Abstract
The medicinal chemistry and pharmacology of the four subtypes of adenosine receptors (ARs) and the eight subtypes of P2Y receptors (P2YRs, activated by a range of purine and pyrimidine mono- and dinucleotides) has recently advanced significantly leading to selective ligands. X-ray crystallographic structures of both agonist- and antagonist-bound forms of the A2AAR have provided unprecedented three-dimensional detail concerning molecular recognition in the binding site and the conformational changes in receptor activation. It is apparent that this ubiquitous cell signaling system has implications for understanding and treating many diseases. ATP and other nucleotides are readily released from intracellular sources under conditions of injury and organ stress, such as hypoxia, ischemia, or mechanical stress, and through channels and vesicular release. Adenosine may be generated extracellularly or by cellular release. Therefore, depending on pathophysiological factors, in a given tissue, there is often a tonic activation of one or more of the ARs or P2YRs that can be modulated by exogenous agents for a beneficial effect. Thus, this field has provided fertile ground for pharmaceutical development, leading to clinical trials of selective receptor ligands as imaging agents or for conditions including cardiac arrhythmias, ischemia/reperfusion injury, diabetes, pain, thrombosis, Parkinson’s disease, rheumatoid arthritis, psoriasis, dry eye disease, pulmonary diseases such as cystic fibrosis, glaucoma, cancer, chronic hepatitis C, and other diseases.
Keywords: GPCR structure; Adenosine receptors; P2Y receptors; Agonists; Antagonists; Clinical trials, nucleosides; Nucleotides
Introduction
There are four subtypes of adenosine receptors (ARs, or alternately P1 receptors), i.e., A1, A2A, A2B, and A3, and eight subtypes of P2Y receptors (P2YRs), i.e., a family of Gq-coupled P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11Rs and a second family of Gi-coupled P2Y12, P2Y13, and P2Y14Rs (Table 1) [1, 2]. The native agonists for these twelve receptors are clearly divided between purine nucleosides (ARs) and purine and pyrimidine nucleotides (P2YRs), although a high concentration of AMP (1 mM) activates the A1AR, independent of P2YR activity [3]. The two A2 subtypes are coupled to Gs protein to stimulate adenylate cyclase, and the other two AR subtypes inhibit adenylate cyclase through Gi protein. In some cells, the A2BAR is dually coupled to Gs and Gq and consequently elevates phosphoinositides, mobilizes calcium and activates phospholipase C and MAPK [111–115]. This signaling pathway appears to be important in mast cells, in which A2A and A2BARs have opposing actions. ARs are the site of action of widely consumed alkylxanthines, which act as competitive antagonists. Knockout mice and selective ligands as pharmacological tools (Figs. 1, 2, and 3) are now available for all AR subtypes and for many of the P2Y subtypes (except for P2Y11 that is absent in the mouse) [4]. Many ligands for these receptors are under consideration for pharmaceutical development.
Table 1.
Properties of ARs and P2Y receptors and key ligands (including radioligands) suitable for cell biological studies (Ado = adenosine)
| Group | Subtype, gene symbol | Chromosome, human, (length, amino acids) | Native agonist (human, pEC50) | Selective agonist (pEC50) | Selective antagonist (pIC50) | G protein |
|---|---|---|---|---|---|---|
| ARs | A1, Adora1 | 1q32.1 (326) | Ado 1 (6.51) | S-ENBA 13 (9.47), CCPA 8 (9.08),dR-PIA 6 (8.82),d CPA 7 (8.65), CHA 11 (8.64),d GR79236 10 (8.51), SPA 5 (6.2) | PSB-36 38 (9.8), KW3902 37 (9.1), SLV320 39 (9.0), DPCPX 36 (8.5)d | Gi, Go |
| A2A, Adora2a | 22q11.23 (412) | Ado 1 (6.14) | ATL-146e 18 (9.30), UK-432097 19 (8.40), CGS21680 16 (7.64)d | ZM241385 44 (9.0),d SCH442416 47 (8.39), SCH58261 46 (8.3) | Gs, Golf | |
| A2B, Adora2b | 17p12 (332) | Ado 1 (4.59) | Bay60-6583 21 (8.0) | PSB-603 57 (9.3),d MRS1754 52 (8.9),d MRE2029-F20 54 (8.8),d PSB-0788 58 (8.6),d MRS1706 53 (8.4), PSB-1115 56 (7.7), CVT-6883 55 (7.7), PSB-298 (7.2)d | Gs, Gq | |
| A3, Adora3 | 1p13.2 (isoform 1: 347) | Ado 1 (6.53), inosine (6.60) | IB-MECA 23 (8.85), Cl-IB-MECA 24 (8.74), I-AB-MECA (8.50),d CP532,903 25 (8.24) | MRE3008-F20 62 (9.1),d MRS1220 64 (8.8), MRS1334 66 (8.6), VUF5574 67 (8.4), PSB-11 61 (8),d MRS1523 63 (7.7), MRS1191 65 (7.5), MRS3777 69 (7.3) | Gi | |
| P2Y1-like | P2Y1, P2RY1 | 3q24-25 (373) | ADP 70 (5.09) | MRS2365 76 (9.40) | MRS2500 95 (9.02),d MRS2279 94 (8.10),d MRS2179 93 (6.48),d | Gq |
| P2Y2, P2RY2 | 11q13.5 (377) | UTP 79 (8.10), ATP 71 (7.07) | MRS2698 (8.10), MRS2768 90 (5.72) | PSB-716 (5.01) | Gq, Gi | |
| P2Y4, P2RY4 | Xq13 (365) | UTP 79 (5.60)a | MRS4062 83 (7.64) | b | Gq, Gi | |
| P2Y6, P2RY6 | 11q13.5 (328) | UDP 78 (6.52) | 5-iodo-UDP 85 (7.83), PSB-0474 84 (7.15) | MRS2578 109 (7.43) [noncompetitive] | Gq | |
| P2Y11, P2RY11 | 19p31 (374) | ATP 71 (4.77) | NF157 103 (7.35), NF546 104 (6.27)c | NF340 105 (7.14) | Gq, Gs | |
| P2Y12-like | P2Y12, P2RY12 | 3q21-25 (342) | ADP 70 (7.22)d | b | PSB-0739 101 (9.8), AR-C69931MX 98 (9.40), AZ11931285,d PSB-0413 (8.3),d AZD6140 99 (7.90) | Gi |
| P2Y13, P2RY13 | 3q24-25 (354) | ADP 70 (7.94) | b | MRS2211 108 (5.97) | Gi | |
| P2Y14, P2RY14 | 3q24-25 (338) | UDP 78 (6.80),dUDP-glucose 88 (6.45) | MRS2690 89 (7.31), MRS2802 (7.20) | Compound 116 (8.7) | Gi |
aATP acts as an antagonist at the human P2Y4 receptor and as agonist at the rat and mouse P2Y4 receptors
bSelective ligands not yet available
cNF546 activates the P2Y11R, although it belongs to a structural class of antagonists
dUsed as a radioligand, either in [3H], [32P], [33P] or [125I] form, as appropriate. The selectivity of [125I]I-AB-MECA (4-amino analogue of 23) for the A3AR is low, and therefore binding to A1AR is also seen. The listed A3AR antagonist radioligands are suitable for use in primate but not rodent species. Other (nonselective) radioligands are: agonist [3H]NECA 3 for A2AAR, A2BAR or A3AR; agonist [125I]I-APNEA (3-iodo analogue of 4) for the A3AR; agonist [3H] or [33P]2-MeSADP 72 for P2Y1R or P2Y12R. [33P]ADP 70 is also used for binding to the P2Y12R. [3H]UDP 78 (Kd 10 nM) has been used for binding to the P2Y14R. Chemical names: [3H]PSB-0413, 2-propylthioadenosine-5′-adenylic acid (1,1-dichloro-1-phosphonomethyl-1-phosphonyl) anhydride; [3H]PSB-298, [(8-{4-[2-(2-hydroxyethylamino)-2-oxoethoxy]phenyl}-1-propylxanthine]; [125I]AZ11931285 (used at 125 pM), (1S,2R,3S,4R)-2,3-dihydroxy-4-[7-[[(2E)-3-iodoprop-2-en-1-yl]amino]-5-(propylthio)3H-[1–3]triazolo[4,5-d]pyrimidin-3-yl]cyclopentane-carboxylic acid
Fig. 1.
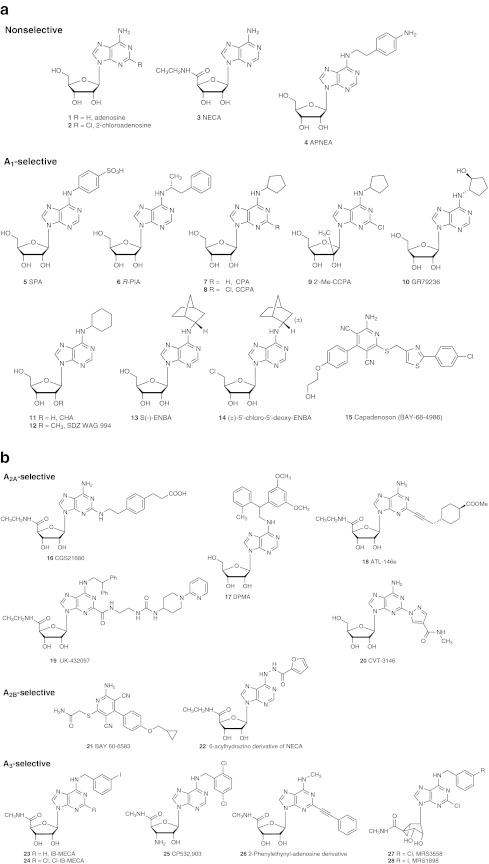
a Nonselective AR and A1AR selective agonists (including nucleosides and a nonnucleoside derivative 15). b A2AAR, A2BAR, and A3AR selective agonists (including nucleosides and a nonnucleoside derivative 21). AR affinites (Table 1) and selectivities of many of these ligands are available [1, 55]
Fig. 2.
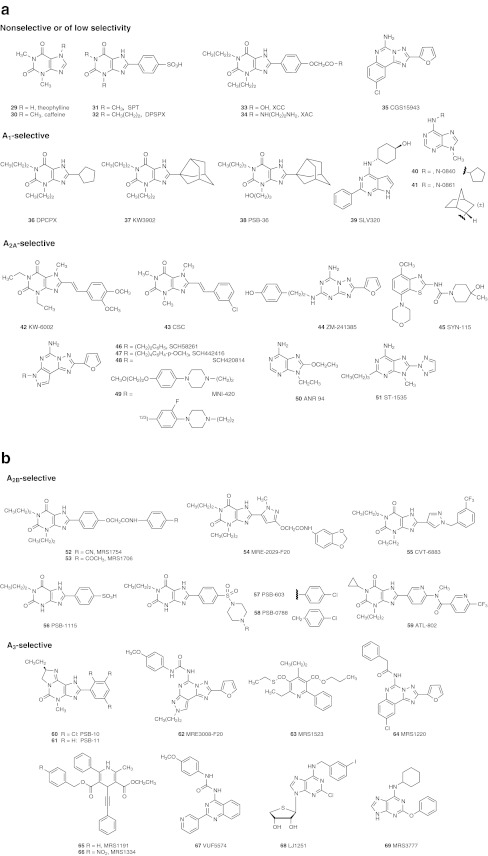
a. Nonselective AR antagonists and A1AR and A2AAR selective antagonists (including xanthines and nonxanthine derivatives 44–51). b A2BAR (xanthines 52–59) and A3AR selective (nonxanthine derivatives 60–69) antagonists. Compound 68 is a truncated nucleoside derivative that displays A3AR antagonist properties. AR affinites (Table 1) and selectivities of many of these ligands are available [1, 42, 55]
Fig. 3.
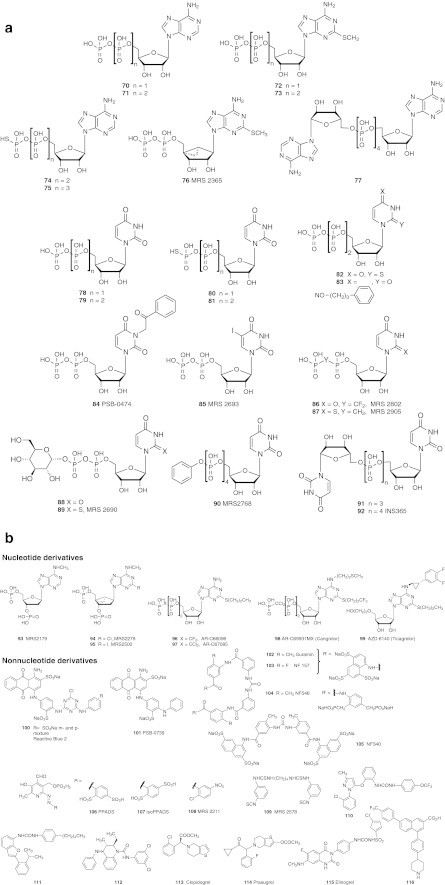
a Nonselective and selective P2YR agonists and related substances (including nucleotide derivatives). b Nonselective and selective P2YR antagonists (including nucleotide and nucleotide derivatives). Note that NF546 104 is a P2Y11R agonist, although it belongs to a structural class of antagonists. Compound 110 is a P2Y1R antagonist containing a novel chemotype, and 111–115 are P2Y12R antagonists. Compound 116 is a P2Y14R antagonist containing a novel chemotype. P2Y potencies of many of these ligands are available in Table 1 or reference [2]
Processing of ARs and P2YRs in the cell has been studied, including posttranslational modification and trafficking, intracellular localization, and the related phenomena of agonist-induced desensitization, internalization, and degradation [5–7]. The mechanisms of release, uptake, and degradation of extracellular nucleosides and nucleotides have also been explored [8, 9]. The ubiquitous presence of pharmacologically active endogenous ligands of ARs and P2YRs warrants careful consideration in experimental design.
Structure of adenosine and P2Y receptors
The ARs and the P2YRs share the overall topological structure typical of G protein-coupled receptors (GPCRs) belonging to family A: seven α-helical domains (TM) that cross the cell membrane and are connected by three extracellular (ELs) and three intracellular (IL) loops, the N terminus in the extracellular part and the C terminus in the intracellular part of the receptor. Nevertheless, from comprehensive sequence comparisons and phylogenetic analyses, it is clear that ARs and P2YRs belong to two different groups of the rhodopsin-like family of GPCRs [10], respectively, the rhodopsin α-group and γ-group of GPCRs [11].
Within the AR family, the average sequence identity between subtypes of the same species is about 47%, which increases to an average of ∼57% if only the TM domains are considered. The residues in the binding cavity involved in ligand recognition are mostly conserved among the AR subtypes and between species, with the A3AR being the most divergent from the other subtypes, as shown in Table 2. Specific variable amino acids in the binding site are most likely involved in the ligand selectivity or the unique pharmacological behavior of each AR subtype.
Table 2.
List of the key residues in the binding pocket of the A2AAR compared with the corresponding residues in other AR subtypes and in ARs of different species (h, human; m, mouse; r, rat)
| Rec/Res | TM2 2.61 | TM3 3.32 | TM3 3.33 | TM3 3.36 | TM3 3.37 | EL2 | EL2 | TM5 5.38 | TM5 5.42 | TM6 6.48 | TM6 6.51 | TM6 6.52 | TM6 6.55 | TM7 7.35 | TM7 7.39 | TM7 7.42 | TM7 7.43 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h_A1 | A66 | V87 | L88 | T91 | Q92 | F171 | E172 | M180 | N184 | W247 | L250 | H251 | N254 | T270 | I274 | T277 | H278 |
| m_A1 | A66 | V87 | L88 | T91 | Q92 | F171 | E172 | M180 | N184 | W247 | L250 | H251 | N254 | I270 | I274 | T277 | H278 |
| r_A1 | A66 | V87 | L88 | T91 | Q92 | F171 | E172 | M180 | N184 | W247 | L250 | H251 | N254 | I270 | I274 | T277 | H278 |
| h_A2A | A63 | V84 | L85 | T88 | Q89 | F168 | E169 | M177 | N181 | W246 | L249 | H250 | N253 | M270 | I274 | S277 | H278 |
| m_A2A | A60 | V81 | L82 | T85 | Q86 | F163 | E164 | M172 | N176 | W241 | L244 | H245 | N248 | M265 | I269 | S272 | H273 |
| r_A2A | A60 | V81 | L82 | T85 | Q86 | F163 | E164 | M172 | N176 | W241 | L244 | H245 | N248 | M265 | I269 | S272 | H273 |
| h_A2B | A64 | V85 | L86 | T89 | Q90 | F173 | E174 | M182 | N186 | W247 | V250 | H251 | N254 | M272 | I276 | S279 | H280 |
| m_A2B | A64 | V85 | L86 | T89 | Q90 | F173 | E174 | M182 | N186 | W247 | V250 | H251 | N254 | M272 | I276 | S279 | H280 |
| r_A2B | A64 | V85 | L86 | T89 | Q90 | F173 | E174 | M182 | N186 | W247 | V250 | H251 | N254 | M272 | I276 | S279 | H280 |
| h_A3 | A69 | L90 | L91 | T94 | H95 | F168 | V169 | M177 | S181 | W243 | L246 | S247 | N250 | L264 | I268 | S271 | H272 |
| m_A3 | A70 | L91 | L92 | T95 | H96 | F169 | R170 | M178 | S182 | W244 | L247 | S248 | N251 | M265 | I269 | S272 | H273 |
| r_A3 | A71 | L92 | L93 | T96 | H97 | F170 | R171 | M179 | S183 | W245 | L248 | S249 | N252 | M266 | I270 | S273 | H274 |
For the ARs, structural information has been available since 2008 with the high resolution X-ray structure of the human A2AAR in complex with the antagonist 4-(2-[7-amino-2-(2-furyl)]1,2,4] triazolo[2,3-a][1,3,5]triazin-5-yl-amino]ethyl)phenol 44 (ZM241385) [12]. The crystal structure of the A2AAR in its inactive conformation gave insight into the ligand recognition mechanism, showing the key residues involved in the ligand binding and the major interactions anchoring the antagonist to the binding site. Many of the site-directed mutagenesis data previously available for the ARs were structurally explained, and new mutational experiments were guided by the knowledge gained from the A2AAR structure, helping to further define the ligand binding cavity of this AR [13].
The antagonist-bound A2AAR structure has since improved widely the modeling approaches to the ARs, suggesting for example the possible binding modes of agonists to the A2AAR [13, 14] or aiding the modeling of the other AR subtypes [15]. Before the release of the A2AAR X-ray structure, other structural templates were used for the modeling of the ARs, as detailed in a recent review by Dal Ben et al. [16]. More recently, new crystal structures of a thermostabilized (by mutagenesis) A2AAR in complex with the xanthine derivative 8-[4-[[[[(2-aminoethyl)amino]carbonyl]methyl]oxy]phenyl]-l,3-dipropylxanthine (XAC, structure not shown) and caffeine 30 were made available [17]. New structural insights into the activation mechanism and the conformational changes that occur upon agonist binding to the A2AAR were revealed with the recent release of new crystal structures of the A2AAR in complex with different agonists, the bulky substituted agonist 2-(3-[1-(pyridin-2-yl)piperidin-4-yl]ureido)ethyl-6-N-(2,2-diphenylethyl)-5′-N-ethylcarboxamidoadenosine-2-carboxamide 19 (UK432097) [18], the native agonist adenosine 1, and the non-selective adenosine-5′-N-ethyluronamide 3 (NECA) [19]. These new crystal structures lack a coupled G protein, but nevertheless, they are helpful in understanding the function of the ARs, and they will aid the drug design approaches for the AR family, thereby also improving the quality of models for other AR subtypes. The conformational changes upon activation of the A2AAR resemble those of opsin, but there are other changes more specific to this receptor structure such as a see-saw movement of TM7 [18]. Homology models of A1AR and A3AR based on the agonist-bound structure of the A2AAR, were recently proposed with docked agonists in the binding site [15].
The crystal structures of the A2AAR revealed a peculiar orientation of the ligand in the binding site of the receptor when compared to the available structures of other GPCRs, e.g., bovine rhodopsin or the β-adrenergic receptors. The binding site for the ligands in the ARs is located near the extracellular portion of the domains TM3, TM5, TM6, and TM7, and the ligands are in a roughly vertical orientation with respect to the plane of the membrane. A crucial residue anchoring the aromatic core of agonists and antagonists in the binding pocket of A2AAR is Asn6.55 (using the numbering convention of Ballesteros and Weinstein [20]), a residue conserved among the AR subtypes and also among different species. His6.52, Thr3.36, Ser7.42, and His7.43 play key roles in the binding of the hydrophilic ribose moiety of nucleoside agonists, while they are less critical in the binding of antagonists. Residues from the extracellular domain EL2 are also involved in anchoring ligands in the binding pocket. Phe168 in A2AAR, a residue conserved among the ARs, interacts through a strong π–π stacking with the aromatic core of agonists and antagonists. Glu169, conserved in A1AR and A2BAR but substituted with a hydrophobic valine in human A3AR, interacts with both agonist and antagonist H-bond donor groups, i.e., the exocyclic amino group of ZM241385, the exocyclic amino groups of NECA and adenosine, or the urea moiety at the C2 position of UK432097. Trp6.48, the so-called “toggle switch” of GPCR activation, is conserved among the ARs and was found in close proximity to the ligands in the A2AAR complexes. While the residues surrounding the ligand core in the A2AAR structure are mostly conserved in the binding pocket of the AR subtypes, other residues are less conserved, and possibly, they might be involved in the selectivity of the receptors for different substituted ligands. Those less conserved residues are located mainly in the most extracellular part of the binding cavity embedding the substitutuent groups projecting from the core of the ligands. For example, Leu267(7.32) in A2AAR is substituted with a serine in A1AR, with a lysine in A2BAR, and a glutamine in A3AR. Met270(7.35) in A2AAR is a threonine in A1AR and a leucine in A3AR. Also in EL3, there are some nonconserved residues, such as His264 of A2AAR, which is substituted with an asparagine in A2BAR and a glutamate in A3AR (Table 2).
Sequence alignments, phylogenetic analysis, and effector coupling of the P2YRs have distinguished two P2YR subfamilies [2, 21]. The P2Y1-like family activates the phospholipase C signaling pathway through coupling with Gq protein. P2Y11R also couples with Gs protein to activate adenylate cyclase. The other family of P2YRs is the P2Y12-like family, which couples to Gi protein to inhibit the adenylate cyclase pathway [23, 24]. The sequence identity between the two subfamilies is quite low, with only 20% identity between P2Y1R and P2Y12R, while the sequence identity is higher between the members within the same subfamily, for example, with a 45% identity between P2Y12R and P2Y14R.
Unlike the ARs, no experimentally determined structural information is yet available for the P2YR family, and so far, the only structural characteristics of the P2YRs have come from structural modeling [21, 25–28]. Mostly, the modeling of P2YRs has focused on identifying the putative binding site and the analysis of the residues involved in the ligand binding and receptor specificity, with the aim to gain information on the ligand recognition mechanism. Site-directed mutagenesis and structure–activity relationship (SAR) analysis have been used to support and guide the modeling of the P2YRs [29–36], which has been used to identify new key residues important for the ligand binding and receptor activation [25, 37–39]. Several models based on different structural templates have been published for many of the P2YRs. The bovine rhodopsin crystal structure was used to build models for P2Y1R [21, 39–41], P2Y2R [31, 35, 42], P2Y4R [42], P2Y6R [43, 44], P2Y11R [45], P2Y12R [21], and P2Y14R [46].
The putative binding pocket of the P2Y1R, suggested by the modeling and supported by the many available mutagenesis data, is located near the extracellular region of TM3, TM6, and TM7. The positively charged residues of P2Y1R Arg3.29, Lys6.55, and Arg7.39, conserved among the P2Y1-like receptors (Table 3), appear to be involved in the coordination of the negatively charged phosphate groups of nucleotide ligands. Hydrophilic residues in the binding pocket were suggested to surround the ribose moiety of the nucleotides, while hydrophobic residues created a favorable environment for the aromatic nucleoside core of the nucleotides derivatives. Position 6.52 is a histidine residue that is conserved across the P2YRs, and mutagenesis studies on P2Y1R, P2Y2R, and P2Y12R have implicated this residue in ligand recognition [28, 33, 35, 47]. Mutagenesis studies on the P2Y1R also showed the crucial role of two disulfide bridges for the correct function of the receptor: a disulfide bridge between TM3 and EL2, conserved among family A GPCRs, and a second disulfide bridge between the N terminus and EL3 [32].
Table 3.
List of the key residues in the putative binding pocket of the human P2YRs
| Rec/Res | TM3 3.29 | TM3 3.32 | TM3 3.33 | TM3 3.37 | TM5 5.47 | TM6 6.48 | TM6 6.51 | TM6 6.52 | TM6 6.55 | TM6 6.58 | TM7 7.35 | TM7 7.36 | TM7 7.39 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Yb1 | R128 | F131 | H132 | Y136 | F226 | Y273 | F276 | H277 | K280 | N283 | Y306 | Q307 | R310 |
| P2Yb2 | R110 | F113 | Y114 | Y118 | F207 | F258 | F261 | H262 | R265 | Y268 | Y288 | K289 | R292 |
| P2Y4 | R112 | F115 | Y116 | Y120 | F209 | F258 | F261 | H262 | R265 | Y268 | Y288 | K289 | R292 |
| P2Y6 | R103 | F106 | Y107 | H111 | F201 | F252 | F255 | H256 | K259 | Y262 | Y283 | K284 | R287 |
| P2Y11 | R106 | F109 | T110 | L114 | C214 | Y261 | Y264 | H265 | R268 | N271 | Y303 | Q304 | R307 |
| P2Y12 | S101 | F104 | Y105 | Y109 | F198 | F249 | F252 | H253 | R256 | Y259 | K280 | E281 | L284 |
| P2Y13 | S99 | F102 | Y103 | Y107 | F217 | F247 | F250 | H251 | R254 | Y278 | K278 | E279 | L282 |
| P2Y14 | A98 | F101 | Y102 | Y106 | F195 | F246 | F249 | H250 | R253 | Y256 | K277 | E278 | L281 |
Residues are denoted in the top row by a numbering convention as described [20]. Residues in bold have been shown using site directed mutagenesis to be important in ligand recognition
Images displaying the role in ligand binding of amino acids in the binding site of various P2YRs are found in references [25–27, 31, 33, 35, 38, 40, 42–47, 49, 50]
Residues in the ELs that affect ligand recognition include: D204, E209, and R287 (in P2Y1R) [31]; R177, R180, and R272 (in P2Y2R) [31]
The P2YR models have been improved and refined continually using updated information from studies of their structural biology and mutagenesis. Recent advances in the structural biology of GPCRs have provided alternative templates to the bovine rhodopsin structure as a basis for the homology modeling. For example, the sequence identity between rhodopsin and human P2Y12R, the site of action of the active metabolite [37] of the blockbuster antithrombotic Clopidogrel [2], is only 16% overall and 19% for only the TM regions, while the sequence identity between human P2Y12R and the chemokine receptor CXCR4 is 22% overall and 26% for the TM domains. The X-ray structures of the human CXCR4 in its inactive state in complex with a small antagonist and a long peptide were released in late 2010 [48]. A sequence comparison between P2Y12R and CXCR4 suggested other structural features of CXCR4 that might be shared by P2Y12R, making the crystal structure of CXCR4 a more suitable template than other available GPCR crystal structures for the modeling of P2Y12 and other P2YRs [49, 50]. A model of P2Y12R based on the CXCR4 crystal structure and guided by the mutagenesis data and SAR studies available on the P2Y12R was recently published [49]. The homology models of P2Y2R and P2Y4R based on the CXCR4 crystal structure were used to explain the selectivity of agonists toward these two P2YR subtypes [50]. The modeling studies of P2Y12R showed how key residues in the binding pocket in TM6 and TM7, Arg6.55, Lys7.35, and Tyr6.58, were involved in the anchoring of the negatively charged phosphate groups of the nucleotide ligands. Those residues are conserved across the P2Y12-like subfamily of P2YRs, as shown in Table 3. Other hydrophobic or aromatic residues from TM1, TM3, TM6, and TM7 were suggested to form a suitable environment for the aromatic core of nucleotides derivatives, while the ribose moiety was surrounded by hydrophilic residues.
Key ligand tools for studying adenosine and P2Y receptors
AR agonists and antagonists
Selective agonist and antagonist ligands for each of the four AR subtypes are now available as pharmacological tools. The medicinal chemistry of the A2BAR is the least developed of the four subtypes, with selective antagonists and a few selective agonists reported only since 2000 [51, 52]. The optimal binding features of AR ligands have also been predicted on the basis of quantitative SAR (QSAR) approaches, such as comparative molecular field analysis (CoMFA) [116–119], although the use of X-ray structural data is now able to provide greater insight than earlier approaches. The selective AR ligands now include compounds that are stable in vivo, high affinity radioligands for binding assays or in vivo imaging by positron emission tomography or single photon emission tomography [53], that bind irreversibly to the receptor affinity probes, fluorescent and other spectroscopic probes, and multivalent conjugates that retain high potency. An overview of SAR is provided below for AR agonists and antagonists.
-
Agonists: The SAR of adenosine derivatives as AR agonists has been well explored [1, 54], and selective agonists and antagonists for all four subtypes have been reported (Figs. 1 and 2 and affinities of selected compounds listed in Table 1). Data on selectivity of AR ligands have been collected [1, 55]. Typically, these agonists are nucleoside derivatives substituted at one or more of the following positions: ribose 5′, adenine C2, and adenine N6. Hydrophobic groups substituted at the adenine C2 position (linked by NH or S) often provide selectivity for the A2AAR (e.g., 16, 18, 20), and hydrophobic groups substituted at the adenine N6 position often provide selectivity for the A1AR (e.g., 5–14). An N6-(4-aminophenylethyl) derivative, APNEA 4, is a nonselective AR agonist with high affinity for both A1 and A3ARs, and has been used as a radioligand in its [125I] 3-iodo form. In exceptional cases, this selectivity pattern may be altered to display A2AAR selectivity, as in 17, or with combined modifications, as in 19. Nonnucleoside agonists of the A1AR are also known, including the clinical candidate Capadenoson 15 [56].
The most common ribose modification that enhances AR potency is a small N-alkyl-uronamide at the 5′ position, as in the potent nonselective agonist NECA 3, an N-ethyl-uronamide. The presence of an N-ethyl-uronamide is typical of A2AR-selective agonists (A2A: 16, 18, and 19; A2B: 22), and a N-methyl-uronamide is typical of A3AR-selective agonists (23–28). The ribose moiety of nucleoside ligands having high AR affinity could also be substituted with a limited set of other modifications [54, 55, 57–60], for example: carbocyclics, including a ring-constrained methanocarba (fused cyclopropyl and cyclopentyl rings as in A3AR-selective 27 and 28), 4′-thio in place of oxo (68), 2′-methyl (9), 2′-methoxy (12), and 3′-amino-3′-deoxy (25). By comparing the AR binding affinities of isomeric bicyclic methanocarba adeonsine analogues that maintain either a North (N), as in 27 and 28, or a South (S) conformation, it was determined that there is a strong preference for the (N) conformation in binding to the A3AR. This bicyclic modification of ribose often enhances the affinity, as well as selectivity, at the A3AR [54]. The (N)-methanocarba modification is also preferred over the (S)-methanocarba modification at the A1AR, but affinity enhancement was not observed. The preference of the (N) over (S) conformation of the ribose moiety was also determined using C methylation at the 2′ and 3′ positions [59].
It is to be noted that some nucleoside derivatives act as full agonists at certain AR subtypes and antagonists or partial agonists at other subtypes. Typically, the efficacy at the A3AR is particularly sensitive to structural modification of the nucleoside derivative. Thus, reducing the flexibility or H-bond donating ability of the ribose moiety, especially around the 5′-amide group, or introducing certain sterically bulky hydrophobic substituents at the N6 or C2 position tends to lower the relative efficacy at the A3AR [61]. Various 8-cycloalkylamino adenosine derivatives or those modified at the ribose hydroxyl positions have reduced efficacy at the A1AR or A2AAR [60]. The introduction of bulky groups at the 5′ position has been shown to reduce efficacy at the A1AR [56].
Selective agonists for the A1AR [54, 57] include: R-PIA 6, CPA 7 and its more selective 2-chloro analogue CCPA 8, and CHA 11 (all of which have been radiolabeled as tracers for binding experiments); SPA 5 (excluded from crossing the blood brain barrier). CPA, CCPA, and CHA are more selective for the A1AR in mouse than in human, in comparison to the A3AR. (S)-ENBA 13 displays high A1AR selectivity (human, rat) in comparison to both A2AAR and A3AR, but also has reduced water solubility. A 4′-truncated (N)-methanocarba nucleoside containing an N6-dicyclopropylmethyl group (not shown) fully activated the A1AR with moderate selectivity [15]. CGS21680 16 and DPMA 17 are A2AAR selective in binding to the rat and mouse A2AARs, but in the human, they bind with similar affinity to the A3AR. The nonnucleoside 3,5-dicyanopyridine derivative 21 and the nucleoside derivative 22 are moderately A2BAR selective. A3AR-selective agonists typically have combined N6 and ribose 5′ modications (23–28). Introduction of certain bulky groups at the 5′ position of A1AR reduced the efficacy in functional assays, to provide partial agonists [57]. N6-Benzyl substitution tends to provide greater between-species consistency in A3AR binding affinity [61], while small N6-alkyl groups, such as methyl (26) are often more potent at the human A3AR than at rat and mouse A3ARs. However, N6-benzyladenosine derivatives are variable in their A1AR binding affinity depending on the substitution pattern, which can reduce A3AR selectivity. The product of enzymatic action of adenosine deaminase, inosine, also activates the A3AR in the micromolar range [1].
-
Antagonists: The prototypical AR antagonists are theophylline 29, caffeine 30 and other naturally occurring xanthines, but these are of micromolar affinity and not subtype-selective antagonists. Both synthetic purine and nonpurine (e.g., nonselective 35, A1AR-selective 39, A2AAR-selective 44–49, A3AR-selective 60–67) heterocycles have been extensively explored as subtype-selective AR antagonists [55]. Purine derivatives as selective AR antagonists include xanthines (e.g., high affinity 8-phenylxanthines 31–34 (including water-soluble and peripherally selective sulfophenyl derivatives 31 and 32), A1AR-selective 8-cycloalkylxanthines 36–38, A2AAR-selective 8-styrylxanthines 42 and 43, A2BAR-selective 8-arylxanthines 52–59) and adenines (e.g., A1AR-selective 39–41, A2AAR-selective 50 and 51, A3AR-selective 69). A3AR-selective nucleoside 68 behaves as an antagonist in functional assays, which is related to the absence of the 5′-hydroxymethyl group that is associated with the conformational change needed to activate the A3AR [15].
The in silico screening of chemical libraries of diverse structure by docking to an X-ray structure or even a homology model is now an accepted method of discovering new chemotypes that bind to a given GPCR. Non-nucleotide antagonists of ARs and P2YRs have been discovered in this manner [62].
P2YRs
Progress in the development of selective agonist and antagonist ligands for P2YRs (Fig. 3, and potencies of selected compounds listed in Table 1) has accelerated in recent years. Detailed SAR analyses are available for activation by nucleotides of most of the P2YRs [2, 63]. One must keep in mind that extracellular nucleotides can be interconverted in situ to different phosphate forms or to the corresponding nucleoside, which may complicate pharmacological studies. In some cases, the addition of an inhibitor of ectonucleotidases or of other enzymes involved in this conversion, or even addition of a purified enzyme, aids in the interpretation of pharmacological data. A challenge is to design P2YR ligands that are stable in vivo. Nevertheless, there are now nucleotide agonists selective for P2Y1, P2Y2, P2Y4, P2Y6, and P2Y14Rs and nucleotide antagonists selective for P2Y1 and P2Y12Rs. The diastereoselectivity of binding of the phosphate groups of nucleotide agonists selective at the P2Y1, P2Y2, P2Y4 and P2Y11 Rs has been characterized [29, 41, 42]. Also, subtype-selective non-nucleotide antagonists have been introduced for P2Y1, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14Rs. Isolated reports have suggested non-nucleotide antagonists of the P2Y2R, but these so far are weakly binding. Chemically diverse library screening is now being applied to the problem of identifying new structural leads for receptor antagonists, e.g., the A2AAR, P2Y12R and P2Y14R [49, 62, 64]. A general description of SAR is provided below for each of the P2Y subtypes.
P2Y1R
One of the earliest potent agonists of the P2Y1R identified was 2-MeSADP 72 (Fig. 3a). However, like the native agonist ADP 70, it also activates the P2Y12R and P2Y13R. There has been a question about the ability of 5′-triphosphate derivatives such as 2-MeSATP 73 to activate the P2Y1R; some studies show it to be an agonist while others demonstrate low efficacy [2].
The introduction of conformationally restricted (i.e., rigid) ribose substitions has established the favored ribose-ring conformation for each of the subtypes of the P2Y1-like subfamily [38, 43]. Principally, this approach has made use of the methanocarba ring system consisting of fused cyclopropane and cyclopentane, as applied earlier to the ARs, in exploring the biologically active conformations of nucleoside and nucleotide derivatives. Thus, the North (N)-methanocarba analog of 2-MeSADP, i.e., MRS 2365 76 is a selective, high affinity agonist of the P2Y1R that does not appreciably activate the other ADP-preferring subtypes, i.e., P2Y12 and P2Y13Rs [65]. The (N)-methanocarba modification is also known to improve the stability of the phosphate esters toward nucleotidases, especially the 5′-monophosphate toward the hydrolytic action of the ectonucleotidase CD73. Borano analogues of the phosphate group have been found in some cases to preserve potency and to enhance selectivity of P2YR agonists, e.g., P2Y1R agonists [66].
Many nucleotide antagonists of the P2Y1R have been introduced. Usually, these are adenine nucleotides containing bisphosphate groups, for example, a ribose 3′,5′-bisphosphate moiety. N6-methyl 2′-deoxyadenosine bisphosphate derivatives MRS 2179 93 and its 2-chloro analogue MRS 2216 (not shown) are selective P2Y1 antagonists [38]. In both agonist and antagonist series, only limited substitution of the N6 position of ADP and other nucleotides, i.e., methyl and ethyl, is tolerated at the P2Y1R. The same (N)-conformational constraint of the ribose moiety that enhances P2Y1R agonist action also favors potency and selectivity in nucleotide antagonists. For example, the ring-constrained (N)-methanocarba nucleotide bisphosphates MRS 2279 94 and MRS 2500 95 are selective, high affinity antagonists of the P2Y1R [67]. Antagonists of the P2Y1R of moderate affinity may also be derived from acyclic nucleotides, such as the bisphosphate derivative MRS 2298 (not shown) [67].
A representative antagonist of the P2Y1R discovered through optimization of a high throughput screening hit is a substituted 1-phenyl-3-methyl pyrazol-5-one 110, which has a Ki of 90 nM and is orally bioavailable [68]. Other structurally diverse antagonists of the P2Y1R have been reported.
P2Y2 and P2Y4Rs
UTP 79 is a native agonist of both P2Y2R and P2Y4R. Another native ligand, ATP 71, activates the P2Y2R, but at the P2Y4R its action is species-dependent, i.e., it acts as an antagonist at the human homologue and agonist at the rat P2Y4R. Synthetic UTP analogues with selectivity for the P2Y2R have been reported, e.g., UTPγS 77, 2-thioUTP 82 and MRS 2698 (not shown), which is 300-fold P2Y2R-selective in comparison to the P2Y4R [35]. Recently, an N4-alkoxyimino derivative of CTP, MRS 4062 83, was found to be a full agonist of the P2Y4R with ∼30-fold selectivity in comparison to the P2Y2R and P2Y6R [50]. Molecular modeling and docking of N4-alkoxyimino derivatives of CTP defined a new subpocket facing the exterior of the P2Y4R that could accommodate steric bulk.
Dinucleoside tetraphosphates, e.g., INS 365 (Diquafosol) 92 and Up4-2′-deoxyC (structure not shown, INS 37217, Denufosol) are moderately potent agonists of both P2Y2R and P2Y4R. In general, dinucleotides are more stable to hydrolysis by nucleotidases than are nucleotides bearing a free terminal phosphate group [69]. MRS 2768 90 (uridine tetraphosphate δ-phenyl ester) is somewhat selective for the P2Y2R but is less potent than other P2Y2R agonists [70].
Several weak antagonists of the P2Y2R that are uracil derivatives, e.g., AR-C126313 and AR-C 118925 (not shown), have been reported but full pharmacological characterization is still lacking [2]. For lack of better antagonists, the anti-infective drug suramin 102 and the large anthraquinone dye Reactive blue 2 100 (RB2), which is a mixture of isomers, are used as partially selective antagonists of the P2Y2R and P2Y4R, respectively. It should be noted that suramin and many other weak P2YR antagonists typically display other activities, such as inhibition of ectonucleotidases, which may complicate the interpretation of experiments [2].
P2Y6R
UDP 78 is the native agonist of the P2Y6R, but was recently found to also activate the P2Y14R [71]. UDPβS 80, 3-phenacyl UDP (PSB 0474) 84, 5-iodo-UDP (MRS 2693) 85 and dinucleoside triphosphates, such as Up3U 91 and INS 48823 (not shown) [2], have been used as moderately selective agonists of the P2Y6R [44]. Probing the conformation of the ribose ring at the P2Y6R by molecular modeling and chemical synthesis of ring-constrained analogues has clearly identified the South (S)-conformation as the receptor-preferred conformation at this subtype [43]. Thus, a rigid bicyclic (S)-methanocarba-UDP (not shown) was more potent than UDP, and the corresponding ring-constrained isomer with a (N)-conformation was inactive. The di-isothiocyanate derivative MRS 2578 109 is a non-competitive P2Y6R antagonist that has limited stability in aqueous medium and presumably reacts irreversibly with the receptor.
P2Y11R
ATPγS 75 is usually used as a potent but nonselective P2Y11R agonist. Few P2Y11R-selective agonists have been reported, but an atypical agonist NF546 104 of the suramin class of antagonists was reported to activate this receptor selectively [72]. However, several reported P2Y12R antagonists, such as 2-propylthio-β,γ-dichloromethylene-ATP (AR-C 67085 97), also act as potent P2Y11R agonists. The suramin derivative NF 157 103 is an antagonist of the P2Y11R, but it is not selective with respect to the nucleotide-gated ion channels P2X1R, P2X2R, and P2X3R. NF340 105 related to suramin is a selective P2Y11R antagonist.
P2Y12R
ADP 70 is the native agonist of the P2Y12R, and another native ligand, ATP 71, acts as a competitive antagonist. Many nucleotide (96–98) and non-nucleotide (111–115) antagonists of the P2Y12R have been reported, because of commercial interest. The thienopyridine Clopidgrel 113 is a blockbuster antithrombotic agent, which must be first activated in two steps by cytochrome P450 in the liver to subsequently irreversibly inhibit the P2Y12R [2, 37, 70]. The recently approved antithrombotic Prasugrel 114 belongs to this thienopyridine family of P2Y12 antagonists. Competitive P2Y12R antagonists that do not require preactivation are also under development, for example, the antithrombotic nucleotide derivative AR-C 69931MX 98 (Cangrelor). An uncharged nucleoside derivative that binds potently to the P2Y12R, AZD 6140 99 (Ticagrelor) was recently approved to reduce cardiovascular death and heart attack in cases of acute coronary syndrome. A major metabolite of 99 that is formed by oxidative loss of the hydroxyethyl side chain also acts as a potent P2Y12R antagonist [22]. A sulfonate derivative related to RB2, PSB-0739 101, is a representative nonnucleotide antagonist of the P2Y12R that displays high affinity and was used in the characterization of the effects of site directed mutagenesis of the receptor and in molecular modeling [34, 49].
P2Y13R
ADP 70 is a native agonist of the P2Y13R, while ATP 71 at high concentrations is at best a weak partial agonist. The pyridoxal phosphate derivative MRS 2211 108, is a selective antagonist of the P2Y13R and related to the nonselective P2 antagonists PPADS 106 and iso-PPADS 107 [73]. However, MRS2211 and other pyridoxal phosphate derivatives also inhibit protein interactions of the 14-3-3 family of intracellular phosphoserine/threonine-recognition proteins [74].
P2Y14R
UDP-glucose 88, other UDP-sugars and UDP 78 are native agonists of the P2Y14R [71]. A synthetic 2-thio analog of UDP-glucose, i.e., MRS 2690 89, is a more potent and selective agonist at the P2Y14R. α,β-difluoromethylene-UDP, MRS 2802 86, and the more potent α,β-methylene-2-thio analogue MRS2905 87 are inactive at the P2Y6R and fully activate the human P2Y14R.
Allosteric modulation of ARs and P2YRs
In addition to orthosteric agonists that bind at the same site on the receptor as the native agonist, allosteric modulators for ARs and P2YRs have been studied. The structure and action of positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) for ARs and P2YR have been recently reviewed [75]. This includes both heterocyclic derivatives and nucleotide analogues that resemble a native P2Y agonist. Some of the PAMs have no action of their own and require the presence of an agonist, either the endogenous ligand or a synthetic agonist, and other PAMs are allosteric agonists that act in the absence of orthosteric ligands. The SAR of PAMs of the A1AR (e.g., the tetrahydrobenzothiophene derivative T-62) and A3AR (e.g., the imidazoquinolinamine derivative LUF6000 and the quinoline derivative LUF6096, structures not shown) has been extensively explored. Recently, AEA061 was reported as a PAM of the A2AAR [76].
Ligands in the clinic and in current clinical trials
The biological role of adenosine and P2YRs has been extensively explored, contributing to the entry of certain selective ligands on a clinical pathway [54, 70, 77, 78]. Table 4 lists those AR and P2YR ligands in the clinic for therapeutic and diagnostic applications, including those currently in clinical trials for chronic diseases, such as inflammatory, ischemic, and neurodegenerative diseases, and for other conditions.
Table 4.
Ligands of ARs or P2Y receptors currently in clinical use or trials (previous clinical trials with selective adenosine and P2Y receptor ligands are listed in refs. [1, 2, 57, 70])
| Ligand | Subtype action | Route | Application | Phase | Company |
|---|---|---|---|---|---|
| Adenosine 1 (Adenocard) | A1 agonist | iv | Paroxysmal supraventricular tachycardia | Approved | Astellas |
| INO-8875 | A1 agonist | Topical | Glaucoma | I–II | Inotek |
| Capadenoson 15, Bay68-4986 | A1 agonist | Oral | Atrial fibrillation | II | Bayer-Schering |
| Adenosine 1 (Adenoscan) | A2A agonist | iv | Myocardial perfusion imaging | Approved | Astellas |
| Apadenoson 18, ATL146e (Stedivaze) | A2A agonist | iv | Myocardial perfusion imaging | III | Forest Laboratories |
| Regadenoson 20, CV-3146 (Lexiscan) | A2A agonist | iv | Myocardial perfusion imaging | Approved | Astellas/Gilead |
| Regadenoson 20, CV-3146 (Lexiscan) | A2A agonist | iv | Sickle cell disease | I | Dana-Farber Cancer Institute |
| IB-MECA 23, CF101 | A3 agonist | Oral | Rheumatoid arthritis, psoriasis, dry eye, glaucoma | II/III | Can-Fite |
| Cl-IB-MECA 24, CF102 | A3 agonist | Oral | Hepatocellular carcinoma, chronic hepatitis C (genotype 1) | II | Can-Fite |
| Caffeine 30 | AR antagonist | iv or oral | Sleep apnea, cancer pain, PD | II/III | Univ. of Texas, McMaster Univ., Nobelpharma, Korea Research, McGill University |
| Theophylline 29 | AR antagonist | Oral | Asthma, COPD | Approved | – |
| Istradefylline 42, KW-6002 | A2A antagonist | Oral | PD | III | Kyowa Hakko |
| KW-6356 | A2A antagonist | PD | Kyowa Hakko (in Asia), Lundbeck (non-Asia) | ||
| Preladenant 46, SCH-420814 | A2A antagonist | Oral | PD | III | Schering |
| Tozadenant 45, SYN-115 | A2A antagonist | Oral | PD, cocaine dependence | IIB | Biotie, NIDA (Synosia Therapeutics) |
| ST-1535 51 | A2A antagonist | Oral | PD | I | Sigma-Tau |
| V81444 | A2A antagonist | Oral | PD | I | Vernalisa |
| DT1133 | A2A antagonist | Oral | PD | Pre-clinical | Domain Therapeutics |
| [11C]-SCH442416 47 | A2A antagonist | iv | PET imaging of PD | I | Institute for Neurodegenerative Disorders |
| [123I]MNI-420 49c | A2A antagonist | iv | SPECT imaging of PD, Huntington’s disease | I | Institute for Neurodegenerative Disorders |
| CVT-6883 55, GS 6201 | A2B antagonist | Oral | Chronic pulmonary and inflammatory diseasesd | I | Gilead |
| Diquafosol 92 (Diquas) | P2Y2 agonist | Local | Dry eye disease | Approved (Japan) | Santen (Inspire) |
| Clopidogrel 113 (Plavix) | P2Y12 antagonist | Oral | Acute coronary syndrome, atherosclerosis | Approved | BMS/Sanofi |
| Prasugrel 114 (Effient) | P2Y12 antagonist | Oral | Acute coronary syndrome, angioplasty | Approved | Lilly/Daiichi Sankyo |
| Ticagrelor 99, AZD6140 (Brilinta) | P2Y12 antagonist | Oral | Acute coronary syndrome | Approved | AstraZeneca |
| Cangrelor 98, AR-C69931MX | P2Y12 antagonist | iv | Coronary artery bypassb | III | The Medicines Co. |
| Elinogrel 115, PRT-060128 | P2Y12 antagonist | Oral or iv | Acute coronary syndrome | II | Portola/Novartis |
PD Parkinson’s disease
aClinical trials of another A2AAR antagonist, vipadenant (V2006/BIIB014), for PD were recently halted by Vernalis and partner Biogen Idec
bEffective at maintaining platelet inhibition in patients on thienopyridines who required bypass surgery
cReference [110]
dClinical trials discontinued or unsuccessful (see also references [1, 2, 57, 70])
AR ligands as clinical candidates and approved drugs
Adenosine may be released from intracellular sources or generated by the action of ectonucleootidases on ATP that is released under stress conditions. Therefore, depending on pathophysiological factors, in a given tissue, there is often a tonic activation of one or more of the ARs that can be modulated by exogenous agents. AR agonists are currently in clinical trials for various conditions, including cardiac arrhythmias, neuropathic pain, myocardial perfusion imaging, cardiac ischemia, autoimmune inflammatory diseases, and cancer [57, 79–84].
The first AR agonist to be approved was adenosine 1 itself (as Adenocard), used as a rapidly metabolized therapeutic treatment of cardiac arrhythmias, specifically paroxysmal supraventricular tachycardia (PSVT), by slowing atrioventricular (AV) nodal conduction, an A1AR effect. Its short half-life upon intravenous infusion (seconds) avoids some side effects, although A2AAR-related side effects still may occur. Other agonists of the A1AR have been in clinical trials for pain and cardiac arrythmias, including atrial fibrillation, supraventricular arrythmias, paroxysmal supraventricular tachycardia, and atrial flutter [54, 57]. CVT-3619 (GS9667, not shown), a partial A1AR agonist, has been in clinical trials for type 2 diabetes [54]. Side effects associated with A1AR agonists applied to cardioprotective and cardiovascular regeneration may be overcome by using partial A1AR agonists such as Capadenoson 15 [85].
Adenosine (as Adenoscan) is used as a pharmacological stress agent for cardiovascular imaging based on its A2AAR-dependent vasodilatory effect in the coronary artery. Also, more advanced A2AAR-selective agonists [79] are either already approved for this purpose (CVT-3146, 20) or in clinical trials (ATL-146e, Apadenson, 18). A2AAR agonists also have anti-inflammatory and anti-ischemic effects, and have been in clinical trials for related conditions, including sickle cell disease by targeting iNKT cells [58, 84]. Selective agonists of A2A, A2B or A3ARs have been shown to have anti-inflammatory effects due to inhibition of the release of pro-inflammatory cytokines and other mechanisms [80, 86, 87]. This also led to former clinical trials of A2AAR agonists for the treatment of chronic and neuropathic pain and diabetic foot ulcers. However, an A2AAR agonist was found ineffective for treating foot ulcers. A2AAR agonists also show beneficial effects in wound healing, because A2A and A2BARs stimulate granulation tissue formation by inducing new matrix production and angiogenesis [88, 89]. A2BAR agonists have been proposed for the treatment of hyperlipidemia and atherosclerosis [90].
A2AAR antagonists (e.g. 42, 45, 48, and 51) are being developed for treatment of Parkinson’s disease (PD) and other disorders of the central nervous system including addiction [79, 91], and several clinical candidates have been radiolabeled for in vivo imaging [53]. In the striatum, a heterodimer of the A2AAR and the D2 dopamine receptor is thought to establish the inverse action of dopamine and adenosine agonists; thus, an A2AAR antagonist would have a net effect similar to a D2 agonist. A2AAR antagonists could also be of interest in preventing fibrosis in the liver and elsewhere [88] or in the treatment of cancer [92]. A2BAR antagonists are under consideration for treating inflammatory diseases, diabetes, and asthma [81, 82], although trials of CVT-6883 55 were unsuccessful.
Native adenosine acting at various AR subtypes has antiischemic activities in multiple organs, for example, a cardioprotective action, either as a preconditioning agent or during ischemia reperfusion. Adenosine and more selective AR agonists, e.g. A3AR agonists such as CP532,903 25, have been considered for treating acute myocardial infarction [80]. One of the first actions discovered for A3AR agonists administered in vivo was cerebroprotection. Also noted were paradoxical effects in which nM concentrations of A3AR agonists prevented apoptosis and high μM concentrations induced apoptosis. The relative lack of cardiovascular side effects of A3AR agonists in comparison to other AR agonists is considered an advantage in application to ischemia. The orally active A3AR agonist CF101 (IB-MECA) 23 is in clinical trials for rheumatoid arthritis, psoriasis, keratoconjunctivitis sicca (dry eye syndrome), and glaucoma [80]. The closely related CF102 (Cl-IB-MECA) 24 is in clinical trials for advanced hepatocellular carcinoma and for patients with chronic hepatitis C genotype 1.
P2YR ligands as clinical candidates and approved drugs
Although the ARs are a mature field of medicinal chemistry, the P2YRs generally lag behind in the development of selective ligands, radioligands and other affinity probes, imaging agents, and clinical candidates. The most successful application in that area is the use of P2Y12R antagonists as antithrombotics, but other disease areas are potentially amenable to treatment using selective P2YR agonists or antagonists [70]. Since some P2Y subtypes have a widespread distribution, there might be substantial side effects, such as those noted to occur in bone [4].
Nucleotides, such as ATP 71 and UTP 79, are readily released from intracellular sources under conditions of injury and organ stress, such as hypoxia, ischemia, or mechanical stress, and through channels and vesicular release. One of the consequences of this release is a proinflammatory effect [2], for example from ATP that accummulates in asthmatic airways. Consistently, antagonists and other ligands of the P2YRs could serve as therapeutic targets for a variety of conditions, including cardiovascular diseases and inflammatory diseases such as asthma and neurodegeneration [70, 93]. It has been suggested that antagonists of P2Y2R, P2Y6R, or P2Y11R might be beneficial in asthma and inflammatory bowel disease [70]. Beneficial effects of P2 receptor antagonists have been observed in a stroke model [94]. The effects of various P2YR ligands on apoptosis in cell culture and in the central nervous system have been explored [95–98], suggesting application to a variety of diseases, from cancer to diabetes to ischemia.
P2YRs are widespread in hematopoietic cells, and therefore the effects of extracellular nucleotides and their antagonists are being studied in the immune/inflammatory system. The platelet expresses two P2YRs, i.e., P2Y1R and P2Y12R, both of which have to be activated in order for ADP to have a prothrombotic effect [23]. Therefore, blocking either of these receptors produces an antithrombotic effect. P2Y12R antagonists, three of which are already approved as agents for acute coronary syndrome and for prevention of secondary thrombotic events, have been described above. The antithrombotic action of MRS 2500 95 by selectively blocking the P2Y1R is evident in vivo in the mouse and other species, suggesting this receptor subtype as a clinical target. Furthermore, genetic deletion of the P2Y1R is associated with fewer atherosclerotic lesions in ApoE−/− mice. Bone marrow reconstitution has demonstrated the involvement of non-hematopoietic-derived cells, probably the endothelial cells [99].
Several agonists of the P2Y2R have been in clinical trials for cystic fibrosis and other pulmonary conditions. Activation of the P2Y2R on epithelial cells in the airways and the eye promotes chloride secretion, independently of the genetically defective transporter in cystic fibrosis. However, the P2Y2R agonist Up4-2′-deoxyC (Denufosol) was denied approval for the treatment of cystic fibrosis due to the failure to reproduce the positive results of the TIGER-1 study in the longer duration TIGER-2 trial. A P2Y2R agonist of low selectivity, Up4U 92 (Diquafosol), has been approved in Japan but not the U.S. for the treatment of dry eye disease [100]. P2Y2R activation has also been shown to protect rat fetal cardiomyocytes against ischemia [101]. P2Y4R activation by UTP promotes chloride and water secretion by intestinal epithelial cells, suggesting the use of agonists of this subtype in treating chronic constipation [102].
Pancreatic islets express both the P2Y1R and P2Y6R, both of which are coupled to Gq and promote insulin release. The use of P2Y1R agonists in diabetes has been proposed, and relatively stable nucleotide analogues that activate this subtype have been applied in vivo [69]. Furthermore, agonists of the P2Y6R have been shown to have beneficial antiapoptotic effects on pancreatic islets cells in culture, suggesting their possible application to diabetes [95] Endogenous UDP activating the P2Y6R is involved in the autocrine potentiation of insulin secretion [103]. However, there are significant side effects of activation of the P2Y6R, such as a proinflammatory effect, atherosclerotic plaques, cardiac fibrosis and possibly a loss of bone mass [4, 70, 104].
P2Y11R activation mediates ATP-induced semi-maturation of human monocyte-derived dendritic cells and increases the release of interleukin-8 from human monocyte-derived dendritic cells, suggesting use of ligands of this subtype in immune modulation [72, 105]. Semi-maturation of dendritic cells is characterized by an increased expression of co-stimulatory molecules with no stimulation of interleukin-12 secretion, leading to a Th2 response or tolerance.
The activation and migration of microglia in the brain are modulated by P2YRs [106, 107]. ADP activating the microglial P2Y12R induces a “find-me” signal (to induce migration), and UDP activating the microglial P2Y6R induces an “eat-me” signal (to induce phagocytosis). These findings suggest application of P2Y12R or P2Y6R ligands to neuropathic pain and neurodegenerative diseases. Indeed, intrathecal administration of P2Y12R antagonist AR-C69931MX 98 prevented the development of tactile allodynia [106].
Activation of the P2Y13R by ADP promotes reverse cholesterol transport in hepatocytes with the endocytosis of HDL particles [108]. Thus, activation of P2Y13R might be a new target for treatment of dyslipidemia and atherosclerosis.
Modulation of the P2Y14R has potential for the treatment of immune and inflammatory disorders, pain, asthma, gastric disorders, central nervous system diseases, and glaucoma. Non-nucleotide antagonists of the P2Y14R, e.g., 116, and prodrug derivatives to increase their bioavailability have been proposed [64]. Intracellular UDP sugars, many of which would activate the cell-surface P2Y14R, are substrates for protein glycosylation, and are released as the proteins are trafficked to the surface [71], where they may fulfill a cell signaling role. The role of P2Y receptors in stem cell differentiation has been explored; P2Y4 and P2Y14Rs appear to regulate the onset of mesenchymal differentiation, and the downregulation of P2Y1 and P2Y2Rs are markers for early osteogenic differentiation [109].
Conclusions
There have been significant recent advances in the structural biology of purine receptors and in the medicinal chemistry of selective ligands and their pharmacology. Potent purine and pyrimidine analogues have aided in the characterization of regulation of many physiological and pathophysiological processes. It is apparent that this ubiquitous cell signaling system has implications for understanding and treating many diseases. Thus, this field has provided fertile ground for pharmaceutical development.
Acknowledgements
Supported by the NIDDK Intramural Research Program, National Institutes of Health.
Glossary
- AR
Adenosine receptor
- EL
Extracellular loop
- GPCR
G protein-coupled receptor
- IL
Intracellular loop
- TM
Transmembrane helix
- NECA
Adenosine-5′-N-ethyluronamide
- PD
Parkinson’s disease
- PET
Positron emission tomography
- SAR
Structure–activity relationship
- SPECT
Single photon emission tomography
- UDPG
Uridine-5′-diphosphoglucose
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller C. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, King BF, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII. Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ. The nucleotide AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;PMID:22215671. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orriss I, Syberg S, Wang N, Robaye B, Gartland A, Jorgensen N, Arnett T, Boeynaems JM. Bone phenotypes of P2 receptor knockout mice. Front Biosci (Schol Ed) 2011;3:1038–1046. doi: 10.2741/208. [DOI] [PubMed] [Google Scholar]
- 5.Klaasse EC, IJzerman AP, Grip WJ, Beukers MW. Internalization and desensitization of adenosine receptors. Purinergic Signal. 2008;4:21–37. doi: 10.1007/s11302-007-9086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hechler B, Gachet C. P2 receptors and platelet function. Purinergic Signal. 2011;4:293–303. doi: 10.1007/s11302-011-9247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo W, Wang Y, Reiser G. Proteinase-activated receptors, nucleotide P2Y receptors, and μ-opioid receptor-1B are under the control of the type I transmembrane proteins p23 and p24A in post-Golgi trafficking. J Neurochem. 2011;117:71–81. doi: 10.1111/j.1471-4159.2011.07173.x. [DOI] [PubMed] [Google Scholar]
- 8.Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol. 2011;61:221–261. doi: 10.1016/B978-0-12-385526-8.00008-4. [DOI] [PubMed] [Google Scholar]
- 9.Kukulski F, Levesque SA, Sevigny J. Impact of ectoenzymes on P2 and P1 receptor signaling. Adv Pharmacol. 2011;61:263–299. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 10.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 11.Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schiöth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–273. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, IJzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaakola VP, Lane JR, Lin JY, Katritch V, IJzerman AP, Stevens RC. Ligand binding and subtype selectivity of the human A2A adenosine receptor. Identification and characterization of essential amino acid residues. J Biol Chem. 2010;285:13032–13044. doi: 10.1074/jbc.M109.096974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov AA, Barak D, Jacobson KA. Evaluation of homology modeling of G protein-coupled receptors in light of the A2A adenosine receptor crystallographic structure. J Med Chem. 2009;52:3284–3292. doi: 10.1021/jm801533x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tosh DK, Phan K, Deflorian F, Wei Q, Gao ZG, Jacobson KA. Truncated (N)-methanocarba nucleosides as A1 adenosine receptor agonists and partial agonists: overcoming lack of a recognition element. ACS Med Chem Lett. 2011;2:626–631. doi: 10.1021/ml200114q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dal Ben D, Lambertucci C, Marucci G, Volpini R, Cristalli G. Adenosine receptor modeling: what does the A2A crystal structure tell us? Curr Top in Med Chem. 2010;10:993–1018. doi: 10.2174/156802610791293145. [DOI] [PubMed] [Google Scholar]
- 17.Doré AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F, Tate CG, Weir M, Marshall FH. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Struct. 2011;19:1283–1293. doi: 10.1016/j.str.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–327. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–525. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G protein-coupled receptors. In: Conn PM, Sealfon SC, editors. Methods in Neurosciences, Vol. 25. USA: Academic; 1995. pp. 366–428. [Google Scholar]
- 21.Costanzi S, Mamedova L, Gao ZG, Jacobson KA. Architecture of P2Y nucleotide receptors: Structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem. 2004;47:5393–5404. doi: 10.1021/jm049914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres B, Zambon AC, Insel PA. P2Y11 receptors activate adenylyl cyclase and contribute to nucleotide-promoted cAMP formation in MDCK-D1 cells. J Biol Chem. 2002;277:7761–7765. doi: 10.1074/jbc.M110352200. [DOI] [PubMed] [Google Scholar]
- 23.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 24.Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM. Identification of a novel human ADP receptor coupled to Gi. J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- 25.Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. Human P2Y1 receptor: molecular modeling and site-directed mutagenesis as tools to identify agonist and antagonist recognition sites. J Med Chem. 1998;41:1456–1466. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee AM, Fischer B, Galen PJ, Jacobson KA. Modelling the P2Y purinoceptor using rhodopsin as template. Drug Design Discov. 1995;13:133–154. [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Q, Guo D, Lee BX, Rhee AM, Kim YC, Nicholas RA, Schachter J, Harden TK, Jacobson KA. A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol Pharmacol. 1997;52:499–507. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erb L, Garrad R, Wang Y, Quinn T, Turner JT, Weisman GA. Site-directed mutagenesis of P2U purinoceptors. Positively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J Biol Chem. 1995;270:4185–4188. doi: 10.1074/jbc.270.52.30845. [DOI] [PubMed] [Google Scholar]
- 29.Ecke D, Fischer B, Reiser G. Diastereoselectivity of the P2Y11 nucleotide receptor: mutational analysis. Br J Pharmacol. 2008;155:1250–1255. doi: 10.1038/bjp.2008.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo D, Kügelgen I, Moro S, Kim YC, Jacobson KA. Evidence for the recognition of non-nucleotide antagonists within the transmembrane domains of the human P2Y1 receptor. Drug Devel Res. 2002;57:173–181. doi: 10.1002/ddr.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillmann P, Ko GY, Spinrath A, Raulf A, Kügelgen I, Wolff SC, Nicholas RA, Kostenis E, Höltje HD, Müller CE. Key determinants of nucleotide-activated G protein-coupled P2Y2 receptor function revealed by chemical and pharmacological experiments, mutagenesis and homology modeling. J Med Chem. 2009;52:2762–2775. doi: 10.1021/jm801442p. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann C, Moro S, Nicholas RA, Harden TK, Jacobson KA. The role of amino acids in extracellular loops of the human P2Y1 receptor in surface expression and activation processes. J Biol Chem. 1999;274:14639–14647. doi: 10.1074/jbc.274.21.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann K, Sixel U, Pasquale F, Kügelgen I. Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y12-receptor. Biochem Pharmacol. 2008;76:1201–1213. doi: 10.1016/j.bcp.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann K, Baqi Y, Morena MS, Glänzel M, Müller CE, Kügelgen I. Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J Pharm Exp Therap. 2009;331:648–655. doi: 10.1124/jpet.109.156687. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov AA, Ko H, Cosyn L, Maddileti S, Besada P, Fricks I, Costanzi S, Harden TK, Calenbergh S, Jacobson KA. Molecular modeling of the human P2Y2 receptor and design of a selective agonist, 2′-amino-2′-deoxy-2-thiouridine 5′-triphosphate. J Med Chem. 2007;50:1166–1176. doi: 10.1021/jm060903o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi AD, Zambon AC, Insel PA, Nicholas RA. An arginine/glutamine difference at the juxtaposition of transmembrane domain 6 and the third extracellular loop contributes to the markedly different nucleotide selectivities of human and canine P2Y11 receptors. Mol Pharmacol. 2001;60:1375–1382. doi: 10.1124/mol.60.6.1375. [DOI] [PubMed] [Google Scholar]
- 37.Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–1521. doi: 10.1124/dmd.110.032250. [DOI] [PubMed] [Google Scholar]
- 38.Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, Boyer JL, Jacobson KA. Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. J Med Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moro S, Jacobson KA. Molecular modeling as a tool to investigate molecular recognition in P2Y receptors. Curr Pharmaceut Design. 2002;8:99–110. doi: 10.2174/1381612023392892. [DOI] [PubMed] [Google Scholar]
- 40.Major DT, Fischer B. Molecular recognition in purinergic receptors. 1. A comprehensive computational study of the hP2Y1-receptor. J Med Chem. 2004;47:4391–4404. doi: 10.1021/jm049772m. [DOI] [PubMed] [Google Scholar]
- 41.Major DT, Nahum V, Wang Y, Reiser G, Fischer B. Molecular recognition in purinergic receptors. 2. Diastereoselectivity of the hP2Y1-receptor. J Med Chem. 2004;47:4405–4416. doi: 10.1021/jm049771u. [DOI] [PubMed] [Google Scholar]
- 42.Jacobson KA, Costanzi S, Ivanov AA, Tchilibon S, Besada P, Gao ZG, Maddileti S, Harden TK. Structure activity and molecular modeling analyses of ribose- and base-modified uridine 5′-triphosphate analogues at the human P2Y2 and P2Y4 receptors. Biochem Pharmacol. 2006;71:540–549. doi: 10.1016/j.bcp.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costanzi S, Joshi BV, Maddileti S, Mamedova L, Gonzalez-Moa MJ, Marquez VE, Harden TK, Jacobson KA. Human P2Y6 receptor: molecular modeling leads to the rational design of a novel agonist based on a unique conformational preference. J Med Chem. 2005;48:8108–8111. doi: 10.1021/jm050911p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Besada P, Shin DH, Costanzi S, Ko H, Mathé C, Gagneron J, Gosselin G, Maddileti S, Harden TK, Jacobson KA. Structure–activity relationships of uridine 5′-diphosphate analogues at the human P2Y6 receptor. J Med Chem. 2006;49:5532–5543. doi: 10.1021/jm060485n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zylberg J, Ecke D, Fischer B, Reiser G. Structure and ligand-binding site characteristics of the human P2Y11 nucleotide receptor deduced from computational modelling and mutational analysis. Biochem J. 2007;405:277–286. doi: 10.1042/BJ20061728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanov AA, Fricks I, Harden TK, Jacobson KA. Molecular dynamics simulation of the P2Y14 receptor. Ligand docking and identification of a putative binding site of the distal hexose moiety. Bioorg Med Chem Lett. 2007;17:761–766. doi: 10.1016/j.bmcl.2006.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao Y, Zhang L, Jin J, Ashby B, Kunapuli SP. Mutational analysis of residues important for ligand interaction with the human P2Y12 receptor. Eur J Pharmacol. 2010;644:10–16. doi: 10.1016/j.ejphar.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu B, Chien EYT, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deflorian F, Jacobson KA. Comparison of three GPCR structural templates for modeling of the P2Y12 nucleotide receptor. J Comput Aided Mol Des. 2011;25:329–338. doi: 10.1007/s10822-011-9423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maruoka H, Jayasekara MP, Barrett MO, Franklin DA, Castro S, Kim N, Costanzi S, Harden TK, Jacobson KA. Pyrimidine nucleotides with 4-alkyloxyimino and terminal tetraphosphate δ-ester modifications as selective agonists of the P2Y4 receptor. J Med Chem. 2011;54:4018–4033. doi: 10.1021/jm101591j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalla RV, Zablocki J, Tabrizi MA, Baraldi PG (2009) Recent developments in A2B adenosine receptor ligands. In: HEP adenosine receptors in health and disease, Springer, Wilson C, Mustafa J (eds) Handb Exp Pharmacol 193:99–121 [DOI] [PubMed]
- 52.Baraldi PG, Tabrizi MA, Fruttarolo F, Romagnoli R, Preti D. Recent improvements in the development of A2B adenosine receptor agonists. Purinergic Signal. 2010;5:3–19. doi: 10.1007/s11302-009-9140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauer A, Ishiwata K. Adenosine receptor ligands and PET imaging of the CNS. Handb Exp Pharmacol. 2009;193:617–642. doi: 10.1007/978-3-540-89615-9_19. [DOI] [PubMed] [Google Scholar]
- 54.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nature Rev Drug Disc. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Müller C, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. BBA—Biomembranes. 2011;1808:1290–1308. doi: 10.1016/j.bbamem.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiesman WF, Elzein E, Zablocki J. A1 adenosine receptor antagonists, agonists, and allosteric enhancers. Handb Exp Pharmacol. 2009;193:25–58. doi: 10.1007/978-3-540-89615-9_2. [DOI] [PubMed] [Google Scholar]
- 57.Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs. 2008;17:1901–1910. doi: 10.1517/13543780802497284. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Do CW, Avila MY, Peterson-Yatorno K, Stone RA, Gao ZG, Joshi BV, Besada P, Jeong LS, Jacobson KA, Civan MM. Nucleoside-derived antagonists to A3 adenosine receptors lower mouse intraocular pressure and act across species. Exp Eye Res. 2010;90:146–154. doi: 10.1016/j.exer.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franchetti P, Cappellacci L, Vita P, Petrelli R, Lavecchia A, Kachler S, Klotz KN, Marabese I, Luongo L, Maione S, Grifantini M. N6-Cycloalkyl- and N6-bicycloalkyl-C5′(C2′)-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice. J Med Chem. 2009;52:3430–3430. doi: 10.1021/jm900498h. [DOI] [PubMed] [Google Scholar]
- 60.Wenden EM, Carnielli M, Roelen HCPF, Lorenzen A, Frijtag Drabbe Künzel JK, IJzerman AP. Ribose-modified adenosine analogs as potential partial agonists for the adenosine receptor. J Med Chem. 1998;41:102–108. doi: 10.1021/jm970508l. [DOI] [PubMed] [Google Scholar]
- 61.Jacobson KA, Klutz AM, Tosh DK, Ivanov AA, Preti D, Baraldi PG (2009) Medicinal chemistry of the A3 adenosine receptor: agonists, antagonists, and receptor engineering. In: HEP adenosine receptors in health and disease, Springer, WilsonC, Mustafa J (eds) Handb Exp Pharmacol 193:123–159 [DOI] [PMC free article] [PubMed]
- 62.van der Horst E, van der Pijl R, Mulder-Krieger T, Bender A, IJzerman AP (2011) Substructure-based virtual screening for adenosine A2A receptor ligands. Chem Med Chem 6:2301–2311 [DOI] [PubMed]
- 63.Brunschweiger A, Müller CE. P2 receptors activated by uracil nucleotides—an update. Curr Med Chem. 2006;13:289–312. doi: 10.2174/092986706775476052. [DOI] [PubMed] [Google Scholar]
- 64.Robichaud J, Fournier JF, Gagne S, Gauthier JY, Hamel M, Han Y, Hénault M, Kargman S, Levesque JF, Mamane Y, Mancini J, Morin N, Mulrooney E, Wu J, Black WC. Applying the pro-drug approach to afford highly bioavailable antagonists of P2Y14. Bioorg Med Chem Lett. 2011;21:4366–4368. doi: 10.1016/j.bmcl.2010.12.113. [DOI] [PubMed] [Google Scholar]
- 65.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analogue. J Pharm Exp Therap. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yelovitch S, Camden J, Weisman GA, Fischer B (2012) Boranophosphate isoster controls P2Y-receptor subtype selectivity and metabolic stability of dinucleoside polyphosphate analogues. J Med Chem 55:437-448 [DOI] [PMC free article] [PubMed]
- 67.Cattaneo M, Lecchi A, Joshi BV, Ohno M, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfefferkorn JA, Choi C, Winters T, Kennedy R, Chi L, Perrin LA, Ping YW, McClanahan T, Schroeder R, Leininger MT, Geyer A, Schefzick S, Atherton J. P2Y1 receptor antagonists as novel antithrombotic agents. Bioorg Med Chem Lett. 2008;18:3338–3343. doi: 10.1016/j.bmcl.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 69.Eliahu S, Barr HM, Camden J, Weisman GA, Fischer B. A novel insulin secretagogue based on a dinucleoside polyphosphate scaffold. J Med Chem. 2010;53:2472–2481. doi: 10.1021/jm901621h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacobson KA, Boeynaems J-M. P2Y nucleotide receptors: promise of therapeutic applications. Drug Disc Today. 2010;15:570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harden TK, Sesma JI, Fricks IP, Lazarowski ER. Signaling and pharmacological properties of the P2Y14 receptor. Acta Physiol. 2010;199:149–160. doi: 10.1111/j.1748-1716.2010.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meis S, Hamacher A, Hongwiset D, Marzian C, Wiese M, Eckstein N, Royer HD, Communi D, Boeynaems JM, Hausmann R, Schmalzing G, Kassack MU. NF546 [4,4′-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)-carbonylimino))-bis(1,3-xylene-alpha, alpha′-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells. J Pharm Exp Therap. 2010;332:238–247. doi: 10.1124/jpet.109.157750. [DOI] [PubMed] [Google Scholar]
- 73.Felix R, Martin S, Pinion S, Crawford DJ (2012) Development of a comprehensive set of P2 receptor pharmacological research compounds. Purinerg Signal 8(Suppl 1):101–112 [DOI] [PMC free article] [PubMed]
- 74.Zhao J, Du Y, Horton JR, Upadhyay AK, Lou B, Bai Y, Zhang X, Du L, Li M, Wang B, Zhang L, Barbieri JT, Khuri FR, Cheng X, Fu H. Discovery and structural characterization of a small molecule 14-3-3 protein–protein interaction inhibitor. Proc Natl Acad Sci USA. 2011;108:16212–16216. doi: 10.1073/pnas.1100012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacobson KA, Gao ZG, Göblyös A, Ijzerman AP. Allosteric modulators of purine and pyrimidine receptors. Adv Pharmacol. 2011;61:187–221. doi: 10.1016/B978-0-12-385526-8.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Welihinda AA, Amento EP.Functional characterization of an allosteric enhancer of the adenosine A2a receptor that inhibits pro-inflammatory cytokine production. [abstract] Arthritis Rheum 201163Suppl10181221305513 [Google Scholar]
- 77.Gessi S, Merighi S, Varani K, Borea PA. Adenosine receptors in health and disease. Adv Pharmacol. 2011;61:41–75. doi: 10.1016/B978-0-12-385526-8.00002-3. [DOI] [PubMed] [Google Scholar]
- 78.Erlinge D. P2Y receptors in health and disease. Adv Pharmacol. 2011;61:417–439. doi: 10.1016/B978-0-12-385526-8.00013-8. [DOI] [PubMed] [Google Scholar]
- 79.Cristalli G, Müller CE, Volpini R. Recent developments in adenosine A2A receptor ligands. Handb Exp Pharmacol. 2009;193:59–98. doi: 10.1007/978-3-540-89615-9_3. [DOI] [PubMed] [Google Scholar]
- 80.Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA (2012) Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. doi:10.1016/j.drudis.2011.10.007 [DOI] [PMC free article] [PubMed]
- 81.Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 82.Feoktistov I, Biaggioni I. Role of adenosine A2B receptors in inflammation. Adv Pharmacol. 2011;61:115–144. doi: 10.1016/B978-0-12-385526-8.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Field JJ, Nathan DG, Linden J. Targeting iNKT cells for the treatment of sickle cell disease. Clin Immunol. 2011;140:177–183. doi: 10.1016/j.clim.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Albrecht-Küpper BE, Leineweber K, Nell PG (2012) Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signal 8(Suppl 1):S91–S99 [DOI] [PMC free article] [PubMed]
- 86.Morello S, Sorrentino R, Pinto A. Adenosine A2a receptor agonists as regulators of inflammation: pharmacology and therapeutic opportunities. J Receptor Ligand Channel Res. 2009;2:11–17. [Google Scholar]
- 87.Aherne CM, Kewley EM, Eltzschig HK. The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta. 2011;1808:1329–1339. doi: 10.1016/j.bbamem.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feoktistov I, Biaggioni I, Cronstein BN. Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb Exp Pharmacol. 2009;193:383–397. doi: 10.1007/978-3-540-89615-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cronstein BN (2011) Adenosine receptors and fibrosis: a translational review. F1000 Biology Reports 3:21 [DOI] [PMC free article] [PubMed]
- 90.Koupenova M, Johnson-Cox H, Vezeridis A, Gavras H, Yang D, Zannis V, Ravid K (2012) The A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation 125:354–363 [DOI] [PMC free article] [PubMed]
- 91.Shook BC, Jackson PF. Adenosine A2A receptor antagonists and Parkinson’s disease. ACS Chem Neurosci. 2011;2:555–567. doi: 10.1021/cn2000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohta A, Sitkovsky M. Methylxanthines, inflammation, and cancer: fundamental mechanisms. Handb Exp Pharmacol. 2011;200:469–481. doi: 10.1007/978-3-642-13443-2_19. [DOI] [PubMed] [Google Scholar]
- 93.Kügelgen I, Harden TK. Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv Pharmacol. 2011;61:373–415. doi: 10.1016/B978-0-12-385526-8.00012-6. [DOI] [PubMed] [Google Scholar]
- 94.Lämmer AB, Beck A, Grummich B, Förschler A, Krügel T, Kahn T, Schneider D, Illes P, Franke H, Krügel U. The P2 receptor antagonist PPADS supports recovery from experimental stroke in vivo. PLoS One. 2011;6:e19983. doi: 10.1371/journal.pone.0019983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balasubramanian R, Ruiz de Azua I, Wess J, Jacobson KA. Activation of distinct P2Y receptor subtypes stimulates insulin secretion and cytoprotection in MIN6 mouse pancreatic β cells. Biochem Pharmacol. 2010;79:1317–1326. doi: 10.1016/j.bcp.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan C, Salehi A, Svensson S, Olde B, Erlinge D. ADP receptor P2Y13 induce apoptosis in pancreatic beta-cells. Cell Mol Life Sci. 2010;67:445–453. doi: 10.1007/s00018-009-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franke H, Krügel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 98.Wei Q, Costanzi S, Liu QZ, Gao ZG, Jacobson KA. Activation of the P2Y1 receptor induces apoptosis and inhibits proliferation of prostate cancer cells. Biochem Pharmacol. 2011;82:418–425. doi: 10.1016/j.bcp.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hechler B, Freund M, Ravanat C, Magnenat S, Cazenave JP, Gachet C. Reduced atherosclerotic lesions in P2Y1/apolipoprotein E double-knockout mice: the contribution of non-hematopoietic-derived P2Y1 receptors. Circulation. 2008;118:754–763. doi: 10.1161/CIRCULATIONAHA.108.788927. [DOI] [PubMed] [Google Scholar]
- 100.Gendaszewska-Darmach E, Kucharska M. Nucleotide receptors as targets in the pharmacological enhancement of dermal wound healing. Purinergic Signal. 2011;7:193–206. doi: 10.1007/s11302-011-9233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cohen R, Shainberg A, Hochhauser E, Cheporko Y, Tobar JA, Birk E, Pinhas L, Leipziger J, Don J, Porat E. UTP reduces infarct size and improves mice heart function after myocardial infarct via P2Y2 receptor. Biochem Pharmacol. 2011;82:1126–1133. doi: 10.1016/j.bcp.2011.07.094. [DOI] [PubMed] [Google Scholar]
- 102.Ghanem E, Robaye B, Leal T, Leipziger J, Driessche W, Beauwens R, Boeynaems JM. The role of epithelial P2Y2 and P2Y4 receptors in the regulation of intestinal chloride secretion. Br J Pharmacol. 2005;146:364–369. doi: 10.1038/sj.bjp.0706353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sassmann A, Gier B, Gröne HJ, Drews G, Offermanns S, Wettschureck N. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J Clin Invest. 2010;120:2184–2193. doi: 10.1172/JCI41541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Riegel AK, Faigle M, Zug S, Rosenberger P, Robaye B, Boeynaems JM, Idzko M, Eltzschig HK. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood. 2011;117:2548–2555. doi: 10.1182/blood-2010-10-313957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 106.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a novel mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boeynaems JM, Sirtori CR. The unexpected roles of extracellular ADP and P2Y13 receptor in reverse cholesterol transport. Purinergic Signal. 2010;6:361–363. doi: 10.1007/s11302-010-9211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zippel N, Limbach CA, Ratajski N, Urban C, Pansky A, Luparello C, Kassack MU, Tobiasch E (2011) Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells Dev. doi:10.1089/scd.2010.0576 [DOI] [PubMed]
- 110.Zhang X, Alagille D, Koren AO, Cosgrove K, Seibyl JP, Batis JC, Tamagnan GD (2009) Synthesis of a small library of A2A receptor antagonists for PET/SPECT imaging and in vivo evaluation of [123I]-MNI-420 in nonhuman primate. ACS 238th Natl Meeting, Abstr. MEDI469, Aug. 2009, Washington DC
- 111.Cohen MV, Yang X, Downey JM. A2b adenosine receptors can change their spots. Br J Pharmacol. 2010;159:1595–1597. doi: 10.1111/j.1476-5381.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55:726–734. [PubMed] [Google Scholar]
- 113.Gao Z, Chen T, Weber MJ, Linden J. A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells. Cross-talk between cyclic AMP and protein kinase c pathways. J Biol Chem. 1999;274:5972–5980. doi: 10.1074/jbc.274.9.5972. [DOI] [PubMed] [Google Scholar]
- 114.Linden J, Auchampach JA, Jin X, Figler RA. The structure and function of A1 and A2B adenosine receptors. Life Sci. 1998;62:1519–1524. doi: 10.1016/S0024-3205(98)00100-3. [DOI] [PubMed] [Google Scholar]
- 115.Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I. Cross-talk between Gs- and Gq-coupled pathways in regulation of interleukin-4 by A2B adenosine receptors in human mast cells. Mol Pharmacol. 2006;70:727–735. doi: 10.1124/mol.106.022780. [DOI] [PubMed] [Google Scholar]
- 116.Rieger JM, Brown ML, Sullivan GW, Linden J, Macdonald TL. Design, synthesis, and evaluation of novel A2A adenosine receptor agonists. J Med Chem. 2001;44:531–539. doi: 10.1021/jm0003642. [DOI] [PubMed] [Google Scholar]
- 117.Baraldi PG, Baraldi S, Saponaro G, Preti D, Romagnoli R, Piccagli L, Cavalli A, Recanatini M, Moorman AR, Zaid AN, Katia Varani KA, Borea PA, Tabrizi MA. Novel 1,3-dipropyl-8-(3-benzimidazol-2-yl-methoxy-1-methylpyrazol-5-yl)xanthines as potent and selective A2B adenosine receptor antagonists. J Med Chem. 2012;55:797–811. doi: 10.1021/jm201292w. [DOI] [PubMed] [Google Scholar]
- 118.Ivanov AA, Wang B, Klutz AM, Chen VL, Gao ZG, Jacobson KA. Probing distal regions of the A2B adenosine receptor by quantitative structure–activity relationship modeling of known and novel agonists. J Med Chem. 2008;51:2088–2099. doi: 10.1021/jm701442d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fossa P, Pestarino M, Menozzi G, Mosti L, Schenone S, Ranise A, Bondavalli F, Trincavelli ML, Lucacchini A, Martini C. New pyrazolo[3,4-b]pyridones as selective A1 adenosine receptor antagonists: synthesis, biological evaluation and molecular modelling studies. Org Biomol Chem. 2005;3:2262–2270. doi: 10.1039/b502831k. [DOI] [PubMed] [Google Scholar]