Abstract
Since 1929, when it was discovered that ATP is a substrate for muscle contraction, the knowledge about this purine nucleotide has been greatly expanded. Many aspects of cell metabolism revolve around ATP production and consumption. It is important to understand the concepts of glucose and oxygen consumption in aerobic and anaerobic life and to link bioenergetics with the vast amount of reactions occurring within cells. ATP is universally seen as the energy exchange factor that connects anabolism and catabolism but also fuels processes such as motile contraction, phosphorylations, and active transport. It is also a signalling molecule in the purinergic signalling mechanisms. In this review, we will discuss all the main mechanisms of ATP production linked to ADP phosphorylation as well the regulation of these mechanisms during stress conditions and in connection with calcium signalling events. Recent advances regarding ATP storage and its special significance for purinergic signalling will also be reviewed.
Keywords: ATP synthesis, ATP storage, Mitochondria, Calcium
Introduction
Within cells, energy is provided by oxidation of “metabolic fuels” such as carbohydrates, lipids, and proteins. It is then used to sustain energy-dependent processes, such as the synthesis of macromolecules, muscle contraction, active ion transport, or thermogenesis. The oxidation process results in free energy production that can be stored in phosphoanhydrine “high-energy bonds” within molecules such as nucleoside diphosphate and nucleoside triphosphate (i.e., adenosine 5′ diphosphate and adenosine 5′ trisphosphate, ADP, and ATP, respectively), phosphoenolpyruvate, carbamoyl phosphate, 2,3-bisphosphoglycerate, and other phosphagens like phosphoarginine, or phosphocreatine. Among them, ATP is the effective central link—the exchange coin—between energy-producing and the energy-demanding processes that effectively involve formation, hydrolysis, or transfer of the terminal phosphate group.
In general, the main energy source for cellular metabolism is glucose, which is catabolized in the three subsequent processes—glycolysis, tricarboxylic acid cycle (TCA or Krebs cycle), and finally oxidative phosphorylation—to produce ATP. In the first process, when glucose is converted into pyruvate, the amount of ATP produced is low. Subsequently, pyruvate is converted to acetyl coenzyme A (acetyl-CoA) which enters the TCA cycle, enabling the production of NADH. Finally, NADH is used by the respiratory chain complexes to generate a proton gradient across the inner mitochondrial membrane, necessary for the production of large amounts of ATP by mitochondrial ATP synthase. In addition, it should be mentioned that acetyl-CoA can be generated also by lipid and protein catabolism.
The aim of this work is to provide an overview of the principles governing ATP production and describe cellular mechanisms that sense levels of ATP and regulate its synthesis. Metabolic alterations that promote the sustaining of cancer progression, as well as methods for monitoring ATP levels and production are also reviewed here.
Basic principles of ATP-producing pathways
Glycolysis
Glycolysis is a process by which glucose is partially converted through a series of enzyme-catalyzed reactions into two molecules of pyruvate. Some mammalian cell types (erythrocytes, sperm) and tissues (brain, renal medulla) are able to survive only (or mostly) on the energy derived from glycolysis. The steps comprising the processes leading to the breakdown of the six-carbon glucose into two three-carbon pyruvate molecules can be divided into two phases: the preparatory phase and the so-called “payoff” phase (Fig. 1).
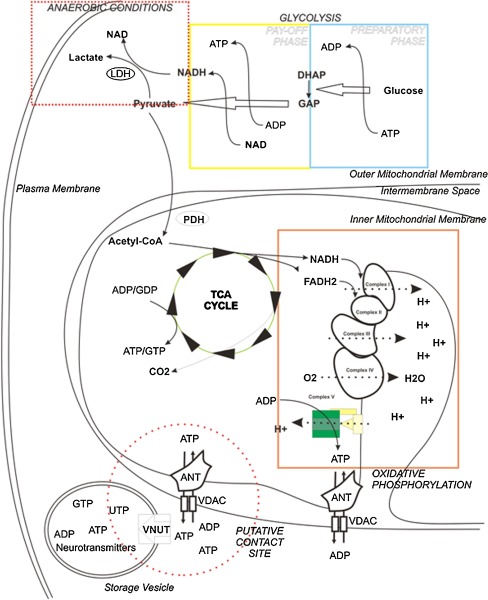
Fig. 1.
ATP management within the cell. Schematic representation of mechanisms of ATP synthesis and storage inside the cell. Glycolysis is represented in the yellow and blue boxes, the TCA cycle by the green circle, and oxidative phosphorylation in the orange box. Reduction of pyruvate to lactate is represented inside the red dotted rectangle. Hypothetical contacts between ATP storage vesicles and mitochondria, with preferential ATP transfer, are shown within the red dotted circle
In the first phase, glucose is phosphorylated at the hydroxyl group on C-6 by hexokinase (HK) generating glucose 6-phosphate. This event is fundamental to “trap” the hexose within the cell. In fact, the existence of a transporter of phosphorylated hexose has not been reported in mammalian cells. In this way, the phosphorylation of glucose shifts the equilibrium of glucose concentration, preventing its escape. Several types of HKs have been found, each with specific features. In the case of HK IV (glucokinase), known to be liver-specific, it is the insensitivity to glucose 6-phosphate inhibition that allows its direct regulation by the levels of glucose in the blood [1]. Recently, there has been increased interest in the mitochondria-associated HK (mtHK). mtHK is able to promote cell survival through an AKT-mediated pathway. This was one of the first mechanisms suggested to couple metabolism to cell fate [2] because of its ability to participate in mitochondrial dynamics during apoptosis and especially due to its involvement in the formation of the mitochondrial permeability transition pore.
Subsequently, glucose 6-phosphate is converted to fructose 6-phosphate by glucose 6-phosphate isomerase. This isomerization is fundamental for the subsequent step in which C-1 is once again phosphorylated, resulting in the formation of fructose 1,6-bisphosphate. Aldolase is then able to split fructose 1,6-bisphosphate into two three-carbon molecules: dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GAP). This step represents the real “lysis” phase.
Until now, the glycolytic pathway consumed ATP instead of producing it. This should be interpreted as an investment raising the free-energy content of the intermediates, and the real yield of the process starts from here, with the beginning of the second phase.
DHAP is isomerized by triosephosphate isomerase to form a second molecule of GAP. The carbon chain of the entire glucose is thus converted into two molecules of GAP. Each of these molecules is oxidized and phosphorylated by inorganic phosphate to form 1,3-bisphosphoglycerate. During this process, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) uses nicotinamide adenine dinucleotide (NAD+) as cofactor and releases NADH for each molecule of GAP. The resulting NADH will directly feed into the respiratory chain to propel mitochondrial ATP synthesis. It is noteworthy that GAPDH is also able to regulate several processes which are not part of the glycolytic pathway. These include the regulation of apoptosis, membrane fusion, microtubule bundling, RNA export, DNA replication, and repair [3].
Some energy is released through the conversion of 1,3-bisphosphoglycerate into two molecules of pyruvate by the sequential steps performed by phosphoglycerate kinase (PGK), phosphoglicerate mutase, enolase, and pyruvate kinase. The conversions of 1,3-bisphosphoglycerate to 3-phosphoglycerate (by PGK) and phosphoenolpyruvate to pyruvate (by pyruvate kinase) are the steps that promote ATP synthesis from ADP in glycolysis. The last step is also a fundamental regulator of the whole process. Pyruvate kinase (PK) undergoes allosteric regulation by fructose 1,6-bisphosphate that promotes PK activity and boosts the rate of glycolysis [4]. Allosteric regulation and tissue expression characterize several isoforms of the PK enzyme, i.e., the isoform M2, usually expressed during embryogenesis, has been found as a special promoter of tumorigenesis. This isoform is characterized by a high affinity to phosphoenolpyruvate, and it has been associated with favoring the conversion of pyruvate to lactate instead of its entry in the TCA cycle [5, 6].
Thus, the second phase of glycolysis provides four molecules of ATP and two of NADH per molecule of glucose, paying the investment of the preparatory phase. The final balance of this process is then: two molecules of ATP, two of NADH (that could directly feed into the respiratory chain), and two of pyruvate. The latter enters the TCA cycle and undergoes complete oxidation in aerobic conditions.
During anaerobic conditions (such as what occurs in muscles during a burst of extreme activity, when oxygen is not obtained fast enough from the blood), the low oxygen amounts do not allow the complete and efficient oxidation of pyruvate. During these conditions, NADH (produced in large amounts from the citric acid cycle; see next section) cannot be reoxidized to NAD, thus limiting the activity of GAPDH and glucose consumption. Pyruvate is then reduced to lactate with the consumption of one NADH in a process called lactic fermentation catalyzed by lactate dehydrogenase. In this way, the two molecules of NADH produced in glycolysis are consumed in lactic fermentation to restore the NAD reservoir, and the final balance of one glucose degradation is two molecules of ATP. This condition occurs also in aerobic conditions in erythrocytes (that have no mitochondria) or in many cancer cells as was originally observed by doctor Otto Warburg in 1930, and which led to the widely accepted Warburg effect theory [7].
Citric acid cycle
The TCA, also known as the citric acid cycle, was elucidated by Sir Hans Krebs in 1940 when he concluded, “the oxidation of a triose equivalent involves one complete citric acid cycle” [8]. The “triose” deriving from glycolysis is completely oxidized into three molecules of CO2 during a sequence of reactions that allow the reduction of cofactors NAD and flavin adenine nucleotide (FAD), providing energy for the respiratory chain in the form of electrons. In 1949, it was demonstrated by Kennedy and Lehningher that the entire cycle occurs inside mitochondria [9] (Fig. 1).
The starting material for the citric acid cycle is directly provided by the pyruvate coming from glycolysis through the activity of the pyruvate dehydrogenase complex. This enzymatic complex, composed of multiple copies of the three enzymes pyruvate dehydrogenase (E1), dihydrolipoyl transacetylase (E2), and dihydrolipoyl dehydrogenase (E3), oxidizes pyruvate to acetyl-CoA and CO2 in an irreversible reaction in which the carboxyl group is removed from pyruvate as a molecule of CO2. This reaction is strictly related to the cycle, even if is not comprised in it. The acetyl group introduces two carbons in each turn of the cycle; these carbons will then leave the cycle as CO2.
The first reaction of the citric acid cycle is the condensation of one acetyl-CoA and a molecule of citrate to generate oxaloacetate and is catalyzed by citrate synthase. Citrate is then transformed into isocitrate by aconitase through the formation of cis-aconitate. This step is reversible and could lead to the formation of both citrate and isocitrate. Only the fast consumption of isocitrate by its dehydrogenase can force the reaction to the proper direction. Isocitrate dehydrogenase catalyzes the first irreversible oxidation leading to the decarboxylation of isocitrate, generating CO2 and α-ketoglutarate. The second carbon leaves the cycle in the following step, when the newly generated α-ketoglutarate is immediately decarboxylated by the α-ketoglutarate dehydrogenase complex in a reaction similar to the pyruvate decarboxylation. In fact, both these complexes share high similarities in enzyme amino acid composition and in the organization of the different subunits. Energy released from both oxidations is used to generate NADH from NAD that directly feeds into the respiratory chain.
The following step is catalyzed by succinyl–Coa synthetase and utilizes the energy derived from the CoA removal to phosphorylate GDP (or ADP) to GTP (or ATP). Selectivity for the nucleotide is determined by the isozyme involved. It has been well established that at least two isozymes of succinyl–CoA synthetase are expressed in animal tissues [10], and the proportion between them seems to be tissue-specific.
The succinate generated in the previous step is the four-carbon compound that is then converted, by three sequential reactions, to oxaloacetate to conclude the cycle. The first of these steps is the oxidation of succinate to fumarate by succinate dehydrogenase. This enzyme, tightly bound to the inner mitochondrial membrane (IMM), catalyzes FAD reduction to FADH2 that provides electrons for the respiratory chain. Fumarate is then hydrated by fumarate hydratase to l-malate. It is particularly interesting that both succinate dehydrogenase and fumarate hydratase are oncosuppressor genes. It has been demonstrated that inactivation of these oncosuppressors leads to the accumulation of succinate and fumarate that spread in the cytosol and promote hypoxia-inducible factor 1α (HIF1α) accumulation by inactivating prolyl hydroxylase enzymes (promoter of HIF1α degradation); HIF1α in turn promotes a pseudo-hypoxic condition that favors tumor development [11]. The last event that completes the citric acid cycle is the oxidation of l-malate to oxaloacetate. This reaction is performed by l-malate dehydrogenase which induces the reduction of another molecule of NAD to NADH. The resulting molecule of oxaloacetate is suitable for starting another cycle through condensation with an acetyl group.
During all these processes, only one molecule of ATP (or GTP) is produced, but three molecules of NADH and one of FADH2 (plus one molecule of NADH from pyruvate dehydrogenase), which provide electrons for respiratory chain, are also generated and subsequently result in the production of large amounts of ATP (discussed later).
Respiratory chain and oxidative phosphorylation
Respiratory chain comprises a series of components (complexes) conducting electron transfer across the membrane and involved in oxidative phosphorylation (OXPHOS), a process which occurs in aerobic conditions. In eukaryotic cells, electron transport occurs in mitochondria and chloroplasts, whereas in bacteria it is carried out across the plasma membrane. As mentioned, the electron transfer is considered a part OXPHOS, the process through which ADP is phosphorylated into ATP by dint of energy derived from the oxidation of nutrients.
Four protein complexes and ATP synthase, all bound to the IMM, as well as two shuttles are the known players of one of the trickiest mechanisms resolved in biochemistry (Fig. 1). The first of these complexes is the NADH/ubiquinone oxidoreductase (complex I) which removes electrons from NADH (produced in the citric acid cycle) and passes them on to the first shuttle, ubiquinone, a liposoluble cofactor located within the phospholipid bilayer of the IMM. Succinate dehydrogenase (or complex II) is another entrance site for electrons into the respiratory chain. In this case, electrons derived from the oxidation of succinate are passed through FAD to ubiquinone. Once ubiquinone is reduced to ubiquinol, it is able to pass electrons to the third complex, ubiquinone/cytochrome c oxidoreductase. Here, electrons are moved through several heme groups from the liposoluble shuttle ubiquinone to the water-soluble shuttle cytochrome c. Cytochrome c is a small protein (about 12.5 kDa), located in the intermembrane space (IMS), which can accommodate one electron in its heme group. Despite its water solubility, cytochrome c is usually bound to the external surface of the IMM due to the interaction with the cardiolipin [12]. This interaction (crucial in the determination of the cell fate) helps the shuttle to reach its electron acceptor, complex IV. Cytochrome c oxidase is the last complex of the electron transport. Electrons from cytochrome c are accumulated in copper centers and passed to oxygen through heme groups. Oxygen is then reduced to water. This constitutes the bulk of oxygen consumption in all aerobic life.
Electron transport through complexes I, III, and IV induces the pumping of protons from the matrix to the IMS. Specifically, for every two electrons coming from one molecule of NADH, four H+ are moved by complex I, four by complex III, and two by complex IV. The second respiratory complex does not generate any proton movement [13]. The respiratory chain in active mitochondria generates a large difference in [H+] across the IMM, resulting in the generation of an electrical potential (about −180 to −200 mV) and variation in the pH of about 0.75. A constant proton motive force drives the ATP synthesis through the last step of OXPHOS, the ATP synthase. Understanding the activity and organization of this enzyme won researchers more than one Nobel Prize. First, Peter Mitchell in 1978 received his prize for the formulation of the chemiosmotic theory. Initially, he hypothesized how an enzymatic activity could at the same time involve ion transport (proton transport through the IMM) and a chemical reaction (ATP phosphorylation). Almost two decades later, in 1997, the Nobel Prize was awarded to Paul Boyer and John Walker who elucidated the mechanism of action of ATP synthase, here briefly reviewed. ATP synthase could be divided in two main components: F0 that allows the channelling of protons and F1 that catalyzes ATP phosphorylation. The F0 is embedded in the IMM, while the F1 resides in the mitochondrial matrix and is bound to the F0 through a γ subunit (which drives conformational changes) and a b2δ dimer (that holds F0 and F1 together). The protons flow from the intermembrane space to the matrix through the F0 inducing its rotation; the movement is transmitted from the γ subunit to the F1 causing conformational rearrangements. The F1 has a trimeric structure consisting of αβ dimers. This structure allows three different conformational states which is able to bind ADP + Pi, ATP, or remain unbound. The sequential changes are linked to the binding of substrates, phosphorylation, and release of ATP. The three available dimers are never in the same conformational state, and, what is more, the conformational changes in one dimer drive rearrangements in the other (for a more detailed explanation, refer to [14]). It has been calculated that, for the synthesis of one ATP molecule, four protons are required (three for the ATP synthase rearrangements and one for ATP, ADP, and Pi transport [15]). Once synthesized, ATP can locate inside mitochondrial matrix or be transported into the IMS by the nucleotide exchanger adenine nucleotide translocase (ANT) which passively exchanges ATP with ADP. Once in the IMS, ATP can freely pass the OMM through the voltage-dependent anion channel (VDAC).
ATP production is strongly regulated upon environmental stresses
Phosphorylation of ATP is strongly modulated by environmental stresses, such as hypoxia or heat shock. It has also been demonstrated, both in vitro and in vivo, that intracellular ATP levels are implicated in the regulation of fundamental cellular processes, such as growth, development, and death/survival decisions.
In 1964, Daniel Atkinson [16] proposed the energy-charge hypothesis, which stated that regulatory enzymes involved in fundamental pathways for a correct development and survival of the cell, would be sensitive to the energy charge, that is, to ATP levels. To confirm this hypothesis, a series of studies on enzymes was conducted. The results have demonstrated how these metabolic enzymes are indeed regulated by adenine nucleotides and, more specifically, that they are allosterically activated by AMP and inhibited by ATP (Fig. 1).
With these observations in mind (that show the primary importance in maintaining appropriate ratios of ATP/ADP and ATP/AMP), it is possible to assume the presence of a protein-kinase cascade (Fig. 2) that operates in accordance with a sophisticated mechanism, fundamentally based on cellular levels of ATP [17].
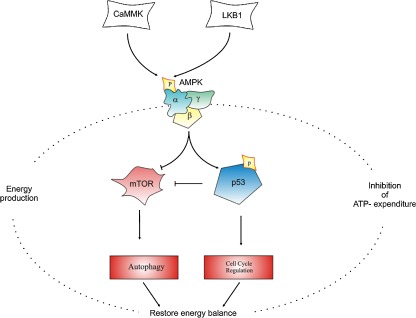
Fig. 2.
Protein-kinase cascade involved in fundamental pathways sensitive to the energy charge. The activity of AMPK is related to the phosphorylation of several downstream substrates capable of altering gene and protein expression, in response to a situation of metabolic and energy crisis due to a deep alteration in ATP synthesis and production. AMPK is a heterotrimeric complex composed of catalytic α-subunits and regulatory β- and γ-subunits, where the major regulatory phosphorylation site is Thr172, phosphorylated by LKB1 (Liver Kinase B1) and CaMKK (calmodulin-dependent protein kinase kinase), respectively. AMPK acts to conserve energy by directing metabolism towards ATP production while inhibiting pathways that utilize ATP. Its major downstream targets are mTOR (mammalian target of rapamycin) and p53, modulating autophagy and cell cycle regulation processes in order to restore the intracellular energy balance
A key molecular player involved in the maintenance of these biological processes is 5′ AMP-activated protein kinase (AMPK). This Ser/Thr protein kinase is sensitive to the cellular AMP/ATP ratio. This enzyme was shown to be activated by AMP and able to activate/inactivate proteins involved in cholesterol and lipid synthesis [18]. Generally, the activity of AMPK is related to the phosphorylation of several downstream substrates in response to a situation of metabolic and energy crisis due to an alteration of ATP synthesis, with subsequent regulation of gene expression. The overall effect of this activation is extra work for ATP-generating pathways (such as glycolysis and fatty acid oxidation), and thus, it causes the inhibition of anabolic (ATP-utilizing) processes like protein and lipid synthesis. AMPK is a heterotrimeric complex composed of catalytic α-subunits and regulatory β- and γ-subunits [19]. The α-subunits contain the catalytic domain in the N terminus and the domain necessary for the interaction with the β- and γ-subunits in the C terminus. It has been demonstrated that Thr172 is the major regulatory phosphorylation site of the α-subunits and that it is essential for the catalytic activity [20]. The β-subunit accounts for a region targeting AMPK to glycogen particles [21], and the γ-subunit is committed to the detection of the AMP/ATP ratio through four particular domains of cystathionine β-synthase [22]. It has been suggested that an elevation in the AMP/ATP ratio due to a decrease in intracellular ATP levels (with consequent increase of AMP) induces a conformational change in the AMPK complex, improving the ability of the α-subunit to serve as a substrate for an upstream kinase. During the last decade, two of the newly identified kinases have been shown to phosphorylate the α-subunit at the crucial Thr172/LKB1 (liver kinase B1) and calmodulin-dependent protein kinase kinase (CaMKK) [23].
LKB1, a tumor suppressor with an evident role in stress and damage response, was initially discovered as a serine–threonine kinase mutated in Peutz–Jeghers syndrome [24]. This kinase regulates cell growth and cell death, and these features have been correlated with the tumor suppressor protein p53, which is known to physically interact with it. Less is known about other LKB1 substrates; recently, it has been shown that AMPK is one of its best-characterized substrates. Originally, during investigations aimed at clarifying the mechanisms of activation in yeast AMPK ortholog snf1, three different kinases were identified as upstream Pak1, Tos3, and Elm1. Subsequent works found that LKB1 was the Ser/Thr protein kinase with the kinase domain closest to the snf1 upstream kinase, confirming the existence of an LKB1–AMPK pathway. Different biochemical assays have shown the ability of LKB1 to phosphorylate the α-subunit of AMPK in Thr172 [25, 26].
During studies in LKB1-deficient mice, an alternative upstream kinase that activates AMPK by phosphorylation in Thr172 on the α-subunit was discovered. It was demonstrated that the CaMKK, particularly the β-isoform, phosphorylates and activates AMPK [27]. This study suggests a second signalling pathway (apart from the one mediated by LKB1 and changes in the cellular AMP/ATP ratio), capable of activating AMPK. In this pathway, an increase in cytosolic Ca2+ drives the activation of CaMKK which acts on AMPK, promoting its phosphorylation and consequent activation. In order to confirm this activity, a CaMKK inhibitor was applied which antagonized the AMPK activation. Additionally, the concomitant use of the Ca2+ ionophore A23187 (able to activate AMPK) and siRNAs selectively targeted at α- and β-isoforms of CaMKK suggested that CaMKKβ is the principal candidate for the phosphorylation of AMPK. The rise of cellular Ca2+ is accompanied by an increased demand for ATP, due to the activation of pumps that equilibrate cytosolic ions. The consequent activation of AMPK by CaMKK increases glucose uptake by GLUT1 and, together with the effects of Ca2+ on mitochondrial dehydrogenases (discussed later), leads to the generation of ATP.
Once activated, AMPK acts to conserve energy by directing metabolism towards ATP production while inhibiting pathways that utilize ATP. Furthermore, AMPK is one of the major cellular energy sensors, also able to regulate a correct metabolic homeostasis. This kinase is involved in the preservation of energy in different cellular events, such as metabolic syndrome, a combination of metabolic disorders that increase the risk of cardiovascular disease and diabetes [28]. Recently, great attention has been paid to the possible link between AMPK and cancer. Indeed, it has already been demonstrated that this kinase plays an important role in mediating tumorigenic effects of the tumor suppressor LKB1.
One major downstream target of AMPK is mammalian target of rapamycin (mTOR), a member of the phosphatidylinositol 3-kinase protein family recognized as a central regulator in an array of diverse and vital cellular processes such as: regulation of growth through maintenance of the appropriate balance between anabolic processes (i.e., macromolecular synthesis and nutrient storage), as well as catabolic processes (i.e., through regulation of autophagy when large parts of cytoplasm and organelles undergo degradations and recycling) [29]. The signalling components upstream and downstream of mTOR are frequently altered in a wide variety of human tumors. Mutations in several tumor suppressor genes (like TSC1, TSC2, LKB1, PTEN, VHL, NF1, and PKD1) trigger the development of different diseases [30]. In the conditions of glucose deprivation and hypoxia, ATP levels are lowered, which results in the inhibition of mTOR, in a mechanism that sees also an increase in the AMP/ATP ratio and therefore the activation of AMPK. Activated AMPK can directly phosphorylate tuberous sclerosis protein 2 (TSC2), which in turn suppresses the activity of the small Ras homolog enriched in brain GTPase (RHEB). Inactive RHEB leads to the inactivation of mTOR, which is an essential mechanism for cell survival under glucose deprivation conditions [30].
However, judging from the observations that the relationship between AMPK and mTOR remains conserved in all eukaryotes (including organisms lacking TSC2 orthologs such as Caenorhabditis elegans and Saccharomyces cerevisiae) and the fact that cells lacking TSC2 remain responsive to energy stress, it is possible to speculate about the presence of additional AMPK substrates capable of modulating mTOR activity. Recently, a critical mTOR binding partner was identified and named Raptor (regulatory associated protein of mTOR) [31]. The phosphorylation of Raptor by AMPK is required for the suppression of mTOR activity by energy stress. The presence of a direct regulation of mTOR mediated by AMPK suggests a direct control of the “fuel gauge” kinase AMPK in the regulation of mTOR-dependent cellular processes. This discovery also opens the possibility of employing an AMPK agonist to treat tumors exhibiting hyperactivation of mTOR [32].
In physiological conditions, the most important tumor suppressor gene, p53, is rapidly ubiquinated and degraded. However, phosphorylation of p53 by AMPK stabilizes the protein with a consequent promotion of cell cycle arrest and anti-tumorigenic effect mediated by the expression of p21 that arrests the cell cycle in G1 and G2. One can also consider the AMPK-p53 connection as a possible cell cycle checkpoint in a situation of low nutrient availability and energy stress. What is more, it is tempting to envision the use of AMPK-activators as anticancer drugs [33].
Several reports [34–36] have shown how p53 inhibits mTOR to repress cell growth and proliferation beyond genotoxic stress. Furthermore, p53 enhances the phosphorylation of AMPKα subunit, promoting AMPK activity and, as was mentioned above, repressing the activity of mTOR.
Upon DNA damage and oxidative stress, p53 promotes the expression of Sestrin-1 and Sestrin-2, which in turn promote AMPK activation with the final goal of negatively regulating cell growth through the mTOR pathway, supporting further the role of AMPK in cancer development [34].
The ATP/ADP ratio regulation of metabolism occurs also within the mitochondrial matrix. It has already been reported that the addition of ADP to isolated mitochondria results in an increase of mitochondrial respiration (state 3) which is maintained for a short period of time, after which it is inhibited (state 4). This effect was clarified in 1997, with experiments that demonstrated that ATP produced in state 3 is able to bind to complex IV, allosterically inhibiting respiration [37]. Three years later, it was shown that, in freshly isolated mitochondria, ATP was able to induce a cAMP-dependent phosphorylation of subunits II and Vb of cytochrome c mediated by protein kinase A (PKA). Moreover, these phosphorylated sites (which seem to be facing the “cytosolic” side of the IMM) can be dephosphorylated in a calcium-dependent manner by protein phosphatase 1 [38]. Another phosphorylation site was identified and published in the work of Lee et al. [39]. The authors described how complex IV inhibition could be mediated by another cAMP-dependent activity, this time, in subunit I. On the other hand, a PKA phosphorylation site was recently found on the matrix side of subunit IV. In this case, by dint of phosphorylation site prediction and mutagenesis techniques, it was not only possible to hypothesize about the amino acid residue responsible for ATP allosteric inhibition, but it was also demonstrated that the phosphorylation in that site blocks allosteric inhibition induced by ATP [40].
These reports suggest that a complicated network of phosphorylation-dependent regulatory processes occur at the level of respiratory complex IV. Elucidation of these mechanisms will facilitate the understanding of the connection between metabolic states within the cell and its ability to adapt to stress conditions.
Calcium-dependent regulation
New experimental tools introduced in the last years have enormously expanded our ability to monitor the dynamics of mitochondrial events in the living cell. These organelles have been recognized as fascinating structures, involved in many aspects of mammalian physiology and pathophysiology. They play subtle roles in glucose homeostasis [41, 42], act as oxygen-sensors in the regulation of respiration [43, 44], and are pivotal in the pathways to both necrotic and apoptotic cell death [45]. Mitochondria also take up calcium, impacting the spatiotemporal dynamics of intracellular calcium signals [46], but their central and ubiquitous task is clearly the production of ATP.
Using a recombinant ATP indicator (a mitochondria-targeted chimera of the photoprotein luciferase), it was demonstrated that an increase in mitochondrial ATP levels ran parallel to an increase in mitochondrial calcium concentration ([Ca2+]m) evoked in stimulated cells. Moreover, the [ATP] increase was prevented by the use of Ca2+ chelators, such as the 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid [47]. Recent work, together with previous considerations which emerged in the 1970s and 1980s [50, 52, 53], have revealed a considerable amount of data regarding the relationship of Ca2+ regulation to mitochondrial ATP production.
Mitochondria can accumulate Ca2+ in a respiration-dependent manner via the ruthenium red-sensitive Ca2+ uniporter (mCU) [48, 49], or by RaM (rapid mode of Ca2+ uptake [50]), when mitochondria are predominantly found in close interaction with endoplasmic reticulum membranes [51]. The electrochemical gradient, generated by the combination of the mitochondrial inner membrane potential and the low concentration of Ca2+ in the matrix, serves as the energy source for both the Ca2+-transporters. Mitochondrial calcium homeostasis is maintained by different efflux carriers: the Na+/Ca2+ exchanger (mNCX) which exchanges one Ca2+ for three Na+ ions [52], Ca2+-proton exchanger (that release calcium following the addition of acid with efflux stoichiometry of 2 protons/1 Ca2+ [53]) and the mitochondrial permeability transition pore, a multicomplex pore involved in the apoptotic response [54]. Accrued data on the mitochondrial calcium accumulation machinery has had an enormous impact on our understanding of the major function of this organelle, ATP production.
The first results were obtained by Denton and McCormack, who showed that free intramitochondrial Ca2+-activated mitochondrial dehydrogenases, leading to increased NADH and thus ATP production [55, 56]. The regulation of oxidative phosphorylation by Ca2+ appears extremely complex, but different targets have already emerged: dehydrogenase activity, F1–F0–ATPase and mitochondrial substrate-transport (Fig. 3).
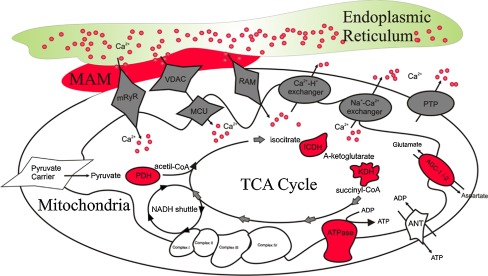
Fig. 3.
Mitochondrial Ca2+ dynamics and stimulation of the TCA cycle and oxidative phosphorylation during mitochondrial ATP production. The direct transfer of Ca2+ from the ER to mitochondria supports oxidative phosphorylation during mitochondrial ATP production. This is an example of metabolic crosstalk between these organelles and characterizes the importance of ER/SR–mitochondria contacts. Schematic mitochondrial Ca2+ uptake and efflux mechanisms are displayed in grey. All enzymes and protein complexes stimulated or influenced by Ca2+ during mitochondrial ATP production are displayed in red. (MCU, mitochondrial calcium uniporter; mRyR, mitochondrial ryanodine receptor; RAM, rapid mode; VDAC, voltage-dependent anionic channel; PTP, permeability transition pore; ANT, adenine nucleotide transporter; PDH, pyruvate dehydrogenase; ICDH, isocitrate dehydrogenase; KDH, a-ketogluterate dehydrogenase; AGC1/2, aralar and citrin aspartate/glutamate carriers; MAM, mitochondrial–endoplasmic reticulum associated membranes)
Three mitochondrial dehydrogenases are sensitive to Ca2+: pyruvate dehydrogenase (PDH, [55]), isocitrate dehydrogenase (ICDH, [57]), and α-ketoglutarate dehydrogenase (KDH, [58]). All three exhibit different calcium-dependent mechanisms, suggesting that the origins or the specific signalling of their activation are very diverse. PDH is activated by dephosphorylation via a calcium-dependent phosphatase [59]. Differently, ICDH and KDH directly bind calcium ions, resulting in alterations in the kinetics of both substrates and inhibitory metabolites [60, 61]. However, several studies have demonstrated that these Ca2+-dependent dehydrogenases are not the only molecular route for the Ca2+-dependent ATP synthase. The first suggestion came from Harris, showing that F1–F0–ATPase might be directly affected by Ca2+ in heart cells [62]. Balaban successively confirmed this in vivo on canine hearts [63]. Territo et al. demonstrated that Ca2+ directly actives F1–F0–ATPase with a Km of 200 nM, increasing the velocity of ATP production [64]. No molecular mechanism as to how Ca2+ may activate the ATPase has been described until now, although the involvement of calcium-dependent proteins or post-translational modifications that may regulate ATPase have been proposed to induce the change [65–69]. Also, calcium-regulated mitochondrial carriers play an interesting role in this complex scenery. Aralar and citrin are aspartate/glutamate carriers (AGC) present in excitable and non-excitable cells, respectively. Both were shown to possess EF-hand-based regulatory sites in the portion of the molecule exposed in the IMS [70]; both are involved in the malate–aspartate NADH shuttle across the IMM, equilibrating the NAD/NADH ratio from cytosol to matrix. These carriers are the target of cytosolic calcium and could alter mitochondrial metabolism without entering the matrix. Interestingly, overexpression of wild-type AGCs (but not mutants lacking the Ca2+-binding sites) enhanced the upregulation of ATP production upon cell stimulation, confirming that different Ca2+-effectors (enzymes, carriers) located in different mitochondrial fractions (matrix, intermembrane space) may cooperate in ATP-producing capacity [71]. Thus, a [Ca2+]m increase, with a simultaneous [Ca2+]c rise, evoked by extracellular stimuli, could convey activation signals to the organelle, in order to boost ATP synthase. This is partially sustained in experiments in beating hearts from guinea-pig perfused with 10 μM RU360 (which inhibits Ca2+-uptake into mitochondria), where no effect on contraction was observed [72]. This observation implies that ATP levels were maintained even though no increase in [Ca2+]m should have occurred, suggesting thus that increase in [Ca2+]m may play a role under specific conditions of increased workload or signalling. Whereas under basal conditions there are alternative mechanisms coupling ATP supply to demand, this balanced activation of oxidative phosphorylation by Ca2+ plays a pivotal role in balancing the rate of ATP production in the cell. It has been shown in some cell types that, during prolonged cytosolic calcium elevations, the uniporter exhibits inactivation, reducing its Ca2+-uptake capacity [73, 74]. A possible explanation for this was proposed by Moreau, where the Ca2+-dependent inactivation was mediated by acidification of mitochondrial pH [75]. This suggests a molecular mechanism whereby ATP synthesis can regulate mitochondrial calcium signalling: through the H+ flow, generated during ATP production, by ATPase into the matrix. This mechanism could represent not only the critical step that prevents mitochondrial Ca2+ overload, but it could also be a trigger for apoptosis too.
Recently, it was proposed that constitutive low-level InsP3R-mediated Ca2+ release is essential for maintaining optimal conditions for mitochondrial bioenergetics [76]. This ongoing Ca2+ transfer from ER to mitochondria supports oxidative phosphorylation, at least in part by providing sufficient reducing equivalents. The reduction in mitochondrial Ca2+-uptake results in reduced ATP production and activation of AMPK, which promotes autophagy as a pro-survival mechanism. An intriguing prospect in inter-organelle communication was also disclosed by the studies of Kaasik et al. [77], extending the importance of ER/SR–mitochondria contacts as examples of metabolic crosstalk between these organelles. Moreover, it was observed in CK knockout mice that a structural reorganization of mitochondria, myofilaments, and SR leads to the development of more intimate contacts between these organelles and functional units ensuring direct ATP supply [78].
It seems clear that the relationships between Ca2+ and ATP vary significantly in different cell or tissue types, although similar mechanisms are probably involved. These variations seem to be promoted by a different spatial organisation of mitochondria (close interaction between ER and mitochondria membranes), temporal demands in ATP production, mitochondrial Ca2+ concentrations, mitochondrial pH, and redox state that occur in different and specific contexts (Fig. 3).
ATP storage
ATP usually reaches high concentrations within cells, in the millimolar range. Nonetheless, because of the high rate of ATP-dependent processes, together with its low stability in water, ATP content could quickly be depleted if it were not immediately replenished by glycolysis and oxidative phosphorylation. Hence, ATP cannot be stored easily within cells, and the storage of carbon sources for ATP production (such as triglycerides or glycogen) is the best choice for energy maintenance. Surprisingly, in 1974, Dowdall [79] and co-workers found a considerable amount of ATP (together with acetylcholine) in cholinergic vesicles from the electric organ of Torpedo marmorata. Several similar findings were made in subsequent years in other mammalian and non-mammalian species. The common feature is that ATP can be stored in large dense core vesicles together with neurotransmitters. It was found co-stored with acetylcholine in guinea pig cortex, calf superior cervical ganglion, and motor nerve terminals of rat diaphragm [76]; with noradrenaline, it was found in human blood vessels, smooth muscles, and endothelial cells [80]; it was also found in adrenal chromaffin cells together with serotonin, with neuropeptide Y and glutamate in astrocytes, and with dopamine in neuron-differentiated PC12 [81–84]. Moreover, in other cell types in the nervous tissue, particularly astrocytes, ATP was found also in small synaptic-like vesicles [85].
The co-storage of ATP with neurotransmitters support the idea that ATP is a fundamental mediator of purinergic neurotransmission in sympathetic and parasympathetic nerves, where it can induce several purinergic responses (i.e., control of autonomic functions, neural glial interactions, pain and vessel tone control). In fact, this storage is a selective form of exocytotic signalling in which ATP participates as a neurotransmitter. The release of ATP from vesicular stores is controlled by cytoplasmic inositol triphosphate 3 (IP3)-mediated calcium signalling, as well as other common exocytosis mechanisms [86]. ATP itself is then able to induce IP3-mediated calcium release in several cell types [87, 88]. In astrocytes, it was proposed that ATP could also be independently stored in vesicles that respond to a selected control mechanism, independent from any other neurotransmitter [81]. It was also demonstrated that ATP exocytosis is able to promote calcium waves across astrocyte layers and in communication with other cell types, such as Muller cells [89], in a way independent from tight junction. Stored ATP exocytosis occurs by different mechanisms. In HUVEC cells, it was demonstrated that ATP exocytosis could be induced by sheer stress [90]. In these conditions, a rapid release of all vesicles is observed, but this is partially blocked by extracellular calcium removal, suggesting that calcium-independent mechanisms could exist. Similar ATP release could be induced also in astrocytes [81] and glial cells of the retina [89].
The ATP concentration within these stores appears significantly different, dependent on the cell type, but it can reach high levels of around 150–200 mM [91]. Also, other nucleotides were found to be co-compartmentalized, especially GTP, UTP, and ADP, suggesting that the transport inside vesicles is not directly due to a nucleotide exchanger. Moreover, these activities of ATP transport were originally described by Bankston and Guidotti as a membrane potential-dependent activity that requires positive potential inside the vesicles [83]; it was only recently, however, that a vesicular nucleoside transporter was identified [92].
An isoform of the SLC17 phosphate transporter family, SLC17A9, was found to be expressed in all mouse organs but especially in adrenal glands and the thyroid. Through electron microscopy, the protein was found to associate with chromaffin granules in the medulla, and, if bound to liposomes, it was able to induce radioactive ATP uptake only after previous induction of a positive membrane potential inside. Even if specific mechanisms for the homeostasis of ATP storage are identified in detail (such as vesicle import and exocytosis), the source of ATP and its links to cellular metabolism remain unclear. Not much data have been published about the contact between catecholamine-storage vesicles and mitochondria in the adrenal medulla of rats. As previously mentioned, mitochondria can be found in close connection with other organelles, favoring the exchange of ions and other molecules. When in contact with the endoplasmic reticulum, for example, mitochondria favor ATP delivery to the SERCA and calcium pumping activity in the ER lumen [93]. One can imagine a similar mechanism occurring in vesicles containing ATP, in order to maintain an elevated ATP concentration. More than one report supports this idea, showing how the import of ATP into vesicles could be totally or partially inhibited by the ANT blocker atractyloside [83, 92, 94]. This protein is located in the IMM, in close proximity to the ATP synthase and exchanges ATP with ADP between the mitochondrial matrix and the IMS.
These observations suggest that a preferential ATP delivery route between mitochondria and ATP-containing vesicles could exist, even if it has not been properly demonstrated yet. If verified, this could provide a direct connection between cell metabolism and ATP storage that could be extended to the whole purinergic signalling mediated by ATP exocytosis (Fig. 1).
Recently, other reports suggest a link between extracellular ATP and mitochondria. This is the case of the previously mentioned ATP synthase. It has been reported how this complex could localize to the plasma membrane in endothelial cells, hepatocytes, adipocytes, as well as some tumor cells. Especially, it appears to be concentrated in structure called caveole. At this site, ATP synthase has been suggested to promote ATP synthesis and also as proton channels and ligand receptor providing a role for numerous biological processes including cell malignancy [95]. Specificity for ATP synthesis reaction has been suggested by monitoring extracellular ATP levels in presence of oligomycin or other selective inhibitors; nonetheless, some authors suggest that the presence of others nucleotide-converting enzymes such as adeylate kinase (AK) or nucleoside diphosphate kinase (NDPK) could generate artifacts leading to a misinterpretation of a role for ATP synthase on plasma membrane (for a more detailed review, see [96]). The mechanism of translocation of ATP synthase to plasma membrane has still to be elucidated, but more than one author provides evidences suggesting that it could translocate directly from IMM [97, 98].
The concomitant presence of ectopic ATP synthase, AK, and NDPK focuses attention on the concept of near-equilibrium and phosphotransfer networks [99]. This concept is based on the idea that the bulk of ATP, synthesized within mitochondrial cristae, does not easily diffuse to the whole cell. It has been then hypothesized that sequential reactions (specially catalyzed by AK, NDPK, and creatine kinase) allow a facilitated transport of phosphoryl groups between adenine nucleotides resulting in virtually more efficient ATP diffusion [100]. Recently, it has been reviewed the possibility that the same preferential reaction network could exist on the plasma membrane surface allowing the generation of a pericellular layer of adenosine nucleotides [96]. In this optic, it could be imagined that vesicular transport or ATP synthase act as source of ATP while ectopic AK or NDPK exert a phosphotransfer network that allow maintenance of ATP in the extracellular space. It would be interesting to investigate if similar networks exist within ATP storage vesicles and if they are involved in preferential ATP storage or ATP level maintenance.
Extracellular adenosine nucleotides are able to induce activation of AMPK through a P2Y-dependent pathway. This phenomenon has been already shown in HUVEC cells, elicited by ATP and ADP, and astrocytes after stimulation with ADP only [101, 102]. In the latter, authors also shown how ADP exposure for 24 h induced an AMPK-dependent increase of mitochondrial membrane potential and intracellular ATP synthesis. These data suggest a possible feedback mechanism by which extracellular adenosine nucleotides are able to promote ATP generation, possibly to increase levels of viable ATP and sustain the purinergic signal execution.
Finally, the common co-presence with adenosine nucleotides of other molecules during purinergic signals should be mentioned. ATP, especially, is often stored and released in the co-presence of NAD+ [85, 103]. For a long time, extracellular NAD+ has been addressed as a key signal of cell lysis with potent activation properties on several immune system cells [104–106] and as an inducer of intracellular calcium signals [107]. Its regulated co-storage and co-release with ATP lets us propose a more refined role in purinergic signaling, and ATP and NAD+, especially, appear to show synergistic activity by activation of several members of the purinergic receptor family, such as P2X7 and P2Y [108]. This extracellular cooperation appear surprising if considering the fact that NAD+, as ATP, in its intracellular location has a metabolic activity (see previews chapters); nonetheless, in contrast to ATP, clear studies about extracellular NAD+ and regulation of metabolism (or even during its cooperation with ATP) are still lacking. It should be considered that the P2X7 receptor has been widely characterized has a regulator of inflammasome complex, especially during ATP stimulation (for review, see [109]) and that, recently, the NLRP3 inflammasome components have been found located on mitochondria [110] where, apparently, they can sense mitochondrial dysfunctions through ROS production. These observations suggest that the purinergic signals mediated by ATP and NAD+ can sense the mitochondria and possibly verify the whole metabolic status of the cell.
The link between inflammasome and metabolism could gain a special meaning. In fact, NLRP3 inflammasome could be activated by necrosis leading to the sterile inflammation condition [111, 112]. Moreover, it is well known that extracellular ATP is able to induce either apoptosis or necrosis [113–115] and that intracellular ATP is involved in the main switch between necrosis and apoptosis [116]. It could be then imagined that, during purinergic activation, inflammasome senses mitochondria and participates in the decision between necrotic or apoptotic cell death.
Methods for measurements of intracellular ATP
Several soluble coupled multi-enzyme systems for adenine nucleotide assays (AMP, ADP, and ATP) were based on the reactions catalyzed by adenylate kinase, pyruvate kinase, and firefly luciferase [117]. At present, the best method to measure intracellular ATP is using the firefly luciferase, an enzyme that causes the oxidation of luciferin (an oxidizable substrate), which is quantifiable since the energy produced releases a photon of light (bioluminescence).
A method for measuring the concentration of the cytosolic free ATP utilizes the microinjection of purified luciferase [118]. However, in this manner, the targeting of the reporter to the lumen of organelles (or its attachment to intracellular membranes) is impossible [45]. This problem was solved through recombinant DNA technology. To measure the concentration of ATP in the mitochondrial matrix, a specifically targeted chimera of the ATP-sensitive photoprotein luciferase was designed: Luciferase cDNA [119] was delivered to the mitochondrial matrix using the targeting sequence of subunit VIII of cytochrome c oxidase, COX8, [120]; in the absence of this targeting sequence, luciferase remains in the cytoplasm. Luminescence is dependent on the concentration of luciferin (usually, between 20 and 200 μM is used [47]), and the light emitted is proportional to the ATP concentration in the sample. The affinity of luciferase for ATP is in the micromolar range in vitro but is much lower in vivo [121], and this assay can detect ATP concentrations ranging from micromolar to picomolar levels. Recombinant luciferase has thus been used repeatedly to study changes in ATP concentration due to cell stimulation with specific agonists, such as hormones that move intracellular calcium or changes in fuel supply to the cell [45, 122, 123].
Considering the difficulty in using recombinant DNAs in peculiar cell systems, such as myocytes, tracebulae, or even organs (i.e., whole hearts) that are refractory to transection or infection, other strategies were developed [124–126]. In these cases, the NADH/NADPH ratio was used as a valuable, indirect indicator of cell energy status. As previously described, NADH is one of the principal substrates of OXPHOS and thus of ATP synthesis. On the basis of its fluorescent properties, NADH levels can be easily monitored with a microscope, a fluorimeter, or a plate reader, equipped with a filter set for 4′,6-diamidino-2-phenylindole. In fact, NADH has its maximum absorption peak at 365 nm, with emission at 450 nm. Unfortunately, this is a very indirect method of monitoring ATP synthesis and can suffer from artefacts. Another difficulty is that NADPH, which is an analogue to NADH for fluorescent properties, is involved in different intracellular pathways, such as the pentose phosphate pathways. Additionally, the respiratory chain is also driven by FADH2, and it should also be noted that respiratory chain activity is not perfectly proportional to ATP synthesis, especially because of the uncoupling proteins.
The above-mentioned methods are able to provide time-resolved experiments with high speed (as shown for the calcium-stimulated mitochondrial ATP production [47]) but miss information related to the intracellular localization of ATP.
This shortcoming was addressed with quinacrine-based ATP staining. Quinacrine is a fluorescent dye with anti-malarial properties, derivative of the quinoline–acridine compounds. It is known to stain ATP when stored in high concentrations which makes it very useful for the detection of ATP storage vesicles [127]. It was used to visualize purine vesicles in several cultured cells like paraneurons [128], astrocytes [129], pancreatic acini [130], HUVEC [90], and Jurkat [131], but also in tissues like intestinal nerves [132], marginal cells of cochlea [133], or chromaffin cells [134].
Differently from the previous methods, quinacrine does not allow the measurement of ATP concentration in a wide dynamic range. It is, however, useful to monitor purine vesicles using confocal fluorescence microscopy, both in living or fixed cells, allowing morphological descriptions and the visualization of live exocytosis.
There is also a sensitive method for ATP detection that is based on a label-free DNA aptamer as the recognition element and ethidium bromide as the signal reporter. Aptamers are single-stranded DNA, RNA, or even modified nucleic acid molecules that have the ability to form defined tertiary structures upon specific target binding [135]. This method generally requires a fluorophore-labelled DNA aptamer and a quencher-labelled complementary DNA or a dual-labelled aptamer beacon with a quencher at one end and a fluorophore at the other end. When ATP is present in solution, there is a conformational change in the aptamer duplex; an aptamer/ATP complex is formed, and the number of duplexes in solution decreases [136].
Recently, a new method with high sensitivity has been proposed to detect basal levels of extracellular ATP. This method, based on radio thin-layer chromatography, is able to detect low amounts of ATP generated to basal levels in integer lymphocytes. Coupled to confocal microscopy and quinacrine staining, this new application allows the measure of micromolar pericellular ATP pools [131].
Definitely, combinations of each of these methods should be considered when performing experiments related to the precise measurement of ATP synthesis, consumption, or storage.
Acknowledgments
The authors apologize for any excessive bias and the inevitable omissions. The authors are also deeply indebted to past collaborators. This research was supported by the Ministry of Science and Higher Education, Poland, grant NN407 075 137 to MRW; the Italian Ministry of Health to A.R.; and the Italian Association for Cancer Research (AIRC), Telethon (GGP09128), local funds from the University of Ferrara, the Italian Ministry of Education, University and Research (COFIN, FIRB and Futuro in Ricerca), the Italian Cystic Fibrosis Research Foundation, and Italian Ministry of Health to P.P.
SM was supported by a FIRC fellowship; AB was supported by a research fellowship Fondazione Italiana Sclerosi Multipla (FISM)-Cod. 2010/B/1; SP was supported by a training fellowship FISM-Cod. 2010/B/13; JMS was supported by PhD fellowship from The Foundation for Polish Science (FNP), UE, European Regional Development Fund and Operational Programme “Innovative economy”.
References
- 1.Pollard-Knight D, Cornish-Bowden A. Mechanism of liver glucokinase. Mol Cell Biochem. 1982;44(2):71–80. doi: 10.1007/BF00226892. [DOI] [PubMed] [Google Scholar]
- 2.Robey RB, Hay N. Mitochondrial hexokinases: guardians of the mitochondria. Cell Cycle. 2005;4(5):654–658. doi: 10.4161/cc.4.5.1678. [DOI] [PubMed] [Google Scholar]
- 3.Sirover MA. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1999;1432(2):159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- 4.Jurica MS, et al. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998;6(2):195–210. doi: 10.1016/s0969-2126(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 5.Mazurek S (2011) Pyruvate kinase type M2: A key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43:969–980 [DOI] [PubMed]
- 6.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 7.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krebs HA. The citric acid cycle and the Szent-Gyorgyi cycle in pigeon breast muscle. Biochem J. 1940;34(5):775–779. doi: 10.1042/bj0340775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy EP, Lehninger AL. Oxidation of fatty acids and tricarboxylic acid cycle intermediates by isolated rat liver mitochondria. J Biol Chem. 1949;179(2):957–972. [PubMed] [Google Scholar]
- 10.Lambeth DO, et al. Expression of two succinyl–CoA synthetases with different nucleotide specificities in mammalian tissues. J Biol Chem. 2004;279(35):36621–36624. doi: 10.1074/jbc.M406884200. [DOI] [PubMed] [Google Scholar]
- 11.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25(34):4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 12.Quinn PJ, Dawson RM. Interactions of cytochrome c and [14C] Biochem J. 1969;115(1):65–75. doi: 10.1042/bj1150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010;12(8):961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- 14.Boyer PD. Catalytic site forms and controls in ATP synthase catalysis. Biochim Biophys Acta. 2000;1458(2–3):252–262. doi: 10.1016/s0005-2728(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson SJ. ATP synthase: from sequence to ring size to the P/O ratio. Proc Natl Acad Sci USA. 2010;107(39):16755–16756. doi: 10.1073/pnas.1012260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramaiah A, Hathaway JA, Atkinson DE. Adenylate as a metabolic regulator. Effect on yeast phosphofructokinase kinetics. J Biol Chem. 1964;239:3619–3622. [PubMed] [Google Scholar]
- 17.Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem. 1997;246(2):259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 18.Carling D, et al. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989;186(1–2):129–136. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 19.Hardie DG. AMPK and SNF1: snuffing out stress. Cell Metab. 2007;6(5):339–340. doi: 10.1016/j.cmet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crute BE, et al. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273(52):35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 21.Hudson ER, et al. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13(10):861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 22.Scott JW, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113(2):274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci. 2008;65(23):3737–3755. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen M, et al. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89(3):777–798. doi: 10.1152/physrev.00026.2008. [DOI] [PubMed] [Google Scholar]
- 25.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101(10):3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13(22):2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Hawley SA, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9(5):407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Dennis PB, et al. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294(5544):1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 30.Rosner M, et al. The mTOR pathway and its role in human genetic diseases. Mutat Res. 2008;659(3):284–292. doi: 10.1016/j.mrrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardie DG. AMPK and Raptor: matching cell growth to energy supply. Mol Cell. 2008;30(3):263–265. doi: 10.1016/j.molcel.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Zeng PY, Berger SL. LKB1 is recruited to the p21/WAF1 promoter by p53 to mediate transcriptional activation. Cancer Res. 2006;66(22):10701–10708. doi: 10.1158/0008-5472.CAN-06-0999. [DOI] [PubMed] [Google Scholar]
- 34.Budanov AV, Karin M. p53 Target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang WB, et al. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem. 2010;285(52):40461–40471. doi: 10.1074/jbc.M110.164046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beffy P, et al. Altered signal transduction pathways and induction of autophagy in human myotonic dystrophy type 1 myoblasts. Int J Biochem Cell Biol. 2010;42(12):1973–1983. doi: 10.1016/j.biocel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Arnold S, Kadenbach B. Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. Eur J Biochem. 1997;249(1):350–354. doi: 10.1111/j.1432-1033.1997.t01-1-00350.x. [DOI] [PubMed] [Google Scholar]
- 38.Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466(1):130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- 39.Lee I, et al. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J Biol Chem. 2005;280(7):6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- 40.Acin-Perez R, et al. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13(6):712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21(7):288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- 42.Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- 43.Duchen MR, Biscoe TJ. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J Physiol. 1992;450:13–31. doi: 10.1113/jphysiol.1992.sp019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duchen MR, Biscoe TJ. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rimessi A, et al. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta. 2008;1777(7–8):808–816. doi: 10.1016/j.bbabio.2008.05.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pozzan T, Rizzuto R. High tide of calcium in mitochondria. Nat Cell Biol. 2000;2(2):E25–E27. doi: 10.1038/35000095. [DOI] [PubMed] [Google Scholar]
- 47.Jouaville LS, et al. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96(24):13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matlib MA, et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273(17):10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 49.De Stefani D et al (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476:336–340 [DOI] [PMC free article] [PubMed]
- 50.Sparagna GC, et al. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270(46):27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- 51.Rizzuto R, et al. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358(6384):325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 52.Carafoli E, et al. The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol. 1974;6(4):361–371. doi: 10.1016/0022-2828(74)90077-7. [DOI] [PubMed] [Google Scholar]
- 53.Fiskum G, Lehninger AL. Regulated release of Ca2+ from respiring mitochondria by Ca2+/2H+ antiport. J Biol Chem. 1979;254(14):6236–6239. [PubMed] [Google Scholar]
- 54.Baumgartner HK, et al. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J Biol Chem. 2009;284(31):20796–20803. doi: 10.1074/jbc.M109.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 57.Denton RM, Richards DA, Chin JG. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCormack JG, Denton RM. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kerbey AL, et al. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rutter GA, Denton RM. The binding of Ca2+ ions to pig heart NAD+−isocitrate dehydrogenase and the 2-oxoglutarate dehydrogenase complex. Biochem J. 1989;263(2):453–462. doi: 10.1042/bj2630453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawlis VB, Roche TE. Effect of micromolar Ca2+ on NADH inhibition of bovine kidney alpha-ketoglutarate dehydrogenase complex and possible role of Ca2+ in signal amplification. Mol Cell Biochem. 1980;32(3):147–152. doi: 10.1007/BF00227441. [DOI] [PubMed] [Google Scholar]
- 62.Harris DA. Regulation of the mitochondrial ATP synthase in rat heart. Biochem Soc Trans. 1993;21(Pt 3(3)):778–781. doi: 10.1042/bst0210778. [DOI] [PubMed] [Google Scholar]
- 63.Scholz TD, Balaban RS. Mitochondrial F1-ATPase activity of canine myocardium: effects of hypoxia and stimulation. Am J Physiol. 1994;266(6 Pt 2):H2396–H2403. doi: 10.1152/ajpheart.1994.266.6.H2396. [DOI] [PubMed] [Google Scholar]
- 64.Territo PR, et al. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol. 2000;278(2):C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 65.Hubbard MJ, McHugh NJ. Mitochondrial ATP synthase F1-beta-subunit is a calcium-binding protein. FEBS Lett. 1996;391(3):323–329. doi: 10.1016/0014-5793(96)00767-3. [DOI] [PubMed] [Google Scholar]
- 66.Sun J, et al. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101(11):1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 67.Azarashvili TS, et al. Phosphorylation of a peptide related to subunit c of the F0F1-ATPase/ATP synthase and relationship to permeability transition pore opening in mitochondria. J Bioenerg Biomembr. 2002;34(4):279–284. doi: 10.1023/a:1020204518513. [DOI] [PubMed] [Google Scholar]
- 68.Taylor SW, et al. Oxidative post-translational modification of tryptophan residues in cardiac mitochondrial proteins. J Biol Chem. 2003;278(22):19587–19590. doi: 10.1074/jbc.C300135200. [DOI] [PubMed] [Google Scholar]
- 69.Anello M, et al. Glucosamine-induced alterations of mitochondrial function in pancreatic beta-cells: possible role of protein glycosylation. Am J Physiol Endocrinol Metab. 2004;287(4):E602–E608. doi: 10.1152/ajpendo.00320.2003. [DOI] [PubMed] [Google Scholar]
- 70.Arco A, Satrustegui J. Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J Biol Chem. 1998;273(36):23327–23334. doi: 10.1074/jbc.273.36.23327. [DOI] [PubMed] [Google Scholar]
- 71.Lasorsa FM, et al. Recombinant expression of the Ca(2+)-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J Biol Chem. 2003;278(40):38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- 72.Jo H, Noma A, Matsuoka S. Calcium-mediated coupling between mitochondrial substrate dehydrogenation and cardiac workload in single guinea-pig ventricular myocytes. J Mol Cell Cardiol. 2006;40(3):394–404. doi: 10.1016/j.yjmcc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Collins TJ, et al. Mitochondrial Ca(2+) uptake depends on the spatial and temporal profile of cytosolic Ca(2+) signals. J Biol Chem. 2001;276(28):26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 74.Maechler P, et al. Desensitization of mitochondrial Ca2+ and insulin secretion responses in the beta cell. J Biol Chem. 1998;273(33):20770–20778. doi: 10.1074/jbc.273.33.20770. [DOI] [PubMed] [Google Scholar]
- 75.Moreau B, Parekh AB. Ca2+-dependent inactivation of the mitochondrial Ca2+ uniporter involves proton flux through the ATP synthase. Curr Biol. 2008;18(11):855–859. doi: 10.1016/j.cub.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 76.Cardenas C, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142(2):270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaasik A, et al. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res. 2001;89(2):153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- 78.Crozatier B, et al. Role of creatine kinase in cardiac excitation-contraction coupling: studies in creatine kinase-deficient mice. FASEB J. 2002;16(7):653–660. doi: 10.1096/fj.01-0652com. [DOI] [PubMed] [Google Scholar]
- 79.Whittaker VP, et al. Proteins of cholinergic synaptic vesicles from the electric organ of Torpedo: characterization of a low molecular weight acidic protein. Brain Res. 1974;75(1):115–131. doi: 10.1016/0006-8993(74)90774-4. [DOI] [PubMed] [Google Scholar]
- 80.Pablo Huidobro-Toro J, Veronica Donoso M. Sympathetic co-transmission: the coordinated action of ATP and noradrenaline and their modulation by neuropeptide Y in human vascular neuroeffector junctions. Eur J Pharmacol. 2004;500(1–3):27–35. doi: 10.1016/j.ejphar.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Coco S, et al. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278(2):1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 82.Zimmermann H. ATP and acetylcholine, equal brethren. Neurochem Int. 2008;52(4–5):634–648. doi: 10.1016/j.neuint.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271(29):17132–17138. doi: 10.1074/jbc.271.29.17132. [DOI] [PubMed] [Google Scholar]
- 84.Haanes KA, Novak I. ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J. 2010;429(2):303–311. doi: 10.1042/BJ20091337. [DOI] [PubMed] [Google Scholar]
- 85.Yamboliev IA, et al. Storage and secretion of beta-NAD, ATP and dopamine in NGF-differentiated rat pheochromocytoma PC12 cells. Eur J Neurosci. 2009;30(5):756–768. doi: 10.1111/j.1460-9568.2009.06869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cotrina ML, et al. ATP-mediated glia signaling. J Neurosci. 2000;20(8):2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guthrie PB, et al. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19(2):520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giorgi C, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330(6008):1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21(7):2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38(6):900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Hillarp NA. Adenosinephosphates and inorganic phosphate in the adrenaline and noradrenaline containing granules of the adrenal medulla. Acta Physiol Scand. 1958;42(3–4):321–332. doi: 10.1111/j.1748-1716.1958.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 92.Sawada K, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105(15):5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landolfi B, et al. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured in situ in intact cells. J Cell Biol. 1998;142(5):1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luqmani YA. Nucleotide uptake by isolated cholinergic synaptic vesicles: evidence for a carrier of adenosine 5′-triphosphate. Neuroscience. 1981;6(6):1011–1021. doi: 10.1016/0306-4522(81)90067-1. [DOI] [PubMed] [Google Scholar]
- 95.Champagne E, et al. Ecto-F1F0 ATP synthase/F1 ATPase: metabolic and immunological functions. Curr Opin Lipidol. 2006;17(3):279–284. doi: 10.1097/01.mol.0000226120.27931.76. [DOI] [PubMed] [Google Scholar]
- 96.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 97.Wang T, et al. Cholesterol loading increases the translocation of ATP synthase beta chain into membrane caveolae in vascular endothelial cells. Biochim Biophys Acta. 2006;1761(10):1182–1190. doi: 10.1016/j.bbalip.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Ma Z, et al. Mitochondrial F1Fo-ATP synthase translocates to cell surface in hepatocytes and has high activity in tumor-like acidic and hypoxic environment. Acta Biochim Biophys Sin (Shanghai) 2010;42(8):530–537. doi: 10.1093/abbs/gmq063. [DOI] [PubMed] [Google Scholar]
- 99.Yegutkin GG, Henttinen T, Jalkanen S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15(1):251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- 100.Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206(Pt 12):2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 101.Cui J, et al. Prevention of extracellular ADP-induced ATP accumulation of the cultured rat spinal astrocytes via P2Y(1)-mediated inhibition of AMPK. Neurosci Lett. 2011;503(3):244–249. doi: 10.1016/j.neulet.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 102.Silva CG, et al. Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters. Circ Res. 2006;98(5):e39–e47. doi: 10.1161/01.RES.0000215436.92414.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scheuplein F, et al. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J Immunol. 2009;182(5):2898–2908. doi: 10.4049/jimmunol.0801711. [DOI] [PubMed] [Google Scholar]
- 104.Koch-Nolte F, et al. Compartmentation of NAD+-dependent signalling. FEBS Lett. 2011;585(11):1651–1656. doi: 10.1016/j.febslet.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 105.Hubert S, et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2–P2X7 pathway. J Exp Med. 2010;207(12):2561–2568. doi: 10.1084/jem.20091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heiss K, et al. High sensitivity of intestinal CD8+ T cells to nucleotides indicates P2X7 as a regulator for intestinal T cell responses. J Immunol. 2008;181(6):3861–3869. doi: 10.4049/jimmunol.181.6.3861. [DOI] [PubMed] [Google Scholar]
- 107.Klein C, et al. Extracellular NAD(+) induces a rise in [Ca(2+)](i) in activated human monocytes via engagement of P2Y(1) and P2Y(11) receptors. Cell Calcium. 2009;46(4):263–272. doi: 10.1016/j.ceca.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 108.Haag F, et al. Extracellular NAD and ATP: partners in immune cell modulation. Purinergic Signal. 2007;3(1–2):71–81. doi: 10.1007/s11302-006-9038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28(9):465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 110.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 111.Li H, Ambade A, Re F. Cutting edge: necrosis activates the NLRP3 inflammasome. J Immunol. 2009;183(3):1528–1532. doi: 10.4049/jimmunol.0901080. [DOI] [PubMed] [Google Scholar]
- 112.Iyer SS, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106(48):20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng LM, et al. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991;112(2):279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schulze-Lohoff E, et al. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol. 1998;275(6 Pt 2):F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- 115.Jun DJ, et al. Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J Biol Chem. 2007;282(52):37350–37358. doi: 10.1074/jbc.M707915200. [DOI] [PubMed] [Google Scholar]
- 116.Leist M, et al. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185(8):1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brovko L, Romanova NA, Ugarova NN. Bioluminescent assay of bacterial intracellular AMP, ADP, and ATP with the use of a coimmobilized three-enzyme reagent (adenylate kinase, pyruvate kinase, and firefly luciferase) Anal Biochem. 1994;220(2):410–414. doi: 10.1006/abio.1994.1358. [DOI] [PubMed] [Google Scholar]
- 118.Bowers KC, Allshire AP, Cobbold PH. Bioluminescent measurement in single cardiomyocytes of sudden cytosolic ATP depletion coincident with rigor. J Mol Cell Cardiol. 1992;24(3):213–218. doi: 10.1016/0022-2828(92)93159-h. [DOI] [PubMed] [Google Scholar]
- 119.Wet JR, et al. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rizzuto R, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280(5370):1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 121.Gandelman O, et al. Cytoplasmic factors that affect the intensity and stability of bioluminescence from firefly luciferase in living mammalian cells. J Biolumin Chemilumin. 1994;9(6):363–371. doi: 10.1002/bio.1170090603. [DOI] [PubMed] [Google Scholar]
- 122.Denton RM, McCormack JG, Edgell NJ. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rutter GA. Ca2(+)-binding to citrate cycle dehydrogenases. Int J Biochem. 1990;22(10):1081–1088. doi: 10.1016/0020-711x(90)90105-c. [DOI] [PubMed] [Google Scholar]
- 124.White RL, Wittenberg BA. NADH fluorescence of isolated ventricular myocytes: effects of pacing, myoglobin, and oxygen supply. Biophys J. 1993;65(1):196–204. doi: 10.1016/S0006-3495(93)81058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J. 1996;71(2):1024–1035. doi: 10.1016/S0006-3495(96)79303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Katz LA, et al. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol. 1989;256(1 Pt 2):H265–H274. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- 127.Burnstock G, et al. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bock P. Identification of paraneurons by labelling with quinacrine (Atebrin) Arch Histol Jpn. 1980;43(1):35–44. doi: 10.1679/aohc1950.43.35. [DOI] [PubMed] [Google Scholar]
- 129.Pryazhnikov E, Khiroug L. Sub-micromolar increase in [Ca(2+)](i) triggers delayed exocytosis of ATP in cultured astrocytes. Glia. 2008;56(1):38–49. doi: 10.1002/glia.20590. [DOI] [PubMed] [Google Scholar]
- 130.Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276(35):32925–32932. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- 131.Yegutkin GG, et al. The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Mol Biol Cell. 2006;17(8):3378–3385. doi: 10.1091/mbc.E05-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crowe R, Burnstock G. Comparative studies of quinacrine-positive neurones in the myenteric plexus of stomach and intestine of guinea-pig, rabbit and rat. Cell Tissue Res. 1981;221(1):93–107. doi: 10.1007/BF00216573. [DOI] [PubMed] [Google Scholar]
- 133.White PN, et al. Quinacrine staining of marginal cells in the stria vascularis of the guinea-pig cochlea: a possible source of extracellular ATP? Hear Res. 1995;90(1–2):97–105. doi: 10.1016/0378-5955(95)00151-1. [DOI] [PubMed] [Google Scholar]
- 134.Alund M, Olson L. Quinacrine affinity of endocrine cell systems containing dense core vesicles as visualized by fluorescence microscopy. Cell Tissue Res. 1979;204(2):171–186. doi: 10.1007/BF00234631. [DOI] [PubMed] [Google Scholar]
- 135.Savran CA, et al. Micromechanical detection of proteins using aptamer-based receptor molecules. Anal Chem. 2004;76(11):3194–3198. doi: 10.1021/ac049859f. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Liu B. ATP detection using a label-free DNA aptamer and a cationic tetrahedralfluorene. Analyst. 2008;133(11):1593–1598. doi: 10.1039/b806908e. [DOI] [PubMed] [Google Scholar]