Abstract
Behavioral models indicate that persistent small afferent input, as generated by tissue injury, results in a hyperalgesia at the site of injury and a tactile allodynia in areas adjacent to the injury site. Hyperalgesia reflects a sensitization of the peripheral terminal and a central facilitation evoked by the persistent small afferent input. The allodynia reflects a central sensitization. The spinal pharmacology of these pain states has been defined in the unanesthetized rat prepared with spinal catheters for injection and dialysis. After tissue injury, excitatory transmitters (e.g., glutamate and substance P) acting though N-methyl-d-aspartate (NMDA) and neurokinin 1 receptors initiate a cascade that evokes release of (i) NO, (ii) cyclooxygenase products, and (iii) activation of several kinases. Spinal dialysis show amino acid and prostanoid release after cutaneous injury. Spinal neurokinin 1, NMDA, and non-NMDA receptors enhance spinal prostaglandin E2 release. Spinal prostaglandins facilitate release of spinal amino acids and peptides. Activation by intrathecal injection of receptors on spinal C fiber terminals (μ,/∂ opiate, α2 adrenergic, neuropeptide Y) prevents release of primary afferent peptides and spinal amino acids and blocks acute and facilitated pain states. Conversely, consistent with their role in facilitated processing, NMDA, cyclooxygenase 2, and NO synthase inhibitors act to diminish only hyperalgesia. Importantly, spinal delivery of several of these agents diminishes human injury pain states. This efficacy emphasizes (i) the role of facilitated states in humans, (ii) shows the importance of spinal systems in human pain processing, and (iii) indicates that these preclinical mechanisms reflect processes that regulate the human pain experience.
Local tissue injury and inflammation yields well-defined escape behaviors in animals and pain reports in humans. Examination of the histochemistry and electrophysiology of spinal systems has revealed considerable detail regarding the elements of systems that are activated by these stimuli. Nevertheless, the functional contribution of different spinal systems in pain processing ultimately must be defined in terms of the systems in which such end points can be measured, e.g., the behavior of the intact organism. We will consider below how certain spinal systems contribute to the observed behavioral states.
Behavioral Effects of Cutaneous Stimuli After Injury
An acute, unconditioned, thermal, or mechanical stimulus sufficient to activate polymodel nociceptive afferents (C fibers) depolarizes populations of dorsal horn wide dynamic range (WDR) neurons that project supraspinally. This output in turn evokes a supraspinally organized escape behavior. The hot plate test (thermal stimulus to the paw) or the local injection of an irritant such as formalin or capsaicin where the unconditioned stimulus evokes a somatotopically directed behavior (e.g., withdrawal or licking) are behavioral paradigms believed to reflect this underlying mechanism (1). The more intense the stimulus, the more robust will be the afferent volley and the more vigorous or shorter latencied is the escape behavior (2).
An acute stimulus of intensity and duration that leads to tissue injury also produces an acute discharge. In addition, the injury leads to the local release of active factors that evoke and sustain persistent activity in the sensory afferents innervating the injured or inflamed tissue (3). Thus, in contrast to the acute response, injury leads to persistent activity in populations of small afferents and also may activate afferent populations that are excited only in the presence of local factors generated by the injury (e.g., silent “nociceptors”) (4). Electrophysiological studies have shown that the persistent activation of spinal WDR neurons by small, but not large, afferents, will lead: (i) a progressive enhancement of the WDR response to each subsequent input, and (ii) an increase in the dimensions of the peripheral receptive field to which the spinal neuron will respond (5). This electrophysiological observation parallels behavioral changes in which the animal displays an enhanced response to a given stimulus or a reduction in the intensity of the stimulus required to evoke an escape response. Thus, the injection of an irritant (formalin) into one hind paw evokes a high frequency barrage during the first 10–20 min followed by a modest ongoing discharge over the next hour (6). Coincident with the initial afferent barrage, WDR neurons display an initial burst of activity followed by a period of quiescence and then a progressive enhanced barrage (7). In rats, injection of formalin results in a prominent licking and flinching of the injected paw with the incidence of flinching showing a biphasic time course that parallels that reported for the discharge of spinal WDR neurons (see Fig. 1). The first-phase behavior is the result of an initial intense afferent barrage. The second-phase behavior is believed to represent the induction of a state of spinal facilitation in which the diminished formalin-initiated afferent input yields a prominent response.
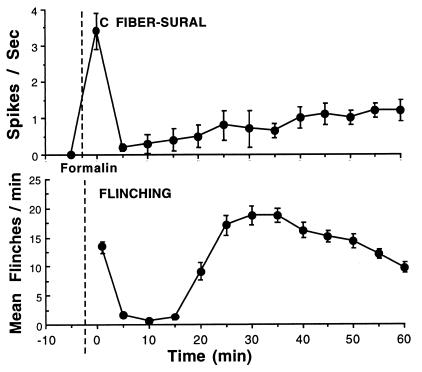
Figure 1.
(Upper) C fiber activity recorded in situ in rats from single sural nerve fibers, identified by their conduction velocity and modality as C fibers. Immediately after formalin injection (as indicated by the dashed line) into their receptive fields, high activity was observed in high-threshold C nociceptive afferent fibers (as well as in A beta and A delta fibers, data not shown). At later intervals, activity was observed in all mechanically sensitive C fibers, at rates that were less (1/2–2/3) than those achieved initially (adapted from ref. 6). (Lower) Frequency of flinching as measured by an automated motion detector is plotted at 5-min intervals after the injection of formalin into the paw at the time indicated by the vertical dashed line. As indicated, the flinching behavior displays a biphasic occurrence (phase 1 and phase 2). The data represent the mean ± SEM of eight rats.
Alternately, after a mild local burn, there is a decreased nociceptive threshold to heat within the burn area (a 1° thermal hyperalgesia) and a pain response generated by light touch applied to uninjured skin regions adjacent to the area of injury (a 2° tactile allodynia) (see Fig. 2) (8). Importantly, at least the initiating component of this hyperalgesia reflects on small afferent input. The intradermal injection of capsaicin, an agent known to selectively activate C fibers, can induce a 2° allodynia in humans and animals (9). This altered sensory condition persists after the termination of the pain produced by the capsaicin injection and extends anatomically beyond the local site in which the capsaicin was shown to exert an effect.
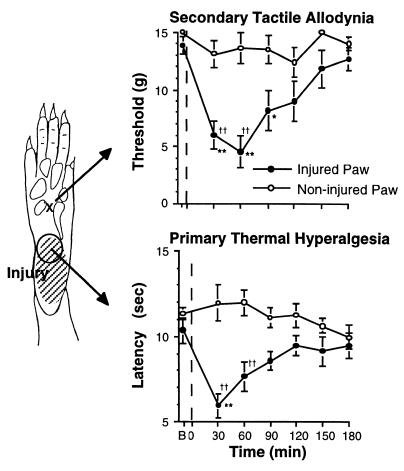
Figure 2.
Time course of change in mechanical threshold necessary to evoke acute withdrawal (Upper) or the thermal escape latency to evoke withdrawal (Lower) in the normal (noninjured) and injured paw. The injury was induced with the exposure of the shaded area indicated in the paw diagram (Left) to a 52°C thermal stimulus applied for 45 sec at the time indicated in the graphs by the vertical dashed line. As indicated, the test stimuli were applied to the sites as indicated. Only a modest change in tactile thresholds were observed at the injury site, and no change in thermal escape thresholds were noted in the off injury site (data not shown). Hence the lower response latency corresponds to a 1° thermal hyperalgesia and 2° tactile allodynia. Contralateral paws showed no systematic change. Mechanical thresholds were determined with Von Frey hairs, and the thermal escape thresholds were assessed with Hargreaves apparatus (10). All points represent mean ± SEM of five animals. B, baseline threshold.
Role of Spinal and Peripheral Systems in the Post-Tissue Injury Pain State
The behavioral sequelae outlined above, showing a hyperalgesic/allodynic state after tissue injury, may result from a peripheral sensitization secondary to the injury and/or to a change in central processing initiated by the persistent small afferent input generated by the injury. Blockade of spinal activation by the spinal delivery of a local anesthetic (11) or a selective blockade of small afferent input by the intrathecal infusion of a short-lasting opiate during the initial period of injury (12) will attenuate the second phase of the formalin response and abolish the 2° tactile allodynia, but not the 1° hyperalgesia observed secondary to a mild thermal injury (N.N.-T. and T.L.Y., unpublished observations). Importantly, as the pain behavior observed during the second phase after formalin injection is blocked by the injection of local anesthetic into the paw (13), it is clear that the exaggerated responding indeed depends on the concurrent low-level afferent input observed during the second phase of the formalin test (see Fig. 1). These findings thus support the hypothesis that (i) the initial injury-induced afferent barrage generated in an opiate-sensitive spinal system initiates a cascade that supports the 2° allodynia observed after injury, and (ii) the 1° hyperalgesia is mediated in part by a peripheral sensitization of small opiate-sensitive C fibers.
Characterization of Several Spinal Components Leading to Postinjury Pain States
Based on immunohistochemistry and electrophysiology, several points are evident regarding the biology of several spinal systems that may mediate the consequences of small afferent activation. (i) Populations of C fibers jointly contain peptides such as substance P (sP) and calcitonin gene-related peptide (CGRP), as well as amino acids such as glutamate. (ii) Small afferent activation will evoke the Ca2+-dependent spinal release of these products. (iii) Focusing on sP and glutamate, these agents evoke excitation of second-order neurons through an effect mediated by the tachykinin neurokinin 1 (NK-1) and the glutamatergic α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)/N-methyl-d-aspartate (NMDA) receptors, respectively. In situ hybridization shows labeling for NK-1 and NMDA receptor units in the dorsal gray matter, particularly in the substantia gelatinosa where small afferents are known to terminate. (iv) Electrophysiologically, NK-1 and AMPA receptor antagonists will diminish small afferent-evoked excitation. NMDA antagonists do not appear to reduce monosynaptically mediated afferent-evoked excitation and thus are not believed to be immediately postsynaptic to the primary afferent terminal, though some binding may be on the C-fiber terminal itself (see refs. 5 and 14 for references).
Spinal Pharmacology of Facilitated Processing
The preceding section emphasizes that after tissue injury there is an excitation of small sensory afferents and the production of a behaviorally defined state of hyperalgesia and allodynia. The overview of connectivity suggests elements that define a portion of the organization of spinal systems that encode activity generated by small afferent input. The contribution of these several spinal systems in nociceptive processing can be determined by considering the effects of systematically altering spinal pharmacology on pain behavior generated by acute high intensity and tissue injurious stimuli. Modifications of spinal pharmacology can be accomplished by the spinal delivery of pharmacological agents in animal behavior models by using chronically implanted catheter systems as noted above (15).
Regulation of Spinal Terminal Excitability.
Based on the described role of small afferents activated by tissue injury, it is reasonable to hypothesize that regulation of small afferent terminal excitability will diminish afferent-evoked pain behavior. Such regulation should be achieved by receptors having a presynaptic inhibitory effect on spinal C-fiber terminals as defined by: (i) presence of receptor binding on terminals of C fibers [e.g., receptor mRNA in the dorsal root ganglia (DRG), particularly in small DRG cell bodies, binding, or receptor protein in the spinal substantia gelatinosa]; (ii) negative coupling of receptor to the opening of voltage-sensitive Ca2+ channels, and (iii) their ability to block release of small afferent transmitters (sP or CGRP). Mu and delta opioid (16), alpha 2 agonist (17), and neuropeptide Y (18) receptor systems possess such a presynaptic distribution and effect (see Fig. 3). In addition to the presynaptic action of these agents, binding, receptor protein, and/or mRNA is in dorsal horn neurons. These postsynaptic receptors are coupled to Gi/o-protein and increase potassium conductance, serving to hyperpolarize those membranes and directly block depolarization of that neuron (1). This joint action, reducing small afferent excitatory input and diminishing postsynaptic excitability, maximizes the likelihood of a selective effect on acute nociceptive processing. The functional importance to pain processing of this concurrent spinal action is demonstrated by the dose-dependent and pharmacologically specific blockade of the acute response to an acute high intensity or injurious thermal (hot plate and tail flick), mechanical (paw pressure), or chemical (intradermal formalin) stimuli produced when these agents are delivered intrathecally in a variety of animal models (see ref. 1). Consistent with the electrophysiology, at doses that alter pain behavior there is no effect on the response to proprioceptive stimuli or on motor function.
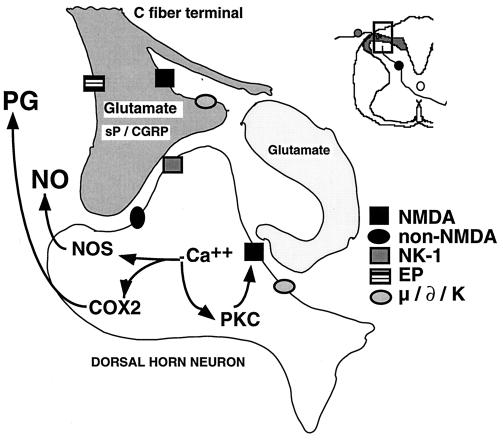
Figure 3.
Schematic summarizes the organization of several dorsal horn systems that contribute to the processing of nociceptive information. Primary afferent C fibers release peptide (e.g., sP/CGRP, etc.) and excitatory amino acid (glutamate) products. Small DRG as well as some postsynaptic elements contain NOS) and are able on depolarization to release NO. Peptides and excitatory amino acids evoke excitation in second-order neurons. For glutamate, direct monosynaptic excitation is mediated by non-NMDA receptors (i.e., acute primary afferent excitation of WDR neurons is not mediated by the NMDA or NK-1 receptor). Interneurons excited by afferent barrage induce excitation in second-order neuron via a NMDA receptor, which leads to an increase in intracellular Ca2+, activation of phospholipase A2, NOS, and phosphorylating enzymes. COX products (PG) and NO are formed and released. These agents diffuse extracellularly and facilitate transmitter release (retrograde transmission) from primary and nonprimary afferent terminals by either a direct cellular action (e.g., NO) or by an interaction with a specific class of receptors [e.g., PG type E (EP) receptors for prostanoids]. Phosphorylation of intracellular protein (e.g., enzymes and receptors such as NMDA) leads to additional enhanced sensitivity. See text for other details.
sP.
The spinal delivery of NK-1 receptor agonists results in a mild acute “pain behavior” and a subsequent reduced response latency to thermal stimuli (thermal hyperalgesia). Blockade of the NK-1 receptor by intrathecal antagonists (19) or down-regulation of NK-1 receptor expression by intrathecal treatment with NK-1 receptor mRNA antisense (20) has no effect on acute nociceptive thresholds, but reduces the second phase of the formalin response. Intrathecal injection of NK-1 antagonists after phase 1 reduces their effect on the second phase (19).
Glutamate Receptors.
Repetitive small afferent input (as that which occurs after tissue injury) will evoke spinal glutamate release (see Fig. 4) (21, 22). The spinal delivery of agonists for the ionotrophic glutamate receptors (NMDA/AMPA) will evoke a potent spontaneous pain behavior and a subsequent thermal hyperalgesia and tactile allodynia (23). Blockade of spinal AMPA receptors by intrathecal antagonists (24) will elevate acute nociceptive thresholds, as well as the first and second phase of the formalin test. In contrast, intrathecal NMDA antagonists have little effect on acute nociception, but diminish the second phase of the formalin test (25). As with the NK-1 antagonists, NMDA antagonists in the formalin model show a diminished effect on phase two when delivered after phase 1 (26), which reflects the fact that after an acute injurious stimulus, such as with formalin injection, there is an initiating barrage of activity leading to transmitter release (see Fig. 4). This release, for example of glutamate, sP, and prostanoids, leads to biochemical changes within the spinal cord that must persist after the initial occupancy of the NMDA (or NK-1 receptor) receptor during phase 1 has passed.
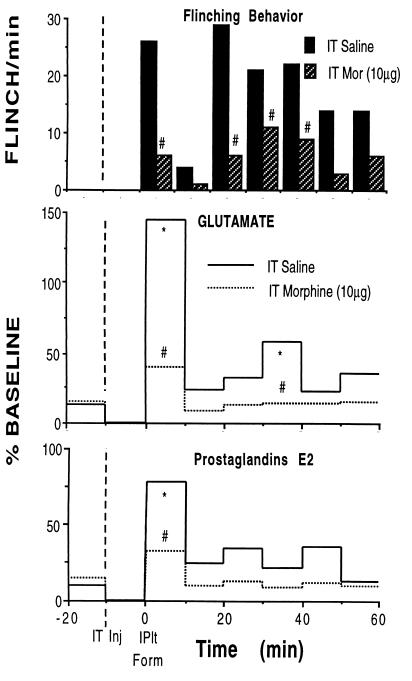
Figure 4.
Time course of flinching behavior (Top) and concurrent assessment of lumbar spinal glutamate (Middle), and PGs E2 (Bottom) release measured in unanesthetized rats before and after the injection of formalin (5%/50 μl) into the left hind paw of the rat at time = 0. At −10 min, the rats received an intrathecal (IT) injection of saline (vehicle: n = 4) or morphine (10 μg; n = 7). Release was assessed by using a chronically implanted loop dialysis probe (27), and the intrathecal injection was through a chronic intrathecal catheter. Each line presents the mean of four and seven rats, respectively. Error bars deleted for clarity. ∗, P < 0.05 versus formalin; #, P < 0.05 vs. IT saline.
Prostaglandins (PGs).
PGs are released in vivo from the spinal cord by a peripherally injurious stimulus that is associated with small afferent activation (see Fig. 4) (28) and by the direct spinal delivery of NK-1 and glutamate receptor agonists (22, 29). Prostanoid receptors are present in the dorsal horn and on DRG (30). Activation of prostanoid receptors has been shown to increase the opening of voltage-sensitive Ca channels and to enhance primary afferent peptide release (31). Consistent with these events, intrathecal PG receptor agonists will evoke a hyperalgesia and allodynia (32). The spinal delivery of cyclooxygenase (COX) inhibitors or antagonists has no effect on acute nociceptive processing, but will reduce the phase 2 of the formalin test at doses that are inactive when given systemically (33, 34). Consistent with the observation that NMDA/NK-1 antagonists can block certain hyperalgesic states, spinal NMDA agonists can evoke a hyperalgesic state and this hyperalgesia also can be blocked by spinal COX inhibitors (23). Several COX isozymes have been identified (35). Current evidence indicates that COX-2 is constitutive in the spinal cord (but only in a few peripheral organs). The intrathecal delivery of selective COX inhibitors suggests that the hyperalgesia induced by intrathecal NK1/NMDA is mediated in part by the local release of PGs. In recent work, we have shown that spinal COX2 inhibitors are also effective in models of inflammatory hyperalgesia (ref. 36 and D. M. Dirig and T.L.Y., unpublished observations).
NO.
NO is synthesized by NO synthase (NOS). Evidence of in vivo NO release from cord secondary to repetitive afferent stimulation and by intrathecal NMDA has been presented (21). The hyperalgesia induced by intrathecal NMDA and the second phase of the formalin test has been shown to be reduced by intrathecal competitive NOS inhibitors (37).
Kinases and Phosphorylation.
Increasing intracellular Ca2+I, though the inosital triphosphate pathway by activation of NK receptor and/or by the influx of Ca2+ through voltage-gated Ca2+ channels or ionophores (NMDA receptor) (38), activates kinases that phosphorylate and phosphatases, which dephosphorylate local proteins. Phosphorylating enzyme systems consist of several classes of kinases that are distinguished by structure and the pharmacology of their inhibitors. In the spinal dorsal horn, cAMP-dependent kinase (39) and camkinase II (40) have been observed in the spinal dorsal horn and DRG. Protein kinase (PK) C consists of a large family of isozymes. In the spinal cord PKCα (41) and PKCγ (42) are limited to the spinal gray, with PKCγ being reported to be largely in cells in lamina I and II inner of the dorsal horn. Although it is possible that any or all of the phosphorylating enzymes noted above may play a role, the use of inhibitors for PKA and PKC have shown the particular importance of this family of kinases in regulating spinal facilitation. Many hyperalgesic states are mediated by a spinal NMDA receptor. The NMDA receptor is multiply phosphorylated by PKA and PKC (43). Intrathecal delivery of PKC inhibitors has been shown to stereospecifically diminish the hyperalgesic effects of intrathecal NMDA. In addition, the augmented activity in dorsal horn neurons after intradermal mustard oil (44, 45) or spinal NMDA (46) is reduced by the local spinal delivery of PKC inhibitors. PKA and PKC inhibitors, but not inactive isomers, will diminish capsaicin-evoked hyperpathia (45) and the second phase of the formalin test (47, 48).
System Interactions
As reviewed above, after local injury, the behaviorally defined components of post-tissue injury pain states reflect an increased receptive field and a left shift in the stimulus response curve for spinal dorsal horn neurons, which is evoked initially and then sustained in part by persistent small afferent input. The contribution of these changes in spinal function to the behaviorally relevant nociceptive state is substantiated by comparing the pharmacology associated with the effects on the behavior of the unanesthetized animal with the effects of the drugs on the underlying electrophysiology. Table 1 provides a summary of the effects of several classes of agonists and antagonists given intrathecally in rats on acute pain behavior (as measured by thermal escape) and facilitated processing (as defined by their effects on the second phase of the formalin test). Based on such observations, it is possible to formulate a heuristic picture of the organization of several pharmacologically defined spinal systems that mediate the response of the animal to a strong and injurious stimulus. Thus, repetitive afferent input increases excitatory amino acid and peptide release from primary afferents that serve to initially depolarize dorsal horn neurons. Persistent depolarization serves to increase intracellular calcium, activating a variety of intracellular enzymes (COX-2 and NOS) and various kinases (PKC). PGs and NO are released spinally and serve to acutely enhance the subsequent release of afferent peptides and glutamate. Activation of local kinases serves to phosphorylate membrane receptors and channels. As an example, the NMDA receptor when phosphorylated displays an enhanced calcium flux (see Fig. 3). The role of these system-level changes in spinal nociceptive processing in pain behavior is supported by the analgesic effects of spinally delivered agents known to reduce small afferent transmitter release (μ, ∂ opioid, and α2 adrenergic agonists) and the antihyperalgesic actions of spinally delivered NK-1 and NMDA receptor antagonists, as well as inhibitors of spinal COX-2, NOS, and PKC.
Table 1.
Spinal drug action in nociceptive processing in animals models and human pain states
| Agonists | Rat acute thermal escape | Rat phase 2 formalin | Human pain states | Reference |
|---|---|---|---|---|
| μ agonist | + | + | Morphine | (16) |
| ∂ agonist | + | + | DADL | (16, 49) |
| α2 agonist | + | + | Clonidine | (17, 50) |
| Aden A-1 agonist | +/− | + | R-PIA/adenosine | (51–53) |
| GABA-A/B agonist | +/− | + | Baclofen178 | (54, 55) |
| GABAPENTIN | 0 | + | (56) | |
| NMDA antagonist | 0 | + | Ketamine/CPP | (25, 57, 58) |
| AMPA antagonist | + | + | (24, 59) | |
| Metab Glu-antagonist | +/− | + | (60) | |
| NK-1 agonist | 0 | + | (20) | |
| COX inhibitor | 0 | + | Lysine acetylsalicylate | (23, 61) |
| EP-antagonist | 0 | + | (34) | |
| NOS inhibitor | 0 | + | (37) | |
| AChase inhibitor (mus) | + | + | Neostigmine | (62–66) |
| N-Ca Ch blocker | 0 | + | Ziconotide (SNX-111) | (67, 68) |
GABA, γ-aminobutyric acid; DADL, d-Ala2-d-Leu5-encephalin; R-PIA, R(−)N6-(2-phenylisopropyl) adenosine; CPP, (±)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid; EP, PG type E.
Used intrathecally for spasticity.
Several additional points should be considered in interpreting these data. The above comments are limited to several specific components of dorsal horn biology. Other systems that no doubt play an important role in spinal nociceptive processing, such as the purinergic receptors (69) and the metabotrophic glutamate receptors (70) are not considered. In each case, the effects of manipulations associated with a single system, e.g., NK-1, glutamate, or AMPA, are considered. In the case of the primary afferent, terminals are known to routinely contain and likely release combinations of amino acids (glutamate), peptides (CGRP, sP, vasoactive intestinal polypeptide; ref. 71) and peptides (such as growth factors, ref. 72). The net combination of these drug effects are poorly studied. In the case of sP and NMDA, both contribute to the postsynaptic action (73). This observed excitation is consistent with (i) the ability of either agent to activate the second-order neurons, (ii) a depolarization by sP serving to remove the Mg2+ block, and (iii) sP activating by local kinases to phosphorylate the NMDA channel. Agents that block the opening of Ca2+ channels in primary afferents likely block all transmitter release from that terminal, which accounts for the potent antinociception that is produced by these agents in contrast to that produced by antagonists for specific receptors (e.g., NK-1 or glutamate). The present comments focus primarily on the events that occur in the interval around the injury period. Over extended intervals of hours to days there is an up-regulation of receptors (NK-1) (74) and enzymes [COX (75) and NOS (76)], leading to additional changes in system function. The evidence presented here clearly reflects the functional complexity of the events that occur secondary to a focal injury, leading to a persistent small afferent barrage. The fact that such stimuli will lead to a local 1° hyperalgesia and 2° tactile allodynia raises the likelihood that specific components of the post-tissue injury pain state may have distinct components. Thus, as noted, after a mild, local tissue injury a 1° hyperalgesia and a 2° tactile allodynia are noted. Treatment with spinal opiates during the injury phase will prevent the appearance of the allodynia, but not the hyperalgesia. This finding suggests that the allodynia after an acute injury depends on a cascade that is initiated, but not sustained, by the injury stimulus. In contrast, the hyperalgesia does not appear to depend on that cascade to be made manifest. Such differences may reflect on the clinical phenomena of preemptive analgesia (77). In pre-emptive analgesia the patient receiving opiates during the surgery is hypothesized to require less analgesic postoperatively. To the extent that the postoperative pain state reflects the allodynic component noted here, that would indeed be true. To the extent that the postoperative state involves a hyperalgesic mechanisms, the differences produced by intraoperative opiates might be slight. Finally, the early discussions on the events that occur during the periods immediately after injury focused on the phenomena as if it were a unitary phenomena. The initial observations, for example, that demonstrated that dorsal horn “wind-up” and several inflammatory models all were diminished by spinal NMDA receptor antagonists supported such homogeneity. It is now clear that variations in mechanism can be defined even with acute injury stimulus conditions. Thus, on examining the allodynia observed after the healing of a skin incision (59) or the hyperalgesia induced by a local burn (N.N.-T. and T.L.Y., unpublished observations), the hyperpathia was noted to be poorly diminished by NMDA receptor antagonists.
Human Spinal Processing
Although the above work is of importance in defining the biology of spinal processing in the mechanistic sense, such preclinical insights also appear to be relevant to our understanding of spinal system function in humans. Two points can be made: (i) comparability of the behavioral components and (ii) parallels in pharmacological activity.
Comparability of Behavioral Pain Components in Humans and Animal Models.
As in the preclinical models, after focal tissue injury (whether experimental or pathological) in humans, there is a clearly defined 1° hyperalgesia and 2° off-site tactile allodynia (78) Though as yet poorly studied, it is clear that the postoperative or postinjury pain state in humans possess the same complexity (see refs. 79 and 80). Still, the typical postoperative pain evaluation typically is limited to a univariate assessment (e.g., visual analogue score or postoperative narcotic consumption). Although clinically practical, such limited surveys may well obscure the benefits or actions of a drug that influences one of the components of the pain state.
Comparability of Spinal Pharmacology in Humans and Animal Models.
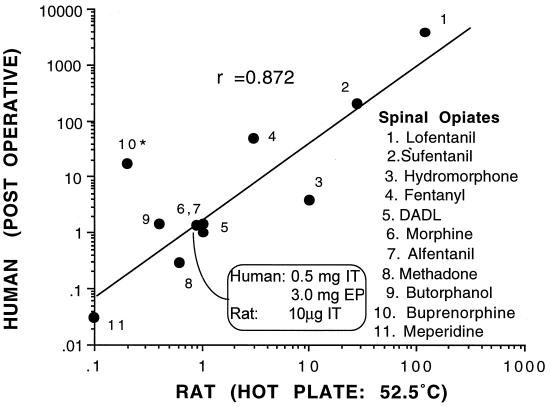
The pharmacology and activity of drug effects at the spinal level as defined in rodent systems have been shown to be extraordinarily predictive of the activity in human pain states. The best evaluated pharmacology is that of the opiates that have been widely examined in both humans and animals. As presented in Fig. 5, plotting the spinal potency of such agents relative to morphine in rats (intrathecal) and humans (epidural or intrathecal) reveals a high correlation. More importantly, a variety of nonopioid agents have been delivered intrathecally or epidurally in animal models and then in humans. Table 1 summarizes such work in which humans have received the respective novel class of agent. Importantly, it should be noted that agents, which, unlike opiates, have little effect on acute pain behavior (e.g., thermal escape) are indeed active in human clinical pain states.
Figure 5.
Graph plots the relative intrathecal potency of several opioids as defined in rats on the hot plate test versus the relative potency when given epidurally (EP) or intrathecally (IT) in human postoperative or cancer pain. The axis plot the potency of the agent (in μg for rat or mg in human) relative to the potency of morphine given in that model. These calculations are based on a standard analgesic dose for intrathecal morphine in rats on the hot plate (10 μg) and after intrathecal (0.5 mg) or epidural (3 mg) delivery for pain in humans. Human data are based on reported doses necessary to produce an “adequate” clinical analgesia (data derived from ref. 16). DADL, d-Ala2-d-Leu5-encephalin.
These results jointly (i) support the importance of spinal processing in human pain states, (ii) emphasize the functional and pharmacological comparability of these systems across species, and (iii) provide an important source of targets for the development of novel clinically relevant analgesics processing can be expected to yield even greater rewards.
ABBREVIATIONS
- WDR
wide dynamic range
- NMDA
N-methyl-d-aspartate
- COX
cyclooxygenase
- NK
neurokinin
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- PK
protein kinase
- NOS
NO synthase
- PG
prostaglandin
- sP
substance P
- CGRP
calcitonin gene-related peptide
- DRG
dorsal root ganglia
References
- 1.Yaksh T L, Lynch III C, Zapol W M, Maze M, Biebuyck JF, Saidman L J, editors. Anesthesia: Biologic Foundations. Philadelphia: Lippincott; 1997. pp. 685–718. [Google Scholar]
- 2.Dirig D M, Yaksh T L. Pain. 1995;62:321–328. doi: 10.1016/0304-3959(95)00006-E. [DOI] [PubMed] [Google Scholar]
- 3.Handwerker H O. Agents Actions. 1991;32:91–99. doi: 10.1007/978-3-0348-7405-2_12. [DOI] [PubMed] [Google Scholar]
- 4.Messlinger K. Anaesthesist. 1997;46:142–153. doi: 10.1007/s001010050384. [DOI] [PubMed] [Google Scholar]
- 5.Dickenson A H, Stanfa L C, Chapman V, Yaksh T L. In: Anesthesia: Biologic Foundations. Yaksh T L, Lynch III C, Zapol W M, Maze M, Biebuyck J F, Saidman L J, editors. Philadelphia: Lippincott; 1997. pp. 611–624. [Google Scholar]
- 6.Puig S, Sorkin L S. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 7.Dickenson A H, Sullivan A F. Pain. 1987;30:349–360. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 8.Nozaki-Taguchi N, Yaksh T L. Neurosci Lett. 1998;254:25–28. doi: 10.1016/s0304-3940(98)00648-x. [DOI] [PubMed] [Google Scholar]
- 9.Simone D A, Sorkin L S, Oh U, Chung J M, Owens C, LaMotte R H, Willis W D. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- 10.Dirig D M, Salami A, Rathbun M L, Ozaki G T, Yaksh T L. J Neurosci Methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- 11.Yashpal K, Katz J, Coderre T J. Anesthesiology. 1996;84:1119–1128. doi: 10.1097/00000542-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Buerkle H, Marsala M, Yaksh T L. Br J Anaesth. 1998;80:348–353. doi: 10.1093/bja/80.3.348. [DOI] [PubMed] [Google Scholar]
- 13.Taylor B K, Peterson M A, Basbaum A I. J Neurosci. 1995;15:7575–7584. doi: 10.1523/JNEUROSCI.15-11-07575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox G L, Seybold V. In: Anesthesia: Biologic Foundations. Yaksh T L, Lynch III C, Zapol W M, Maze M, Biebuyck J F, Saidman L J, editors. Philadelphia: Lippincott; 1997. pp. 557–576. [Google Scholar]
- 15.Yaksh T L, Rudy T A. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 16.Yaksh T L. In: Handbook of Experimental Pharmacology. Herz A, editor. 104/II. Berlin: Springer; 1993. pp. 53–90. [Google Scholar]
- 17.Yaksh T L, Jage J, Takano Y. Baillière’s Clin Anaesthesiol. 1993;7:597–614. [Google Scholar]
- 18.Hua X-Y, Boublik J H, Spicer M A, Rivier J E, Brown M R, Yaksh T L. J Pharmacol Exp Ther. 1991;258:243–258. [PubMed] [Google Scholar]
- 19.Yamamoto T, Yaksh T L. Life Sci. 1991;49:1955–1963. doi: 10.1016/0024-3205(91)90637-q. [DOI] [PubMed] [Google Scholar]
- 20.Hua X-Y, Chen P, Polgar E, Nagy I, Marsala M, Phillips E, Wollaston L, Urban L, Yaksh T L, Webb M. J Neurochem. 1998;70:688–698. doi: 10.1046/j.1471-4159.1998.70020688.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang L C, Marsala M, Yaksh T L. Pain. 1996;67:345–354. doi: 10.1016/0304-3959(96)03106-5. [DOI] [PubMed] [Google Scholar]
- 22.Hua X-Y, Chen P, Marsala M, Yaksh T L. Neuroscience. 1999;89:525–534. doi: 10.1016/s0306-4522(98)00488-6. [DOI] [PubMed] [Google Scholar]
- 23.Malmberg A B, Yaksh T L. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama T, Yaksh T L, Weber E. Anesthesiology. 1998;89:715–722. doi: 10.1097/00000542-199809000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Chaplan S R, Malmberg A B, Yaksh T L. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- 26.Yamamoto T, Yaksh T L. Anesthesiology. 1992;77:757–763. doi: 10.1097/00000542-199210000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Marsala M, Malmberg A B, Yaksh T L. J Neurosci Methods. 1995;62:43–53. doi: 10.1016/0165-0270(95)00053-4. [DOI] [PubMed] [Google Scholar]
- 28.Malmberg A B, Yaksh T L. J Neurosci. 1995;15:768–776. doi: 10.1523/JNEUROSCI.15-04-02768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L C, Marsala M, Yaksh T L. Neuroscience. 1996;75:453–461. doi: 10.1016/0306-4522(96)00294-1. [DOI] [PubMed] [Google Scholar]
- 30.Coleman R A, Smith W L, Narumiya S. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 31.Hingtgen C M, Vasko M R. Brain Res. 1994;655:51–60. doi: 10.1016/0006-8993(94)91596-2. [DOI] [PubMed] [Google Scholar]
- 32.Minami T, Uda R, Horiguchi S, Ito S, Hyodo M, Hayaishi O. Pain. 1994;57:217–223. doi: 10.1016/0304-3959(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 33.Malmberg A B, Yaksh T L. J Pharmacol Exp Ther. 1992;263:264–275. [PubMed] [Google Scholar]
- 34.Malmberg A B, Rafferty M F, Yaksh T L. Neurosci Lett. 1994;173:193–196. doi: 10.1016/0304-3940(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 35.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Proc Natl Acad Sci USA. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T, Nozaki-Taguchi N. NeuroReport. 1997;8:2179–2182. doi: 10.1097/00001756-199707070-00018. [DOI] [PubMed] [Google Scholar]
- 37.Malmberg A B, Yaksh T L. Pain. 1993;54:291–300. doi: 10.1016/0304-3959(93)90028-N. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh A, Greenberg M E. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 39.Malmberg A B, Brandon E P, Idzerda R L, Liu H, McKnight G S, Basbaum A I. J Neurosci. 1997;17:7462–7470. doi: 10.1523/JNEUROSCI.17-19-07462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kocsis J D, Rand M N, Lankford K L, Waxman S G. J Neurobiol. 1994;25:252–264. doi: 10.1002/neu.480250306. [DOI] [PubMed] [Google Scholar]
- 41.Roberts R E, McLean W G. Brain Res. 1997;754:147–156. doi: 10.1016/s0006-8993(97)00062-0. [DOI] [PubMed] [Google Scholar]
- 42.Malmberg A B, Chen C, Tonegawa S, Basbaum A I. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 43.Leonard A S, Hell J W. J Biol Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- 44.Munro F E, Fleetwood-Walker S M, Mitchell R. Neurosci Lett. 1994;170:199–202. doi: 10.1016/0304-3940(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 45.Sluka K A, Willis W D. Pain. 1997;71:165–178. doi: 10.1016/s0304-3959(97)03371-x. [DOI] [PubMed] [Google Scholar]
- 46.Cerne R, Rusin K I, Randic M. Neurosci Lett. 1993;161:124–128. doi: 10.1016/0304-3940(93)90275-p. [DOI] [PubMed] [Google Scholar]
- 47.Coderre T J, Yashpal K. Eur J Neurosci. 1994;6:1328–1334. doi: 10.1111/j.1460-9568.1994.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 48.Yashpal K, Pitcher G M, Parent A, Quirion R, Coderre T J. J Neurosci. 1995;15:3263–3272. doi: 10.1523/JNEUROSCI.15-05-03263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onofrio B M, Yaksh T L. Lancet. 1983;1:1386–1387. doi: 10.1016/s0140-6736(83)92170-0. [DOI] [PubMed] [Google Scholar]
- 50.Eisenach J C, De Kock M, Klimscha W. Anesthesiology. 1996;85:655–674. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 51.Poon A, Sawynok J. Pain. 1998;74:235–245. doi: 10.1016/s0304-3959(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 52.Karlsten R, Gordh T., Jr Anesth Analg. 1995;80:844–847. doi: 10.1097/00000539-199504000-00037. [DOI] [PubMed] [Google Scholar]
- 53.Rane K, Segerdahl M, Goiny M, Sollevi A. Anesthesiology. 1998;5:1108–1115. doi: 10.1097/00000542-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Dirig D M, Yaksh T L. Pharmacol Exp Ther. 1995;275:219–227. [PubMed] [Google Scholar]
- 55.Porter B. Br J Nursing. 1997;6:253–260. doi: 10.12968/bjon.1997.6.5.253. [DOI] [PubMed] [Google Scholar]
- 56.Partridge B J, Chaplan S R, Sakamoto E, Yaksh T L. Anesthesiology. 1998;88:196–205. doi: 10.1097/00000542-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 57.Abdel-Ghaffar M E, Abdulatif M A, al-Ghamdi A, Mowafi H, Anwar A. Can J Anaes. 1998;45:103–109. doi: 10.1007/BF03013246. [DOI] [PubMed] [Google Scholar]
- 58.Kristensen J D, Svensson B, Gordh T., Jr Pain. 1992;51:249–253. doi: 10.1016/0304-3959(92)90266-E. [DOI] [PubMed] [Google Scholar]
- 59.Brennan T J. Anesthesiology. 1998;89:1049–1051. doi: 10.1097/00000542-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Fisher K, Coderre T J. Pain. 1996;68:255–263. doi: 10.1016/s0304-3959(96)03212-5. [DOI] [PubMed] [Google Scholar]
- 61.Devoghel J C. J Int Med Res. 1983;11:90–91. doi: 10.1177/030006058301100205. [DOI] [PubMed] [Google Scholar]
- 62.Prado W A, Goncalves A S. Braz J Med Biol Res. 1997;30:1225–1231. doi: 10.1590/s0100-879x1997001000014. [DOI] [PubMed] [Google Scholar]
- 63.Naguib M, Yaksh T L. Anesthesiology. 1994;80:1338–1348. doi: 10.1097/00000542-199406000-00022. [DOI] [PubMed] [Google Scholar]
- 64.Naguib M, Yaksh T L. Anesth Analg. 1997;85:847–853. doi: 10.1097/00000539-199710000-00025. [DOI] [PubMed] [Google Scholar]
- 65.Hood D D, Eisenach J C, Tuttle R. Anesthesiology. 1995;82:331–343. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Lauretti G R, Lima I C. Anest Analg. 1996;82:617–620. doi: 10.1097/00000539-199603000-00033. [DOI] [PubMed] [Google Scholar]
- 67.Malmberg A B, Yaksh T L. J Neurosci. 1994;14:4882–4890. doi: 10.1523/JNEUROSCI.14-08-04882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brose W G, Gutlove D P, Luther R R, Bowersox S S, McGuire D. Clin J Pain. 1997;13:256–259. doi: 10.1097/00002508-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 69.Driessen B, Reimann W, Selve N, Friderichs E, Bultmann R. Brain Res. 1994;666:182–188. doi: 10.1016/0006-8993(94)90770-6. [DOI] [PubMed] [Google Scholar]
- 70.Young M R, Blackburn-Munro G, Dickinson T, Johnson M J, Anderson H, Nakalembe I, Fleetwood-Walker S M. J Neurosci. 1998;18:10180–10188. doi: 10.1523/JNEUROSCI.18-23-10180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levine J D, Fields H L, Basbaum A I. J Neurosci. 1993;13:2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michael G J, Averill S, Nitkunan A, Rattray M, Bennett D L, Yan Q, Priestley J V. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chapman V, Buritova J, Honore P, Besson J M. J Neurophysiol. 1996;76:1817–1827. doi: 10.1152/jn.1996.76.3.1817. [DOI] [PubMed] [Google Scholar]
- 74.Kar S, Rees R G, Quirion R. Eur J Neurosci. 1994;6:345–354. doi: 10.1111/j.1460-9568.1994.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 75.Beiche F, Scheuerer S, Brune K, Geisslinger G, Goppelt-Struebe M. FEBS Lett. 1996;390:165–169. doi: 10.1016/0014-5793(96)00604-7. [DOI] [PubMed] [Google Scholar]
- 76.Wu J, Lin Q, Lu Y, Willis W D, Westlund K N. Exp Brain Res. 1998;118:457–465. doi: 10.1007/s002210050302. [DOI] [PubMed] [Google Scholar]
- 77.McQuay H J. Ann Med. 1995;27:249–256. doi: 10.3109/07853899509031967. [DOI] [PubMed] [Google Scholar]
- 78.Handwerker H O, Kobal G. Physiol Rev. 1993;73:639–671. doi: 10.1152/physrev.1993.73.3.639. [DOI] [PubMed] [Google Scholar]
- 79.O’Connor T C, Abram S E. In: Anesthesia: Biologic Foundations. Yaksh T L, Lynch III C, Zapol W M, Maze M, Biebuyck J F, Saidman L J, editors. Philadelphia: Lippincott; 1997. pp. 747–758. [Google Scholar]
- 80.Silbert B S, Osgoodm P F, Carr D B. In: Anesthesia: Biologic Foundations. Yaksh T L, Lynch III C, Zapol W M, Maze M, Biebuyck J F, Saidman L J, editors. Philadelphia: Lippincott; 1997. pp. 759–773. [Google Scholar]