaced microscopic observation [23] particularly as the kinetics of uptake of fluorescent targets by phagocytes can be followed by instruments capable of time-resolved measurements in a stirred cuvette at 37°C [24]. These assays of phagocytosis by flow cytometry usually include measurements of fluorescence particle uptake by cells pre-incubated with cytochalasin D (CytD), an inhibitor of F-actin polymerization and phagocytic cup formation which allows the assay to distinguish engulfment of particles from adhesion. Particle size is also important in flow studies which generally employ fluorescent particles of 1–3 μm in diameter.
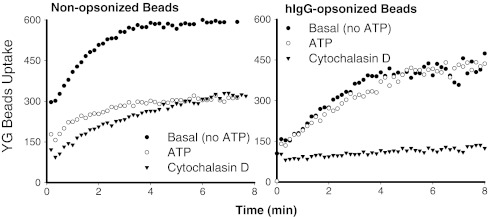
The kinetics of phagocytosis of 1 μm carboxylated fluorescent beads by fresh human monocytes can be defined by the difference in bead uptake with and without CytD. This component of particle uptake shows a similar magnitude and time course whether the beads are non-opsonized or coated with human IgG (Fig. 1). This similarity in engulfment kinetics between the two types of beads suggests a common rate-limiting step for internalization of the particle, most likely related to the cytoskeletal rearrangements necessary for phagocytic cup formation. Previous studies have also shown that apoptotic recognition can be dissociated from the engulfment step [5, 25].
Fig. 1.
Typical time courses of uptake of 1.0 μm yellow-green (YG) beads by fresh human monocytes from the same subject. Cells were labeled with allophycocyanin conjugated anti-CD14 mAb and pre-treated with 1 mM ATP for 15 min or 20 μM CytD for 30 min before the addition of YG beads. The linear mean channel of fluorescence intensity for each gated CD14+ monocyte population over successive 10-s intervals was analyzed by WinMDI software and plotted against time
P2X7 mediates rapid uptake of beads and bacteria in the absence of serum
Pre-incubation of monocytes with ATP for 15–20 min produces a major inhibition of phagocytosis of non-opsonized beads by fresh human monocytes while having no effect on uptake of human IgG-coated beads mediated by the Fc-receptor (Fig. 1). ATP is the physiological ligand for a number of purinergic receptors expressed on cells of monocyte-macrophage lineage which include P2X4, P2X7, and a number of P2Y receptors. A reliable method for establishing the phagocytic role of a receptor is to express it on a non-phagocytic cell type (such as HEK-293, an epithelial cell line) and show it confers phagocytic capacity on the recipient cell. Transfection of HEK-293 cells with P2X7-DsRed constructs conferred a brisk uptake of beads by those cells expressing high amounts of P2X7-DsRed, whereas cells with low P2X7 expression took up few or no beads [24]. Moreover, pre-incubation of the P2X7-transfected cells with mAb (clone L4) to the ectodomain of P2X7 inhibited the uptake of the fluorescent beads down to the level observed with CytD [24]. A second approach to the phagocytic role of P2X7 involved peritoneal macrophages from C57BL/6 mice with and without deletion of the P2X7 gene [26], which confirmed that attenuation of phagocytosis by ATP was mediated via the P2X7 receptor. Exposure of wild type P2X7+/+ macrophages to ATP, either in vitro or by intraperitoneal injection of ATP in vivo reduced the subsequent uptake of beads by these mononuclear phagocytes when the beads were injected 30 min after the ATP. In contrast, exposure of P2X7−/− peritoneal macrophages to ATP had no effect on the subsequent uptake of beads by macrophages either in vitro or after instillation of beads into the peritoneal cavity [24]. It should be noted that the total phagocytic ability of peritoneal macrophages was similar for wild type and P2X7−/− animals, presumably due to upregulation of other scavenger receptors, an effect which has also been observed in CD204 (class A scavenger receptor type I, II)-deficient mouse and CD36 knockout mouse [27, 28]. These data are useful in showing that the inhibitory effect of extracellular ATP on particle uptake by phagocytes can be used to define the contribution made by the P2X7 uptake pathway.
Both live and heat-killed bacteria can be phagocytosed by cells expressing P2X7, although the uptake process is slower and can only be demonstrated in the absence of ambient ATP and serum [24]. Numerous receptors have been shown to mediate bacterial uptake by phagocytes and the contribution of P2X7-mediated phagocytosis to the uptake of bacteria may be limited to serum-free compartments of the body such as the central nervous system. However, P2X7 receptors may play a role in linking innate and adaptive immunity after phagocytosis has occurred. Confocal microscopy shows P2X7 in the phagosome membrane surrounding engulfed particles or bacteria [29, 30] and recent reports suggest that P2X7 together with pannexin-1 may facilitate the delivery of bacterial products from phagosome lumen into the cytosol, where they can activate inflammasome assembly [31, 32]. Moreover P2X7 has a role in facilitating the fusion of phagosome with lysosome by a process involving stimulation of phospholipase D activity presumably on the phagolysosomal membrane [33, 34].
P2X7 is a scavenger receptor for apoptotic cells in the absence of its ligand ATP
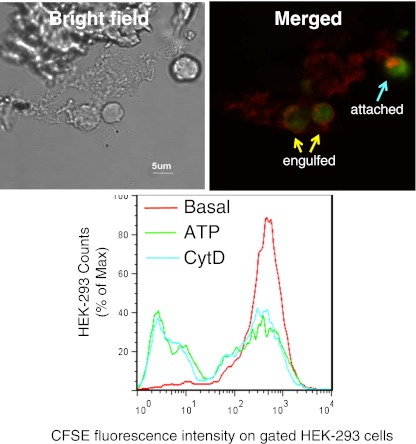
Our recent study suggests that P2X7 expressed on the phagocyte surface directly recognizes newly exposed epitopes on apoptotic lymphocytes and that the P2X7-nonmuscle myosin complex regulates the subsequent engulfment of these targets. Both macrophages (which highly express P2X7) and P2X7-transfected epithelial HEK-293 cells phagocytose apoptotic target cells and confocal images clearly show an accumulation of P2X7 at the point of attachment between the phagocytic effector cell and the captured apoptotic target (Fig. 2). This engulfment process for apoptotic cells is slower than for beads, and incubations of 2–3 h are required to demonstrate substantial uptake of apoptotic material. As found for beads and bacterial targets, exposure of macrophages to ATP added either in vitro or by intraperitoneal injection into mice in vivo inhibited the subsequent uptake of apoptotic lymphocytes by human or murine macrophages, respectively [29]. This effect of ATP pre-incubation to inhibit phagocytosis of non-opsonized targets is a hallmark of the P2X7-mediated phagocytic pathway.
Fig. 2.
Phagocytosis of apoptotic HPB cells by HEK-293 cells transfected with P2X7: upper panel bright field image (left) and confocal image (right) showing a HEK-293 cell transfected with DsRed tagged P2X7 (red) containing two engulfed CFSE-labeled HPB cells and another which is tethered or attached to the HEK cell. Images were acquired by an Olympus IX81 confocal microscope (×600). Lower panel: a typical flow cytometry histogram showing phagocytosis of CFSE-labeled HPB cells by P2X7-DsRed transfected HEK-293 cells. HEK-293 cells were labeled with BODIPY 630/650-SE first and pre-treated with 1 mM ATP or 20 μM CytD for 15 min prior to incubation with HPB cells. BODIPY+/DsRed++ HEK-293 cells were gated for analysis. From Gu et al. [29]
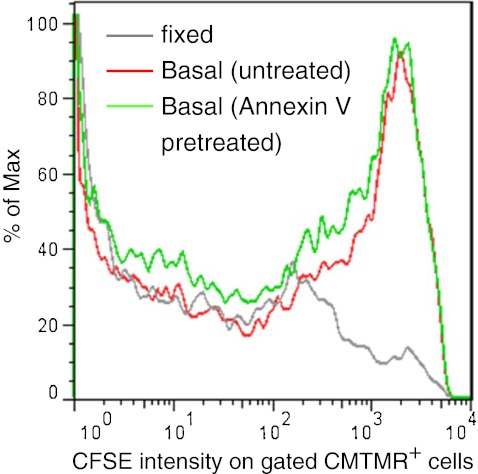
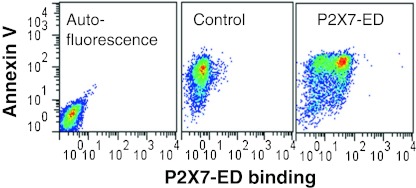
We explored whether PS exposure, the “eat-me” signal on the surface of apoptotic cells might be the interacting epitope recognized by P2X7 on the phagocyte. Fresh human monocytes were differentiated into macrophages (with LPS and interferon-γ) and were labeled with CMTMR+. Lymphocytes from the same donor were induced into apoptosis with staurosporine and labeled with a different fluorophore CFSE+. The apoptotic lymphocytes were then pre-treated with and without annexin V before they were seeded onto the adherent macrophage layer. After 3 h incubation, the macrophages were analyzed by flow cytometry which showed these cells had engulfed substantial numbers of apoptotic lymphocytes and that annexin V had little or no inhibitory effect (Fig. 3). In contrast, a recombinant peptide P2X7 extracellular domain bound strongly to apoptotic cells but not viable cells (Fig. 4), suggesting that P2X7 binds to a motif other than PS on the apoptotic surface.
Fig. 3.
Pretreatment of apoptotic lymphocytes with purified Annexin V does not inhibit their phagocytosis by autologous monocyte-derived macrophages. Human lymphocytes were induced into apoptotic death by overnight incubation with 1 μM staurosporine then labeled with CFSE, and incubated with or without 20 μg/mL purified Annexin V for 30 min at room temperature, Macrophages were labeled with CMTMR prior to the addition of CFSE-labeled apoptotic autologous lymphocytes. Mixed cells were incubated for 3 h at 37°C before fixation and assay by flow cytometry on gated CMTMR+ macrophages
Fig. 4.
Recombinant P2X7 extracellular domain (P2X7-ED) binds to the surface of apoptotic cells but not to viable cells. Human neuronal SH-SY5Y cells were induced into apoptosis with 0.2 μM staurosporine. Cells were resuspended in Na+ medium with 3 mM CaCl2, and incubated with P2X7-ED at a concentration of 15 μg/mL for 30 min, followed by two washes and incubation with anti-5×His mAb, and subsequently with FITC-conjugated goat anti-mouse IgG, APC-conjugated Annexin V, and 7-AAD. All events were gated on the low 7-AAD population to exclude necrotic cells. From Gu et al. [29]
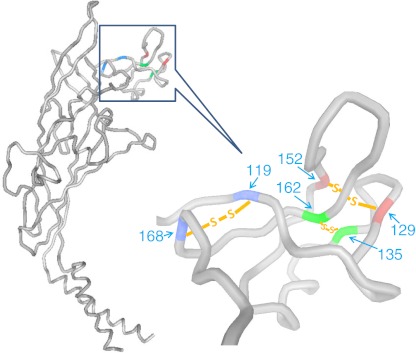
The extracellular domain of P2X7 contains about 290 residues and bears strong homology to the P2X4 receptor. Both receptors contain 10 cysteine residues arranged in five disulfide bonds which are conserved in all members of the P2X receptor family and across species [35]. The recent publication of the crystal structure of the zebrafish P2X4 receptor in the closed conformation reveals an ectodomain likened to a dolphin arising from the membrane with a curved body, dorsal fin, ventral flipper and an exposed “nose” containing three of the five disulfide bridges [36, 37]. These features are visible in the backbone of the P2X7 ectodomain modeled on the Gouaux structure (Fig. 5) and show that peptide sequences around three of the five disulfide bonds are highly exposed to the medium and adjoining cell surfaces (Fig. 5). The peptide regions in the P2X7 ectodomain which directly interact with particle surfaces has been explored by binding short peptide sequences (12–14 mer) tagged with biotin to each type of target particle.
Fig. 5.
Predicted extracellular and transmembrane domains of human P2X7 structure. The P2X7 model was based on the crystal structure of zebrafish P2X4 in the closed state (Ref. [36]) using the online Protein Homology/analogY Recognition Engine (PHYRE, V2.0). The six conserved cysteine residues in the exposed “dolphin nose” region (residues 115–168) form three disulfide bonds shown between the matching colors
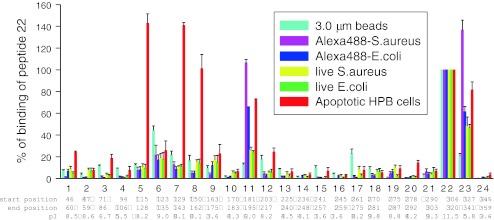
Two dozen biotin-labeled peptides (12–14 mer) which covered the entire sequence of the P2X7 extracellular domain were synthesized and their binding profile to a range of targets (beads, bacteria, apoptotic lymphocytes) was measured using a detection method based on a second incubation with FITC-conjugated streptavidin. Peptide 22 (residues 306–320) was unique in having a strong positive charge (pI, 11.5) and this peptide bound non-selectively to all targets examined (Fig. 6). This peptide also bound to a range of negatively charged phospholipids including phosphatidylserine in a lipid strip binding assay [29]. Three peptides (peptides # 5, # 7, #8) covering the region of residues 115 to 162 showed strong and selective binding to apoptotic cells but to no other particles examined. The importance of this P2X7 region (115 to162) was confirmed by binding studies of shorter peptides (9 or 10 residues) which revealed that those peptides in the 115–162 region which bound strongly always contained one or two cysteine residues. None of the peptides bound to viable cells and moreover substitution of alanine for cysteine in peptides #5, #7, and #8 abolished binding of the peptide to apoptotic cells [29]. These results suggest that the exposed “nose” of the P2X7 molecule which includes the region 115–162 (Fig. 5) is a critical interacting region on the macrophage for recognition of epitopes on the apoptotic cell surface and these epitopes are not present on viable cells. The data also implicate thiol-disulfide exchange reactions as a potential molecular mechanism to tether the apoptotic cell to the macrophage and this concept was supported by the effect of small thiol-reactive compounds on the phagocytosis of apoptotic lymphocytes by monocyte-derived macrophages. Pretreatment of macrophages with dithiolthreonine, glutathione or N-acetyl-l-cysteine abolished the phagocytosis of apoptotic lymphocytes by macrophages [29]. Clearly, a cysteine-rich molecule on the apoptotic target must participate in the putative mixed disulfide bonds linking target to P2X7 on the phagocyte. Which cysteine-rich proteins could become exposed on the cell surface early in apoptosis is unknown, but ecto-calreticulin has been proposed possibly in association with a protein disulfide isomerase of the endoplasmic reticulum [38]. Some macrophage SRs such as SR-A1, MARCO, CD-163, and SP α belong to the scavenger receptor cysteine-rich superfamily (SRCR-SF), a conserved family of receptors containing one or more repeats of a cysteine-rich extracellular domain containing six to eight cysteines arranged in a disulfide-bonded pattern [39]. The macrophage P2X7 receptor may be related to this scavenger superfamily but has developed a divergent role in the recognition and removal of apoptotic cells in the absence of extracellular ATP and serum.
Fig. 6.
The binding of short peptides mimicking P2X7 extracellular domain sequences to a range of phagocytic targets. 24 biotin-tagged peptides were incubated at a concentration of 10 μg/mL with either YG beads (3 μm, 5 μL), Alexa 488 conjugated Staphylococcus aureus or Escherichia coli (2 mg/mL, 5 μL), live S. aureus or E. coli (OD690 = 0.9, 10 μL each) or apoptotic HPB cells (5 × 106/mL, 200 μL) for 30 min, followed by washing and incubation with HRP-labeled streptavidin for 30 min. Particles were washed twice, and resuspended in 100 μL of HRP-labeled streptavidin (Jackson ImmunoResearch Lab, 1:2,000 diluted with PBS containing 1% BSA) for 30 min. After two washes, 500 μL of SuperSignal West Pico was added and the chemiluminescence was measured by a Glo 20/20 luminometer (Promega). From Gu et al. [29]
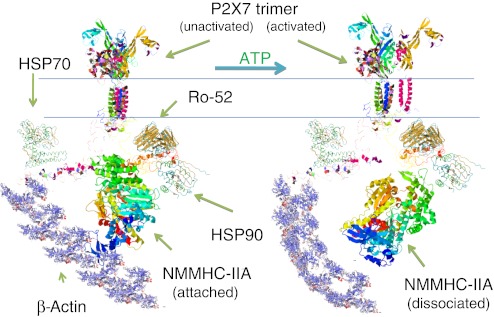
Since the extracellular cysteines are highly conserved in all P2X family members, other P2X receptors may also be capable of recognizing apoptotic cells. However, whether they could function as a scavenger receptor for apoptotic cells like P2X7 may depend on their association with cytoskeletal structures, the nonmuscle myosin of which provides the energy to rearrange the cytoskeleton and internalize the tethered cell. Nonmuscle myosin heavy chain (NMMHC)-IIA has been shown to play a role in phagocytosis of both non-opsonized [24] and IgG-opsonized particles [40], and its expression level is one of the rate-limiting factors for phagocytosis [41]. Inhibition of NMMHC ATPase by S-(−)-blebbistatin abolishes both micropinocytosis and phagocytosis [24, 40], showing energy of ATP hydrolysis is required for these cytoskeletal rearrangements. Our previous study has shown that unactivated P2X7 and NMMHC-IIA form a tight molecular complex, which also includes heat shock protein 70 and 90, Ro 52, protein tyrosine kinase and β-actin [42] (Fig. 6). This tight complex between P2X7 and NMMHC-IIA is required for P2X7 mediated phagocytosis since extracellular ATP causes slow dissociation of NMMHC-IIA from the complex and this inhibits further internalization of non-opsonized particles. Whether other P2X receptors also associate with nonmuscle myosin is unknown (Fig. 7).
Fig. 7.
Schematic illustration of the P2X7 membrane complex showing ATP causes dissociation of nonmuscle myosin from the complex. P2X7 in its inactivated state forms a tight molecular complex with NMMHC-IIA. Once P2X7 is activated by extracellular ATP, NMMHC-IIA slowly dissociates from the complex over 10–15 min. In contrast, Ro-52 remains associated with P2X7. The full-length P2X7 structure modeling is predicted by I-TASSER online server (http://zhanglab.ccmb.med.umich.edu/I-TASSER/). The other protein structures are obtained from RCSB PDB (http://www.pdb.org/pdb/home/home.do) and EMBL-EBI (http://www.ebi.ac.uk/Databases/structure.html)
Conclusions
The P2X7-membrane complex is able to mediate the phagocytosis of a broad range of non-opsonized particles including beads, bacteria, and apoptotic cells suggesting an important role for this receptor in innate immunity. P2X7 is also regulated by its physiological ligand, extracellular ATP, a short-range signaling molecule which opens the P2X7 cation channel/pore and activates downstream signaling pathways leading to inflammasome formation and secretion of proinflammatory cytokines from cells of monocyte-macrophage origin. P2X7 however has a ubiquitous distribution in many other cell types and tissues throughout the body, although its role in other cell types is unknown. Since extracellular ATP concentrations are usually at sub-micromolar levels, it is possible that the major physiological role of P2X7 may be in the recognition and engulfment of apoptotic cells and non-opsonized particles. However, in certain regions of the body, ambient ATP concentrations may increase to the micromolar levels allowing P2X7 activation e.g., inflammatory foci, in restricted spaces such as eye, bone/joint, brain or in tumor nodules. These two alternate functional modes of the P2X7 receptor have widely different functional outcomes and signaling pathways, which will be important in physiology and pathophysiology of disease.
Acknowledgments
We thank the National Health & Medical Research Council of Australia and the Leukaemia Foundation of Australia for financial support.
References
- 1.Chen G, Zhuchenko O, Kuspa A. Immune-like phagocyte activity in the social amoeba. Science. 2007;317(5838):678–681. doi: 10.1126/science.1143991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126(17):3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 3.Brodsky IE, Medzhitov R. Targeting of immune signalling networks by bacterial pathogens. Nat Cell Biol. 2009;11(5):521–526. doi: 10.1038/ncb0509-521. [DOI] [PubMed] [Google Scholar]
- 4.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7(12):964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 5.Cvetanovic M, Mitchell JE, Patel V, et al. Specific recognition of apoptotic cells reveals a ubiquitous and unconventional innate immunity. J Biol Chem. 2006;281(29):20055–20067. doi: 10.1074/jbc.M603920200. [DOI] [PubMed] [Google Scholar]
- 6.Vergeer M, Korporaal SJ, Franssen R, et al. Genetic variant of the scavenger receptor BI in humans. N Engl J Med. 2011;364(2):136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- 7.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 8.Baranova IN, Bocharov AV, Vishnyakova TG, et al. CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J Biol Chem. 2010;285(11):8492–8506. doi: 10.1074/jbc.M109.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart LM, Deng J, Silver JM, et al. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170(3):477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabriek BO, Bruggen R, Deng DM, et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113(4):887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 11.Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40(2):195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 12.Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43(3):207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 14.Santiago C, Ballesteros A, Martinez-Munoz L, et al. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27(6):941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi N, Karisola P, Pena-Cruz V, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27(6):927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D, Tosello-Trampont AC, Elliott MR, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450(7168):430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Jung MY, Kim HJ, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15(1):192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 18.Scott RS, McMahon EJ, Pop SM, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 19.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem. 2000;127(3):411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- 20.Hanayama R, Tanaka M, Miyasaka K, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304(5674):1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 21.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Touret N, Paroutis P, Terebiznik M, et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005;123(1):157–170. doi: 10.1016/j.cell.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Steinkamp JA, Wilson JS, Saunders GC, Stewart CC. Phagocytosis: flow cytometric quantitation with fluorescent microspheres. Science. 1982;215(4528):64–66. doi: 10.1126/science.7053559. [DOI] [PubMed] [Google Scholar]
- 24.Gu BJ, Saunders BM, Jursik C, Wiley JS. The P2X7-nonmuscle myosin membrane complex regulates phagocytosis of non-opsonized particles and bacteria by a pathway attenuated by extracellular ATP. Blood. 2010;115(8):1621–1631. doi: 10.1182/blood-2009-11-251744. [DOI] [PubMed] [Google Scholar]
- 25.Cvetanovic M, Ucker DS. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol. 2004;172(2):880–889. doi: 10.4049/jimmunol.172.2.880. [DOI] [PubMed] [Google Scholar]
- 26.Solle M, Labasi J, Perregaux DG, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276(1):125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 27.Komohara Y, Terasaki Y, Kaikita K, Suzuki H, Kodama T, Takeya M. Clearance of apoptotic cells is not impaired in mouse embryos deficient in class A scavenger receptor types I and II (CD204) Dev Dyn. 2005;232(1):67–74. doi: 10.1002/dvdy.20206. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203(12):2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu BJ, Saunders BM, Petrou S, Wiley JS. P2X7 is a scavenger receptor for apoptotic cells in the absence of its ligand extracellular ATP. J Immunol. 2011;187(5):2365–2375. doi: 10.4049/jimmunol.1101178. [DOI] [PubMed] [Google Scholar]
- 30.Kuehnel MP, Rybin V, Anand PK, Anes E, Griffiths G. Lipids regulate P2X7-receptor-dependent actin assembly by phagosomes via ADP translocation and ATP synthesis in the phagosome lumen. J Cell Sci. 2009;122(Pt 4):499–504. doi: 10.1242/jcs.034199. [DOI] [PubMed] [Google Scholar]
- 31.Marina-Garcia N, Franchi L, Kim YG, et al. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180(6):4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 32.McDermott MF, Tschopp J. From inflammasomes to fevers, crystals and hypertension: how basic research explains inflammatory diseases. Trends in molecular medicine. 2007;13(9):381–388. doi: 10.1016/j.molmed.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Kusner DJ, Barton JA. ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J Immunol. 2001;167(6):3308–3315. doi: 10.4049/jimmunol.167.6.3308. [DOI] [PubMed] [Google Scholar]
- 34.Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X(7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol. 2001;167(6):3300–3307. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- 35.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 36.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460(7255):592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young MT. P2X receptors: dawn of the post-structure era. Trends Biochem Sci. 2010;35(2):83–90. doi: 10.1016/j.tibs.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obeid M, Tesniere A, Panaretakis T, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 39.Freeman M, Ashkenas J, Rees DJ, et al. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci U S A. 1990;87(22):8810–8814. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shu S, Liu X, Korn ED. Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc Natl Acad Sci USA. 2005;102(5):1472–1477. doi: 10.1073/pnas.0409528102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180(5):989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS. Extracellular ATP dissociates nonmuscle myosin from P2X7 complex: this dissociation regulates P2X7 pore formation. Am J Physiol Cell Physiol. 2009;297(2):C430–C439. doi: 10.1152/ajpcell.00079.2009. [DOI] [PubMed] [Google Scholar]